In Vivo Model of Osteoarthritis to Compare Allogenic Amniotic Epithelial Stem Cells and Autologous Adipose Derived Cells
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
- (1)
- autologous culture expanded ADSCs (12 joints);
- (2)
- allogenic culture expanded AECs (12 joints);
- (3)
- autologous SVF (12 joints);
- (4)
- 0.9% NaCl (Control) (12 joints).
2.2. Treatments
- (1)
- A1 (right: SVF, left: AECs);
- (2)
- A2 (left: SVF, right: AECs);
- (3)
- B1 (right: ADSCs, left: 0.9% NaCl);
- (4)
- B2 (left: ADSCs, right: 0.9% NaCl).
2.3. Surgical Procedure
2.4. Evaluations
ADSC and AEC Characterization
2.5. Gross Evaluation
2.6. Histology
2.7. Histomorphometry
2.8. Immunohistochemistry
2.9. Biochemistry of the Synovial Fluid
2.10. Statistical Analysis
3. Results
3.1. ADSCs and AECs Characterization
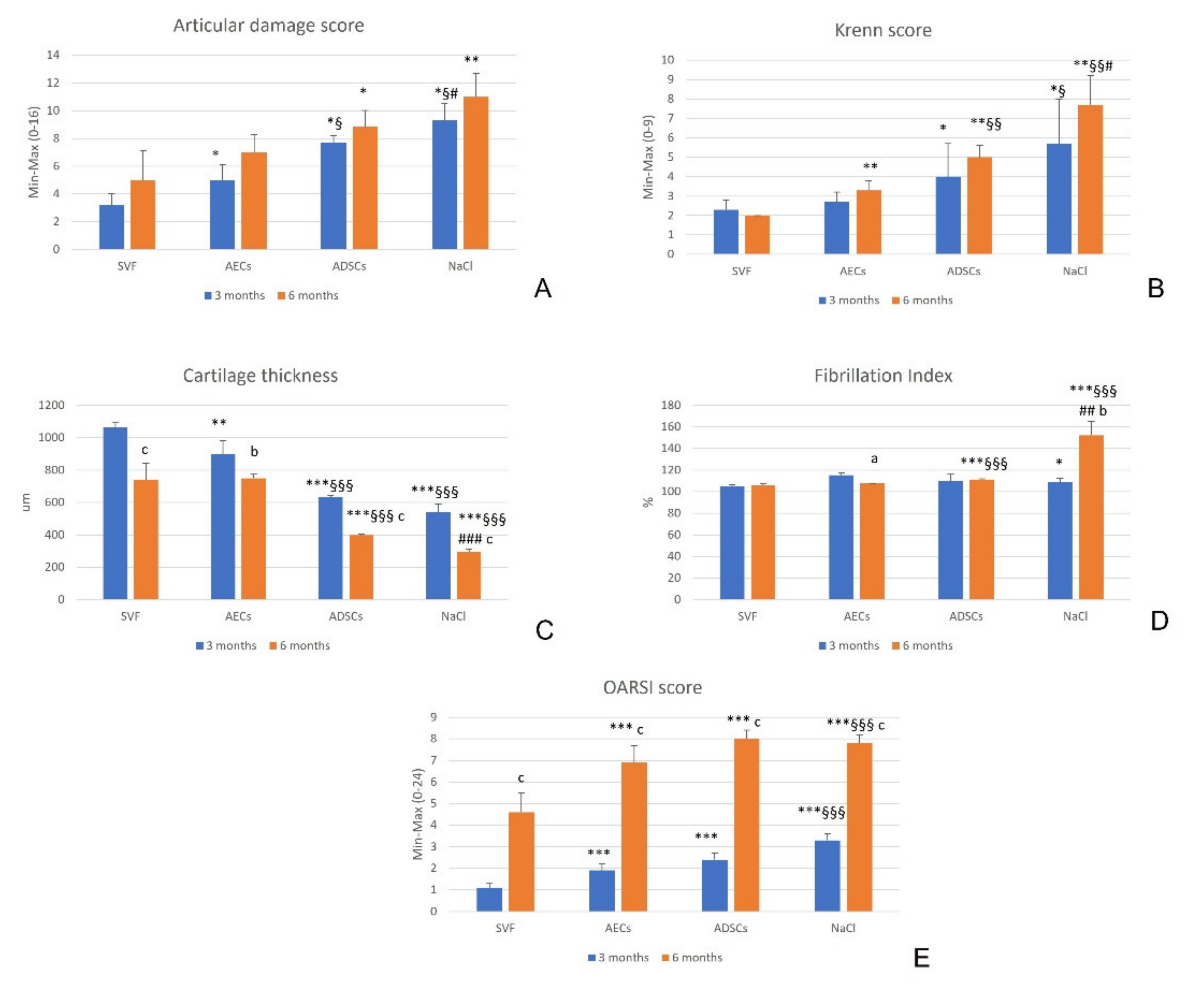
3.2. Clinical and Gross Evaluations
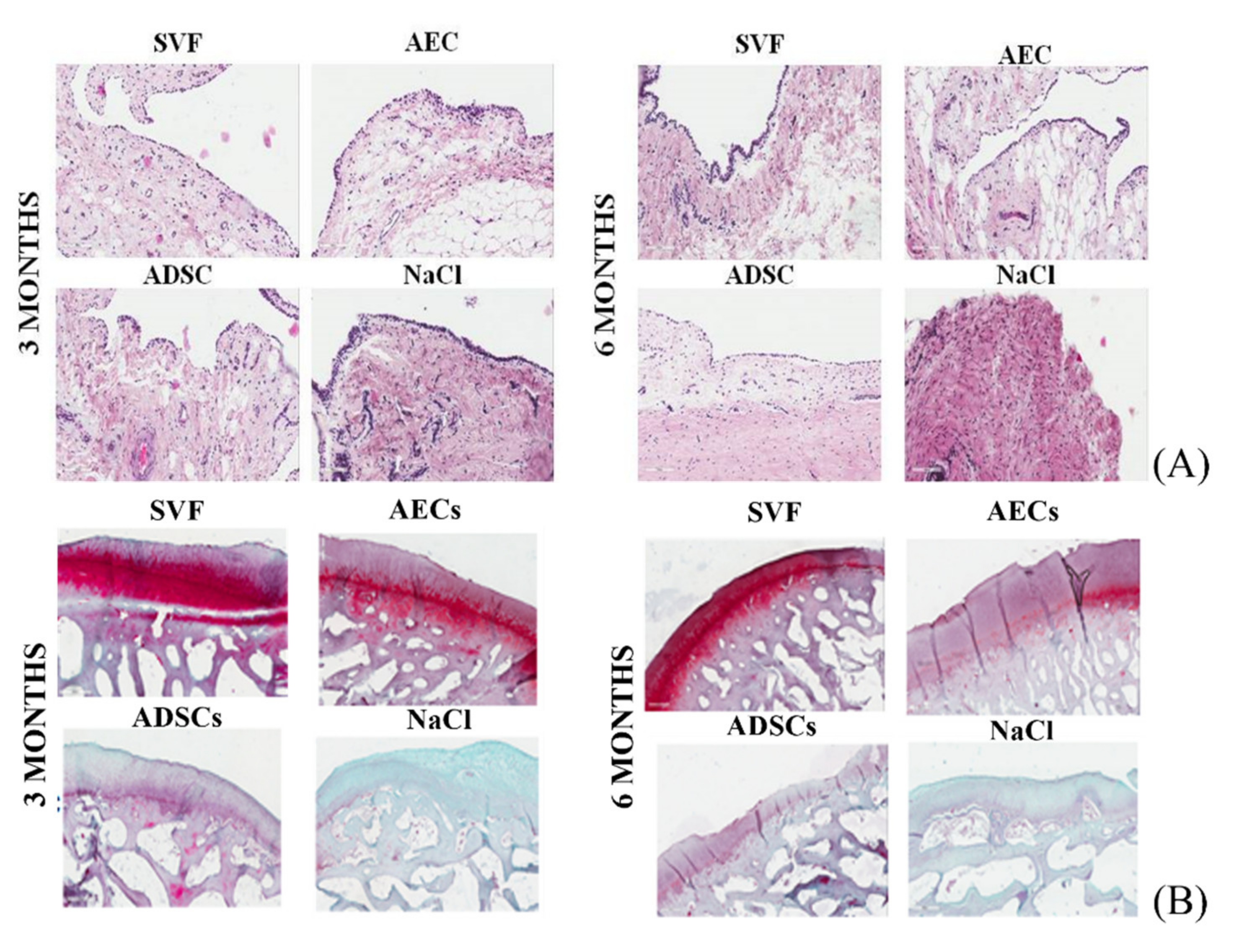
3.3. Synovial Membrane
3.4. Cartilage
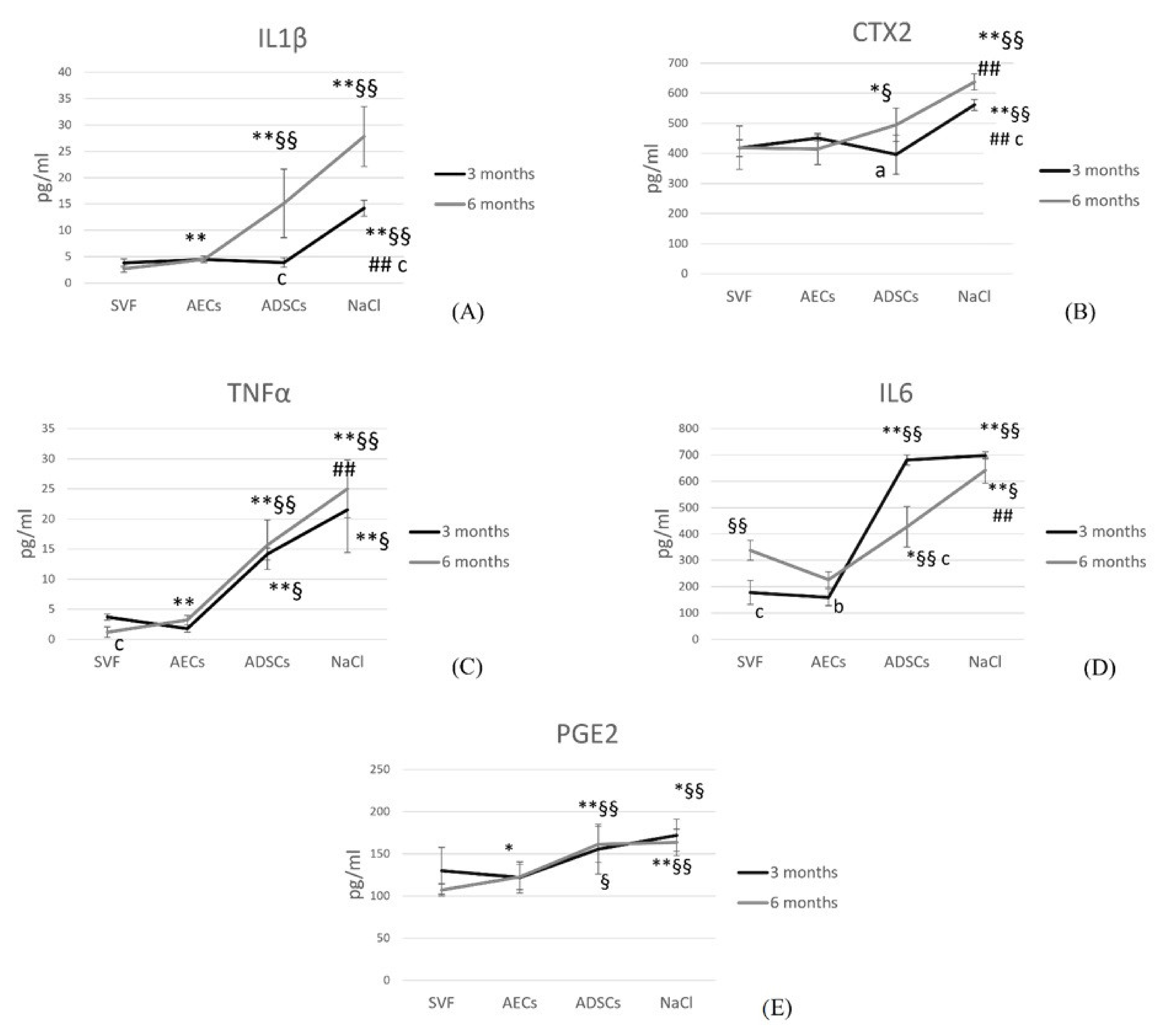
3.5. Synovial Fluid
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abramoff, B.; Caldera, F.E. Osteoarthritis: Pathology, Diagnosis, and Treatment Options. Med. Clin. N. Am. 2020, 104, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Filardo, G.; Drobnic, M.; Madry, H.; Jelic, M.; van Dijk, N.; Della Villa, S. Non-surgical management of early knee osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 436–449. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.S.; Monk, A.P.; Hopewell, S.; Bayliss, L.E.; Jackson, W.; Beard, D.J.; Price, A.J. Surgical interventions for symptomatic mild to moderate knee osteoarthritis. Cochrane Database Syst. Rev. 2019, 7, CD012128. [Google Scholar] [CrossRef] [PubMed]
- Rashid, H.; Kwoh, C.K. Should Platelet-Rich Plasma or Stem Cell Therapy Be Used to Treat Osteoarthritis? Rheum. Dis. Clin. N. Am. 2019, 45, 417–438. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.T.; Feng, Y.; Jia, H.H.; Zhao, M.; Yu, H. Application of mesenchymal stem cell therapy for the treatment of osteoarthritis of the knee: A concise review. World J. Stem Cells 2019, 11, 222–235. [Google Scholar] [CrossRef]
- Lv, X.; He, J.; Zhang, X.; Luo, X.; He, N.; Sun, Z.; Xia, H.; Liu, V.; Zhang, L.; Lin, X.; et al. Comparative Efficacy of Autologous Stromal Vascular Fraction and Autologous Adipose-derived Mesenchymal Stem Cells Combined with Hyaluronic Acid for the Treatment of Sheep Osteoarthritis. Cell Transplant. 2018, 27, 1111–1125. [Google Scholar] [CrossRef]
- Freitag, J.; Bates, D.; Wickham, J.; Shah, K.; Huguenin, L.; Tenen, A.; Paterson, K.; Boyd, R. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: A randomized controlled trial. Regen. Med. 2019, 14, 213–230. [Google Scholar] [CrossRef]
- Veronesi, F.; Maglio, M.; Tschon, M.; Nicoli Aldini, N.; Fini, M. Adipose-derived mesenchymal stem cells for cartilage tissue engineering: State-of-the-art in in vivo studies. J. Biomed. Mater. Res. A 2014, 102, 2448–2466. [Google Scholar] [CrossRef]
- Schiavone Panni, A.; Vasso, M.; Braile, A.; Toro, G.; De Cicco, A.; Viggiano, D.; Lepore, F. Preliminary results of autologous adipose-derived stem cells in early knee osteoarthritis: Identification of a subpopulation with greater response. Int. Orthop. 2019, 43, 7–13. [Google Scholar] [CrossRef]
- Van Pham, P.; Bui, K.H.T.; Ngo, D.Q.; Khuat, L.T.; Phan, N.K. Transplantation of Nonexpanded Adipose Stromal Vascular Fraction and Platelet-Rich Plasma for Articular Cartilage Injury Treatment in Mice Model. J. Med. Eng. 2013, 2013, 832396. [Google Scholar] [CrossRef]
- Roato, I.; Belisario, D.C.; Compagno, M.; Lena, A.; Bistolfi, A.; Maccari, L.; Mussano, F.; Genova, T.; Godio, L.; Perale, G.; et al. Concentrated adipose tissue infusion for the treatment of knee osteoarthritis: Clinical and histological observations. Int. Orthop. 2019, 43, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Chen, J.; Zhang, S.; Zhao, C.; Bi, M.; Chen, X.; Bi, Q. Intra-articular injection of autologous adipose-derived stromal vascular fractions for knee osteoarthritis: A double-blind randomized self-controlled trial. Int. Orthop. 2019, 43, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.D.X.; Wu, C.M.; Dubey, N.K.; Deng, Y.H.; Su, C.W.; Pham, T.T.; Le, P.B.T.; Sestili, P.; Deng, W.P. Time- and Kellgren–Lawrence Grade-Dependent Changes in Intra-Articularly Transplanted Stromal Vascular Fraction in Osteoarthritic Patients. Cells 2019, 8, 308. [Google Scholar] [CrossRef] [PubMed]
- Biferali, B.; Proietti, D.; Mozzetta, C.; Madaro, L. Fibro-Adipogenic Progenitors Cross-Talk in Skeletal Muscle: The Social Network. Front. Physiol. 2019, 10, 1074. [Google Scholar] [CrossRef] [PubMed]
- Muttini, A.; Valbonetti, L.; Abate, M.; Colosimo, A.; Curini, V.; Mauro, A.; Berardinelli, P.; Russo, V.; Cocciolone, D.; Marchisio, M.; et al. Ovine amniotic epithelial cells: In vitro characterization and transplantation into equine superficial digital flexor tendon spontaneous defects. Res. Vet. Sci. 2013, 94, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Magatti, M.; Caruso, M.; De Munari, S.; Vertua, E.; De, D.; Manuelpillai, U.; Parolini, O. Human amniotic membrane-derived, mesenchymal and epithelial cells exert different effects on monocyte-derived dendritic cell differentiation and function. Cell Transplant. 2015, 24, 1733–1752. [Google Scholar] [CrossRef]
- Barboni, B.; Mangano, C.; Valbonetti, L.; Marruchella, G.; Berardinelli, P.; Martelli, A.; Muttini, A.; Mauro, A.; Bedini, R.; Turriani, M.; et al. Synthetic Bone Substitute Engineered with Amniotic Epithelial Cells Enhances Bone Regeneration after Maxillary Sinus Augmentation. PLoS ONE 2013, 8, e63256. [Google Scholar] [CrossRef]
- Jiawen, S.; Jianjun, Z.; Jiewen, D.; Dedong, Y.; Hongbo, Y.; Jun, S.; Xudong, W.; Shen, S.G.F.; Lihe, G. Osteogenic Differentiation of Human Amniotic Epithelial Cells and Its Application in Alveolar Defect Restoration. Stem Cell Translat. Med. 2014, 3, 1504–1513. [Google Scholar] [CrossRef]
- Muttini, A.; Russo, V.; Rossi, E.; Mattioli, M.; Barboni, B.; Tosi, U.; Maffulli, N.; Valbonetti, L.; Abate, M. Pilot experimental study on amniotic epithelial mesenchymal cell transplantation in natural occurring tendinopathy in horses. Ultrasonographic and histological comparison. Muscles Ligaments Tendons. J. 2015, 5, 5–11. [Google Scholar] [CrossRef]
- Mauro, A.; Russo, V.; Di Marcantonio, L.; Berardinelli, P.; Martelli, A.; Muttini, A.; Mattioli, M.; Barboni, B. M1 and M2 macrophage recruitment during tendon regeneration induced by amniotic epithelial cell allotransplantation in ovine. Res. Vet. Sci. 2016, 105, 92–102. [Google Scholar] [CrossRef]
- Barboni, B.; Russo, V.; Gatta, V.; Bernabò, N.; Berardinelli, P.; Mauro, A.; Martelli, A.; Valbonetti, L.; Muttini, A.; Di Giacinto, O.; et al. Therapeutic potential of hAECs for early Achilles tendon defect repair through regeneration. J. Tissue Eng. Regen. Med. 2018, 12, e1594–e1608. [Google Scholar] [CrossRef] [PubMed]
- Muinos-Lopez, E.; Hermida-Gomez, T.; Fuentes-Boquete, I.; de Toro-Santos, J.; Blanco, F.J.; Díaz-Prado, S.M. Human Amniotic Mesenchymal Stromal Cells as Favorable Source for Cartilage Repair. Tissue Eng. Part A 2017, 23, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yu, G.; Cao, C.; Pang, J.; Chen, X. Bone morphogenetic protein-7 promotes chondrogenesis in human amniotic epithelial cells. Int. Orthop. 2011, 35, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Delling, U.; Brehm, W.; Ludewig, E.; Winter, K.; Jülke, H. Longitudinal evaluation of effects of intra-articular mesenchymal stromal cell administration for the treatment of osteoarthritis in an ovine model. Cell Transplant. 2015, 24, 2391–2407. [Google Scholar] [CrossRef] [PubMed]
- Canciello, A.; Greco, L.; Russo, V.; Barboni, B. Amniotic Epithelial Cell Culture. Methods Mol. Biol. 2018, 1817, 67–78. [Google Scholar]
- Barboni, B.; Russo, V.; Curini, V.; Mauro, A.; Martelli, A.; Muttini, A.; Bernabò, N.; Valbonetti, L.; Marchisio, M.; Di Giacinto, O.; et al. Achilles tendon regeneration can be improved by amniotic epithelial cell allotransplantation. Cell Transplant. 2012, 21, 2377–2395. [Google Scholar] [CrossRef]
- Barboni, B.; Russo, V.; Berardinelli, P.; Mauro, A.; Valbonetti, L.; Sanyal, H.; Canciello, A.; Greco, L.; Muttini, A.; Gatta, V.; et al. Placental Stem Cells from Domestic Animals: Translational Potential and Clinical Relevance. Cell Transplant. 2018, 27, 93–116. [Google Scholar] [CrossRef]
- Citeroni, M.R.; Mauro, A.; Ciardulli, M.C.; Di Mattia, M.; El Khatib, M.; Russo, V.; Turriani, M.; Santer, M.; Della Porta, G.; Maffulli, N.; et al. Amnion-Derived Teno-Inductive Secretomes: A Novel Approach to Foster Tendon Differentiation and Regeneration in an Ovine Model. Front. Bioeng. Biotechnol. 2021, 11, 649288. [Google Scholar] [CrossRef]
- Little, C.B.; Smith, M.M.; Cake, M.A.; Read, R.A.; Murphy, M.J.; Barry, F.P. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in sheep and goats. Osteoarthr. Cartil. 2010, 18, S80–S92. [Google Scholar] [CrossRef]
- Pritzker, K.P.; Gay, S.; Jimenez, S.A.; Ostergaard, K.; Pelletier, J.P.; Revell, P.A.; Salter, D.; van den Berg, W.B. Osteoarthritis cartilage histopathology: Grading and staging. Osteoarthr. Cartil. 2006, 14, 13–29. [Google Scholar] [CrossRef]
- Pastoureau, P.C.; Hunziker, E.B.; Pelletier, J.P. Cartilage, bone and synovial histomorphometry in animal models of osteoarthritis. Osteoarthr. Cartil. 2010, 18, S106–S112. [Google Scholar] [CrossRef] [PubMed]
- Najm, A.; Le Goff, B.; Venet, G.; Garraud, T.; Amiaud, J.; Biha, N.; Charrier, C.; Touchais, S.; Crenn, V.; Blanchard, F.; et al. IMSYC immunologic synovitis score: A new score for synovial membrane characterization in inflammatory and non-inflammatory arthritis. Jt. Bone Spine 2019, 86, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Andia, I.; Maffulli, N. New biotechnologies for musculoskeletal injuries. Surgeon 2019, 17, 244–255. [Google Scholar] [CrossRef]
- Di Matteo, B.; El Araby, M.M.; D’Angelo, A.; Iacono, F.; Nannini, A.; Vitale, N.D.; Marcacci, M.; Respizzi, S.; Kon, E. Adipose-Derived Stem Cell Treatments and Formulations. Clin. Sports Med. 2019, 38, 61–78. [Google Scholar] [CrossRef]
- Jo, C.H.; Lee, Y.G.; Shin, W.H.; Kim, H.; Chai, J.W.; Jeong, E.C.; Kim, J.E.; Shim, H.; Shin, J.S.; Shin, I.S.; et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A proof of-concept clinical trial. Stem Cells 2014, 32, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Filardo, G.; Tschon, M.; Perdisa, F.; Brogini, S.; Cavallo, C.; Desando, G.; Giavaresi, G.; Grigolo, B.; Martini, L.; Nicoli Aldini, N.; et al. Micro-fragmentation is a valid alternative to cell expansion and enzymatic digestion of adipose tissue for the treatment of knee osteoarthritis: A comparative preclinical study. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 773–781. [Google Scholar] [CrossRef]
- Veronesi, F.; Berni, M.; Marchiori, G.; Cassiolas, G.; Muttini, A.; Barboni, B.; Martini, L.; Fini, M.; Lopomo, N.F.; Marcacci, M.; et al. Evaluation of cartilage biomechanics and knee joint microenvironment after different cell-based treatments in a sheep model of early osteoarthritis. Int. Orthop. 2021, 45, 427–435. [Google Scholar] [CrossRef]
- Bansal, H.; Comella, K.; Leon, J.; Verma, P.; Agrawal, D.; Koka, P.; Ichim, T. Intra-articular injection in the knee of adipose derived stromal cells (stromal vascular fraction) and platelet rich plasma for osteoarthritis. J. Transl. Med. 2017, 15, 141. [Google Scholar] [CrossRef]
- Gibbs, N.; Diamond, R.; Sekyere, E.O.; Thomas, W.D. Management of knee osteoarthritis by combined stromal vascular fraction cell therapy, platelet-rich plasma, and musculoskeletal exercises: A case series. J. Pain. Res. 2015, 8, 799–806. [Google Scholar] [CrossRef]
- Barton, K.I.; Shekarforoush, M.; Heard, B.J.; Sevick, J.L.; Vakil, P.; Atarod, M.; Martin, R.; Achari, Y.; Hart, D.A.; Frank, C.B.; et al. Use of Pre-Clinical Surgically Induced Models to Understand Biomechanical and Biological Consequences of PTOA Development. J. Orthop. Res. 2017, 35, 454–465. [Google Scholar] [CrossRef]
- Chevrier, A.; Nelea, M.; Hurtig, M.B.; Hoemann, C.D.; Buschmann, M.D. Meniscus structure in human, sheep, and rabbit for animal models of meniscus repair. J. Orthop. Res. 2009, 27, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Cake, M.A.; Read, R.A.; Corfield, G.; Daniel, A.; Burkhardt, D.; Smith, M.M.; Little, C.B. Comparison of gait and pathology outcomes of three meniscal procedures for induction of knee osteoarthritis in sheep. Osteoarthr. Cartil. 2013, 21, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Van Tienen, T.G.; Heijkants, R.G.; de Groot, J.H.; Pennings, A.J.; Schouten, A.J.; Veth, R.P.H.; Buma, P. Replacement of the knee meniscus by a porous polymer implant: A study in dogs. Am. J. Sports Med. 2006, 34, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Spadari, A.; Romagnoli, N.; Predieri, P.G.; Borghetti, P.; Cantoni, A.M.; Corradi, A. Effects of intraarticular treatment with stanozolol on synovial membrane and cartilage in an ovine model of osteoarthritis. Res. Vet. Sci. 2013, 94, 379–387. [Google Scholar] [CrossRef][Green Version]
- Gelse, K.; Körber, L.; Schöne, M.; Raum, K.; Koch, P.; Pachowsky, M.; Welsch, G.; Breiter, R. Transplantation of Chemically Processed Decellularized Meniscal Allografts: A Pilot Sheep Study. Cartilage 2017, 8, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, F.; Vandenbulcke, F.; Ashmore, K.; Di Matteo, B.; Nicoli Aldini, N.; Martini, L.; Fini, M.; Kon, E. Meniscectomy-induced osteoarthritis in the sheep model for the investigation of therapeutic strategies: A systematic review. Int. Orthop. 2020, 44, 779–793. [Google Scholar] [CrossRef]
- De Girolamo, L.; Kon, E.; Filardo, G.; Marmotti, A.G.; Soler, F.; Peretti, G.M.; Vannini, F.; Madry, H.; Chubinskaya, S. Regenerative approaches for the treatment of early OA. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 1826–1835. [Google Scholar] [CrossRef]



| Gross Articular Damage Score | |
|---|---|
| Normal | 0 |
| Fibrillations and fissures | 2 |
| Small erosions up to the subchondral bone (<5 mm diameter) | 3 |
| Large erosions up to the subchondral bone (>5 mm diameter) | 4 |
| Sum | 0–16 |
| Enlargement of the Cell Layer of the Synovial Lining | |
|---|---|
| 1 layer | 0 |
| 2–3 layers | 1 |
| 4–5 layers, few multinucleated cells | 2 |
| 5 layers, the layer can be ulcerated and presence of multinucleated cells | 3 |
| Cell density of synovial stroma | |
| Normal cellularity | 0 |
| Cellularity slightly increased | 1 |
| Cellularity moderately increased, multinucleated cells may be present | 2 |
| Cellularity greatly increased, giant multinucleated cells, cloth formation and rheumatoid granuloma | 3 |
| Inflammatory infiltrate | |
| No inflammatory infiltrate | 0 |
| Few perivascular lymphocytes or plasma cells | 1 |
| Numerous lymphocytes or plasma cells, which form follicular aggregates | 2 |
| Dense inflammatory infiltrate or numerous follicular aggregates | 3 |
| Sum | |
| No synovitis | 0 or 1 |
| Low degree of synovitis | 2–4 |
| High degree of synovitis | 5–9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veronesi, F.; Fini, M.; Martini, L.; Berardinelli, P.; Russo, V.; Filardo, G.; Di Matteo, B.; Marcacci, M.; Kon, E. In Vivo Model of Osteoarthritis to Compare Allogenic Amniotic Epithelial Stem Cells and Autologous Adipose Derived Cells. Biology 2022, 11, 681. https://doi.org/10.3390/biology11050681
Veronesi F, Fini M, Martini L, Berardinelli P, Russo V, Filardo G, Di Matteo B, Marcacci M, Kon E. In Vivo Model of Osteoarthritis to Compare Allogenic Amniotic Epithelial Stem Cells and Autologous Adipose Derived Cells. Biology. 2022; 11(5):681. https://doi.org/10.3390/biology11050681
Chicago/Turabian StyleVeronesi, Francesca, Milena Fini, Lucia Martini, Paolo Berardinelli, Valentina Russo, Giuseppe Filardo, Berardo Di Matteo, Maurilio Marcacci, and Elizaveta Kon. 2022. "In Vivo Model of Osteoarthritis to Compare Allogenic Amniotic Epithelial Stem Cells and Autologous Adipose Derived Cells" Biology 11, no. 5: 681. https://doi.org/10.3390/biology11050681
APA StyleVeronesi, F., Fini, M., Martini, L., Berardinelli, P., Russo, V., Filardo, G., Di Matteo, B., Marcacci, M., & Kon, E. (2022). In Vivo Model of Osteoarthritis to Compare Allogenic Amniotic Epithelial Stem Cells and Autologous Adipose Derived Cells. Biology, 11(5), 681. https://doi.org/10.3390/biology11050681











