1. Introduction
Tolerant food sharing among non-kin is considered a hallmark of human cooperative nature [
1,
2]. In its broadest sense, food sharing can be defined as the joint use of a food source and can take two forms: (1) offering food to another individual and (2) cofeeding, which is feeding in proximity around a food source and often requires tolerance to the proximity of others. In most animal species, food sharing occurs between kin [
3,
4,
5] and mates [
6,
7,
8] as the costs associated with sharing are directly offset by the fitness benefits gained through kin selection and any produced offspring [
9]. However, humans belong to one of the few species that also regularly share food outside of the kin or mating context [
1,
2]. This raises an evolutionary question, since this type of food sharing does not provide obvious fitness benefits [
10], but rather results in the loss of a resource or increased competition around the food source [
1,
2]. This contradiction can be explained by the concept of fitness interdependence, which is defined as the degree to which the fitness of two or more individuals is influenced by their interactions, both positive and negative [
11,
12]. Interactions among both kin and non-kin can thus result in higher fitness, for example, through cooperative hunting or increased protection against predators [
13]. The level of tolerance around food sources would then reflect a balance between within-group competition on the one hand and fitness interdependence on the other, as individuals rely on each other for reproduction and survival [
11,
12,
13,
14].
In humans, the level of tolerant food sharing among non-kin is, among other things, dependent on the quality of the social relationship between the individuals participating in the transfer. For example, human children prefer sharing with a friend over sharing with a familiar child that is not a friend [
15]. Non-human primates can develop very similar close long-lasting social relationships with unrelated individuals that are referred to as friendships [
16,
17,
18], and similar to humans, high-quality social bonds predict more tolerant sharing [
19,
20,
21,
22,
23]. However, social bonds in these studies are typically portrayed by single measures such as grooming or proximity that often fail to capture the complexity of primate social relationships [
21,
24,
25,
26]. A multidimensional model of dyadic relationship quality is available for primates and is based on dimension reduction methods such as factor analysis that consider a variety of behavioral characteristics of the relationship but reduce them to fewer dimensions in an objective manner [
27,
28,
29,
30]. The benefit of this method is that both positive and negative aspects of social relationships are taken into consideration while keeping the number of components that will be tested low, which increases power of statistical analyses in small sample sizes inherent to many primate behavioral studies.
Here, we investigated the role of relationship quality on Dyadic Cofeeding Tolerance in zoo-housed bonobos (
Pan paniscus) and chimpanzees (
Pan troglodytes). Given that they are the two primate species most closely related to humans [
31], both are keystone species for investigating the mechanisms underlying the evolutionary origins of human cofeeding tolerance [
32,
33,
34]. Similar to humans, food sharing in the
Pan species is not limited to kin or mates, but also takes place between unrelated group members [
6,
10,
35,
36,
37], which is rare among primates and makes bonobos and chimpanzees good models to investigate the impact of both kin and non-kin fitness interdependency interactions on tolerance [
38]. Interestingly, previous studies on cofeeding tolerance have reported contrasting results in
Pan. While in general, bonobos are portrayed as more peaceful and egalitarian than chimpanzees [
35,
39,
40,
41,
42,
43,
44], this does not always result in higher tolerance during cofeeding tests [
36,
45,
46,
47,
48,
49]. Results from cofeeding studies are difficult to generalize though, as differences in results could be due to the variety of methodologies used (e.g., dyadic cofeeding or codrinking tests, measures of food transfers in a group setting, cofeeding tests in a group setting [
35,
46,
48,
50,
51]), or the fact that within-species variation in cofeeding tolerance might be greater than between-species variation [
45,
52,
53]. Multigroup studies using identical experimental set-ups in both species are thus warranted but are currently lacking. Finally, for both species, multidimensional relationship quality models were successfully constructed in the past that show great overlap between the two species [
16,
28,
29,
30]. Two factors of relationship quality were consistently found in both species: Value and Incompatibility. Relationship Value refers to the benefits an individual gains from his social partner, such as grooming or agonistic support [
16,
28,
29,
30]. The second component, Incompatibility, is a measure of the general nature of the interactions between the two partners and typically reflects levels of aggression and counter-intervention during agonistic interactions. In both species, measures of relationship quality were influenced by variables such as sex, kinship, age difference, familiarity and personality [
16,
28,
29,
30] (for an overview see
Table 1).
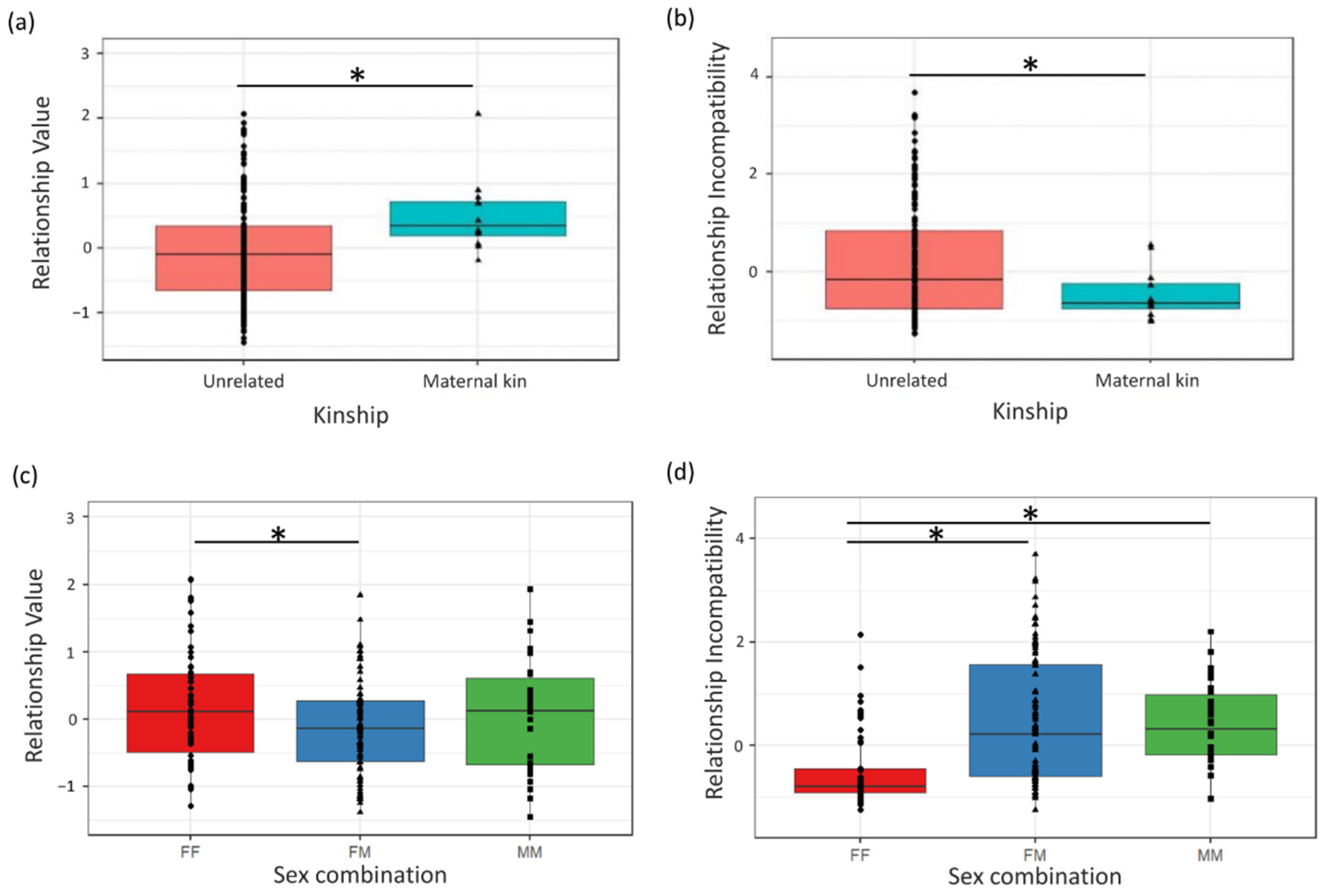
Previous work in
Pan demonstrated that chimpanzees shared more food with group members with whom they had more valuable relationships, and that bonobos tolerated more food transfers from group members with whom they had more affiliative relationships [
36]. However, these relationship quality components were constructed for a small sample of 11 chimpanzees and 6 bonobos, and the number of behavioral variables entered in the factor analysis was limited compared to the later, more elaborate chimpanzee and bonobo relationship quality models [
28,
29,
30]. Therefore, in this study, we included a larger multigroup sample of zoo-housed chimpanzees (N = 22) and bonobos (N = 21). In contrast to previous studies, we constructed a de novo relationship quality model by analyzing identical behavioral variables for both species and including individuals from both species in the same factor analysis, hereafter referred to as “the
Pan model” as both species are combined into one model. This ensured that behavioral loadings on measures of relationship quality were comparable for bonobos and chimpanzees, and that scores of both species could be included in the same statistical model to test for potential species differences on the relationship between cofeeding tolerance and relationship quality. To validate the model, we compared the resulting
Pan relationship quality model to previously described models for chimpanzees and bonobos and tested how scores on components differed between dyads of different sex combinations, age differences and kin relationships. If the resulting components of our
Pan model were valid, we predicted that sex, age and kinship influenced relationship quality in species-specific patterns, as shown in previous studies (
Table 1).
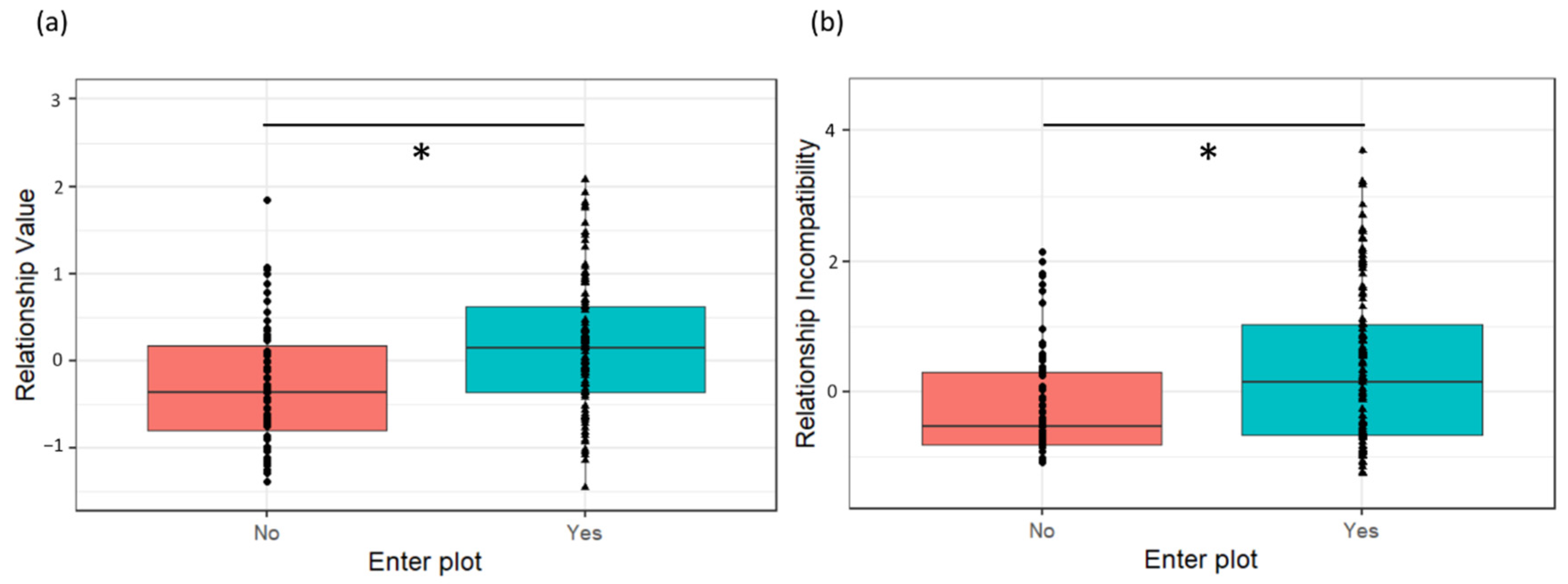
Second, we propose a novel approach to quantify a multidimensional model for Dyadic Cofeeding Tolerance by using factor analysis to construct composite measures that consider both positive and negative food interactions [
14]. In this way, we aimed to capture diverse aspects of cofeeding tolerance that could associate differently with specific relationship quality components. While measures of relationship quality were assessed based on dyadic behavioral data observed in a naturalistic condition throughout the apes’ daily lives [
28,
29,
30], cofeeding tolerance was quantified using a validated group cofeeding paradigm: the resource plot experiment [
45,
52]. In this paradigm, a group of animals was provided with a desirable, non-monopolizable food resource that was adjusted to the group size in a designated feeding area in their enclosure. In previous studies, cofeeding tolerance was typically determined at the group-level by quantifying a single measure: the proportion of the group cofeeding in the plot [
45,
53,
54]. Here, we used the same paradigm to determine novel composite tolerance measures at the dyadic level to identify what dyadic strategies might underly previously reported group differences in tolerance [
45,
53,
54]. The benefit of this approach is that it takes into account multiple facets of complex social relationships while reducing the number of variables to avoid overfitting of models on datasets with relatively small sample sizes. To do so, we scored five dyadic measures of positive and negative food-related behaviors in and around the feeding area during the experiment: proximity in the feeding area, frequency of aggression, frequency of tolerant food transfers, frequency of negative food-related behavior and presence of a dyad in the feeding area [
14]. To allow for direct comparison of the results between bonobos and chimpanzees, an identical methodology was used in all groups of both species.
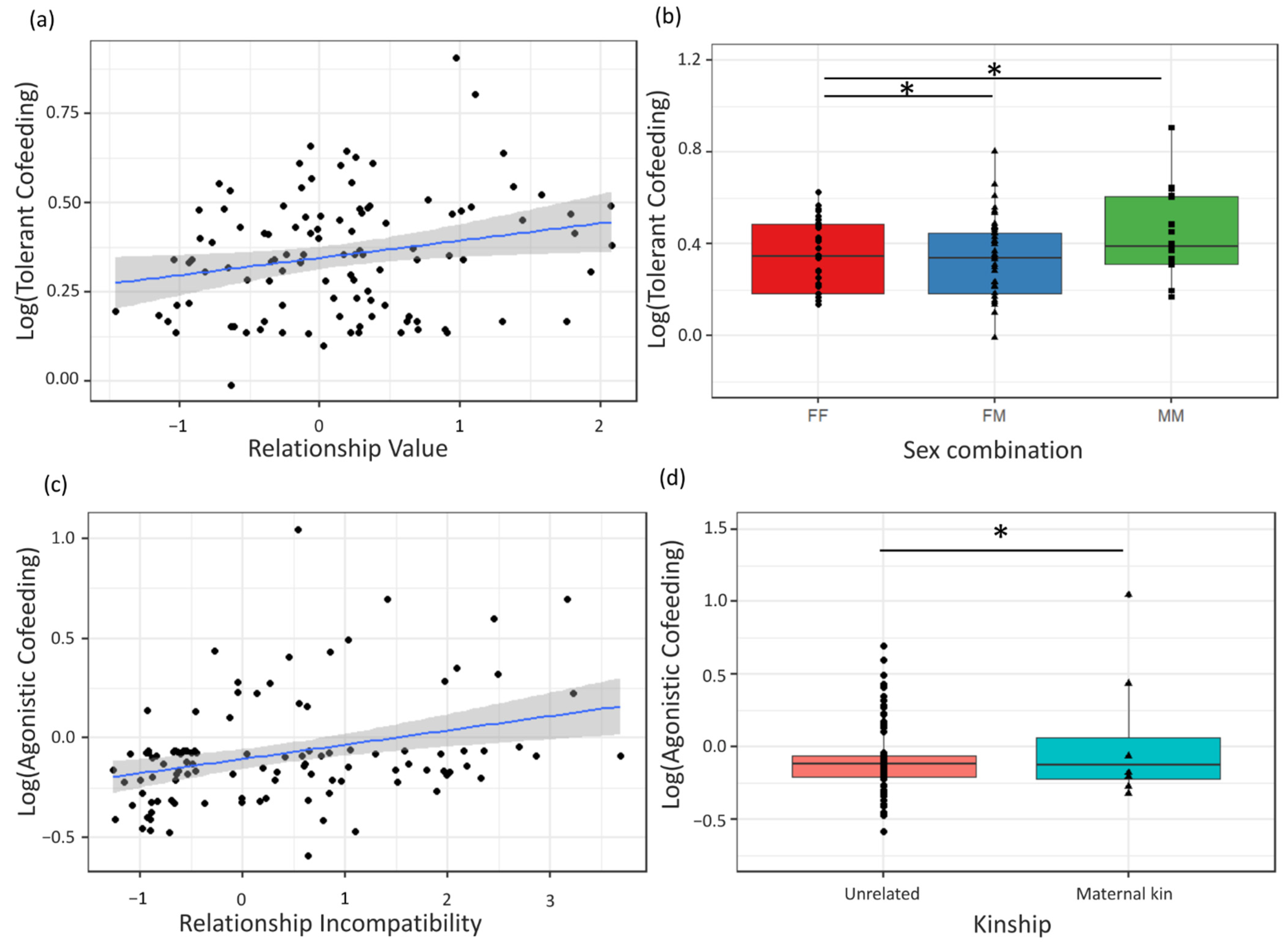
The aim was to test if scores on dimensions of Dyadic Cofeeding Tolerance differed between species, between dyads with different sex combination, age difference, maternal kinship and relationship quality scores. We expected that bonobos would not systematically differ from chimpanzees, in line with previous results using similar feeding experiments, and that sex, age and kinship effects on cofeeding tolerance would follow species-specific patterns similar to what is shown for relationship quality components. We expected to find an association between measures of Dyadic Cofeeding Tolerance and relationship quality similar to previous studies [
8,
19,
21,
26,
36], with higher cofeeding tolerance present in dyads with higher Relationship Values and lower Incompatibilities. Since Relationship Value was consistently higher in maternal kin dyads in both
Pan species [
28,
29,
30], the resulting higher fitness interdependence in these dyads was predicted to result in higher Dyadic Cofeeding Tolerance. For dyads with high Incompatibility and thus high levels of competition [
28,
29,
30], interdependence should be lower, and Dyadic Cofeeding Tolerance was expected to be lower.
5. Conclusions
Our results show that bonobos and chimpanzees do not differ consistently in Dyadic Cofeeding Tolerance, which supports earlier findings of within-species variation in cofeeding tolerance being larger than between-species variation in bonobos and chimpanzees [
45]. It is important to note that the lack of clear species differences in Dyadic Cofeeding Tolerance might be attributable to the reduced importance of fitness interdependence in captive
Pan populations due to more similar socio-ecological conditions versus wild populations. In the wild, bonobos and chimpanzees are both male philopatric species and experience similar predation risks [
77,
78]. However, resource distribution and food availability are believed to be responsible for differences in between- and within-group competition and levels of kin-driven fitness interdependence [
77,
78]. Generally speaking, in chimpanzees, seasonality is higher, and resources are more clumped, which results in higher competition between females, who each occupy and compete for high-quality core areas and show lower tolerance towards immigrating non-kin females and commit female infanticide [
79,
80]. Chimpanzee males are related because of male philopatry and form largely kin-driven alliances and strong social bonds to defend a communal territory with its resident females against males from neighboring communities. This results in strong between-group competition, including border patrols, intercommunity killing, raids and infanticide [
81,
82,
83]. In bonobos, seasonality in food availability is generally considered to be lower and resources are less clumped, resulting in lower between-and within-group competition [
41,
84]. Relationships between bonobo communities are more relaxed; no intercommunity killing or infanticide has been described, and resident bonobo females are less hostile towards immigrating females [
85]. Bonobo females form alliances with non-related females and form coalitions that allow for female codominance [
41,
86]. While male bonobos are related to each other because of male philopatry, similar to chimpanzee males, bonds between male bonobos are weak and they do not form coalitions or border patrols [
66,
67]. This shows that compared to chimpanzees, fitness interdependence in wild bonobos is less kin-driven. At a group level, this is expected to result in higher fitness interdependence in wild bonobo populations, and therefore higher levels of overall group cofeeding tolerance compared to wild chimpanzee populations.
Despite this reported species duality, bonobos and chimpanzees seem to show behavioral plasticity. Western chimpanzee populations seem to experience less food seasonality, resulting in more relaxed relationships between females compared to eastern populations [
87], and within wild bonobo communities, between-group competition increases when food abundance is lower [
88], and some populations seem to experience more seasonality in food availability, but how this relates to tolerance within and between groups is unknown [
89]. This flexibility within
Pan species likely explains why our study, as well as other captive work, did not find the predicted species differences in cofeeding tolerance, as resource distribution in captive populations is similar and intergroup competition is lacking, which reduces the need for male alliance formation in zoo-housed chimpanzees.
The quality of social bonds also predicted Dyadic Cofeeding Tolerance in an identical manner in both
Pan species and provides a mechanism that could explain previously reported between-group variation in feeding tolerance in
Pan [
45,
52], as the strength of social relationships can vary between populations depending on demographics [
52] and ecological conditions [
79]. Our results indicate that cofeeding tolerance in
Pan is flexible and likely regulated through changing levels of mutual interdependence relationships, which are in turn reliant on environmental conditions. This flexibility in tolerance appears to outweigh species-specific physiological restraints on tolerance and highlights the adaptiveness of the two species to changing environments. Our study on zoo-housed chimpanzees and bonobos offers an operational measure to quantify cofeeding tolerance that can be applied to a wider range of populations of chimpanzee and bonobo populations, as well as other primate or mammal species, both in the wild and in zoos or sanctuaries, to see how variation in local socio-ecological circumstances influences fitness interdependence and cofeeding tolerance at the dyadic and group levels. This may ultimately lead to a better understanding of how local environments have shaped the evolution of tolerance in humans and other species.