Effects of Trace Metals and Municipal Wastewater on the Ephemeroptera, Plecoptera, and Trichoptera of a Stream Community
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Locality Description
- (i)
- Site 1 was a stretch of the Obecnický brook in the Brdy highlands above the Obecnice reservoir (third-order watercourse according to Strahler’s system; N 49.7181961, E 13.9110308–N 49.7176411, E 13.9214808; Figure 1 and Figure S1). This site was assumed to be the least affected by human activities, although forestry management has taken place in the area for many years, and there was once here a military firing range. Although increased acidity in this catchment area due to high emissions of sulphur and nitrogen compounds has been reported in the past, this area seems to have partially recovered [33]. The catchment area consists of spruce plantations, forest-free artificial moorlands (the former artillery range), and peat bogs. The brook’s water has very low turbidity, and its rusty brown colour is likely to be caused by humic substances. By comparison, a few spring-fed tributaries had transparent water (an abundant population of acid-sensitive gammarids was observed in one unnamed tributary). Mature spruces grow along the brook edges, along with a few young alders. Particulate organic matter consists mainly of spruce needles, cones, and twigs, as well as leaf litter from the alders, birches, and old unharvested beeches. The brook bottom is densely covered in places by mosses (e.g., Fontinalis antipyretica) and species of Marchantiophyta. Despite not monitoring fish populations by electrofishing, brown trout (Salmo trutta) were observed during the macrozoobenthic sampling (Table S9). Fišer et al. [34] reported the presence of the brook lamprey (Lampetra planeri), brook trout (Salvelinus fontinalis), and European perch (Perca fluviatilis) at this site four years before our study began. Insects were prevalent in the macrozoobenthic samples, although only a few small oligochaetes, crustaceans, and molluscs were found;
- (ii)
- Site 2 consists of a stretch of the Obecnický brook below the Obecnice reservoir (third-order watercourse according to Strahler’s system; N 49.7161703, E 13.9312433–N 49.7161931, E 13.9346444; Figure 1 and Figure S1). Discharge here was usually lower than at Site 1 because of continuous water abstraction from the reservoir. Water retention and discharge manipulation also influence the colour and turbidity, which were both different from those observed at Site 1 (from dark brown with greater turbidity to crystal clear). Site 2 is surrounded by forest where little management occurs, and the brook edges are lined by old alders in the lower part, which increases the site’s heterogeneity by forming wide pools or dividing the brook into several branches (the gradient was also approximately one quarter less). Proportionally more leaf litter from deciduous trees was observed here than at Site 1. The brook bottom is covered in places by mosses (F. antipyretica). Fish stocks consist of brown trout, brook lamprey, and stone loach (Barbatula barbatula) (in decreasing order of abundance; Table S9). Insects prevailed in the macrozoobenthic samples, although small oligochaetes, crustaceans (Gammarus sp.), and molluscs (Pisidium sp. and Ancylus fluviatilis) were relatively abundant;
- (iii)
- Site 3 was on a stretch of the Litavka river below its confluence with the Obecnický brook (fourth-order watercourse according to Strahler’s system; from N 49.7100606, E 13.9884817–N 49.7112883, E 13.9961850; Figure 1 and Figure S2). This site has been heavily affected by the local industrial activity (mining, smelting and processing of silver, iron, lead, zinc, and uranium) that has been active for several centuries [15]. An active industrial complex that recycles, above all, lead waste occupies part of the river’s alluvial plain. Heaps of waste material (especially sodium slag) are still stored several meters from the riverbank. Nevertheless, all mining activity has ceased. Here, the effects of agricultural disturbance are also likely to occur as arable fields cover approximately half of the deforested catchment area (excluding human settlements). The rest of the catchment is covered by pastures and meadows. The effect of the municipal WWs is presumably low because of a relatively small human population upstream. The riparian vegetation has been substantially reduced upstream along the Litavka river (third-order watercourse according to Strahler’s system), which has been channelled in places between artificial banks. In addition, several shallow reservoirs (up to 7 ha), including some small sludge deposits, are situated upstream. We sampled the upper channelled parts with old reinforced banks and little riparian vegetation, as well as a lower unchannelled, naturally heterogenic stretch (morphologically similar to those described for Site 2) with banks lined with old alders and willows (Figure 1). The fish stocks here consist of common minnow (Phoxinus phoxinus), brown trout, and European perch (in descending order of abundance; Table S9). Insects prevailed in the macrozoobenthic samples, and small oligochaetes were relatively abundant, but crustaceans and molluscs were almost totally absent from the macrozoobenthic samples;
- (iv)
- Site 4 was a stretch of the Litavka river downstream from its confluence with the Příbramský brook (fourth-order watercourse according to Strahler’s system; from N 49.7113508, E 14.0093011–N 49.7198211, E 14.0133511; Figure 1 and Figure S2). This site was expected to be the most affected by anthropogenic activities because of the combination of industrial pollution from mining and smelting and contamination by treated municipal WWs from the town of Příbram. Treated WWs are continually pumped into the Příbramský brook approximately 900 m upstream from its confluence with the Litavka river. According to Grabicova et al. [14], Site 4 has the greatest concentrations of psychoactive PhACs of all the inspected localities, probably because of the low dilution possibilities (i.e., a small watercourse receiving relatively large amounts of municipal WWs). As at Site 3, we sampled an upper channelled stretch with artificial banks and little riparian vegetation, as well as a lower unchanneled section. Nonetheless, this morphologically heterogenic stretch only had a narrow and irregular fringe of riparian trees (alders and willows), possibly because of the dynamic migration of the river’s course across its alluvial plain [15]. The fish stock consists of common minnow, common roach (Rutilus rutilus), chub (Squalius cephalus), gudgeon (Gobio gobio), brown trout, European perch, common bream (Abramis brama), common rudd (Scardinius erythrophthalmus), stone loach, and rainbow trout (Oncorhynchus mykiss) (in descending order of abundance; Table S9). The occurrence of limnophilic fish was due to the presence of aquaculture ponds upstream. There was no clear dominance by insects in the macrozoobenthic samples since small oligochaetes, leeches (e.g., Erpobdella octoculata, Helobdella stagnalis), and the water louse (Asellus aquaticus) were very abundant. Molluscs were detected in low numbers.
2.2. Sampling and Analysis of Samples
2.3. Statistical Analyses
3. Results
3.1. Environmental Conditions
3.2. EPT Abundance and Richness
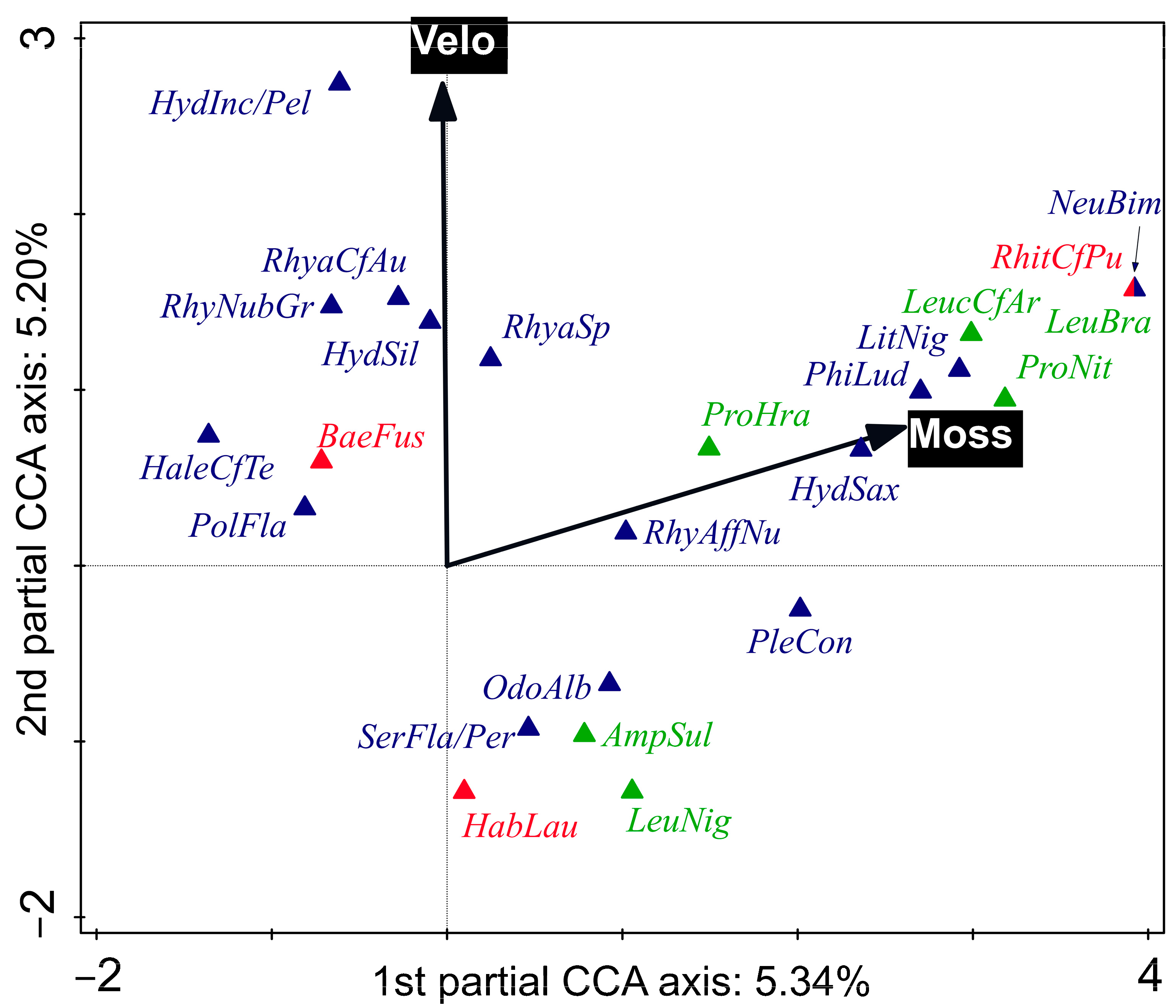
3.3. Shift in EPT Community Composition along Environmental Gradients
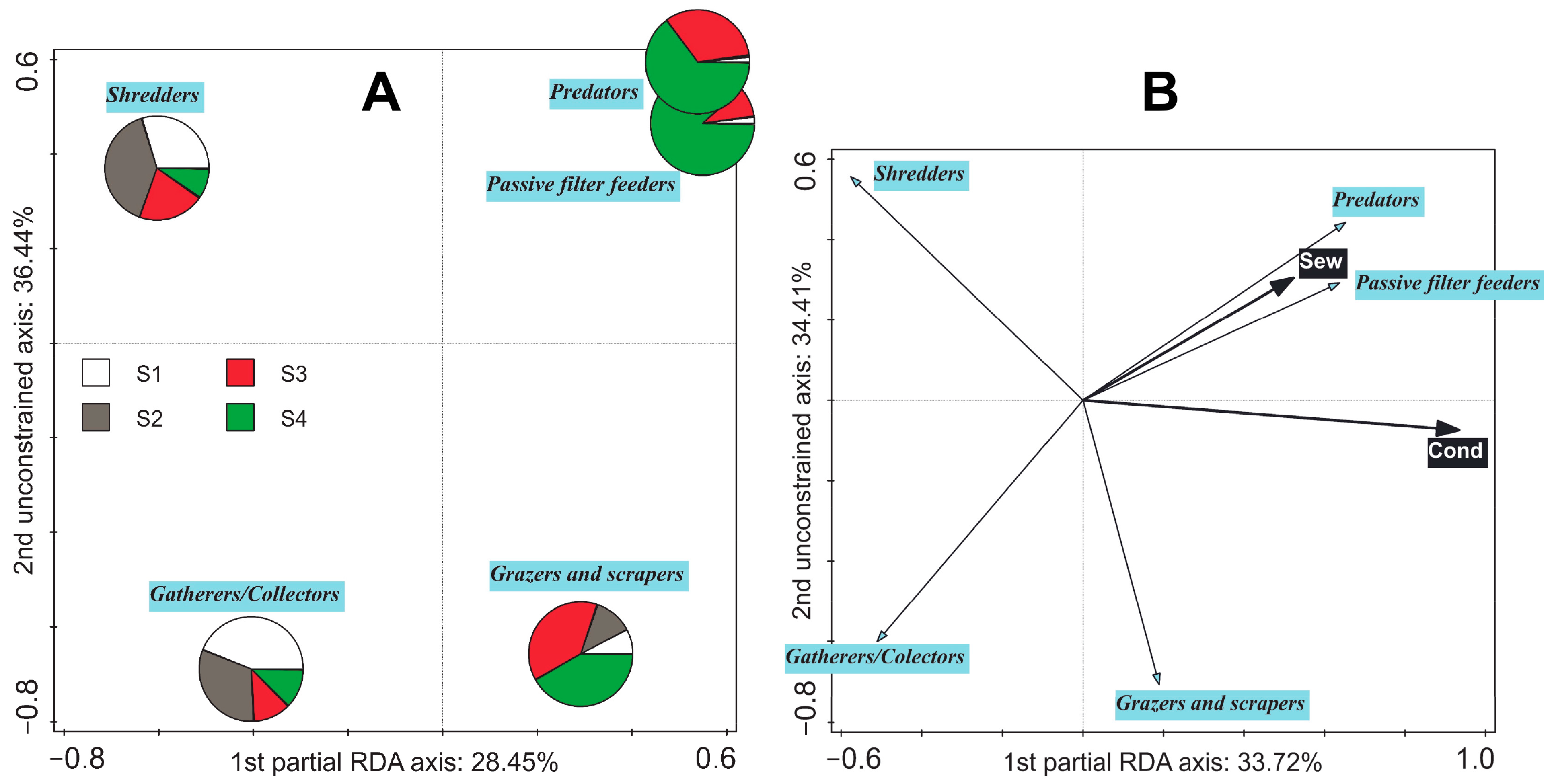
3.4. Shift in Averaged Feeding Strategies along Environmental Gradients
3.5. Malformations and Mortality of Caddisflies
4. Discussion
4.1. Variability in the EPT Community along an Environmental Gradient
4.2. Shift in Averaged Feeding Strategies in the EPT Community along Environmental Gradients
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eriksen, T.E.; Brittain, J.E.; Soli, G.; Jacobsen, D.; Goethals, P.; Friberg, N. A global perspective on the application of riverine macroinvertebrates as biological indicators in Africa, South-Central America, Mexico and Southern Asia. Ecol. Indic. 2021, 126, 107609. [Google Scholar] [CrossRef]
- Carter, J.L.; Resh, V.H.; Hannaford, M.J. Macroinvertebrates as biotic indicators of environmental quality. In Methods in Stream Ecology; Lamberti, G.A., Hauer, F.R., Eds.; Elsevier: London, UK, 2017; Volume 2: Ecosystem Function, pp. 293–318. ISBN 978-0-12-813047-6. [Google Scholar]
- Johnson, R.C.; Jin, H.S.; Carreiro, M.M.; Jack, J.D. Macroinvertebrate community structure, secondary production and trophic-level dynamics in urban streams affected by non-point-source pollution. Freshw. Biol. 2013, 58, 843–857. [Google Scholar] [CrossRef]
- Wagenhoff, A.; Townsend, C.R.; Matthaei, C.D. Macroinvertebrate responses along broad stressor gradients of deposited fine sediment and dissolved nutrients: A stream mesocosm experiment. J. Appl. Ecol. 2012, 49, 892–902. [Google Scholar] [CrossRef]
- Johnson, R.C.; Carreiro, M.M.; Jin, H.S.; Jack, J.D. Within-year temporal variation and life-cycle seasonality affect stream macroinvertebrate community structure and biotic metrics. Ecol. Indic. 2012, 13, 206–214. [Google Scholar] [CrossRef]
- Zwick, P. Historische Dokumente zur Fauna der Elbe bei Dresden vor hundert Jahren. Lauterbornia 1999, 37, 97–112. [Google Scholar]
- Marten, M. Environmental monitoring in Baden-Württemberg with special reference to biocoenotic trend-monitoring of macrozoobenthos in rivers and methodical requirements for evaluation of long-term biocoenotic changes. Aquat. Ecol. 2001, 35, 159–171. [Google Scholar] [CrossRef]
- Štěrba, O.; Měkotová, J.; Benář, V.; Šarapatka, B.; Rychnovská, M.; Kubíček, F.; Řehořek, V. River Landscape and Its Ecosystems, 1st ed.; Univerzita Palackého: Olomouc, Czech Republic, 2008; pp. 295–335. ISBN 978-80-244-2203-9. [Google Scholar]
- Blann, K.L.; Anderson, J.L.; Sands, G.R.; Vondracek, B. Effects of agricultural drainage on aquatic ecosystems: A review. Crit. Rev. Environ. Sci. Technol. 2009, 39, 909–1001. [Google Scholar] [CrossRef]
- Stanford, J.A.; Ward, J.; Liss, W.J.; Frissell, C.A.; Williams, R.N.; Lichatowich, J.A.; Coutant, C.C. A general protocol for restoration of regulated rivers. Regul. Rivers Res. Manag. 1996, 12, 391–413. [Google Scholar] [CrossRef]
- Beermann, A.J.; Elbrecht, V.; Karnatz, S.; Ma, L.; Matthaei, C.D.; Piggott, J.J.; Leese, F. Multiple-stressor effects on stream macroinvertebrate communities: A mesocosm experiment manipulating salinity, fine sediment and flow velocity. Sci. Total Environ. 2018, 610–611, 961–971. [Google Scholar] [CrossRef]
- Diamond, J.M. Ecology—Laboratory, Field and Natural Experiments. Nature 1983, 304, 586–587. [Google Scholar] [CrossRef]
- Directive 2000/60/EC, PE-CONS 3639/1/100 Rev 1; Establishing a Framework for Community Action in the Field of Water Policy; European Commission: Luxemburg, 2000.
- Grabicova, K.; Grabic, R.; Fedorova, G.; Kolarova, J.; Turek, J.; Brooks, B.W.; Randak, T. Psychoactive pharmaceuticals in aquatic systems: A comparative assessment of environmental monitoring approaches for water and fish. Environ. Pollut. 2020, 261, 114150. [Google Scholar] [CrossRef] [PubMed]
- Kotková, K.; Nováková, T.; Tůmová, Š.; Kiss, T.; Popelka, J.; Faměra, M. Migration of risk elements within the floodplain of the Litavka River, the Czech Republic. Geomorphology 2019, 329, 46–57. [Google Scholar] [CrossRef]
- Clements, W.H.; Carlisle, D.M.; Lazorchak, J.M.; Johnson, P.C. Heavy metals structure benthic communities in Colorado mountain streams. Ecol. Appl. 2000, 10, 626–638. [Google Scholar] [CrossRef]
- Qu, X.; Wu, N.; Tang, T.; Cai, Q.; Park, Y.-S. Effects of heavy metals on benthic macroinvertebrate communities in high mountain streams. Ann. De Limnol.-Int. J. Limnol. 2010, 46, 291–302. [Google Scholar] [CrossRef]
- Norris, R.; Lake, P.; Swain, R. Ecological effects of mine effluents on the South Esk River, North-eastren Tasmania. III. Benthic Macroinvertebrates. Mar. Freshw. Res. 1982, 33, 789–809. [Google Scholar] [CrossRef]
- Malmqvist, B.; Hoffsten, P.O. Influence of drainage from old mine deposits on benthic macroinvertebrate communities in central Swedish streams. Water Res. 1999, 33, 2415–2423. [Google Scholar] [CrossRef]
- Hedtke, S.F. Structure and Function of Copper-Stressed Aquatic Microcosms. Aquat. Toxicol. 1984, 5, 227–244. [Google Scholar] [CrossRef]
- Ehrman, J.M.; Barlocher, F.; Wennrich, R.; Krauss, G.J.; Krauss, G. Fungi in a heavy metal precipitating stream in the Mansfeld mining district, Germany. Sci. Total Environ. 2008, 389, 486–496. [Google Scholar] [CrossRef]
- Brix, K.V.; DeForest, D.K.; Adams, W.J. The sensitivity of aquatic insects to divalent metals: A comparative analysis of laboratory and field data. Sci. Total Environ. 2011, 409, 4187–4197. [Google Scholar] [CrossRef]
- DeBruyn, A.M.; Rasmussen, J.B. Quantifying assimilation of sewage-derived organic matter by riverine benthos. Ecol. Appl. 2002, 12, 511–520. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Yu, T.; Li, X.; Yao, J.Q.; Liu, W.G.; Chang, S.L.; Chen, Y.G. The fate and enhanced removal of polycyclic aromatic hydrocarbons in wastewater and sludge treatment system: A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1425–1475. [Google Scholar] [CrossRef]
- Franco, A.A.; Arellano, J.M.; Albendin, G.; Rodriguez-Barroso, R.; Zahedi, S.; Quiroga, M.; Coello, M.D. Mapping microplastics in Cadiz (Spain): Occurrence of microplastics in municipal and industrial wastewaters. J. Water Process Eng. 2020, 38, 101596. [Google Scholar] [CrossRef]
- Gani, K.M.; Tyagi, V.K.; Kazmi, A.A. Occurrence of phthalates in aquatic environment and their removal during wastewater treatment processes: A review. Environ. Sci. Pollut. Res. 2017, 24, 17267–17284. [Google Scholar] [CrossRef] [PubMed]
- Matheri, A.N.; Eloko, N.S.; Ntuli, F.; Ngila, J.C. Influence of pyrolyzed sludge use as an adsorbent in removal of selected trace metals from wastewater treatment. Case Stud. Chem. Environ. Eng. 2020, 2, 100018. [Google Scholar] [CrossRef]
- Soares, A. Wastewater treatment in 2050: Challenges ahead and future vision in a European context. Environ. Sci. Ecotechnol. 2020, 2, 100030. [Google Scholar] [CrossRef]
- Fedorova, G.; Grabic, R.; Grabicova, K.; Turek, J.; Van Nguyen, T.; Randak, T.; Brooks, B.W.; Zlabek, V. Water reuse for aquaculture: Comparative removal efficacy and aquatic hazard reduction of pharmaceuticals by a pond treatment system during a one year study. J. Hazard. Mater. 2022, 421, 126712. [Google Scholar] [CrossRef] [PubMed]
- Grabicova, K.; Grabic, R.; Blaha, M.; Kumar, V.; Cerveny, D.; Fedorova, G.; Randak, T. Presence of pharmaceuticals in benthic fauna living in a small stream affected by effluent from a municipal sewage treatment plant. Water Res. 2015, 72, 145–153. [Google Scholar] [CrossRef]
- Suchowska-Kisielewicz, M.; Nowogonski, I. Influence of storms on the emission of pollutants from sewage into waters. Sci. Rep. 2021, 11, 18788. [Google Scholar] [CrossRef]
- Covich, A.P.; Palmer, M.A.; Crowl, T.A. The role of benthic invertebrate species in freshwater ecosystems: Zoobenthic species influence energy flows and nutrient cycling. BioScience 1999, 49, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Beneš, F.; Horecký, J.; Senoo, T.; Kamasová, L.; Lamačová, A.; Tátosová, J.; Hardekopf, D.W.; Stuchlík, E. Evidence for responses in water chemistry and macroinvertebrates in a strongly acidified mountain stream. Biologia 2017, 72, 1049–1058. [Google Scholar] [CrossRef]
- Fišer, D.; Muška, M.; Vlach, P.; Dort, H.; Ťuláková, A.; Blabolil, P.; Vašek, M.; Kočvara, L. Fish communities of the Brdy Protected Landscape Area, current threats and management suggestions. Bohemia Cent. 2018, 34, 231–272. [Google Scholar]
- Ter Braak, C.; Šmilauer, P. Windows Release; Version 5.12; Software for Mutivariate Data Exploration, Testing, and Summarization; Biometris, Plant Research International: Wageningen, The Netherlands; Germany University & Research: Berlin, Germany; Petr Šmilauer: České Budějovice, Czech Republic, 2012–2019. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Graf, W.; Murphy, J.; Dahl, J.; Zamora-Munoz, C.; López-Rodríguez, M.J. Trichoptera. In Distribution and Ecological Preferences of European Freshwater Organisms; Schmidt-Kloiber, A., Hering, D., Eds.; Pensoft Publishing: Sofia, Bulgaria, 2008; Volume 1, pp. 1–388. ISBN 978-954-642-441-9. [Google Scholar]
- Buffagni, A.; Cazzola, M.; López-Rodríguez, M.J.; Alba-Tercedor, J.; Armanini, D.G. Ephemeroptera. In Distribution and Ecological Preferences of European Freshwater Organisms; Schmidt-Kloiber, A., Hering, D., Eds.; Pensoft Publishing: Sofia, Bulgaria, 2009; Volume 3, pp. 1–254. ISBN 978-954-642-508-9. [Google Scholar]
- Graf, W.; Lorenz, A.W.; Tierno de Figueroa, J.M.; Lücke, S.; López-Rodríguez, M.J.; Davies, C. Plecoptera. In Distribution and Ecological Preferences of European Freshwater Organisms; Schmidt-Kloiber, A., Hering, D., Eds.; Pensoft Publishing: Sofia, Bulgaria, 2009; Volume 2, pp. 1–262. ISBN 978-954-642-479-2. [Google Scholar]
- Waringer, J.; Graf, W. Atlas of Central European Trichoptera Larvae/Atlas der Mitteleuropäischen Köcherfliegenlarven; Erik Mauch Verlag: Dinkelscherben, Germany, 2011; pp. 1–470. ISBN 978-3-00-032177-1. [Google Scholar]
- Burdon, F.J.; Munz, N.A.; Reyes, M.; Focks, A.; Joss, A.; Rasanen, K.; Altermatt, F.; Eggen, R.I.L.; Stamm, C. Agriculture versus wastewater pollution as drivers of macroinvertebrate community structure in streams. Sci. Total Environ. 2019, 659, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- Haidekker, A.; Hering, D. Relationship between benthic insects (Ephemeroptera, Plecoptera, Coleoptera, Trichoptera) and temperature in small and medium-sized streams in Germany: A multivariate study. Aquat. Ecol. 2008, 42, 463–481. [Google Scholar] [CrossRef]
- Moore, R.D.; Spittlehouse, D.; Story, A. Riparian Microclimate and Stream Temperature Response to Forest Harvesting: A Review. J. Am. Water Resour. Assoc. 2005, 41, 813–834. [Google Scholar] [CrossRef]
- Marten, M. Interspecific variation in temperature dependence of egg development of five congeneric stonefly species (Protonemura Kempny, 1898, Nemouridae, Plecoptera). Hydrobiologia 1990, 199, 157–172. [Google Scholar] [CrossRef]
- Humpesch, U.; Elliott, J. Effect of temperature on the hatching time of eggs of three Rhithrogena spp. (Ephemeroptera) from Austrian streams and an English stream and river. J. Anim. Ecol. 1980, 49, 643–661. [Google Scholar] [CrossRef]
- Kiffney, P.M.; Clements, W.H. Effects of Heavy-Metals on a Macroinvertebrate Assemblage from a Rocky-Mountain Stream in Experimental Microcosms. J. N. Am. Benthol. Soc. 1994, 13, 511–523. [Google Scholar] [CrossRef]
- Wesner, J.S.; Kraus, J.M.; Schmidt, T.S.; Walters, D.M.; Clements, W.H. Metamorphosis enhances the effects of metal exposure on the mayfly. Environ. Sci. Technol. 2014, 48, 10415–10422. [Google Scholar] [CrossRef]
- Schmidt, T.S.; Kraus, J.M.; Walters, D.M.; Wanty, R.B. Emergence flux declines disproportionately to larval density along a stream metals gradient. Environ. Sci. Technol. 2013, 47, 8784–8792. [Google Scholar] [CrossRef]
- Bojková, J.; Komprdova, K.; Soldán, T.; Zahrádková, S. Species loss of stoneflies (Plecoptera) in the Czech Republic during the 20th century. Freshw. Biol. 2012, 57, 2550–2567. [Google Scholar] [CrossRef]
- Master, L.L.; Stein, B.A.; Kutner, G.A.; Hammerson, G.A.K. Vanishing assets, conservation status of US species. In Precious Heritage, the Status of Biodiversity in the United States. The Nature Conservancy & Association for Biodiversity Information; Stein, B.A., Kutner, L.S., Adams, J.S., Eds.; Oxford University Press: Oxford, UK, 2000; pp. 93–118. ISBN 978195125191. [Google Scholar]
- Hardekopf, D.W.; Horecky, J.; Kopacek, J.; Stuchlik, E. Predicting long-term recovery of a strongly acidified stream using MAGIC and climate models (Litavka, Czech Republic). Hydrol. Earth Syst. Sci. 2008, 12, 479–490. [Google Scholar] [CrossRef] [Green Version]
- Vuori, K.-M. Direct and indirect effects of iron on river ecosystems. In Proceedings of the Annales Zoologici Fennici, Helsinki, Finland, 1 November 1995; pp. 317–329. [Google Scholar]
- Dangles, O. Functional plasticity of benthic macroinvertebrates: Implications for trophic dynamics in acid streams. Can. J. Fish. Aquat. Sci. 2002, 59, 1563–1573. [Google Scholar] [CrossRef]
- Malaj, E.; Grote, M.; Schafer, R.B.; Brack, W.; von der Ohe, P.C. Physiological sensitivity of freshwater macroinvertebrates to heavy metals. Environ. Toxicol. Chem. 2012, 31, 1754–1764. [Google Scholar] [CrossRef] [PubMed]
- Gattolliat, J.L.; Vincon, G.; Wyler, S.; Pawlowski, J.; Sartori, M. Toward a comprehensive COI DNA barcode library for Swiss Stoneflies (Insecta: Plecoptera) with special emphasis on the genus Leuctra. Zoosymposia 2016, 11, 135–155. [Google Scholar] [CrossRef] [Green Version]
- Skála, I.; Lapšanská, N.; Špaček, J. Macrozoobenthos of brooks in the Brdy Highlands Protected Landscape Area (Czech Republic). Bohemia Cent. 2019, 35, 291–358. [Google Scholar]
- Roux, C.; Tachet, H.; Bournaud, M.; Cellot, B. Stream continuum and metabolic rate in the larvae of five species of Hydropsyche (Trichoptera). Ecography 1992, 15, 70–76. [Google Scholar] [CrossRef]
- Liess, M.; Von Der Ohe, P.C. Analyzing effects of pesticides on invertebrate communities in streams. Environ. Toxicol. Chem. 2005, 24, 954–965. [Google Scholar] [CrossRef]
- Aspin, T.W.H.; Hart, K.; Khamis, K.; Milner, A.M.; O’Callaghan, M.J.; Trimmer, M.; Wang, Z.N.; Williams, G.M.D.; Woodward, G.; Ledger, M.E. Drought intensification alters the composition, body size, and trophic structure of invertebrate assemblages in a stream mesocosm experiment. Freshw. Biol. 2019, 64, 750–760. [Google Scholar] [CrossRef]
- Pitsch, T. Contribution to larval taxonomy, ecology and distribution of the central European species of the genus Philopotamus (Trichoptera: Philopotamidae). In Proceedings of the Fifth International Symposium on Trichoptera, Lyon, France, 21–26 July 1986; pp. 331–335. [Google Scholar] [CrossRef]
- Edington, J. Habitat preferences in net-spinning caddis larvae with special reference to the influence of water velocity. J. Anim. Ecol. 1968, 37, 675–692. [Google Scholar] [CrossRef]
- Reiso, S.; Brittain, J.E. Life cycle, diet and habitat of Polycentropus flavomaculatus, Plectrocnemia conspersa and Rhyacophila nubila (Trichoptera) in Øvre Heimdalen, Jotunheimen Mountains, Norway. Nor. J. Entomol. 2000, 47, 113–124. [Google Scholar]
- Stehle, S.; Schulz, R. Agricultural insecticides threaten surface waters at the global scale. Proc. Natl. Acad. Sci. USA 2015, 112, 5750–5755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Let, M.; Spacek, J.; Ferencik, M.; Kouba, A.; Blaha, M. Insecticides and Drought as a Fatal Combination for a Stream Macroinvertebrate Assemblage in a Catchment Area Exploited by Large-Scale Agriculture. Water 2021, 13, 1352. [Google Scholar] [CrossRef]
- Di Veroli, A.; Santoro, F.; Pallottini, M.; Selvaggi, R.; Scardazza, F.; Cappelletti, D.; Goretti, E. Deformities of chironomid larvae and heavy metal pollution: From laboratory to field studies. Chemosphere 2014, 112, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Ratia, H.; Vuori, K.M.; Oikari, A. Caddis larvae (Trichoptera, Hydropsychidae) indicate delaying recovery of a watercourse polluted by pulp and paper industry. Ecol. Indic. 2012, 15, 217–226. [Google Scholar] [CrossRef] [Green Version]
- Buntha, P.; Traichaiyaporn, S.; Thapanya, D. Food Source for Hydropsychid Larvae during an Algae Bloom in Nan River, Nan Province, Thailand (Trichoptera: Hydropsychidae). Zoosymposia 2020, 18, 9–16. [Google Scholar] [CrossRef]
- Otto, C. Prey size and predation as factors governing the distribution of lotic polycentropodid caddisfly larvae. Oikos 1985, 44, 439–447. [Google Scholar] [CrossRef]
- Englund, G.; Evander, D. Interactions between sculpins, net-spinning caddis larvae and midge larvae. Oikos 1999, 85, 117–126. [Google Scholar] [CrossRef]
- Nakano, D.; Yamamoto, M.; Okino, T. Ecosystem engineering by larvae of net-spinning stream caddisflies creates a habitat on the upper surface of stones for mayfly nymphs with a low resistance to flows. Freshw. Biol. 2005, 50, 1492–1498. [Google Scholar] [CrossRef]
- Glime, J.M. Bryophyte Ecology, Chapter 11—Aquatic Insects. Available online: http://digitalcommons.mtu.edu/bryophyte-ecology2 (accessed on 27 March 2022).
- Krno, I. Longitudinal changes in the structure of macrozoobenthos and its microdistribution in natural and moderately eutrophicated waters of the River Rajcianka (Strázovské vrchy). Acta Fac. Rerum Nat. Univ. Comen. Zool. 1990, 33, 31–48. [Google Scholar]
- McGill, B.J.; Enquist, B.J.; Weiher, E.; Westoby, M. Rebuilding community ecology from functional traits. Trends Ecol. Evol. 2006, 21, 178–185. [Google Scholar] [CrossRef]
- Boulton, A.J.; Peterson, C.G.; Grimm, N.B.; Fisher, S.G. Stability of an Aquatic Macroinvertebrate Community in a Multiyear Hydrologic Disturbance Regime. Ecology 1992, 73, 2192–2207. [Google Scholar] [CrossRef]
- Lima, M.; Firmino, V.C.; de Paiva, C.K.S.; Juen, L.; Brasil, L.S. Land use changes disrupt streams and affect the functional feeding groups of aquatic insects in the Amazon. J. Insect Conserv. 2022, 26, 137–148. [Google Scholar] [CrossRef]
- Grabic, R.; Fick, J.; Lindberg, R.H.; Fedorova, G.; Tysklind, M. Multi-residue method for trace level determination of pharmaceuticals in environmental samples using liquid chromatography coupled to triple quadrupole mass spectrometry. Talanta 2012, 100, 183–195. [Google Scholar] [CrossRef]
- Lindberg, R.H.; Ostman, M.; Olofsson, U.; Grabic, R.; Fick, J. Occurrence and behaviour of 105 active pharmaceutical ingredients in sewage waters of a municipal sewer collection system. Water Res. 2014, 58, 221–229. [Google Scholar] [CrossRef]
- Giddings, E.M.; Hornberger, M.I.; Hadley, H.K. Trace-metal concentrations in sediment and water and health of aquatic macroinvertebrate communities of streams near Park City, Summit County, Utah. In Utah; Science for a Changing World: Salt Lake City, UT, USA, 2001; pp. 9–10. [Google Scholar] [CrossRef]




| Site 1 | Site 2 | Site 3 | Site 4 | |
|---|---|---|---|---|
| n = 4 Mean ± SEM | n = 4 Mean ± SEM | n = 4 Mean ± SEM | n = 4 Mean ± SEM | |
| Temperature (°C) | 12.05 ± 0.34 | 13.33 ± 0.61 | 17.05 ± 1.11 | 16.80 ± 0.68 |
| pH | 7.09 ± 0.24 | 7.35 ± 0.17 | 7.45 ± 0.17 | 7.45 ± 0.09 |
| Oxygen concentration (mg·L–1) | 9.90 ± 0.10 | 9.02 ± 0.18 | 8.91 ± 0.16 | 7.61 ± 0.27 |
| Conductivity (µS·cm–1) | 71.40 ± 5.80 | 84.35 ± 5.98 | 390.60 ± 39.18 | 571.00 ± 56.74 |
| Concentrations in Water Samples | ||||
| Cadmium (µg·L–1) | 0.26 | 0.23 | 7.90 | 3.80 |
| Lead (µg·L–1) | 5.80 | 9.80 | 64.00 | 53.00 |
| Zinc (µg·L–1) | 15.00 | 13.00 | 1200.00 | 530.00 |
| Sum pesticides (ng·L–1) | 132.00 | 70.32 | 462.90 | 877.87 ± 125.71 (n = 3) |
| Sum PhACs (ng·L–1) | 15.17 | 13.40 | 874.58 | 4636.73 ± 254.79 (n = 3) |
| Volume [m3] | ||
|---|---|---|
| 2019 | 2020 | |
| January | 164,799 | 1522 |
| February | 80,750 | 55,224 |
| March | 58,862 | 35,861 |
| April | 183 | 3239 |
| May | 26,930 | 24,749 |
| June | 34,483 | 130,016 |
| July | 13,340 | 11,305 |
| August | 26,333 | 22,382 |
| September | 817 | 12,064 |
| October | 4967 | 67,559 |
| November | 3270 | 5941 |
| December | 71 | 299 |
| Total | 414,805 | 370,161 |
| Site 1 | Site 2 | Site 3 | Site 4 | |
|---|---|---|---|---|
| Velocity (m·s–1) | n = 12 Mean ± SEM | n = 12 Mean ± SEM | n = 12 Mean ± SEM | n = 9 Mean ± SEM |
| Average | 0.19 ± 0.04 | 0.14 ± 0.03 | 0.27 ± 0.04 | 0.29 ± 0.05 |
| Maximum | 0.44 ± 0.09 | 0.32 ± 0.06 | 0.70 ± 0.14 | 0.62 ± 0.10 |
| Minimum | 0.04 ± 0.04 | 0.02 ± 0.03 | 0.04 ± 0.04 | 0.06 ± 0.05 |
| Site 1 | Site 2 | Site 3 | Site 4 | |
|---|---|---|---|---|
| Choriotopic Parameter | n = 12 Mean ± SEM (%) | n = 12 Mean ± SEM (%) | n = 12 Mean ± SEM (%) | n = 12 Mean ± SEM (%) |
| Megalithal Large cobbles, boulders and blocks, bedrock; >40 cm | 5.75 ± 2.63 | 18.29 ± 7.87 | 6.08 ± 2.16 | 18.50 ± 7.30 |
| Macrolithal Coarse blocks, cobbles, gravel and sand; 20–40 cm | 29.92 ± 6.88 | 17.38 ± 5.28 | 19.10 ± 4.62 | 12.60 ± 2.95 |
| Mesolithal Fist to hand-sized cobbles; 6.3–20.0 cm | 16.58 ± 3.75 | 22.29 ± 3.37 | 28.50 ± 2.41 | 19.70 ± 3.48 |
| Microlithal Coarse gravel; 2.0–6.3 cm | 11.08 ± 1.89 | 8.67 ± 2.23 | 18.30 ± 3.29 | 11.20 ± 0.99 |
| Akal Fine to medium-sized gravel; 0.2–2.0 cm | 15.50 ± 3.32 | 5.21 ± 0.93 | 11.60 ± 1.97 | 11.90 ± 2.06 |
| Psammal Sand; <0.2 cm | 3.88 ± 1.12 | 1.21 ± 0.58 | 3.00 ± 1.04 | 4.50 ± 2.00 |
| Xylal Deadwood, cones | 4.79 ± 1.33 | 10.38 ± 2.71 | 2.04 ± 0.82 | 1.63 ± 0.80 |
| Coarse particulate organic matter CPOM; coarse deposits, e.g., leaves | 1.83 ± 0.82 | 2.04 ± 0.81 | 0.88 ± 0.26 | 3.42 ± 1.38 |
| Fine particulate organic matter FPOM; fine deposits | 0.75 ± 0.41 | 3.96 ± 1.54 | 1.25 ± 0.62 | 4.25 ± 1.53 |
| Debris Hard and coarse matter—organic or inorganic | 2.67 ± 0.77 | 6.63 ± 2.04 | 3.13 ± 0.57 | 4.63 ± 1.10 |
| Mosses Fontinalis antipyretica and Marchantiophyta at the Site 1 | 6.25 ± 3.27 | 2.71 ± 1.34 | 1.58 ± 1.21 | 2.13 ± 0.83 |
| Filamentous algae | 0 | 0 | 0 | 2.33 ± 1.37 |
| Roots of riparian trees Alnus sp., Salix sp. or conifers | 0.58 ± 0.42 | 1.25 ± 0.86 | 3.33 ± 1.70 | 0 |
| Submerged riparian vegetation | 0.42 ± 0.40 | 0 | 1.25 ± 0.63 | 0.83 ± 0.54 |
| ‘Wet wipes’ Anthropogenic trash | 0 | 0 | 0 | 1.38 ± 0.61 |
| ‘Metal sheets’ Anthropogenic trash | 0 | 0 | 0 | 1.17 ± 1.12 |
| Factors | ||
|---|---|---|
| Response Variable | Site | Sampling Time |
| EPT abundance | χ23 = 1.19, p = 0.76 | χ23 = 3.96, p = 0.27 |
| EPT richness | F3,44 = 2.82, p = 0.049 | F3,41 = 1.14, p = 0.34 |
| E abundance | χ23 = 1.22, p = 0.75 | χ23 = 7.56, p = 0.055 |
| E richness | F3,44 = 0.14, p = 0.93 | F3,44 = 3.19, p = 0.03 |
| P abundance | χ23 = 56.33, p < 0.001 | χ23 = 9.70, p = 0.02 |
| P richness | χ23 = 124.03, p < 0.001 | χ23 = 8.14, p = 0.04 |
| T abundance | χ23 = 12.59, p = 0.006 | χ23 = 6.11, p = 0.11 |
| T richness | F3,44 = 1.03, p = 0.39 | F3,44 = 0.43, p = 0.73 |
| E family richness | F3,41 = 6.83, p < 0.001 | F3,41 = 2.48, p = 0.074 |
| P family richness | F3,44 = 23.70, p < 0.001 | F3,41 = 1.02, p = 0.39 |
| T family richness | F3,44 = 0.47, p = 0.70 | F3,44 = 0.23, p = 0.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Let, M.; Černý, J.; Nováková, P.; Ložek, F.; Bláha, M. Effects of Trace Metals and Municipal Wastewater on the Ephemeroptera, Plecoptera, and Trichoptera of a Stream Community. Biology 2022, 11, 648. https://doi.org/10.3390/biology11050648
Let M, Černý J, Nováková P, Ložek F, Bláha M. Effects of Trace Metals and Municipal Wastewater on the Ephemeroptera, Plecoptera, and Trichoptera of a Stream Community. Biology. 2022; 11(5):648. https://doi.org/10.3390/biology11050648
Chicago/Turabian StyleLet, Marek, Jan Černý, Petra Nováková, Filip Ložek, and Martin Bláha. 2022. "Effects of Trace Metals and Municipal Wastewater on the Ephemeroptera, Plecoptera, and Trichoptera of a Stream Community" Biology 11, no. 5: 648. https://doi.org/10.3390/biology11050648
APA StyleLet, M., Černý, J., Nováková, P., Ložek, F., & Bláha, M. (2022). Effects of Trace Metals and Municipal Wastewater on the Ephemeroptera, Plecoptera, and Trichoptera of a Stream Community. Biology, 11(5), 648. https://doi.org/10.3390/biology11050648






