Differences in Extracellular NAD+ and NMN Metabolism on the Surface of Vascular Endothelial Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animals Maintenance and Murine Lung Endothelial Cells Isolation
2.3. Cell Culture Conditions of Other Endothelial Cell Types
2.4. Determination of Cell-Surface NAD+ and NMN-Degrading Activities in Cell Cultures
2.5. Immunofluorescence Analysis
2.6. Determination of Particular Ecto-Enzymes Engaged in the Extracellular NAD+ and NMN Catabolism on the Surface of the Endothelial Cells
2.7. Statistical Analysis
3. Results
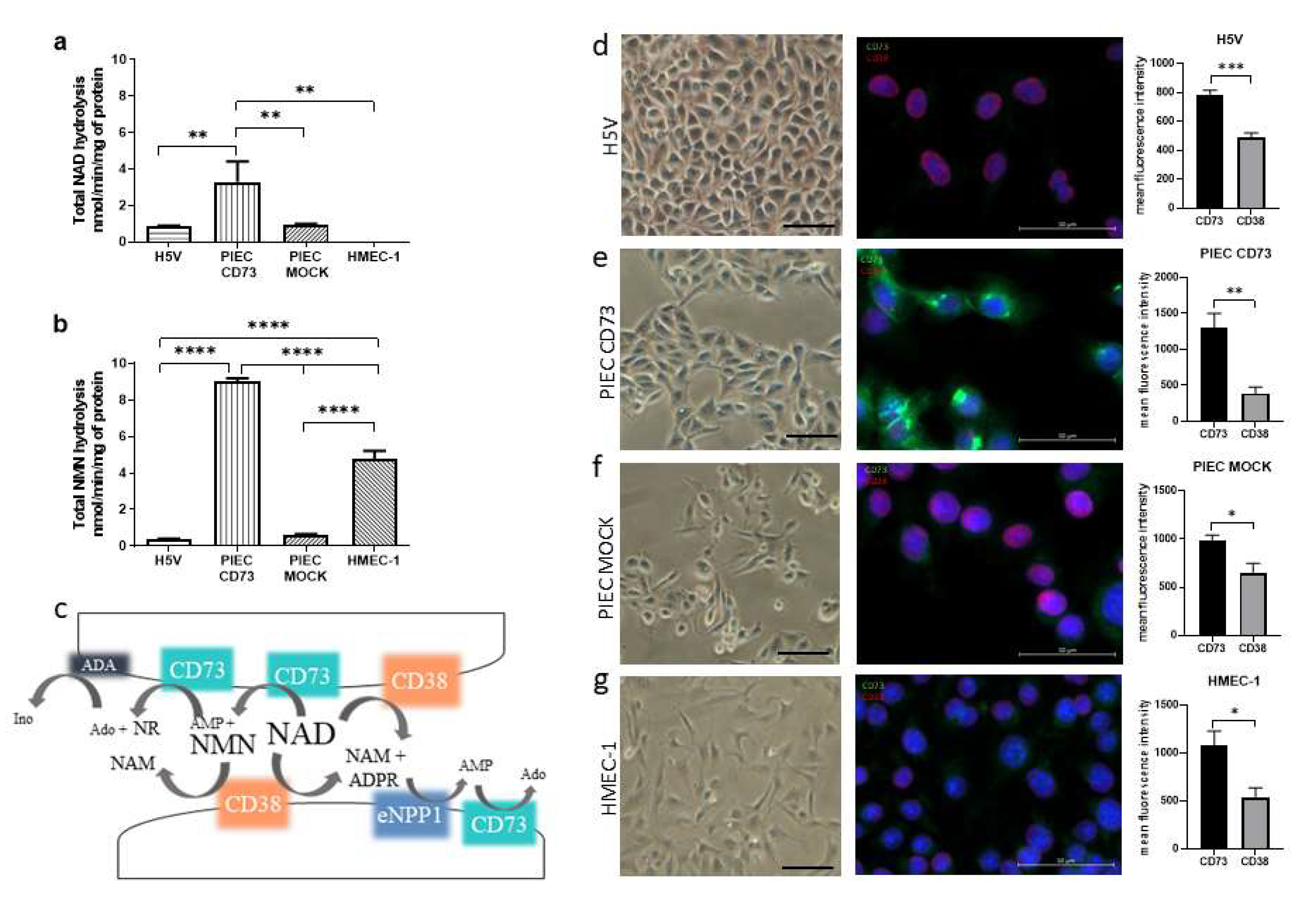
3.1. NAD+ and NMN Hydrolysis Are Different for Various Endothelial Cell Types
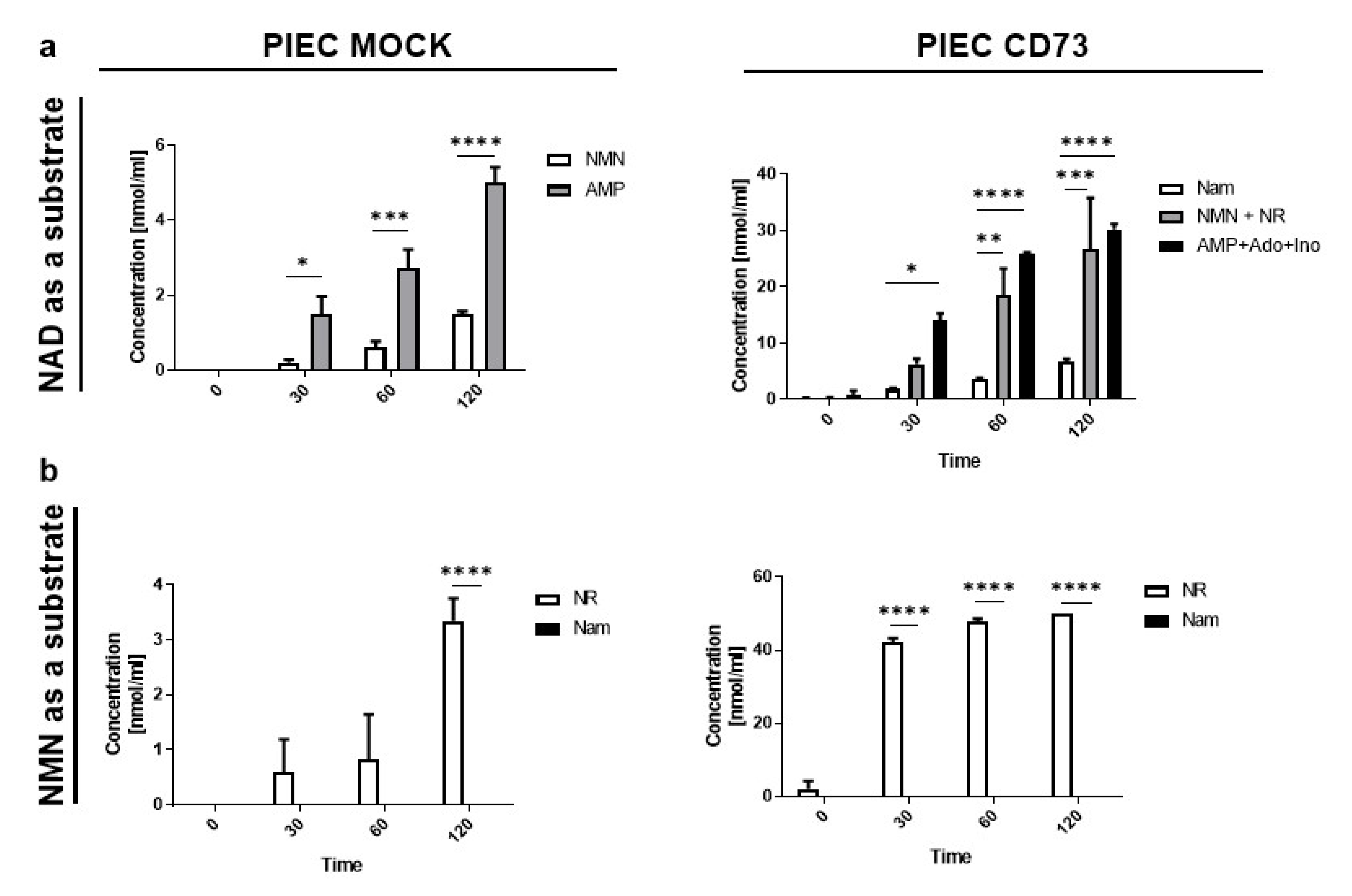
3.2. PIEC Cells Mainly Produce NMN, AMP, and NR
3.3. Nam and ADPR Are the Main Products of NAD+ and NMN Metabolism on the Surface of LECs
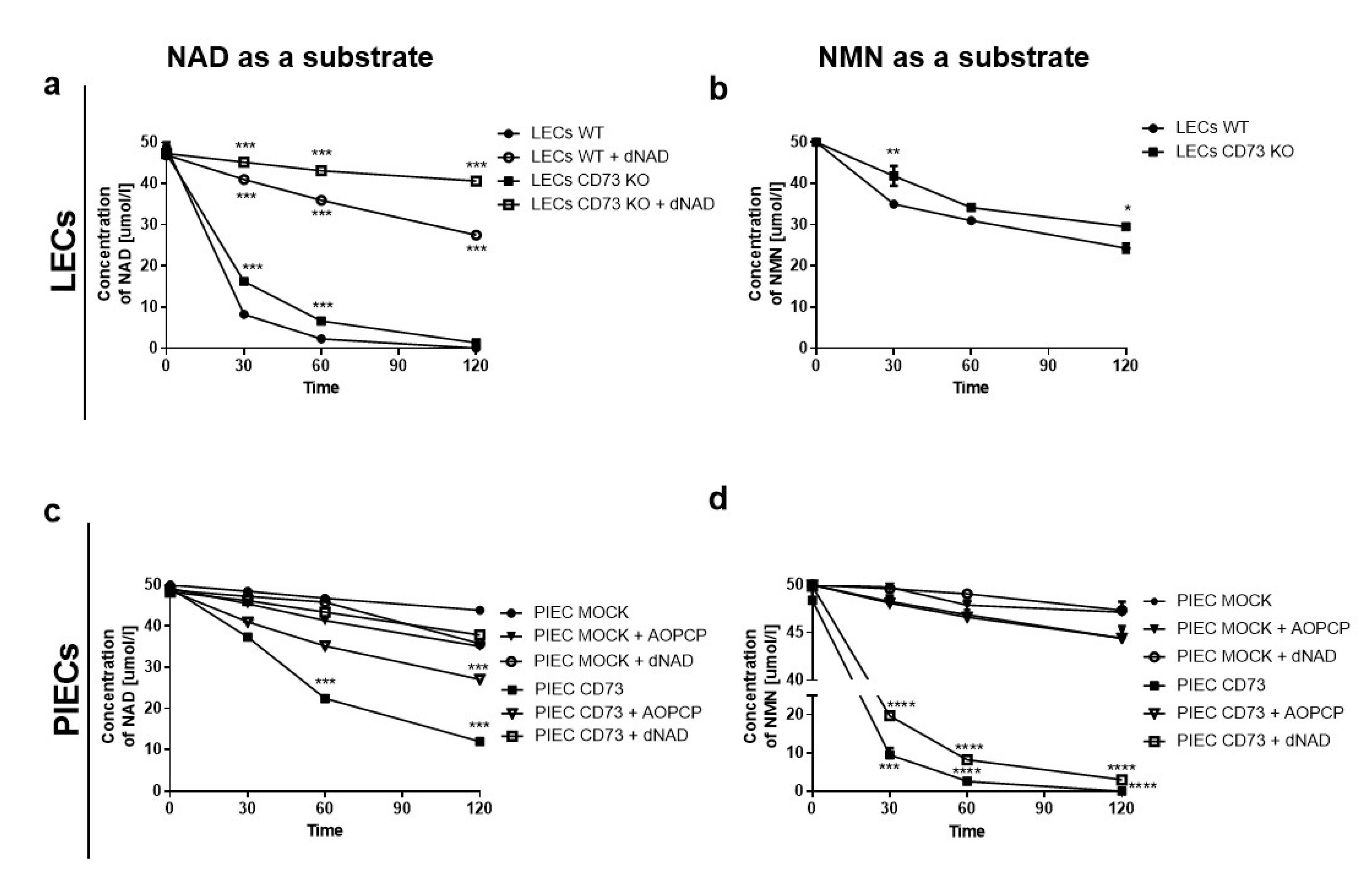
3.4. LEC and PIECs Are Characterized by Different NAD+ and NMN Metabolism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koch-Nolte, F.; Fischer, S.; Haag, F.; Ziegler, M. Compartmentation of NAD+-dependent signalling. FEBS Lett. 2011, 585, 1651–1656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anwar, M.; Aslam, H.M.; Anwar, S. PARP inhibitors. Hered. Cancer Clin. Pract. 2015, 13, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horenstein, A.; Chillemi, A.; Quarona, V.; Zito, A.; Roato, I.; Morandi, F.; Marimpietri, D.; Bolzoni, M.; Toscani, D.; Oldham, R.; et al. NAD+-Metabolizing Ectoenzymes in Remodeling Tumor–Host Interactions: The Human Myeloma Model. Cells 2015, 4, 520–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samiei, N.; Hosseini, S.; Maleki, M.; Moradi, L.; Joghataei, M.T.; Arabian, M. Modulatory Role of SIRT1 and Resistin as Therapeutic Targets in Patients with Aortic Valve Stenosis. Arch. Med. Res. 2019, 50, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Nikiforov, A.; Dölle, C.; Niere, M.; Ziegler, M. Pathways and Subcellular Compartmentation of NAD Biosynthesis in Human Cells. J. Biol. Chem. 2011, 286, 21767–21778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmermann, H.; Zebisch, M.; Sträter, N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012, 8, 437–502. [Google Scholar] [CrossRef] [Green Version]
- Boslett, J.; Hemann, C.; Christofi, F.L.; Zweier, J.L. Characterization of CD38 in the major cell types of the heart: Endothelial cells highly express CD38 with activation by hypoxia-reoxygenation triggering NAD(P)H depletion. Am. J. Physiol. Cell Physiol. 2018, 314, C297–C309. [Google Scholar] [CrossRef]
- De Giorgi, M.; Pelikant-Malecka, I.; Sielicka, A.; Slominska, E.M.; Giovannoni, R.; Cinti, A.; Cerrito, M.G.; Lavitrano, M.; Smolenski, R.T. Functional analysis of expression of human ecto-nucleoside triphosphate diphosphohydrolase-1 and/or ecto-5′-nucleotidase in pig endothelial cells. Nucleosides Nucleotides Nucleic Acids 2014, 33, 313–318. [Google Scholar] [CrossRef]
- Carmeliet, P. Blood vessels and nerves: Common signals, pathways and diseases. Nat. Rev. Genet. 2003, 4, 710–720. [Google Scholar] [CrossRef]
- Eelen, G.; de Zeeuw, P.; Simons, M.; Carmeliet, P. Endothelial Cell Metabolism in Normal and Diseased Vasculature. Circ. Res. 2015, 116, 1231–1244. [Google Scholar] [CrossRef]
- Franses, J.W.; Drosu, N.C.; Gibson, W.J.; Chitalia, V.C.; Edelman, E.R. Dysfunctional endothelial cells directly stimulate cancer inflammation and metastasis. Int. J. Cancer 2013, 133, 1334–1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kutryb-Zajac, B.; Mateuszuk, L.; Zukowska, P.; Jasztal, A.; Zabielska, M.A.; Toczek, M.; Jablonska, P.; Zakrzewska, A.; Sitek, B.; Rogowski, J.; et al. Increased activity of vascular adenosine deaminase in atherosclerosis and therapeutic potential of its inhibition. Cardiovasc. Res. 2016, 112, 590–605. [Google Scholar] [CrossRef] [PubMed]
- Koszalka, P.; Ozüyaman, B.; Huo, Y.; Zernecke, A.; Flögel, U.; Braun, N.; Buchheiser, A.; Decking, U.K.M.; Smith, M.L.; Sévigny, J.; et al. Targeted disruption of cd73/ecto-5’-nucleotidase alters thromboregulation and augments vascular inflammatory response. Circ. Res. 2004, 95, 814–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mierzejewska, P.; Zabielska, M.A.; Kutryb-Zajac, B.; Tomczyk, M.; Koszalka, P.; Smolenski, R.T.; Slominska, E.M. Impaired l-arginine metabolism marks endothelial dysfunction in CD73-deficient mice. Mol. Cell. Biochem. 2019, 458, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, C.; Wu, D.; Ying, W. Extracellular Degradation into Adenosine and the Activities of Adenosine Kinase and AMPK Mediate Extracellular NAD+-produced increases in the Adenylate Pool of BV2 Microglia under Basal Conditions. bioRxiv 2018, 343, 334268. [Google Scholar] [CrossRef]
- Smolenski, R.T.; Lachno, D.R.; Ledingham, S.J.M.; Yacoub, M.H. Determination of sixteen nucleotides, nucleosides and bases using high-performance liquid chromatography and its application to the study of purine metabolism in hearts for transplantation. J. Chromatogr. B Biomed. Sci. Appl. 1990, 527, 414–420. [Google Scholar] [CrossRef]
- Kutryb-Zajac, B.; Jablonska, P.; Serocki, M.; Bulinska, A.; Mierzejewska, P.; Friebe, D.; Alter, C.; Jasztal, A.; Lango, R.; Rogowski, J.; et al. Nucleotide ecto-enzyme metabolic pattern and spatial distribution in calcific aortic valve disease; its relation to pathological changes and clinical presentation. Clin. Res. Cardiol. 2020, 109, 1495. [Google Scholar] [CrossRef] [Green Version]
- Grozio, A.; Sociali, G.; Sturla, L.; Caffa, I.; Soncini, D.; Salis, A.; Raffaelli, N.; De Flora, A.; Nencioni, A.; Bruzzone, S. CD73 Protein as a Source of Extracellular Precursors for Sustained NAD+ Biosynthesis in FK866-treated Tumor Cells. J. Biol. Chem. 2013, 288, 25938–25949. [Google Scholar] [CrossRef] [Green Version]
- Moreschi, I.; Bruzzone, S.; Nicholas, R.A.; Fruscione, F.; Sturla, L.; Benvenuto, F.; Usai, C.; Meis, S.; Kassack, M.U.; Zocchi, E.; et al. Extracellular NAD + Is an Agonist of the Human P2Y 11 Purinergic Receptor in Human Granulocytes. J. Biol. Chem. 2006, 281, 31419–31429. [Google Scholar] [CrossRef] [Green Version]
- Warszta, D.; Nebel, M.; Fliegert, R.; Guse, A.H. NAD derived second messengers: Role in spontaneous diastolic Ca2+ transients in murine cardiac myocytes. DNA Repair (Amst.) 2014, 23, 69–78. [Google Scholar] [CrossRef]
- Bruzzone, S.; Guida, L.; Zocchi, E.; Franco, L.; De Flora, A. Connexin 43 hemi channels mediate Ca 2+ -regulated transmembrane NAD + fluxes in intact cells. FASEB J. 2001, 15, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.; Berg, J.; Mestayer, R.; Braidy, N.; Bennett, J.; Broom, S.; Watson, J. A Pilot Study Investigating Changes in the Human Plasma and Urine NAD+ Metabolome During a 6 Hour Intravenous Infusion of NAD. Front. Aging Neurosci. 2019, 11, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratajczak, J.; Joffraud, M.; Trammell, S.A.J.; Ras, R.; Canela, N.; Boutant, M.; Kulkarni, S.S.; Rodrigues, M.; Redpath, P.; Migaud, M.E.; et al. NRK1 controls nicotinamide mononucleotide and nicotinamide riboside metabolism in mammalian cells. Nat. Commun. 2016, 7, 13103. [Google Scholar] [CrossRef] [PubMed]
- Diguet, N.; Trammell, S.A.J.; Tannous, C.; Deloux, R.; Piquereau, J.; Mougenot, N.; Gouge, A.; Gressette, M.; Manoury, B.; Blanc, J.; et al. Nicotinamide riboside preserves cardiac function in a mouse model of dilated cardiomyopathy. Circulation 2018, 137, 2256–2273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morevati, M.; Egstrand, S.; Nordholm, A.; Mace, M.L.; Andersen, C.B.; Salmani, R.; Lewin, E. Effect of NAD+ boosting on kidney ischemia-reperfusion injury. PLoS ONE 2021, 16, 2554. [Google Scholar] [CrossRef] [PubMed]
- Roboon, J.; Hattori, T.; Ishii, H.; Takarada-Iemata, M.; Nguyen, D.T.; Heer, C.D.; Hori, O. Inhibition of CD38 and supplementation of nicotinamide riboside ameliorate lipopolysaccharide-induced microglial and astrocytic neuroinflammation by increasing NAD. J. Neurochem. 2021, 158, 311–327. [Google Scholar] [CrossRef]
- Mills, K.F.; Yoshida, S.; Stein, L.R.; Grozio, A.; Kubota, S.; Sasaki, Y.; Redpath, P.; Migaud, M.E.; Apte, R.S.; Uchida, K.; et al. Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice. Cell Metab. 2016, 24, 795–806. [Google Scholar] [CrossRef] [Green Version]
- Zapata-Pérez, R.; Tammaro, A.; Schomakers, B.V.; Scantlebery, A.M.L.; Denis, S.; Elfrink, H.L.; Giroud-Gerbetant, J.; Cantó, C.; López-Leonardo, C.; McIntyre, R.L.; et al. Reduced nicotinamide mononucleotide is a new and potent NAD + precursor in mammalian cells and mice. FASEB J. 2021, 35, 1–17. [Google Scholar] [CrossRef]
- Di Stefano, M.; Nascimento-Ferreira, I.; Orsomando, G.; Mori, V.; Gilley, J.; Brown, R.; Janeckova, L.; Vargas, M.E.; Worrell, L.A.; Loreto, A.; et al. A rise in NAD precursor nicotinamide mononucleotide (NMN) after injury promotes axon degeneration. Cell Death Differ. 2015, 22, 731–742. [Google Scholar] [CrossRef]
- Grozio, A.; Mills, K.F.; Yoshino, J.; Bruzzone, S.; Sociali, G.; Tokizane, K.; Lei, H.C.; Cunningham, R.; Sasaki, Y.; Migaud, M.E.; et al. Slc12a8 is a nicotinamide mononucleotide transporter. Nat. Metab. 2019, 1, 47–57. [Google Scholar] [CrossRef]
- Schmidt, M.S.; Brenner, C. Absence of evidence that Slc12a8 encodes a nicotinamide mononucleotide transporter. Nat. Metab. 2019, 1, 660–661. [Google Scholar] [CrossRef] [PubMed]
- Martens, C.R.; Denman, B.A.; Mazzo, M.R.; Armstrong, M.L.; Reisdorph, N.; McQueen, M.B.; Chonchol, M.; Seals, D.R. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD + in healthy middle-aged and older adults. Nat. Commun. 2018, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Mori, N.; Shibata, K. β-nicotinamide mononucleotide, an anti-aging candidate compound, is retained in the body for longer than nicotinamide in rats. J. Nutr. Sci. Vitaminol. (Tokyo) 2016, 62, 272–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knip, M.; Douek, I.F.; Moore, W.P.T.; Gillmor, H.A.; McLean, A.E.M.; Bingley, P.J.; Gale, E.A.M. Safety of high-dose nicotinamide: A review. Diabetologia 2000, 43, 1337–1345. [Google Scholar] [CrossRef] [Green Version]
- Graeff, R.; Guedes, A.; Quintana, R.; Wendt-Hornickle, E.; Baldo, C.; Walseth, T.; O’Grady, S.; Kannan, M. Novel Pathway of Adenosine Generation in the Lungs from NAD +: Relevance to Allergic Airway Disease. Molecules 2020, 25, 4966. [Google Scholar] [CrossRef]
- Guedes, A.G.; Dileepan, M.; Jude, J.A.; Deshpande, D.A.; Walseth, T.F.; Kannan, M.S. Role of CD38/cADPR signaling in obstructive pulmonary diseases. Curr. Opin. Pharmacol. 2020, 51, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, D.A.; Guedes, A.G.P.; Graeff, R.; Dogan, S.; Subramanian, S.; Walseth, T.F.; Kannan, M.S. CD38/cADPR Signaling Pathway in Airway Disease: Regulatory Mechanisms. Mediators Inflamm. 2018, 2018, 2042. [Google Scholar] [CrossRef] [PubMed]
- Bu, X.; Kato, J.; Hong, J.A.; Merino, M.J.; Schrump, D.S.; Lund, F.E.; Moss, J. CD38 knockout suppresses tumorigenesis in mice and clonogenic growth of human lung cancer cells. Carcinogenesis 2018, 39, 242–251. [Google Scholar] [CrossRef] [Green Version]
- Gally, F.; Hartney, J.M.; Janssen, W.J.; Perraud, A.L. CD38 plays a dual role in allergen-induced airway hyperresponsiveness. Am. J. Respir. Cell Mol. Biol. 2009, 40, 433–442. [Google Scholar] [CrossRef] [Green Version]
- Mateuszuk, Ł.; Campagna, R.; Kutryb-Zając, B.; Kuś, K.; Słominska, E.M.; Smolenski, R.T.; Chlopicki, S. Reversal of endothelial dysfunction by nicotinamide mononucleotide via extracellular conversion to nicotinamide riboside. Biochem. Pharmacol. 2020, 178, 4019. [Google Scholar] [CrossRef]
- Camacho-Pereira, J.; Tarragó, M.G.; Chini, C.C.S.; Nin, V.; Escande, C.; Warner, G.M.; Puranik, A.S.; Schoon, R.A.; Reid, J.M.; Galina, A.; et al. CD38 Dictates Age-Related NAD Decline and Mitochondrial Dysfunction through an SIRT3-Dependent Mechanism. Cell Metab. 2016, 23, 1127–1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulikova, V.; Shabalin, K.; Nerinovski, K.; Yakimov, A.; Svetlova, M.; Solovjeva, L.; Kropotov, A.; Khodorkovskiy, M.; Migaud, M.E.; Ziegler, M.; et al. Degradation of Extracellular NAD+ Intermediates in Cultures of Human HEK293 Cells. Metabolites 2019, 9, 293. [Google Scholar] [CrossRef] [Green Version]
- Shabalin, K.; Nerinovski, K.; Yakimov, A.; Kulikova, V.; Svetlova, M.; Solovjeva, L.; Khodorkovskiy, M.; Gambaryan, S.; Cunningham, R.; Migaud, M.E.; et al. NAD Metabolome Analysis in Human Cells Using 1H NMR Spectroscopy. Int. J. Mol. Sci. 2018, 19, 3906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaku, K.; Okabe, K.; Nakagawa, T. Simultaneous measurement of NAD metabolome in aged mice tissue using liquid chromatography tandem-mass spectrometry. Biomed. Chromatogr. 2018, 32, 4205. [Google Scholar] [CrossRef] [PubMed]
- Trammell, S.A.J.; Brenner, C. Targeted, LCMS-based Metabolomics for Quantitative Measurement of NAD(+) Metabolites. Comput. Struct. Biotechnol. J. 2013, 4, e201301012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, H.; Jiang, Y.; Li, J.; Wang, Y.; Nie, M.; Xiao, N.; Hu, Z. Aberrant NAD + metabolism underlies Zika virus-induced microcephaly. Nat. Metab. 2021, 3, 1109–1124. [Google Scholar] [CrossRef]
- Wang, J.; Liu, L.; Ding, Z.; Luo, Q.; Ju, Y.; Song, G. Exogenous NAD+ postpones the d-gal-induced senescence of bone marrow-derived mesenchymal stem cells via sirt1 signaling. Antioxidants 2021, 10, 254. [Google Scholar] [CrossRef]
- Ryu, D.; Zhang, H.; Ropelle, E.R.; Sorrentino, V.; Mázala, D.A.; Mouchiroud, L.; Auwerx, J. NAD+ repletion improves muscle function in muscular dystrophy and counters global PARylation. Sci. Transl. Med. 2016, 8, 5504. [Google Scholar] [CrossRef] [Green Version]
- Shi, B.; Wang, W.; Korman, B.; Kai, L.; Wang, Q.; Wei, J.; Varga, J. Targeting CD38-dependent NAD + metabolism to mitigate multiple organ fibrosis. iScience 2020, 24, 1902. [Google Scholar] [CrossRef]
- Aleo, M.F.; Giudici, M.L.; Sestini, S.; Danesi, P.; Pompucci, G.; Preti, A. Metabolic fate of extracellular NAD in human skin fibroblasts. J. Cell. Biochem. 2000, 80, 360–366. [Google Scholar] [CrossRef]
- García-Rodríguez, S.; Rosal-Vela, A.; Botta, D.; Cumba Garcia, L.M.; Zumaquero, E.; Prados-Maniviesa, V.; Cerezo-Wallis, D.; Lo Buono, N.; Robles-Guirado, J.Á.; Guerrero, S.; et al. CD38 promotes pristane-induced chronic inflammation and increases susceptibility to experimental lupus by an apoptosis-driven and TRPM2-dependent mechanism. Sci. Rep. 2018, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Benzi, A.; Sturla, L.; Heine, M.; Fischer, A.W.; Spinelli, S.; Magnone, M.; Bruzzone, S. CD38 downregulation modulates NAD + and NADP(H) levels in thermogenic adipose tissues. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 8819. [Google Scholar] [CrossRef] [PubMed]
- Jablonska, P.; Kutryb-Zajac, B.; Mierzejewska, P.; Jasztal, A.; Bocian, B.; Lango, R.; Rogowski, J.; Chlopicki, S.; Smolenski, R.T.; Slominska, E.M. The new insight into extracellular NAD + degradation-the contribution of CD38 and CD73 in calcific aortic valve disease. J. Cell. Mol. Med. 2021, 25, 5884–5898. [Google Scholar] [CrossRef] [PubMed]
- Cutler, B.R.; Chua, J.S.; Balagurunathan, K.; Anandh Babu, P.V. Methods to Analyze the Effect of Diet-Derived Metabolites on Endothelial Inflammation and Cell Surface Glycosaminoglycans. Methods Mol. Biol. 2022, 2303, 469–476. [Google Scholar] [CrossRef]
- Canet, F.; Iannantuoni, F.; Marañon AM, D.; Díaz-Pozo, P.; López-Domènech, S.; Vezza, T.; Víctor, V.M. Does Empagliflozin Modulate Leukocyte-Endothelium Interactions, Oxidative Stress, and Inflammation in Type 2 Diabetes? Antioxidants 2021, 10, 1228. [Google Scholar] [CrossRef]
- Armani, G.; Pozzi, E.; Pagani, A.; Porta, C.; Rizzo, M.; Cicognini, D.; Ferraris, E. The heterogeneity of cancer endothelium: The relevance of angiogenesis and endothelial progenitor cells in cancer microenvironment. Microvasc. Res. 2021, 138, 4189. [Google Scholar] [CrossRef]
- Al-Farabi, M.J.; Nugraha, R.A.; Marsudi, B.A.; Azmi, Y. Biomarkers of endothelial dysfunction and outcomes in coronavirus disease 2019 (COVID-19) patients: A systematic review and meta-analysis. Microvasc. Res. 2021, 138, 4224. [Google Scholar] [CrossRef]
- Evans, P.C.; Ed Rainger, G.; Mason, J.C.; Guzik, T.J.; Osto, E.; Stamataki, Z.; Neil, D.; Hoefer, I.E.; Fragiadaki, M.; Waltenberger, J.; et al. Endothelial dysfunction in COVID-19: A position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc. Res. 2020, 116, 2177–2184. [Google Scholar] [CrossRef]
- Altay, O.; Arif, M.; Li, X.; Yang, H.; Aydın, M.; Alkurt, G.; Mardinoglu, A. Combined Metabolic Activators Accelerates Recovery in Mild-to-Moderate COVID-19. Adv. Sci. 2021, 8, 1222. [Google Scholar] [CrossRef]
- Horenstein, A.L.; Faini, A.C.; Malavasi, F. CD38 in the age of COVID-19: A medical perspective. Physiol. Rev. 2021, 101, 1457–1486. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jablonska, P.; Mierzejewska, P.; Tomczyk, M.; Koszalka, P.; Franczak, M.; Kawecka, A.; Kutryb-Zajac, B.; Braczko, A.; Smolenski, R.T.; Slominska, E.M. Differences in Extracellular NAD+ and NMN Metabolism on the Surface of Vascular Endothelial Cells. Biology 2022, 11, 675. https://doi.org/10.3390/biology11050675
Jablonska P, Mierzejewska P, Tomczyk M, Koszalka P, Franczak M, Kawecka A, Kutryb-Zajac B, Braczko A, Smolenski RT, Slominska EM. Differences in Extracellular NAD+ and NMN Metabolism on the Surface of Vascular Endothelial Cells. Biology. 2022; 11(5):675. https://doi.org/10.3390/biology11050675
Chicago/Turabian StyleJablonska, Patrycja, Paulina Mierzejewska, Marta Tomczyk, Patrycja Koszalka, Marika Franczak, Ada Kawecka, Barbara Kutryb-Zajac, Alicja Braczko, Ryszard T. Smolenski, and Ewa M. Slominska. 2022. "Differences in Extracellular NAD+ and NMN Metabolism on the Surface of Vascular Endothelial Cells" Biology 11, no. 5: 675. https://doi.org/10.3390/biology11050675
APA StyleJablonska, P., Mierzejewska, P., Tomczyk, M., Koszalka, P., Franczak, M., Kawecka, A., Kutryb-Zajac, B., Braczko, A., Smolenski, R. T., & Slominska, E. M. (2022). Differences in Extracellular NAD+ and NMN Metabolism on the Surface of Vascular Endothelial Cells. Biology, 11(5), 675. https://doi.org/10.3390/biology11050675








