Autologous Blood Doping Induced Changes in Red Blood Cell Rheologic Parameters, RBC Age Distribution, and Performance
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Overview of Study Design: ABD Procedure, Blood Sampling and Exercise Testing
2.3. Complete Blood Count
2.4. Calculation of RBC Volume
2.5. RBC Deformability and Osmoscan
2.6. Immunohistochemical Staining of Membrane Proteins
2.7. RBC Viscosity and Aggregation
2.8. RBC Electrolyte Concentrations
2.9. Glycerol Levels
2.10. Density Gradient Centrifugation
2.11. Exercise Testing
2.12. Statistical Analysis
3. Results
3.1. Transfusion Volume
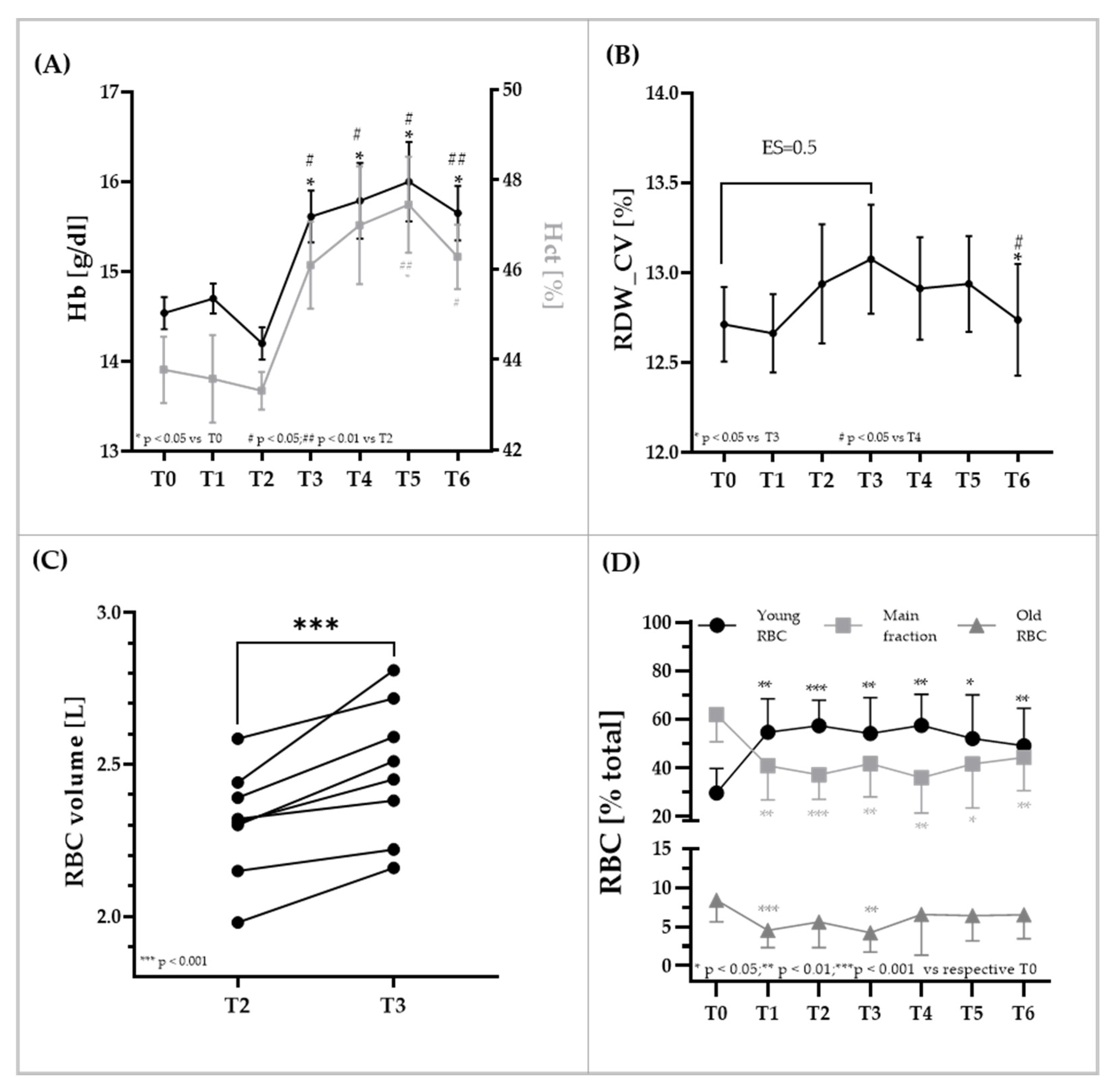
3.2. RBC Parameters
3.3. RBC Rheologic Parameters Aggregation, Viscosity, and Deformability of the Total RBC Population and RBC Deformability Measures of RBC Subpopulations
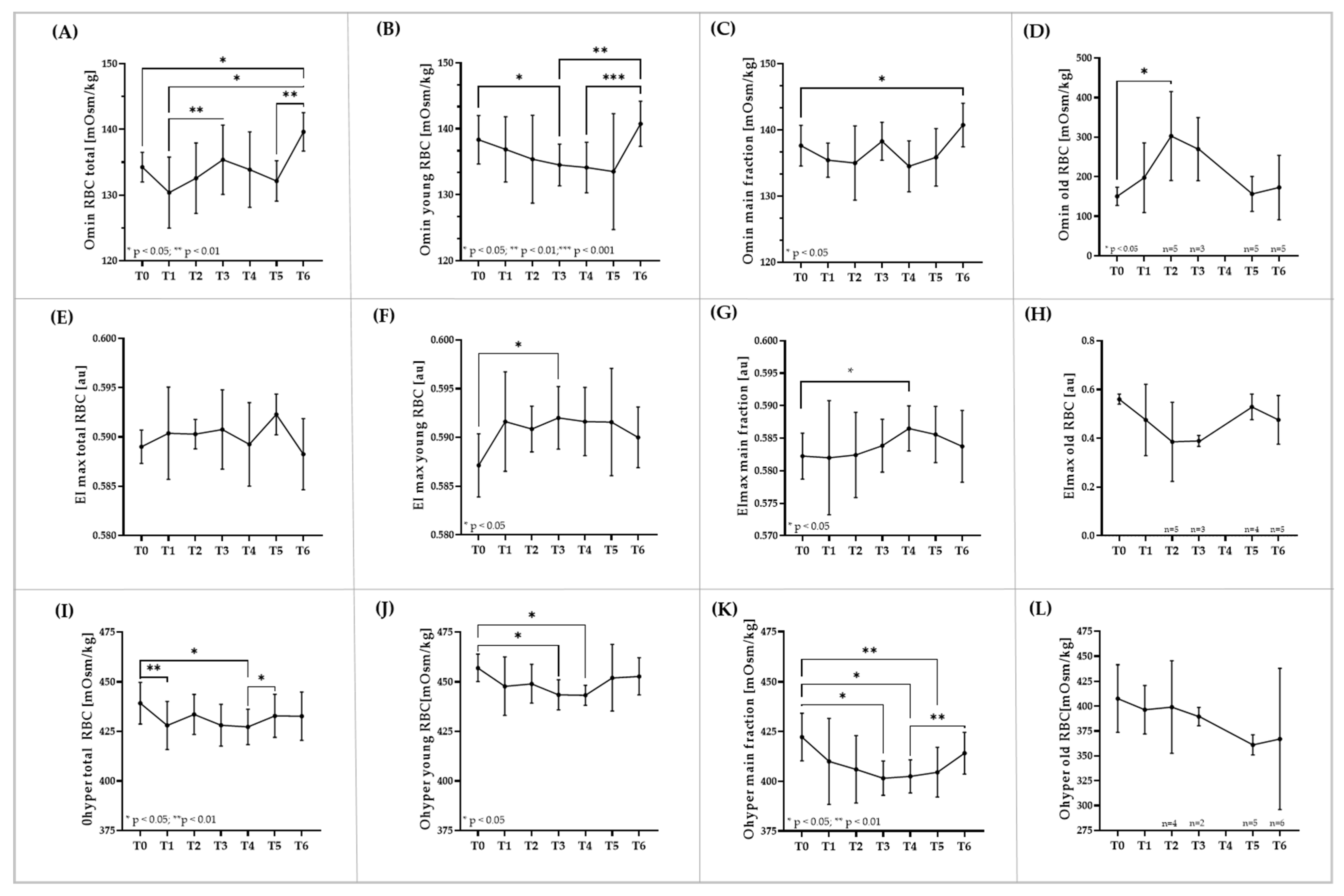
3.4. RBC Osmoscan of Total RBC and RBC Subpopulations
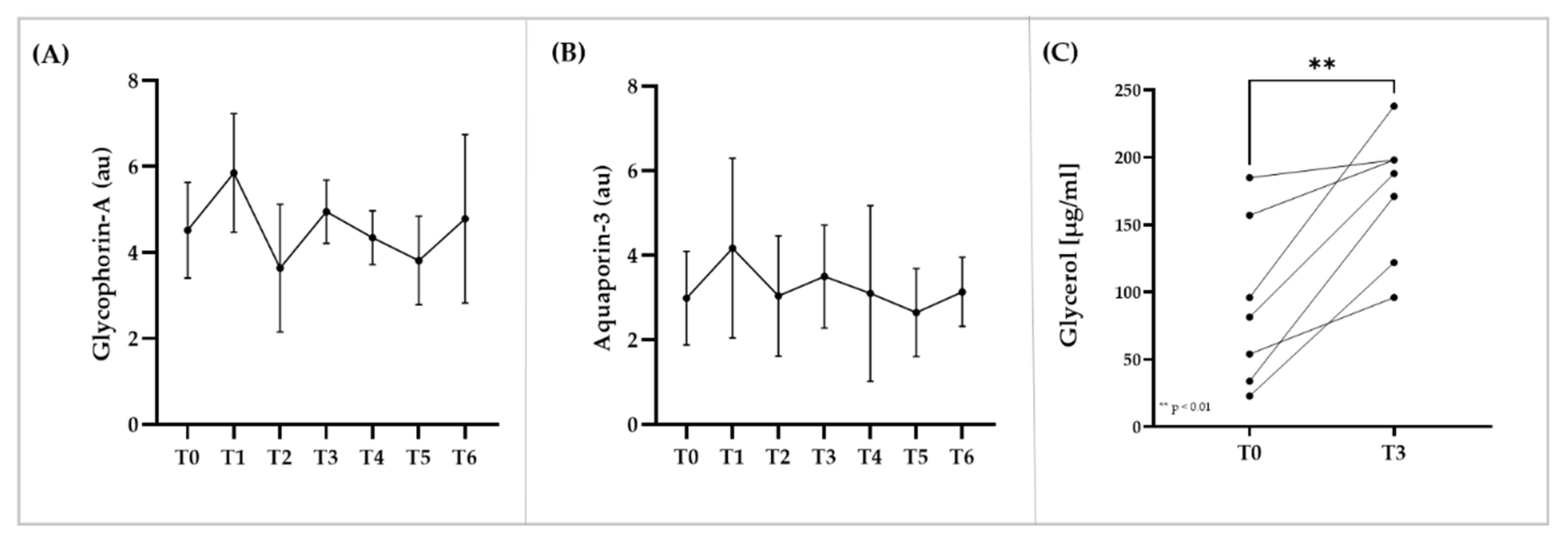
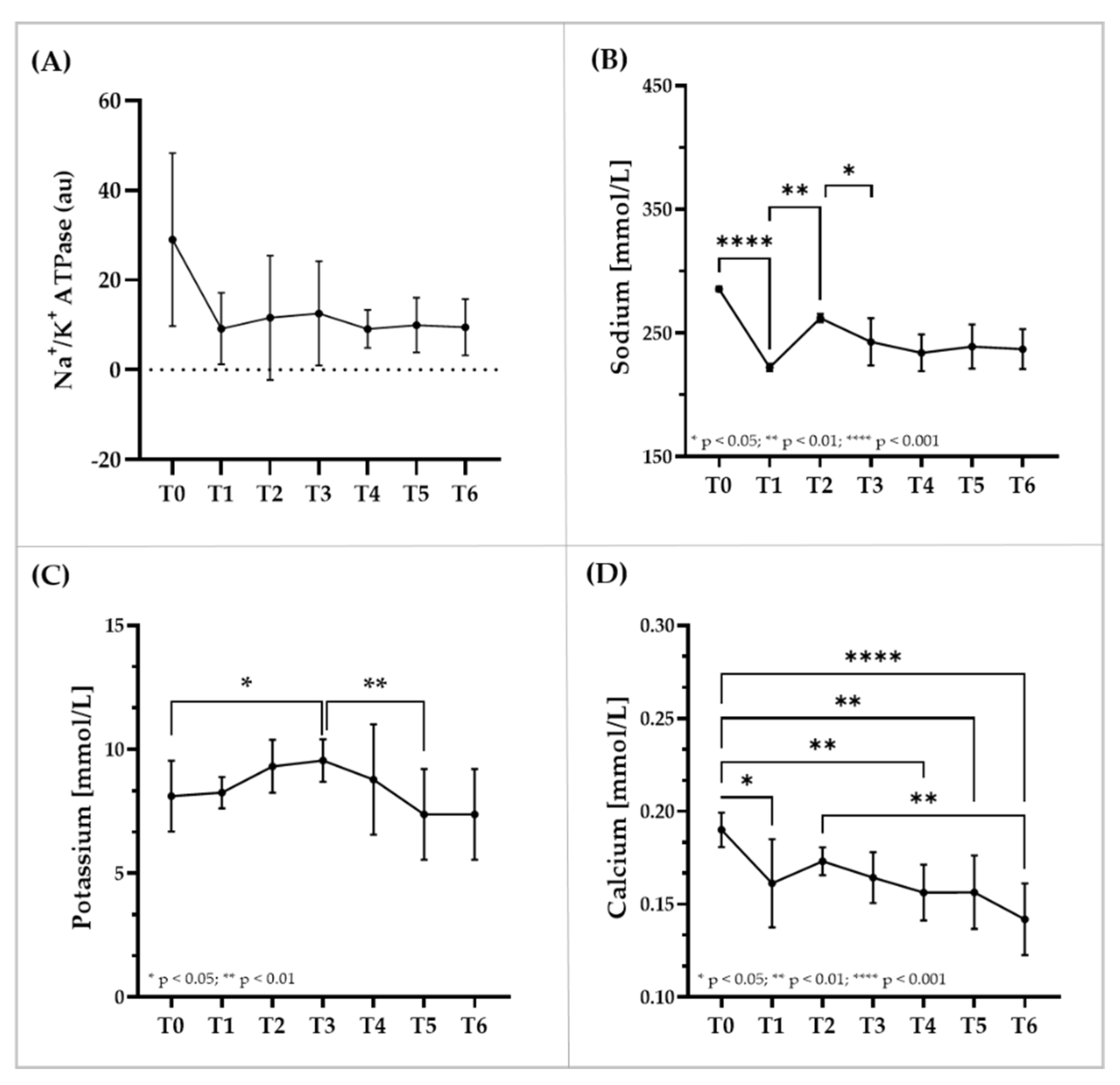
3.5. Immunostaining of RBC Membrane Proteins, Glycerol and Electrolyte Levels within RBC
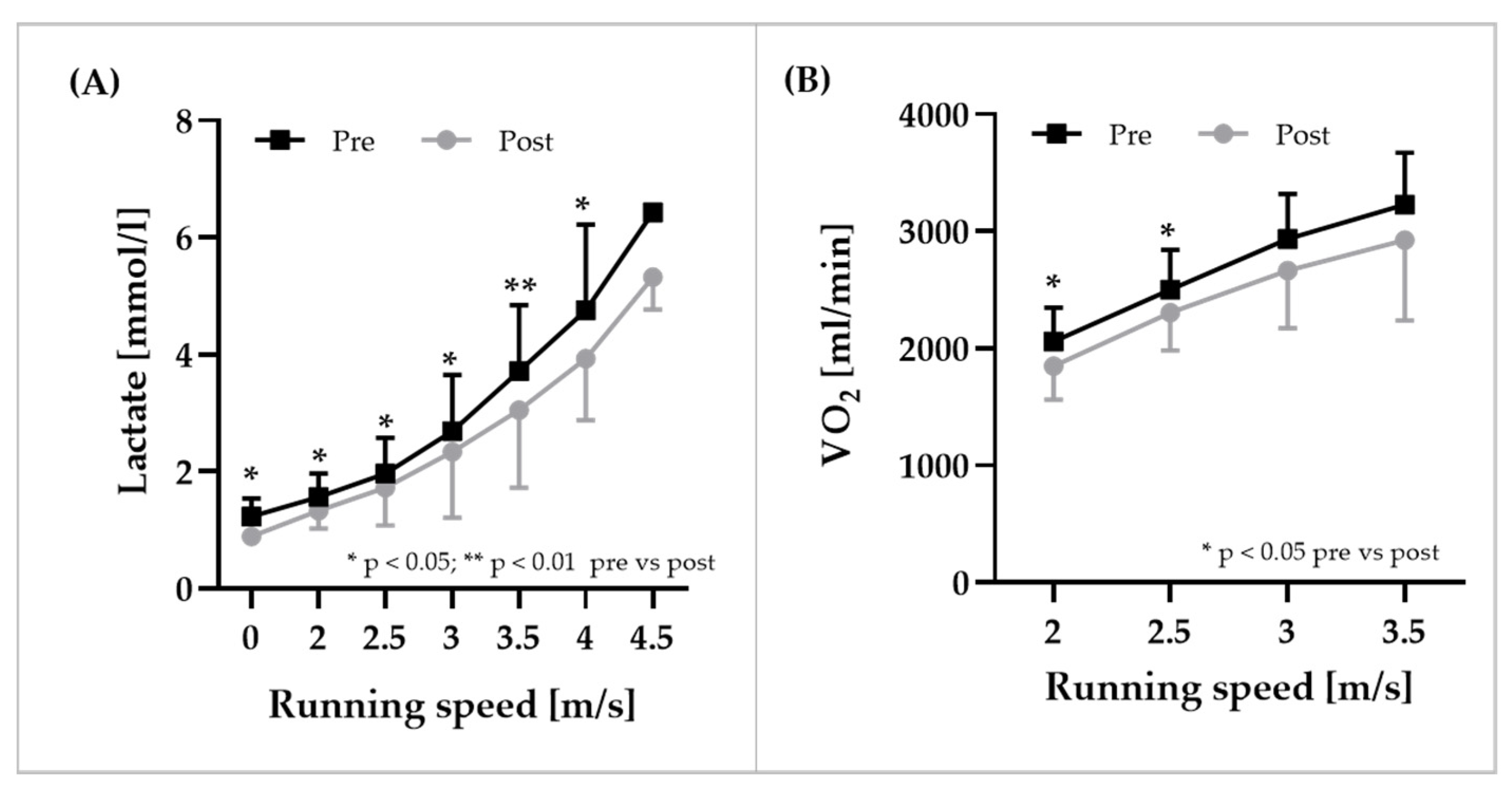
3.6. Exercise Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berglund, B. Development of techniques for the detection of blood doping in sport. Sports Med. 1988, 5, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Eichner, E.R. Better dead than second. J. Lab. Clin. Med. 1992, 120, 359–360. [Google Scholar]
- Lippi, G.; Banfi, G. Blood transfusions in athletes. Old dogmas, new tricks. Clin. Chem. Lab. Med. 2006, 44, 1395–1402. [Google Scholar] [CrossRef] [PubMed]
- Malm, C.B.; Khoo, N.S.; Granlund, I.; Lindstedt, E.; Hult, A. Autologous Doping with Cryopreserved Red Blood Cells—Effects on Physical Performance and Detection by Multivariate Statistics. PLoS ONE 2016, 11, e0156157. [Google Scholar] [CrossRef]
- Solheim, S.A.; Bejder, J.; Breenfeldt Andersen, A.; Mørkeberg, J.; Nordsborg, N.B. Autologous Blood Transfusion Enhances Exercise Performance-Strength of the Evidence and Physiological Mechanisms. Sports Med. Open 2019, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Seeger, B.; Grau, M. Relation between Exercise Performance and Blood Storage Condition and Storage Time in Autologous Blood Doping. Biology 2020, 10, 14. [Google Scholar] [CrossRef]
- Bizjak, D.A.; Grolle, A.; Urena, J.A.N.; Bloch, W.; Deitenbeck, R.; Grau, M. Monitoring of RBC rheology after cryopreservation to detect autologous blood doping in vivo? A pilot study. Clin. Hemorheol. Microcirc. 2020, 76, 367–379. [Google Scholar] [CrossRef]
- Bizjak, D.A.; Jungen, P.; Bloch, W.; Grau, M. Cryopreservation of red blood cells: Effect on rheologic properties and associated metabolic and nitric oxide related parameters. Cryobiology 2018, 84, 59–68. [Google Scholar] [CrossRef]
- Grau, M.; Friederichs, P.; Krehan, S.; Koliamitra, C.; Suhr, F.; Bloch, W. Decrease in red blood cell deformability is associated with a reduction in RBC-NOS activation during storage. Clin. Hemorheol. Microcirc. 2015, 60, 215–229. [Google Scholar] [CrossRef]
- Wang, D.; Sun, J.; Solomon, S.B.; Klein, H.G.; Natanson, C. Transfusion of older stored blood and risk of death: A meta-analysis. Transfusion 2012, 52, 1184–1195. [Google Scholar] [CrossRef]
- Hampton, D.A.; Wiles, C.; Fabricant, L.J.; Kiraly, L.; Differding, J.; Underwood, S.; Le, D.; Watters, J.; Schreiber, M.A. Cryopreserved red blood cells are superior to standard liquid red blood cells. J. Trauma Acute Care Surg. 2014, 77, 20–27; discussion 26–27. [Google Scholar] [CrossRef] [PubMed]
- Leigh-Smith, S. Blood boosting. Br. J. Sports Med. 2004, 38, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Jelkmann, W.; Lundby, C. Blood doping and its detection. Blood 2011, 118, 2395–2404. [Google Scholar] [CrossRef] [PubMed]
- Zorzoli, M.; Pipe, A.; Garnier, P.Y.; Vouillamoz, M.; Dvorak, J. Practical experience with the implementation of an athlete’s biological profile in athletics, cycling, football and swimming. Br. J. Sports Med. 2014, 48, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Berglund, B. High-altitude training. Aspects of haematological adaptation. Sports Med. 1992, 14, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-Y.; Hwang, H.; Park, J.; Lee, S.; Lim, K. The effects of altitude/hypoxic training on oxygen delivery capacity of the blood and aerobic exercise capacity in elite athletes—A meta-analysis. J. Exerc. Nutr. Biochem. 2016, 20, 15–22. [Google Scholar] [CrossRef]
- Salamin, O.; de Angelis, S.; Tissot, J.-D.; Saugy, M.; Leuenberger, N. Autologous Blood Transfusion in Sports: Emerging Biomarkers. Transfus. Med. Rev. 2016, 30, 109–115. [Google Scholar] [CrossRef]
- Mussack, V.; Wittmann, G.; Pfaffl, M.W. On the trail of blood doping-microRNA fingerprints to monitor autologous blood transfusions in vivo. Am. J. Hematol. 2021, 96, 338–353. [Google Scholar] [CrossRef]
- D’Amici, G.M.; Rinalducci, S.; Zolla, L. Proteomic analysis of RBC membrane protein degradation during blood storage. J. Proteome Res. 2007, 6, 3242–3255. [Google Scholar] [CrossRef]
- Leuenberger, N.; Barras, L.; Nicoli, R.; Robinson, N.; Baume, N.; Lion, N.; Barelli, S.; Tissot, J.-D.; Saugy, M. Urinary di-(2-ethylhexyl) phthalate metabolites for detecting transfusion of autologous blood stored in plasticizer-free bags. Transfusion 2016, 56, 571–578. [Google Scholar] [CrossRef]
- Donati, F.; Acciarini, R.; de Benedittis, I.; de La Torre, X.; Pirri, D.; Prete, M.; Stampella, A.; Vernucci, E.; Botre, F. Detecting Autologous Blood Transfusion in Doping Control: Biomarkers of Blood Aging and Storage Measured by Flow Cytofluorimetry. Curr. Pharm. Biotechnol. 2018, 19, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Voss, S.C.; Yassin, M.; Grivel, J.C.; Al Hmissi, S.; Allahverdi, N.; Nashwan, A.; Merenkov, Z.; Abdulla, M.; Al Malki, A.; Raynaud, C.; et al. Red blood cell derived extracellular vesicles during the process of autologous blood doping. Drug Test. Anal. 2021; ahead of print. [Google Scholar] [CrossRef]
- Marcogliese, A.N.; Yee, D.L. Resources for the Hematologist. In Hematology: Basic Principles and Practice, 7th ed.; Hoffman, R., Heslop, H., Weitz, J.I., Anastasi, J., Silberstein, L.E., Salama, M.E., Abutalib, S.A., Eds.; Elsevier: Philadelphia, PA, USA, 2018; pp. e1–e26. ISBN 9780323357623. [Google Scholar]
- Da Costa, L.; Suner, L.; Galimand, J.; Bonnel, A.; Pascreau, T.; Couque, N.; Fenneteau, O.; Mohandas, N. Diagnostic tool for red blood cell membrane disorders: Assessment of a new generation ektacytometer. Blood Cells Mol. Dis. 2016, 56, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Baskurt, O.K.; Hardeman, M.R.; Uyuklu, M.; Ulker, P.; Cengiz, M.; Nemeth, N.; Shin, S.; Alexy, T.; Meiselman, H.J. Comparison of three commercially available ektacytometers with different shearing geometries. Biorheology 2009, 46, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Suhr, F.; Porten, S.; Hertrich, T.; Brixius, K.; Schmidt, A.; Platen, P.; Bloch, W. Intensive exercise induces changes of endothelial nitric oxide synthase pattern in human erythrocytes. Nitric Oxide 2009, 20, 95–103. [Google Scholar] [CrossRef]
- Grau, M.; Pauly, S.; Ali, J.; Walpurgis, K.; Thevis, M.; Bloch, W.; Suhr, F. RBC-NOS-dependent S-nitrosylation of cytoskeletal proteins improves RBC deformability. PLoS ONE 2013, 8, e56759. [Google Scholar] [CrossRef]
- Baskurt, O.K.; Uyuklu, M.; Ulker, P.; Cengiz, M.; Nemeth, N.; Alexy, T.; Shin, S.; Hardeman, M.R.; Meiselman, H.J. Comparison of three instruments for measuring red blood cell aggregation. Clin. Hemorheol. Microcirc. 2009, 43, 283–298. [Google Scholar] [CrossRef]
- Bizjak, D.A.; Brinkmann, C.; Bloch, W.; Grau, M. Increase in Red Blood Cell-Nitric Oxide Synthase Dependent Nitric Oxide Production during Red Blood Cell Aging in Health and Disease: A Study on Age Dependent Changes of Rheologic and Enzymatic Properties in Red Blood Cells. PLoS ONE 2015, 10, e0125206. [Google Scholar] [CrossRef]
- Riebe, D.; Ehrman, J.K.; Liguori, G.; Magal, M. (Eds.) ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2018; ISBN 9781496339072. [Google Scholar]
- Mishra, P.; Pandey, C.M.; Singh, U.; Gupta, A.; Sahu, C.; Keshri, A. Descriptive statistics and normality tests for statistical data. Ann. Card. Anaesth. 2019, 22, 67–72. [Google Scholar] [CrossRef]
- Faiss, R.; Saugy, J.; Zollinger, A.; Robinson, N.; Schuetz, F.; Saugy, M.; Garnier, P.-Y. Prevalence Estimate of Blood Doping in Elite Track and Field Athletes during Two Major International Events. Front. Physiol. 2020, 11, 160. [Google Scholar] [CrossRef]
- Stray-Gundersen, J.; Videman, T.; Penttilä, I.; Lereim, I. Abnormal hematologic profiles in elite cross-country skiers: Blood doping or? Clin. J. Sport Med. 2003, 13, 132–137. [Google Scholar] [CrossRef][Green Version]
- Meurrens, J.; Steiner, T.; Ponette, J.; Janssen, H.A.; Ramaekers, M.; Wehrlin, J.P.; Vandekerckhove, P.; Deldicque, L. Effect of Repeated Whole Blood Donations on Aerobic Capacity and Hemoglobin Mass in Moderately Trained Male Subjects: A Randomized Controlled Trial. Sports Med. Open 2016, 2, 43. [Google Scholar] [CrossRef] [PubMed]
- Schotten, N.; Laarakkers, C.M.M.; Roelofs, R.W.; Origa, R.; van Kraaij, M.G.J.; Swinkels, D.W. EPO and hepcidin plasma concentrations in blood donors and β-thalassemia intermedia are not related to commercially tested plasma ERFE concentrations. Am. J. Hematol. 2017, 92, E29–E31. [Google Scholar] [CrossRef] [PubMed]
- Pottgiesser, T.; Specker, W.; Umhau, M.; Dickhuth, H.-H.; Roecker, K.; Schumacher, Y.O. Recovery of hemoglobin mass after blood donation. Transfusion 2008, 48, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Robinson, N.; Sottas, P.-E.; Mangin, P.; Saugy, M. Bayesian detection of abnormal hematological values to introduce a no-start rule for heterogeneous populations of athletes. Haematologica 2007, 92, 1143–1144. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, Y.O.; Grathwohl, D.; Barturen, J.M.; Wollenweber, M.; Heinrich, L.; Schmid, A.; Huber, G.; Keul, J. Haemoglobin, haematocrit and red blood cell indices in elite cyclists. Are the control values for blood testing valid? Int. J. Sports Med. 2000, 21, 380–385. [Google Scholar] [CrossRef]
- Sennels, H.P.; Jørgensen, H.L.; Hansen, A.-L.S.; Goetze, J.P.; Fahrenkrug, J. Diurnal variation of hematology parameters in healthy young males: The Bispebjerg study of diurnal variations. Scand. J. Clin. Lab. Investig. 2011, 71, 532–541. [Google Scholar] [CrossRef]
- Pallotta, V.; D’Amici, G.M.; D’Alessandro, A.; Rossetti, R.; Zolla, L. Red blood cell processing for cryopreservation: From fresh blood to deglycerolization. Blood Cells Mol. Dis. 2012, 48, 226–232. [Google Scholar] [CrossRef]
- Mairbäurl, H.; Humpeler, E.; Schwaberger, G.; Pessenhofer, H. Training-dependent changes of red cell density and erythrocytic oxygen transport. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1983, 55, 1403–1407. [Google Scholar] [CrossRef]
- Tomschi, F.; Bizjak, D.; Bloch, W.; Latsch, J.; Predel, H.G.; Grau, M. Deformability of different red blood cell populations and viscosity of differently trained young men in response to intensive and moderate running. Clin. Hemorheol. Microcirc. 2018, 69, 503–514. [Google Scholar] [CrossRef]
- Smith, J.A. Exercise, training and red blood cell turnover. Sports Med. 1995, 19, 9–31. [Google Scholar] [CrossRef]
- Smith, J.A.; Martin, D.T.; Telford, R.D.; Ballas, S.K. Greater erythrocyte deformability in world-class endurance athletes. Am. J. Physiol. 1999, 276, H2188-93. [Google Scholar] [CrossRef] [PubMed]
- Bizjak, D.A.; Tomschi, F.; Bales, G.; Nader, E.; Romana, M.; Connes, P.; Bloch, W.; Grau, M. Does endurance training improve red blood cell aging and hemorheology in moderate-trained healthy individuals? J. Sport Health Sci. 2020, 9, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Namvar, A.; Blanch, A.J.; Dixon, M.W.; Carmo, O.M.S.; Liu, B.; Tiash, S.; Looker, O.; Andrew, D.; Chan, L.-J.; Tham, W.-H.; et al. Surface area-to-volume ratio, not cellular viscoelasticity, is the major determinant of red blood cell traversal through small channels. Cell. Microbiol. 2021, 23, e13270. [Google Scholar] [CrossRef]
- van Cromvoirt, A.M.; Fenk, S.; Sadafi, A.; Melnikova, E.V.; Lagutkin, D.A.; Dey, K.; Petrushanko, I.Y.; Hegemann, I.; Goede, J.S.; Bogdanova, A. Donor Age and Red Cell Age Contribute to the Variance in Lorrca Indices in Healthy Donors for Next Generation Ektacytometry: A Pilot Study. Front. Physiol. 2021, 12, 639722. [Google Scholar] [CrossRef] [PubMed]
- Saugel, B.; Klein, M.; Hapfelmeier, A.; Phillip, V.; Schultheiss, C.; Meidert, A.S.; Messer, M.; Schmid, R.M.; Huber, W. Effects of red blood cell transfusion on hemodynamic parameters: A prospective study in intensive care unit patients. Scand. J. Trauma Resusc. Emerg. Med. 2013, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Pichon, A.P.; Connes, P.; Robach, P. Effects of acute and chronic hematocrit modulations on blood viscosity in endurance athletes. Clin. Hemorheol. Microcirc. 2016, 64, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Barath, B.; Somogyi, V.; Tanczos, B.; Varga, A.; Bereczky, Z.; Nemeth, N.; Deak, A. Examination of the relation between red blood cell aggregation and hematocrit in human and various experimental animals. Clin. Hemorheol. Microcirc. 2021, 78, 187–198. [Google Scholar] [CrossRef]
- Brien, A.J.; Simon, T.L. The effects of red blood cell infusion on 10-km race time. JAMA 1987, 257, 2761–2765. [Google Scholar] [CrossRef]
- Çınar, Y. Effect of hematocrit on blood pressure via hyperviscosity. Am. J. Hypertens. 1999, 12, 739–743. [Google Scholar] [CrossRef]
- Chasis, J.A.; Mohandas, N.; Shohet, S.B. Erythrocyte membrane rigidity induced by glycophorin A-ligand interaction. Evidence for a ligand-induced association between glycophorin A and skeletal proteins. J. Clin. Investig. 1985, 75, 1919–1926. [Google Scholar] [CrossRef]
- Sparrow, R.L.; Sran, A.; Healey, G.; Veale, M.F.; Norris, P.J. In vitro measures of membrane changes reveal differences between red blood cells stored in saline-adenine-glucose-mannitol and AS-1 additive solutions: A paired study. Transfusion 2014, 54, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, R.L.; Healey, G.; Patton, K.A.; Veale, M.F. Red blood cell age determines the impact of storage and leukocyte burden on cell adhesion molecules, glycophorin A and the release of annexin V. Transfus. Apher. Sci. 2006, 34, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Roudier, N.; Verbavatz, J.M.; Maurel, C.; Ripoche, P.; Tacnet, F. Evidence for the presence of aquaporin-3 in human red blood cells. J. Biol. Chem. 1998, 273, 8407–8412. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.A.; Nsier, N.; Acker, J.P. Use of supernatant refractive index and supernatant hemoglobin concentration to assess residual glycerol concentration in cryopreserved red blood cells. Clin. Chim. Acta 2009, 408, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.S.; Nahata, M.C.; Hilty, M.D. Glycerol: A review of its pharmacology, pharmacokinetics, adverse reactions, and clinical use. Pharmacotherapy 1981, 1, 147–160. [Google Scholar] [CrossRef]
- Hagström-Toft, E.; Enoksson, S.; Moberg, E.; Bolinder, J.; Arner, P. Absolute concentrations of glycerol and lactate in human skeletal muscle, adipose tissue, and blood. Am. J. Physiol. 1997, 273, E584–E592. [Google Scholar] [CrossRef]
- Jensen, M.D.; Chandramouli, V.; Schumann, W.C.; Ekberg, K.; Previs, S.F.; Gupta, S.; Landau, B.R. Sources of blood glycerol during fasting. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E998–E1004. [Google Scholar] [CrossRef]
- Radosinska, J.; Vrbjar, N. The role of red blood cell deformability and Na,K-ATPase function in selected risk factors of cardiovascular diseases in humans: Focus on hypertension, diabetes mellitus and hypercholesterolemia. Physiol. Res. 2016, 65 (Suppl. 1), S43–S54. [Google Scholar] [CrossRef]
- Beilin, L.J.; Knight, G.J.; Munro-Faure, A.D.; Anderson, J. The sodium, potassium, and water contents of red blood cells of healthy human adults. J. Clin. Investig. 1966, 45, 1817–1825. [Google Scholar] [CrossRef]
- Grau, M.; Cremer, J.M.; Schmeichel, S.; Kunkel, M.; Bloch, W. Comparisons of Blood Parameters, Red Blood Cell Deformability and Circulating Nitric Oxide Between Males and Females Considering Hormonal Contraception: A Longitudinal Gender Study. Front. Physiol. 2018, 9, 1835. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grau, M.; Zollmann, E.; Bros, J.; Seeger, B.; Dietz, T.; Noriega Ureña, J.A.; Grolle, A.; Zacher, J.; Notbohm, H.L.; Suck, G.; et al. Autologous Blood Doping Induced Changes in Red Blood Cell Rheologic Parameters, RBC Age Distribution, and Performance. Biology 2022, 11, 647. https://doi.org/10.3390/biology11050647
Grau M, Zollmann E, Bros J, Seeger B, Dietz T, Noriega Ureña JA, Grolle A, Zacher J, Notbohm HL, Suck G, et al. Autologous Blood Doping Induced Changes in Red Blood Cell Rheologic Parameters, RBC Age Distribution, and Performance. Biology. 2022; 11(5):647. https://doi.org/10.3390/biology11050647
Chicago/Turabian StyleGrau, Marijke, Emily Zollmann, Janina Bros, Benedikt Seeger, Thomas Dietz, Javier Antonio Noriega Ureña, Andreas Grolle, Jonas Zacher, Hannah L. Notbohm, Garnet Suck, and et al. 2022. "Autologous Blood Doping Induced Changes in Red Blood Cell Rheologic Parameters, RBC Age Distribution, and Performance" Biology 11, no. 5: 647. https://doi.org/10.3390/biology11050647
APA StyleGrau, M., Zollmann, E., Bros, J., Seeger, B., Dietz, T., Noriega Ureña, J. A., Grolle, A., Zacher, J., Notbohm, H. L., Suck, G., Bloch, W., & Schumann, M. (2022). Autologous Blood Doping Induced Changes in Red Blood Cell Rheologic Parameters, RBC Age Distribution, and Performance. Biology, 11(5), 647. https://doi.org/10.3390/biology11050647






