Chronic Supplementation of 2S-Hesperidin Improves Acid-Base Status and Decreases Lactate at FatMax, at Ventilatory Threshold 1 and 2 and after an Incremental Test in Amateur Cyclists
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methodology
2.1. Participants
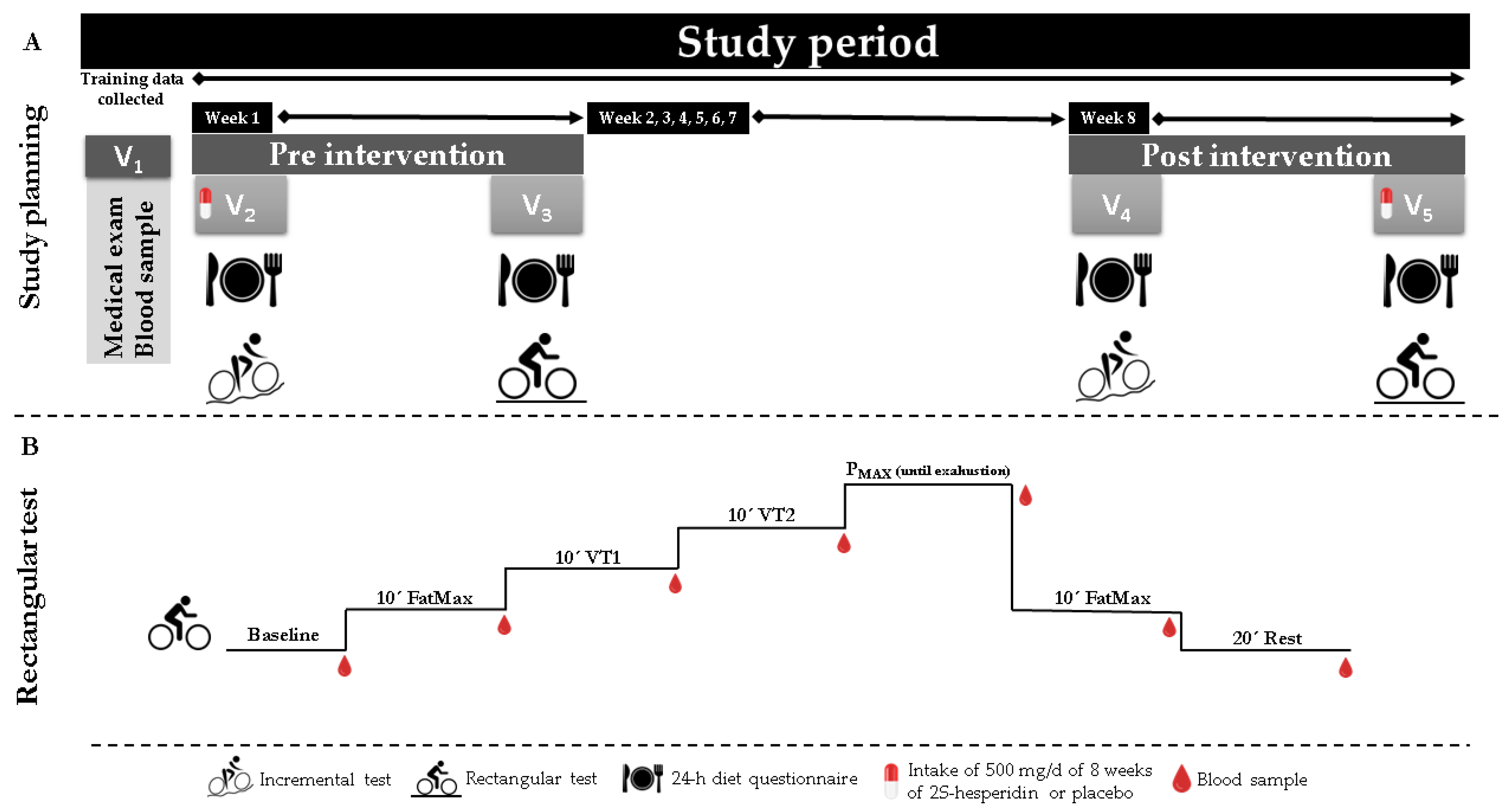
2.2. Study Design
2.3. Procedures
2.4. Testing
2.4.1. Medical Exam
2.4.2. Blood Samples
2.4.3. Maximal Test
2.4.4. Rectangular Test
2.4.5. ABL-90 (Blood Gas Analyzer)
2.5. Statistical Analyses
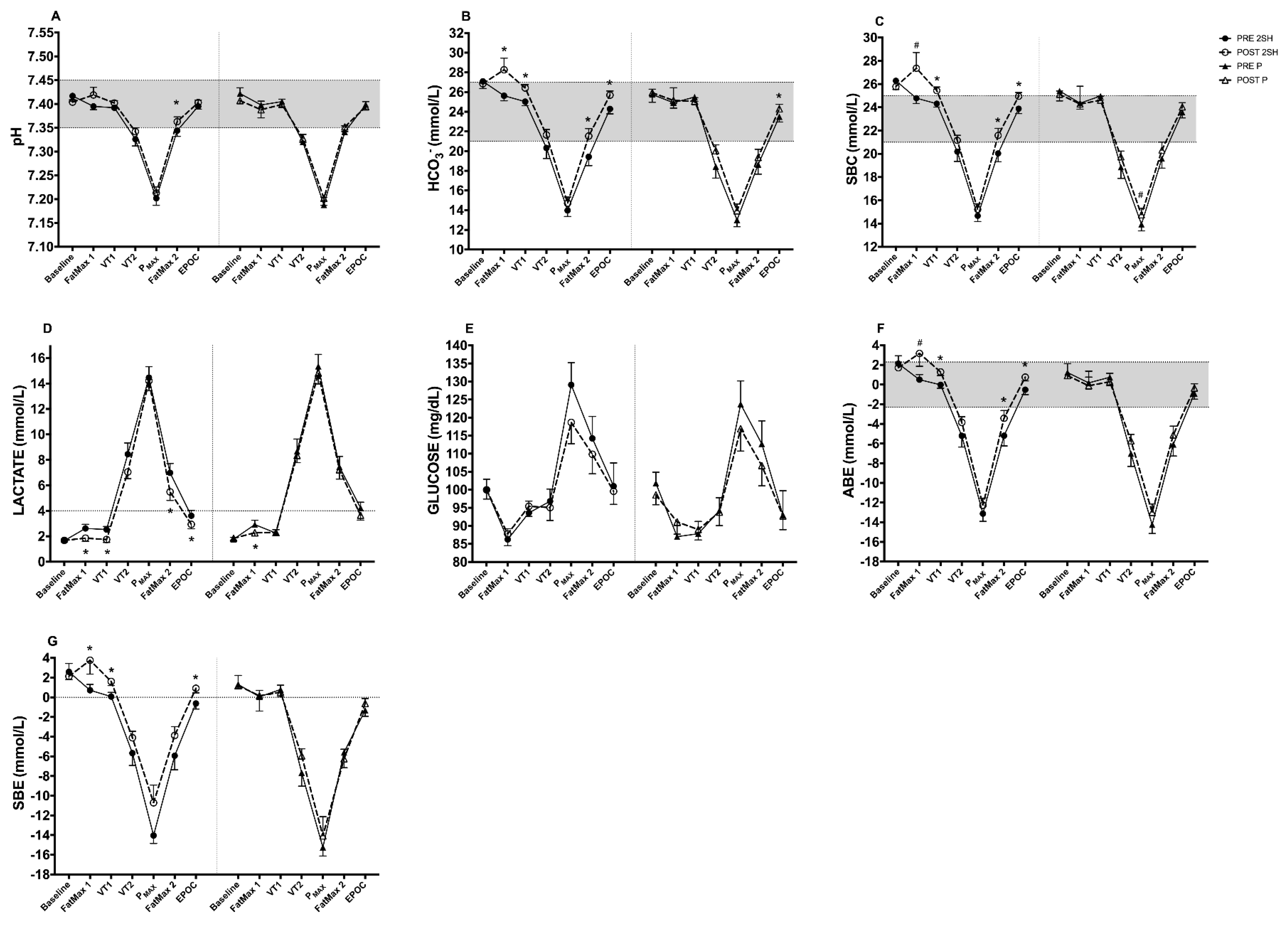
3. Results
3.1. Rectangular Test
3.1.1. Acid-Base Status Biomarkers (Capillary Blood Gases)
3.1.2. Metabolic Biomarker (Capillary Blood)
4. Discussion
4.1. Changes in Acid-Base Status at Baseline
4.2. Changes in Acid-Base Markers at FatMax1 and VT1
4.3. Changes in Acid-Base Markers at VT2
4.4. Changes in Acid-Base Markers at FatMax2
4.5. Changes in Acid-Base Markers at EPOC
4.6. Changes in Acid-Base Markers in AUCs
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stickland, M.K.; Lindinger, M.I.; Olfert, I.M.; Heigenhauser, G.J.; Hopkins, S.R. Pulmonary gas exchange and acid-base balance during exercise. Compr. Physiol. 2013, 3, 693–739. [Google Scholar] [CrossRef]
- Yancy, W.S., Jr.; Olsen, M.K.; Dudley, T.; Westman, E.C. Acid-base analysis of individuals following two weight loss diets. Eur. J. Clin. Nutr. 2007, 61, 1416–1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerretelli, P.; Samaja, M. Acid-base balance at exercise in normoxia and in chronic hypoxia. Revisiting the “lactate paradox”. Eur. J. Appl. Physiol. 2003, 90, 431–448. [Google Scholar] [CrossRef] [PubMed]
- Jubrias, S.A.; Crowther, G.J.; Shankland, E.G.; Gronka, R.K.; Conley, K.E. Acidosis inhibits oxidative phosphorylation in contracting human skeletal muscle in vivo. J. Physiol. 2003, 553, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Walsh, B.; Tiivel, T.; Tonkonogi, M.; Sahlin, K. Increased concentrations of P(i) and lactic acid reduce creatine-stimulated respiration in muscle fibers. J. Appl. Physiol. 2002, 92, 2273–2276. [Google Scholar] [CrossRef] [Green Version]
- Harkema, S.J.; Meyer, R.A. Effect of acidosis on control of respiration in skeletal muscle. Am. J. Physiol. 1997, 272, C491–C500. [Google Scholar] [CrossRef] [PubMed]
- Walter, G.; Vandenborne, K.; McCully, K.K.; Leigh, J.S. Noninvasive measurement of phosphocreatine recovery kinetics in single human muscles. Am. J. Physiol. 1997, 272, C525–C534. [Google Scholar] [CrossRef]
- Peeling, P.; Binnie, M.J.; Goods, P.S.R.; Sim, M.; Burke, L.M. Evidence-Based Supplements for the Enhancement of Athletic Performance. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 178–187. [Google Scholar] [CrossRef] [Green Version]
- Hawley, J.A. Adaptations of skeletal muscle to prolonged, intense endurance training. Clin. Exp. Pharmacol. Physiol. 2002, 29, 218–222. [Google Scholar] [CrossRef]
- Seiler, S. What is best practice for training intensity and duration distribution in endurance athletes? Int. J. Sports Physiol. Perform. 2010, 5, 276–291. [Google Scholar] [CrossRef]
- Rothschild, J.; Earnest, C. Dietary manipulations concurrent to endurance training. J. Funct. Morphol. Kinesiol. 2018, 3, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egan, B.; Zierath, J.R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, A.M. Influence of dietary nitrate on the physiological determinants of exercise performance: A critical review. Appl. Physiol. Nutr. Metab. 2014, 39, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Saunders, B.; Elliott-Sale, K.; Artioli, G.G.; Swinton, P.A.; Dolan, E.; Roschel, H.; Sale, C.; Gualano, B. β-alanine supplementation to improve exercise capacity and performance: A systematic review and meta-analysis. Br. J. Sports Med. 2017, 51, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, M.G.; Kerksick, C.M.; Lamprecht, M.; McAnulty, S.R. Does vitamin C and E supplementation impair the favorable adaptations of regular exercise? Oxidative Med. Cell. Longev. 2012, 2012, 707941. [Google Scholar] [CrossRef] [PubMed]
- Christensen, P.M.; Shirai, Y.; Ritz, C.; Nordsborg, N.B. Caffeine and bicarbonate for speed. A meta-analysis of legal supplements potential for improving intense endurance exercise performance. Front. Physiol. 2017, 8, 240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomcik, K.A.; Camera, D.M.; Bone, J.L.; Ross, M.L.; Jeacocke, N.A.; Tachtsis, B.; Burke, L.M. Effects of creatine and carbohydrate loading on cycling time trial performance. Med. Sci. Sports Exerc. 2018, 50, 141–150. [Google Scholar] [CrossRef]
- Somerville, V.; Bringans, C.; Braakhuis, A. Polyphenols and performance: A systematic review and meta-analysis. Sports Med. 2017, 47, 1589–1599. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [Green Version]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Somerville, V.S.; Braakhuis, A.J.; Hopkins, W.G. Effect of Flavonoids on Upper Respiratory Tract Infections and Immune Function: A Systematic Review and Meta-Analysis. Adv. Nutr. 2016, 7, 488–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ristow, M. Unraveling the truth about antioxidants: Mitohormesis explains ROS-induced health benefits. Nat. Med. 2014, 20, 709–711. [Google Scholar] [CrossRef] [PubMed]
- Mandel, S.; Youdim, M.B. Catechin polyphenols: Neurodegeneration and neuroprotection in neurodegenerative diseases. Free Radic. Biol. Med. 2004, 37, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Neveu, V.; Perez-Jimenez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Clifford, M.N. Flavanones, chalcones and dihydrochalcones—Nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1073–1080. [Google Scholar] [CrossRef]
- Yanez, J.A.; Andrews, P.K.; Davies, N.M. Methods of analysis and separation of chiral flavonoids. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 848, 159–181. [Google Scholar] [CrossRef]
- Aturki, Z.; Brandi, V.; Sinibaldi, M. Separation of Flavanone-7-O-glycoside Diastereomers and Analysis in Citrus Juices by Multidimensional Liquid Chromatography Coupled with Mass Spectrometry. J. Agric. Food Chem. 2004, 52, 5303–5308. [Google Scholar] [CrossRef]
- Biesemann, N.; Ried, J.S.; Ding-Pfennigdorff, D.; Dietrich, A.; Rudolph, C.; Hahn, S.; Hennerici, W.; Asbrand, C.; Leeuw, T.; Strubing, C. High throughput screening of mitochondrial bioenergetics in human differentiated myotubes identifies novel enhancers of muscle performance in aged mice. Sci. Rep. 2018, 8, 9408. [Google Scholar] [CrossRef] [Green Version]
- Estruel-Amades, S.; Massot-Cladera, M.; Garcia-Cerda, P.; Perez-Cano, F.J.; Franch, A.; Castell, M.; Camps-Bossacoma, M. Protective Effect of Hesperidin on the Oxidative Stress Induced by an Exhausting Exercise in Intensively Trained Rats. Nutrients 2019, 11, 783. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Noguera, F.J.; Marín-Pagán, C.; Carlos-Vivas, J.; Alcaraz, P.E. Effects of 8 Weeks of 2S-Hesperidin Supplementation on Performance in Amateur Cyclists. Nutrients 2020, 12, 3911. [Google Scholar] [CrossRef]
- Martinez-Noguera, F.J.; Marin-Pagan, C.; Carlos-Vivas, J.; Rubio-Arias, J.A.; Alcaraz, P.E. Acute Effects of Hesperidin in Oxidant/Antioxidant State Markers and Performance in Amateur Cyclists. Nutrients 2019, 11, 1898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Li, A.; Yang, X. Effect of lemon seed flavonoids on the anti-fatigue and antioxidant effects of exhausted running exercise mice. J. Food Biochem. 2021, 45, e13620. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Zhong, Y.; Li, M.; Chang, Q.; Liao, Y.; Liu, X.; Pan, R. Antioxidant and Anti-Fatigue Constituents of Okra. Nutrients 2015, 7, 8846–8858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarpulla, R.C. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim. Biophys. Acta 2011, 1813, 1269–1278. [Google Scholar] [CrossRef] [Green Version]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez Noguera, F.J.; Alcaraz, P.E.; Carlos Vivas, J.; Chung, L.H.; Marín Cascales, E.; Marín Pagán, C. 8 weeks of 2S-Hesperidin supplementation improves muscle mass and reduces fat in amateur competitive cyclists: Randomized controlled trial. Food Funct. 2021, 12, 3872–3882. [Google Scholar] [CrossRef]
- Urbaniak, G.C.; Plous, S. Research Randomizer (Version 4.0) [Computer Software]. Retrieved on 22 June 2013. Available online: http://www.randomizer.org/ (accessed on 1 February 2022).
- Millet, G.P.; Vleck, V.E.; Bentley, D.J. Physiological Differences between Cycling and Running. Sports Med. 2009, 39, 179–206. [Google Scholar] [CrossRef]
- Edvardsen, E.; Hem, E.; Anderssen, S.A. End criteria for reaching maximal oxygen uptake must be strict and adjusted to sex and age: A cross-sectional study. PLoS ONE 2014, 9, e85276. [Google Scholar] [CrossRef] [Green Version]
- Howley, E.T.; Bassett, D.R., Jr.; Welch, H.G. Criteria for maximal oxygen uptake: Review and commentary. Med. Sci. Sports Exerc. 1995, 27, 1292–1301. [Google Scholar] [CrossRef]
- Wasserman, K.; Beaver, W.L.; Whipp, B.J. Gas exchange theory and the lactic acidosis (anaerobic) threshold. Circulation 1990, 81, II14–II30. [Google Scholar]
- Zhang, J.B.; Lin, J.; Zhao, X.D. Analysis of bias in measurements of potassium, sodium and hemoglobin by an emergency department-based blood gas analyzer relative to hospital laboratory autoanalyzer results. PLoS ONE 2015, 10, e0122383. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Mujika, I.; Padilla, S. Detraining: Loss of training-induced physiological and performance adaptations. Part I: Short term insufficient training stimulus. Sports Med. 2000, 30, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Romijn, J.A.; Coyle, E.F.; Sidossis, L.S.; Gastaldelli, A.; Horowitz, J.F.; Endert, E.; Wolfe, R.R. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am. J. Physiol. 1993, 265, E380–E391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rømer, T.; Thunestvedt Hansen, M.; Frandsen, J.; Larsen, S.; Dela, F.; Wulff Helge, J. The relationship between peak fat oxidation and prolonged double-poling endurance exercise performance. Scand. J. Med. Sci. Sports 2020, 30, 2044–2056. [Google Scholar] [CrossRef] [PubMed]
- Sumi, D.; Kasai, N.; Ito, H.; Goto, K. The Effects of Endurance Exercise in Hypoxia on Acid-Base Balance, Potassium Kinetics, and Exogenous Glucose Oxidation. Front. Physiol. 2019, 10, 504. [Google Scholar] [CrossRef]
- Brooks, G.A.; Mercier, J. Balance of carbohydrate and lipid utilization during exercise: The “crossover” concept. J. Appl. Physiol. 1994, 76, 2253–2261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, K.; Tunaru, S.; Tang, C.; Müller, M.; Gille, A.; Sassmann, A.; Hanson, J.; Offermanns, S. An autocrine lactate loop mediates insulin-dependent inhibition of lipolysis through GPR81. Cell Metab. 2010, 11, 311–319. [Google Scholar] [CrossRef] [Green Version]
- Brooks, G.A. The Science and Translation of Lactate Shuttle Theory. Cell Metab. 2018, 27, 757–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bendahan, D.; Chatel, B.; Jue, T. Comparative NMR and NIRS analysis of oxygen-dependent metabolism in exercising finger flexor muscles. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R740–R753. [Google Scholar] [CrossRef]
- Brooks, G.A. Lactate as a fulcrum of metabolism. Redox Biol. 2020, 35, 101454. [Google Scholar] [CrossRef]
- Cannon, D.T.; Howe, F.A.; Whipp, B.J.; Ward, S.A.; McIntyre, D.J.; Ladroue, C.; Griffiths, J.R.; Kemp, G.J.; Rossiter, H.B. Muscle metabolism and activation heterogeneity by combined 31P chemical shift and T2 imaging, and pulmonary O2 uptake during incremental knee-extensor exercise. J. Appl. Physiol. 2013, 115, 839–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Copp, S.W.; Hirai, D.M.; Musch, T.I.; Poole, D.C. Critical speed in the rat: Implications for hindlimb muscle blood flow distribution and fibre recruitment. J. Physiol. 2010, 588, 5077–5087. [Google Scholar] [CrossRef] [PubMed]
- Moxnes, J.F.; Sandbakk, Ø. The kinetics of lactate production and removal during whole-body exercise. Theor. Biol. Med. Model. 2012, 9, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denham, J.; Scott-Hamilton, J.; Hagstrom, A.D.; Gray, A.J. Cycling Power Outputs Predict Functional Threshold Power and Maximum Oxygen Uptake. J. Strength Cond. Res. 2020, 34, 3489–3497. [Google Scholar] [CrossRef] [PubMed]
- Montero, M.; Lobatón, C.D.; Hernández-Sanmiguel, E.; Santodomingo, J.; Vay, L.; Moreno, A.; Alvarez, J. Direct activation of the mitochondrial calcium uniporter by natural plant flavonoids. Biochem. J. 2004, 384, 19–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duarte, J.; Francisco, V.; Perez-Vizcaino, F. Modulation of nitric oxide by flavonoids. Food Funct. 2014, 5, 1653–1668. [Google Scholar] [CrossRef] [Green Version]
- Si, H.; Wyeth, R.P.; Liu, D. The flavonoid luteolin induces nitric oxide production and arterial relaxation. Eur. J. Nutr. 2014, 53, 269–275. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Tan, J.; Li, H.; Kong, X.; Liu, Y.; Guo, R.; Li, G.; Yang, B.; Pei, M. Cardioprotective effects of total flavonoids from Jinhe Yangxin prescription by activating the PI3K/Akt signaling pathway in myocardial ischemia injury. Biomed. Pharmacother. 2018, 98, 308–317. [Google Scholar] [CrossRef]
- Calbet, J.A.; Lundby, C. Skeletal muscle vasodilatation during maximal exercise in health and disease. J. Physiol. 2012, 590, 6285–6296. [Google Scholar] [CrossRef] [Green Version]
- Cairns, S.P. Lactic acid and exercise performance: Culprit or friend? Sports Med. 2006, 36, 279–291. [Google Scholar] [CrossRef]
- Brooks, G.A. Lactate: Link between glycolytic and oxidative metabolism. Sports Med. 2007, 37, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. Intra- and extra-cellular lactate shuttles. Med. Sci. Sports Exerc. 2000, 32, 790–799. [Google Scholar] [CrossRef]
- Peng, F.; Yin, H.; Du, B.; Niu, K.; Ren, X.; Yang, Y. Anti-fatigue activity of purified flavonoids prepared from chestnut (Castanea mollissima) flower. J. Funct. Foods 2021, 79, 104365. [Google Scholar] [CrossRef]
- Ye, J.; Shen, C.; Huang, Y.; Zhang, X.; Xiao, M. Anti-fatigue activity of sea cucumber peptides prepared from Stichopus japonicus in an endurance swimming rat model. J. Sci. Food Agric. 2017, 97, 4548–4556. [Google Scholar] [CrossRef] [PubMed]
- Perkins, I.C.; Vine, S.A.; Blacker, S.D.; Willems, M.E. New Zealand Blackcurrant Extract Improves High-Intensity Intermittent Running. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 487–493. [Google Scholar] [CrossRef]
- Finsterer, J. Biomarkers of peripheral muscle fatigue during exercise. BMC Musculoskelet. Disord. 2012, 13, 218. [Google Scholar] [CrossRef] [Green Version]
- Fitts, R.H. The Role of Acidosis in Fatigue: Pro Perspective. Med. Sci. Sports Exerc. 2016, 48, 2335–2338. [Google Scholar] [CrossRef]
- Medbø, J.I.; Sejersted, O.M. Acid-base and electrolyte balance after exhausting exercise in endurance-trained and sprint-trained subjects. Acta Physiol. Scand. 1985, 125, 97–109. [Google Scholar] [CrossRef]
- Gough, L.A.; Rimmer, S.; Osler, C.J.; Higgins, M.F. Ingestion of Sodium Bicarbonate (NaHCO3) Following a Fatiguing Bout of Exercise Accelerates Postexercise Acid-Base Balance Recovery and Improves Subsequent High-Intensity Cycling Time to Exhaustion. Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 429–438. [Google Scholar] [CrossRef] [Green Version]
| 2S-Hesperidin | Placebo | p-Value | |
|---|---|---|---|
| Age (years) | 35.0 (9.20) | 32.6 (8.90) | 0.407 |
| Body mass (kg) | 71.0 (6.98) | 70.4 (6.06) | 0.773 |
| Height (cm) | 175.3 (6.20) | 176.5 (6.10) | 0.541 |
| BMI (kg·m−2) | 23.1 (1.53) | 22.6 (1.43) | 0.292 |
| BF (%) | 8.9 (1.63) | 9.0 (1.64) | 0.803 |
| VO2MAX (L·min−1) | 3.99 (0.36) | 3.98 (0.63) | 0.971 |
| VO2MAX (mL·kg−1·min−1) | 57.5 (6.97) | 57.9 (9.53) | 0.880 |
| HRMAX (bpm) | 184.9 (11.11) | 183.2 (8.68) | 0.593 |
| VT1 (%) | 50.9 (5.63) | 50.0 (4.78) | 0.610 |
| VT2 (%) | 84.9 (5.85) | 84.1 (5.70) | 0.644 |
| 2S-Hesperidin | Placebo | ||
|---|---|---|---|
| Total distance (km) | 1121.12 (534.99) | 1082.43 (810.46) | 0.868 |
| HRAVG (bpm) | 144.76 (8.88) | 137.48 (13.11) | 0.067 |
| WAVG (W) | 174.86 (15.79) | 163.47 (32.49) | 0.435 |
| RPE | 6.34 (0.82) | 6.33 (1.16) | 0.975 |
| Pre-Intervention | Post-Intervention | |||||
|---|---|---|---|---|---|---|
| 2S-Hesperidin | Placebo | p-Value | 2S-Hesperidin | Placebo | p-Value | |
| Kcal | 2163.6 (519.02) | 2100.2 (515.77) | 0.708 | 1974.1 (377.97) | 2133.5 (437.98) | 0.237 |
| Kcal/BM | 31.1 (9.34) | 30.2 (8.71) | 0.768 | 27.9 (6.53) | 30.3 (6.46) | 0.249 |
| CHO (g) | 245.7 (73.46) | 222.0 (69.68) | 0.312 | 216.6 (63.47) | 248.3 (58.15) | 0.117 |
| CHO/Kg b.w. | 3.5 (1.31) | 3.2 (1.14) | 0.416 | 3.1 (1.08) | 3.5 (0.94) | 0.173 |
| PRO (g) | 113.5 (25.21) | 115.2 (25.37) | 0.837 | 109.0 (23.05) | 101.5 (23.67) | 0.332 |
| PRO/Kg b.w. | 1.6 (0.41) | 1.7 (0.48) | 0.778 | 1.5 (0.35) | 1.5 (0.42) | 0.596 |
| LP (g) | 80.8 (27.24) | 83.5 (23.65) | 0.739 | 71.5 (17.61) | 71.6 (18.89) | 0.985 |
| LP/Kg b.w. | 1.2 (0.45) | 1.2 (0.37) | 0.758 | 1.0 (0.27) | 1.0 (0.29) | 0.823 |
| 2S-Hesperidin | Placebo | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | FatMax1 | VT1 | VT2 | Pmax | FatMax2 | EPOC | AUC | Baseline | FatMax1 | VT1 | VT2 | Pmax | FatMax2 | EPOC | AUC | ||
| pH | Pre | 7.417 (0.01) | 7.395 (0.01) | 7.392 (0.01) | 7.326 (0.01) | 7.202 (0.02) | 7.344 (0.01) | 7.395 (0.01) | 44.07 (0.13) | 7.422 (0.01) | 7.398 (0.01) | 7.405 (0.01) | 7.320 (0.02) | 7.188 (0.02) | 7.341 (0.01) | 7.399 (0.01) | 44.07 (0.17) |
| Post | 7.404 (0.01) | 7.419 (0.02) | 7.402 (0.01) | 7.342 (0.01) | 7.213 (0.01) | 7.363 (0.01) | 7.403 (0.01) | 44.14 (0.08) | 7.407 (0.01) | 7.388 (0.02) | 7.399 (0.01) | 7.328 (0.01) | 7.202 (0.02) | 7.352 (0.01) | 7.394 (0.01) | 44.07 (0.15) | |
| p-value | 0.248 | 0.131 | 0.121 | 0.254 | 0.364 | 0.028 | 0.213 | 0.016 | 0.238 | 0.564 | 0.317 | 0.572 | 0.292 | 0.231 | 0.412 | 0.895 | |
| ES | 1.11 | 3.21 | 1.87 | 1.07 | 0.69 | 1.48 | 1.25 | 0.54 | 1.15 | 1.15 | 1.11 | 0.46 | 0.79 | 0.78 | 0.77 | 0.00 | |
| Bicarbonate anion (mmol/L) (HCO3−) | Pre | 27.09 (0.73) | 25.63 (0.51) | 25.03 (0.41) | 20.32 (1.07) | 13.98 (0.62) | 19.43 (0.90) | 24.29 (0.50) | 130.72 (10.40) | 25.76 (0.79) | 24.92 (0.55) | 25.49 (0.44) | 18.43 (1.17) | 13.01 (0.68) | 18.64 (0.98) | 23.50 (0.54) | 125.00 (12.27) |
| Post | 26.85 (0.32) | 28.27 (1.18) | 26.42 (0.34) | 21.65 (0.55) | 14.71 (0.63) | 21.53 (0.76) | 25.69 (0.42) | 139.01 (8.94) | 25.94 (0.35) | 25.15 (1.29) | 25.06 (0.37) | 20.04 (0.60) | 13.94 (0.69) | 19.36 (0.83) | 24.30 (0.46) | 128.54 | |
| p-value | 0.744 | 0.040 | <0.001 | 0.200 | 0.204 | 0.003 | 0.001 | 0.012 | 0.828 | 0.864 | 0.231 | 0.156 | 0.136 | 0.304 | 0.045 | 0.122 | |
| ES | 0.31 | 4.85 | 3.20 | 1.16 | 1.08 | 2.19 | 2.63 | 0.74 | 0.20 | 0.38 | 0.89 | 1.27 | 1.26 | 0.69 | 1.36 | 0.26 | |
| Standard bicarbonate (mmol/L) (SBC) | Pre | 26.29 (0.76) | 24.79 (0.46) | 24.31 (0.29) | 20.19 (0.84) | 14.67 (0.49) | 20.04 (0.72) | 23.88 (0.42) | 129.73 (7.73) | 25.41 (0.86) | 24.36 (0.52) | 24.97 (0.34) | 18.85 (0.96) | 13.94 (0.56) | 19.59 (0.82) | 23.56 (0.47) | 126.23 (9.58) |
| Post | 25.78 (0.24) | 27.36 (1.35) | 25.44 (0.31) | 21.16 (0.44) | 15.22 (0.49) | 21.58 (0.61) | 24.95 (0.33) | 136.31 (7.67) | 25.12 (0.28) | 24.28 (1.54) | 24.61 (0.35) | 19.74 (0.50) | 14.76 (0.56) | 20.31 (0.70) | 24.03 (0.37) | 128.30 (8.21) | |
| p-value | 0.516 | 0.076 | 0.001 | 0.222 | 0.165 | 0.006 | 0.001 | 0.017 | 0.744 | 0.960 | 0.267 | 0.322 | 0.072 | 0.227 | 0.164 | 0.111 | |
| ES | 0.63 | 5.26 | 3.59 | 1.08 | 1.04 | 2.01 | 2.39 | 0.79 | 0.31 | 0.14 | 0.98 | 0.85 | 1.34 | 0.80 | 0.90 | 0.20 | |
| Lactate (mmol/L) (Lac) | Pre | 1.66 (0.14) | 2.62 (0.32) | 2.53 (0.24) | 8.45 (0.88) | 14.46 (0.86) | 6.98 (0.74) | 3.61 (0.42) | 36.72 (8.50) | 1.77 (0.15) | 2.92 (0.34) | 2.25 (0.27) | 8.68 (0.96) | 15.35 (0.93) | 7.46 (0.81) | 4.23 (0.45) | 39.32 (6.76) |
| Post | 1.68 (0.13) | 1.85 (0.24) | 1.75 (0.23) | 7.05 (0.52) | 14.21 (0.79) | 5.49 (0.67) | 2.94 (0.35) | 32.10 (6.58) | 1.84 (0.15) | 2.28 (0.26) | 2.30 (0.25) | 8.35 (0.56) | 14.82 (0.86) | 7.22 (0.73) | 3.65 (0.38) | 37.47 (8.54) | |
| p-value | 0.871 | 0.010 | 0.003 | 0.134 | 0.730 | 0.018 | 0.039 | 0.057 | 0.680 | 0.041 | 0.833 | 0.741 | 0.503 | 0.702 | 0.098 | 0.391 | |
| ES | 0.15 | 2.26 | 2.98 | 1.49 | 0.28 | 1.88 | 1.51 | 0.51 | 0.38 | 1.71 | 0.19 | 0.32 | 0.53 | 0.28 | 1.17 | 0.25 | |
| Glucose (mg/dL) (Glu) | Pre | 100.00 (2.91) | 86.25 (2.97) | 93.58 (3.28) | 96.83 (3.37) | 129.08 (6.17) | 114.25 (6.08) | 101.00 (6.45) | 616.46 (68.76) | 101.82 (3.04) | 87.00 (3.10) | 87.82 (3.43) | 94.27 (3.52) | 123.73 (6.44) | 112.73 (6.35) | 93.00 (6.74) | 611.50 (73.23) |
| Post | 100.00 (2.57) | 87.75 (3.24) | 95.42 (2.79) | 95.08 (3.58) | 118.67 (5.92) | 109.83 (5.38) | 99.58 (3.62) | 598.91 (69.45) | 98.55 (2.69) | 91.09 (3.38) | 89.00 (2.91) | 93.82 (3.74) | 116.91 (6.19) | 106.73 (5.62) | 92.73 (3.78) | 591.40 (64.12) | |
| p-value | 1.000 | 0.677 | 0.589 | 0.616 | 0.090 | 0.487 | 0.782 | 0.248 | 0.231 | 0.282 | 0.738 | 0.900 | 0.277 | 0.368 | 0.959 | 0.255 | |
| ES | 0.00 | 0.47 | 0.52 | 0.48 | 1.57 | 0.68 | 0.20 | 0.24 | 0.99 | 1.22 | 0.32 | 0.12 | 0.98 | 0.87 | 0.04 | 0.25 | |
| Actual base excess (mmol/L) (ABE) | Pre | 2.14 (0.79) | 0.50 (0.52) | −0.02 (0.35) | −5.21 (1.14) | −13.13 (0.78) | −5.20 (1.03) | −0.52 (0.50) | 26.57 (8.72) | 1.23 (0.90) | 0.18 (0.59) | 0.75 (0.40) | −7.02 (1.30) | −14.25 (0.89) | −6.09 (1.17) | −0.91 (0.57) | 30.22 (10.04) |
| Post | 1.72 (0.28) | 3.17 (1.31) | 1.28 (0.36) | −3.84 (0.57) | −12.28 (0.74) | −3.42 (0.79) | 0.76 (0.38) | 24.13 (8.72) | 0.92 (0.32) | −0.12 (1.49) | 0.30 (0.41) | −5.71 (0.65) | −13.00 (0.85) | −5.12 (0.90) | −0.35 (0.44) | 26.76 (8.15) | |
| p-value | 0.604 | 0.059 | 0.001 | 0.200 | 0.169 | 0.034 | 0.001 | 0.472 | 0.739 | 0.846 | 0.238 | 0.279 | 0.082 | 0.290 | 0.154 | 0.103 | |
| ES | 0.50 | 4.82 | 3.48 | 1.13 | 1.02 | 1.62 | 2.42 | 0.26 | 0.31 | 0.46 | 1.04 | 0.92 | 1.28 | 0.76 | 0.90 | 0.32 | |
| Standard base excess (mmol/L) (SBE) | Pre | 2.59 (0.85) | 0.72 (0.59) | 0.08 (0.42) | −5.67 (1.25) | −14.04 (0.81) | −5.93 (1.43) | −0.62 (0.57) | 29.22 (9.82) | 1.31 (0.92) | 0.06 (0.64) | 0.79 (0.45) | −7.67 (1.36) | −15.25 (0.88) | −5.59 (1.56) | −1.31 (0.62) | 33.13 (10.89) |
| Post | 2.14 (0.33) | 3.77 (1.41) | 1.61 (0.41) | −4.10 (0.65) | −10.71 (1.79) | −3.88 (0.90) | 0.92 (0.46) | 26.76 (9.37) | 1.21 (0.36) | 0.15 (1.54) | 0.51 (0.45) | −5.94 (0.71) | −14.08 (1.95) | −6.24 (0.97) | −0.62 (0.50) | 29.67 (8.62) | |
| p-value | 0.606 | 0.046 | 0.001 | 0.187 | 0.106 | 0.128 | <0.001 | 0.509 | 0.916 | 0.954 | 0.500 | 0.180 | 0.591 | 0.651 | 0.104 | 0.174 | |
| ES | 0.50 | 4.87 | 3.42 | 1.17 | 3.87 | 1.34 | 2.53 | 0.23 | 0.10 | 0.13 | 0.57 | 1.18 | 1.23 | 0.38 | 1.03 | 0.29 | |
| Between-Group Comparison | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| ΔBaseline | ΔFatMax1 | ΔVT1 | ΔVT2 | ΔPMAX | ΔFatMax2 | ΔEPOC | ΔAUC | ||
| pH | Differences | −0.029 (0.23) | −0.072 (0.32) | 0.198 (0.28) | −0.149 (0.27) | −0.136 (0.36) | 0.055 (0.24) | 0.066 (0.28) | 0.077 (0.03) |
| p-value | 0.90 | 0.82 | 0.49 | 0.58 | 0.71 | 0.83 | 0.81 | 0.02 | |
| Effect size | 0.17 | 2.13 | 2.50 | 0.52 | 0.24 | 0.94 | 2.01 | 1.03 | |
| HCO3− (mmol/L) | Differences | −2.650 (5.33) | −4.495 (4.14) | −4.053 (3.90) | 10.906 (7.43) | 2.786 (19.73) | 2.013 (3.34) | 5.558 (6.17) | 4.752 (3.57) |
| p-value | 0.62 | 0.29 | 0.31 | 0.18 | 0.89 | 0.55 | 0.37 | 0.20 | |
| Effect size | 0.55 | 1.92 | 5.48 | 0.27 | 0.36 | 2.08 | 1.66 | 0.59 | |
| SBC (mmol/L) | Differences | −0.100 (0.16) | 0.348 (0.19) | −0.063 (0.26) | 0.079 (0.47) | 0.777 (0.53) | 0.035 (0.29) | 0.134 (0.17) | 4.522 (2.63) |
| p-value | 0.55 | 0.07 | 0.81 | 0.87 | 0.15 | 0.91 | 0.44 | 0.11 | |
| Effect size | 0.27 | 1.81 | 5.07 | 0.10 | 0.68 | 1.53 | 1.95 | 0.67 | |
| Lac (mmol/L) | Differences | 0.060 (0.70) | −0.118 (0.60) | 0.974 (0.71) | −0.760 (0.96) | 1.055 (0.84) | 0.372 (0.43) | 0.484 (0.80) | −2.772 (3.03) |
| p-value | 0.93 | 0.85 | 0.18 | 0.43 | 0.22 | 0.40 | 0.55 | 0.37 | |
| Effect size | 0.28 | 0.45 | 3.39 | 1.15 | 0.37 | 2.04 | 0.30 | 0.39 | |
| Glu (mg/dL) | Differences | 0.635 (0.74) | 0.435 (0.44) | −0.511 (0.64) | 0.918 (0.93) | −0.872 (0.79) | −0.096 (0.32) | 0.205 (0.62) | 2.558 (21.82) |
| p-value | 0.40 | 0.33 | 0.43 | 0.33 | 0.28 | 0.77 | 0.74 | 0.91 | |
| Effect size | 1.26 | 0.71 | 0.19 | 0.37 | 0.60 | 0.25 | 0.22 | 0.18 | |
| ABE (mmol/L) | Differences | 3.550 (3.23) | 0.853 (4.13) | 7.737 (4.30) | 6.515 (3.91) | −2.786 (7.51) | 2.524 (6.50) | 0.211 (5.42) | 1.020 (4.00) |
| p-value | 0.28 | 0.84 | 0.08 | 0.11 | 0.71 | 0.70 | 0.97 | 0.80 | |
| Effect size | 0.13 | 2.09 | 5.05 | 0.05 | 0.62 | 0.97 | 2.05 | 0.10 | |
| SBE (mmol/L) | Differences | 0.763 (3.65) | −1.609 (3.42) | −8.400 (2.86) | −5.592 (3.81) | 1.642 (8.82) | −6.097 (5.26) | −4.703 (3.58) | 0.994 (4.50) |
| p-value | 0.84 | 0.64 | 0.01 | 0.15 | 0.85 | 0.25 | 0.20 | 0.83 | |
| Effect size | 0.39 | 1.98 | 4.59 | 0.14 | 1.05 | 2.00 | 2.17 | 0.07 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Noguera, F.J.; Alcaraz, P.E.; Carlos-Vivas, J.; Marín-Pagán, C. Chronic Supplementation of 2S-Hesperidin Improves Acid-Base Status and Decreases Lactate at FatMax, at Ventilatory Threshold 1 and 2 and after an Incremental Test in Amateur Cyclists. Biology 2022, 11, 736. https://doi.org/10.3390/biology11050736
Martínez-Noguera FJ, Alcaraz PE, Carlos-Vivas J, Marín-Pagán C. Chronic Supplementation of 2S-Hesperidin Improves Acid-Base Status and Decreases Lactate at FatMax, at Ventilatory Threshold 1 and 2 and after an Incremental Test in Amateur Cyclists. Biology. 2022; 11(5):736. https://doi.org/10.3390/biology11050736
Chicago/Turabian StyleMartínez-Noguera, Francisco Javier, Pedro E. Alcaraz, Jorge Carlos-Vivas, and Cristian Marín-Pagán. 2022. "Chronic Supplementation of 2S-Hesperidin Improves Acid-Base Status and Decreases Lactate at FatMax, at Ventilatory Threshold 1 and 2 and after an Incremental Test in Amateur Cyclists" Biology 11, no. 5: 736. https://doi.org/10.3390/biology11050736
APA StyleMartínez-Noguera, F. J., Alcaraz, P. E., Carlos-Vivas, J., & Marín-Pagán, C. (2022). Chronic Supplementation of 2S-Hesperidin Improves Acid-Base Status and Decreases Lactate at FatMax, at Ventilatory Threshold 1 and 2 and after an Incremental Test in Amateur Cyclists. Biology, 11(5), 736. https://doi.org/10.3390/biology11050736









