Anti-Inflammatory and Antioxidant Properties of Tart Cherry Consumption in the Heart of Obese Rats
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal and Blood Parameters
2.2. Morphological Aspects
2.3. Western Blot and Quantification
2.4. Immunohistochemistry and Image Analysis
2.5. Immunofluorescence and Quantification
2.6. Data Analysis
3. Results
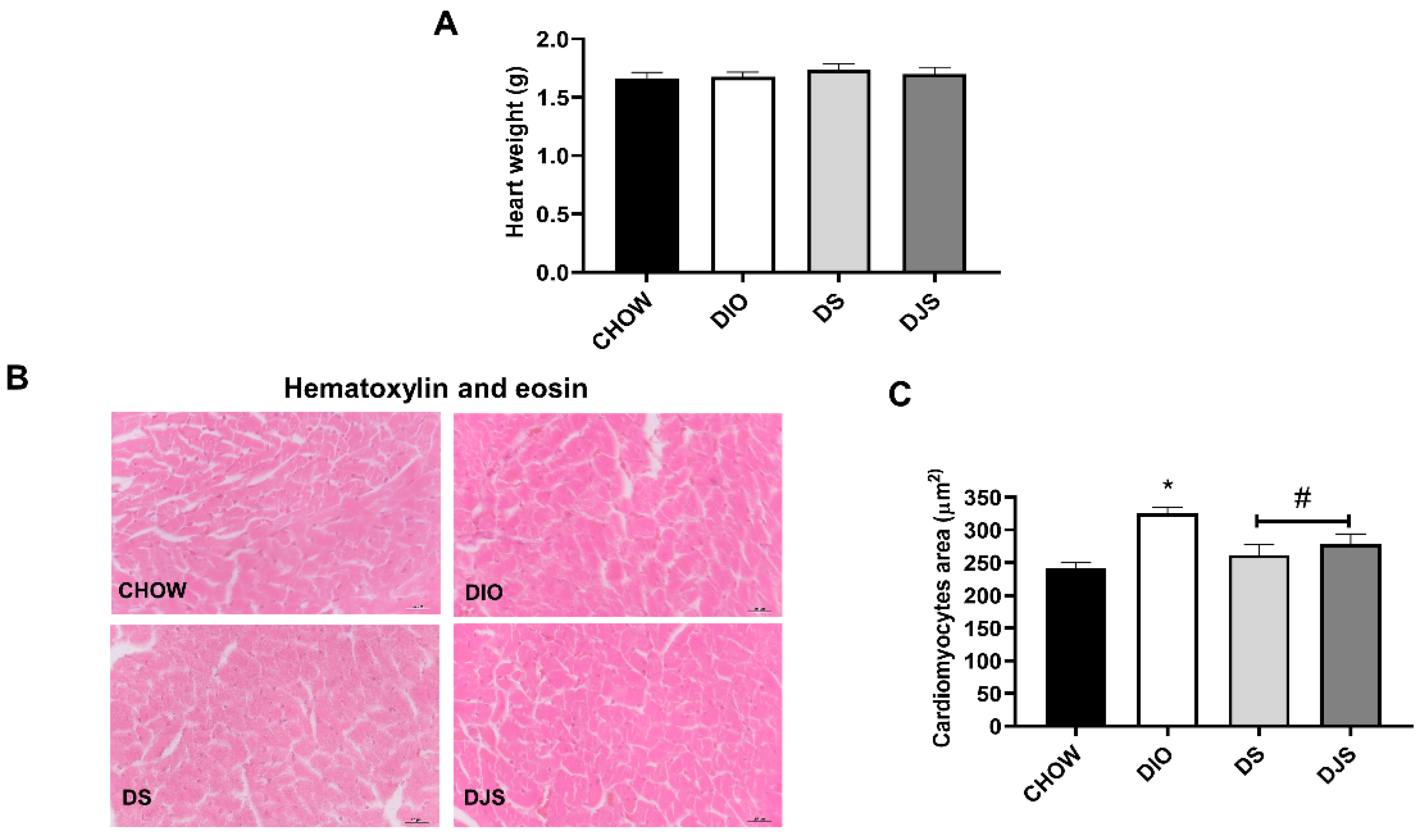
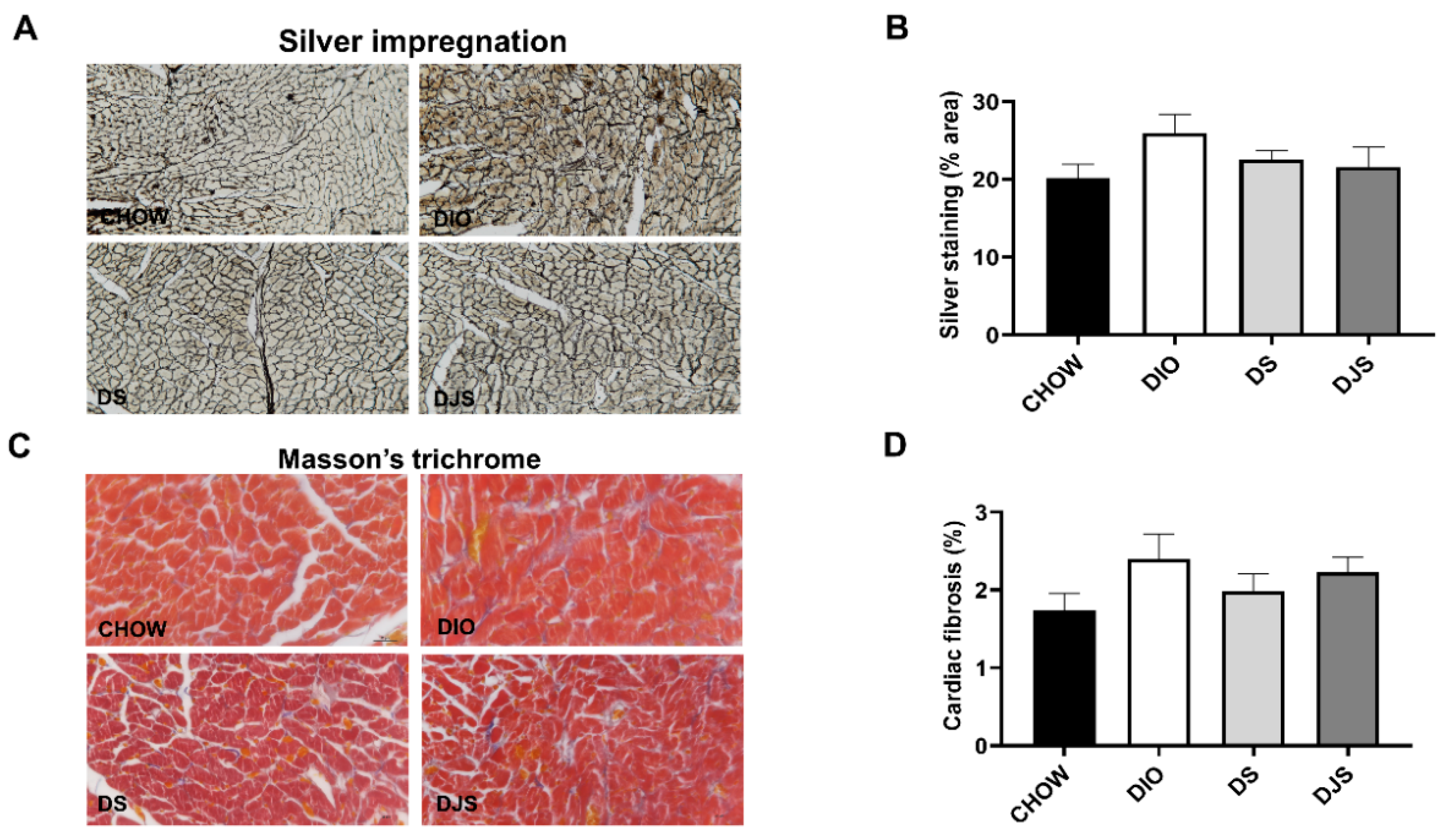
3.1. Weight and Heart Morphology
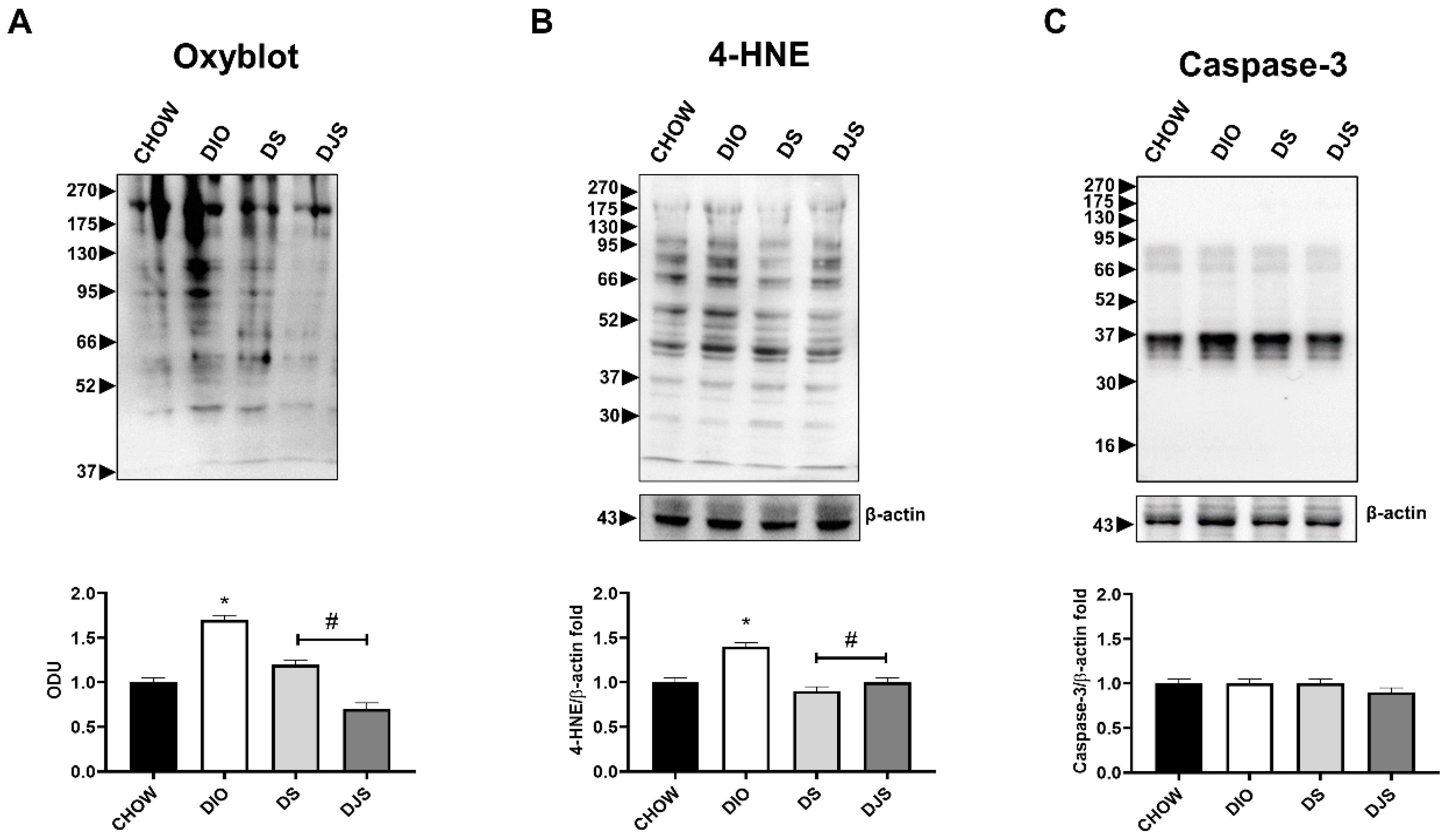
3.2. Oxidative Stress and Apoptosis
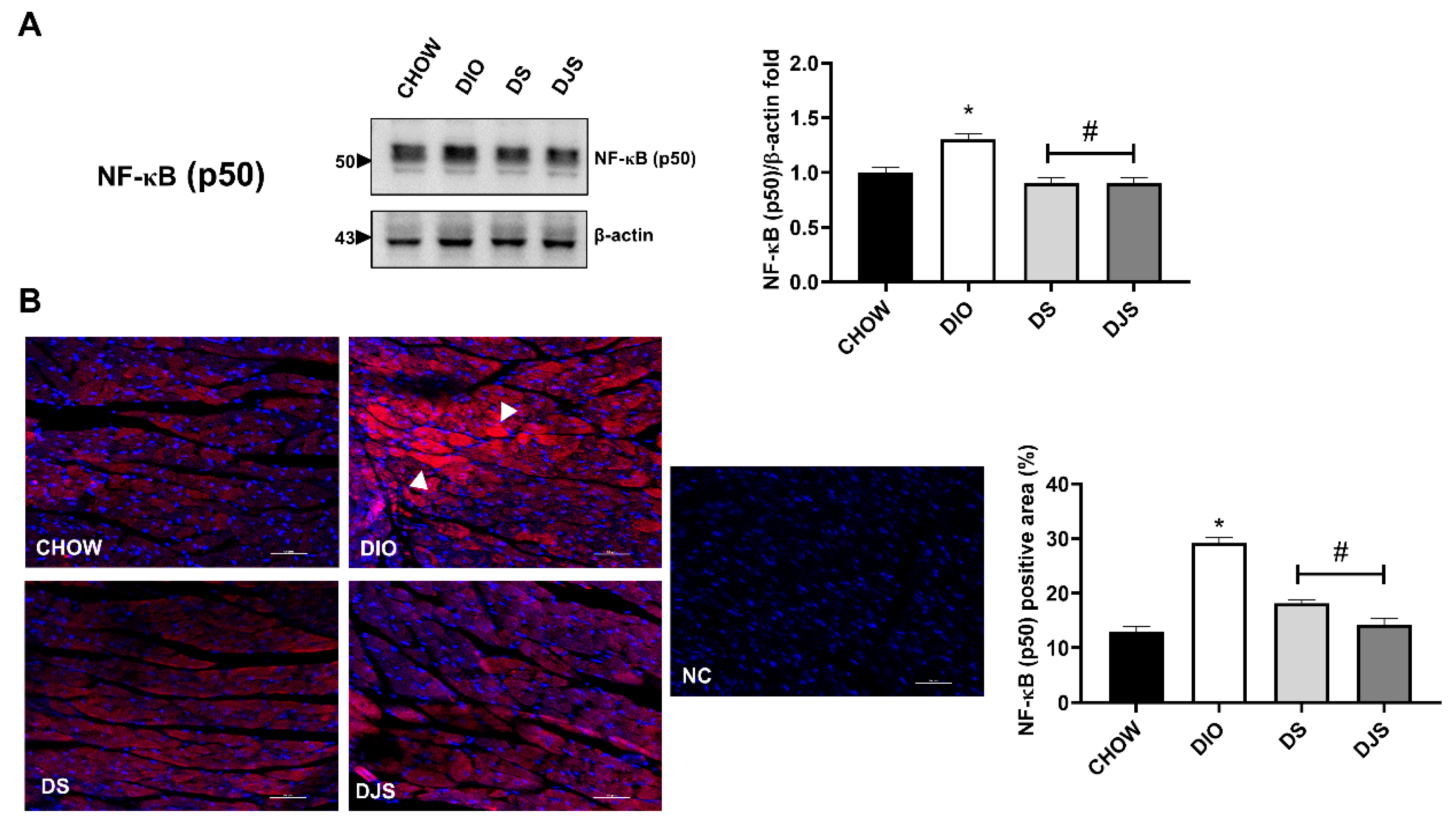
3.3. Inflammation
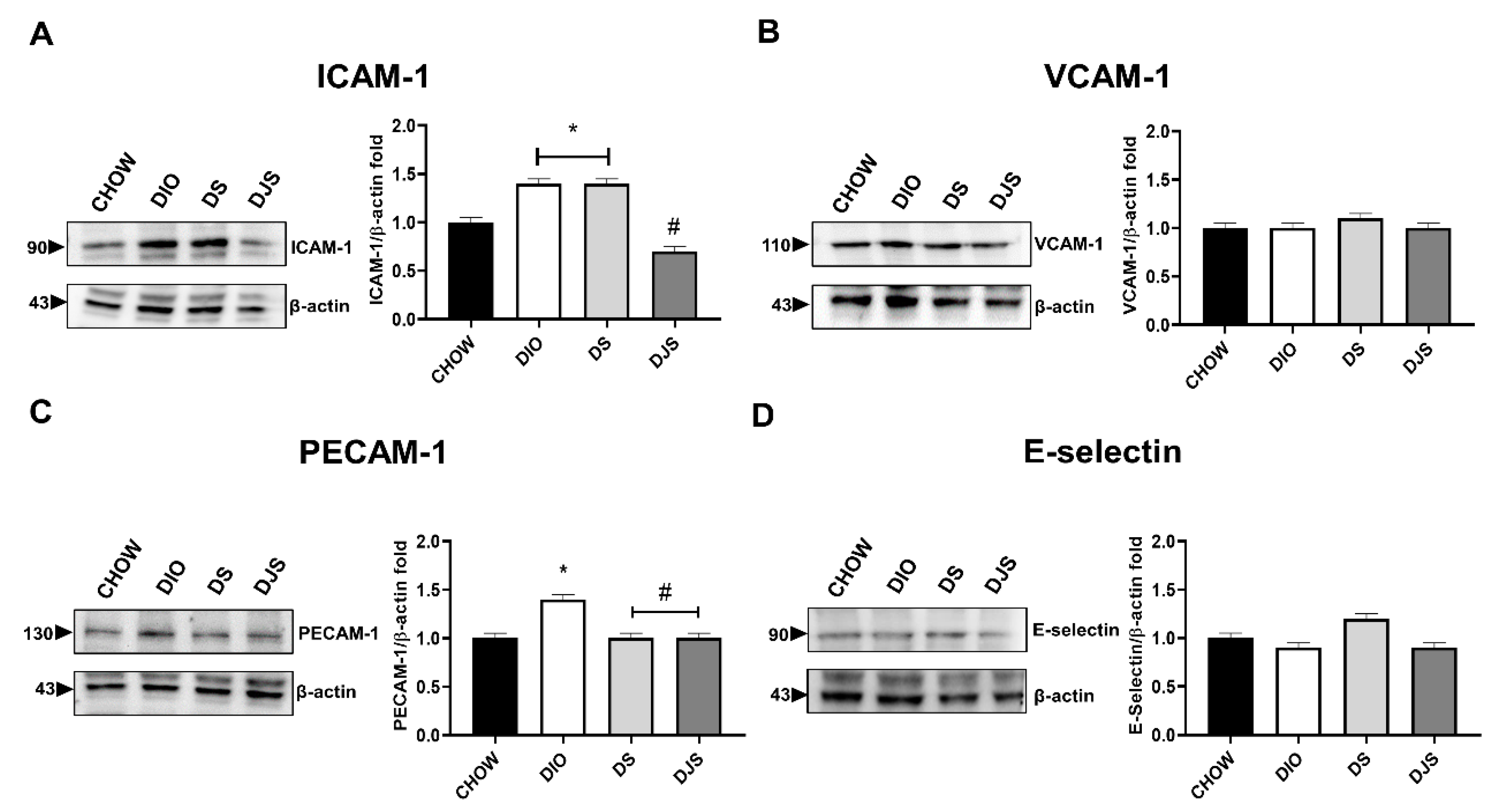
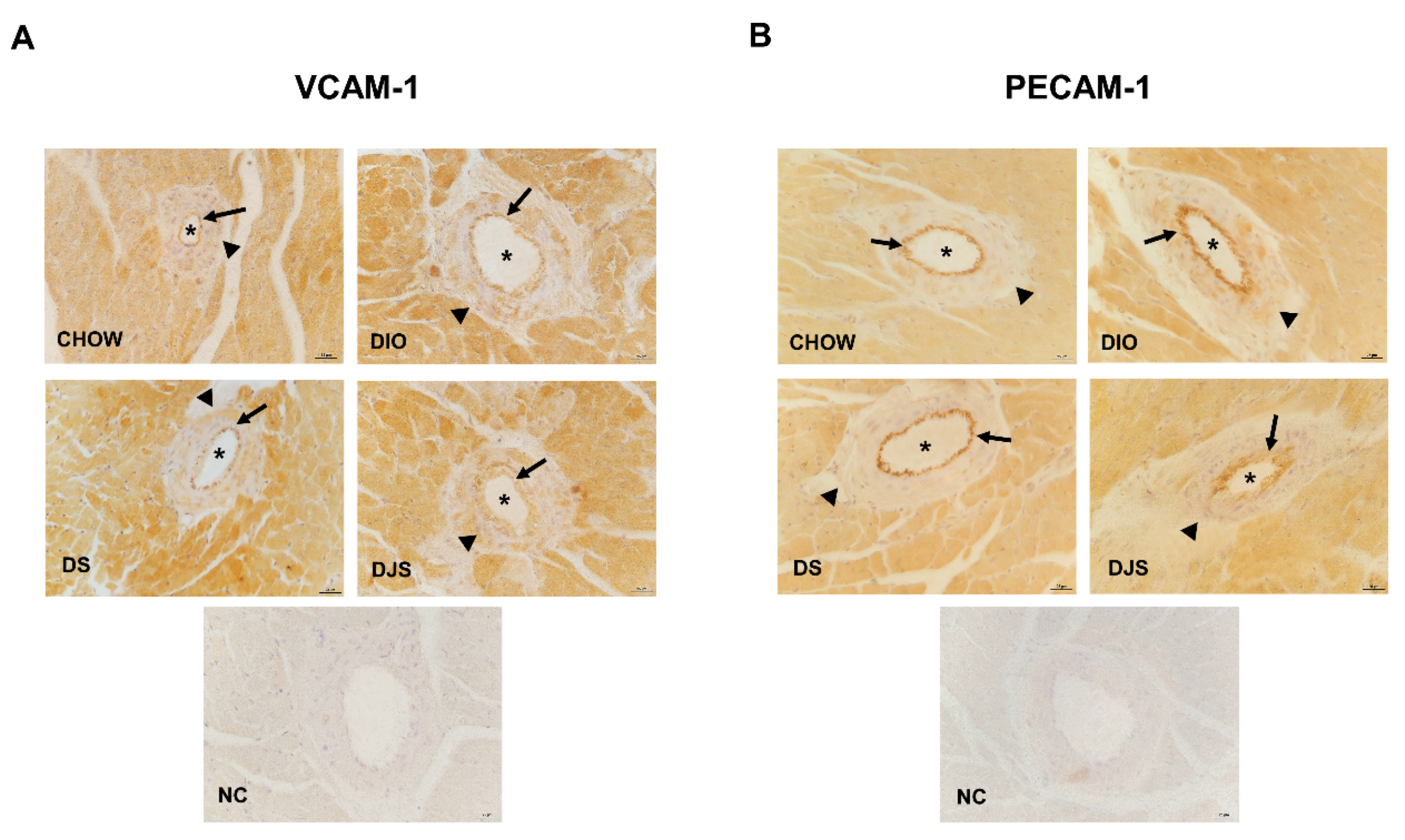
3.3.1. Adhesion Molecules
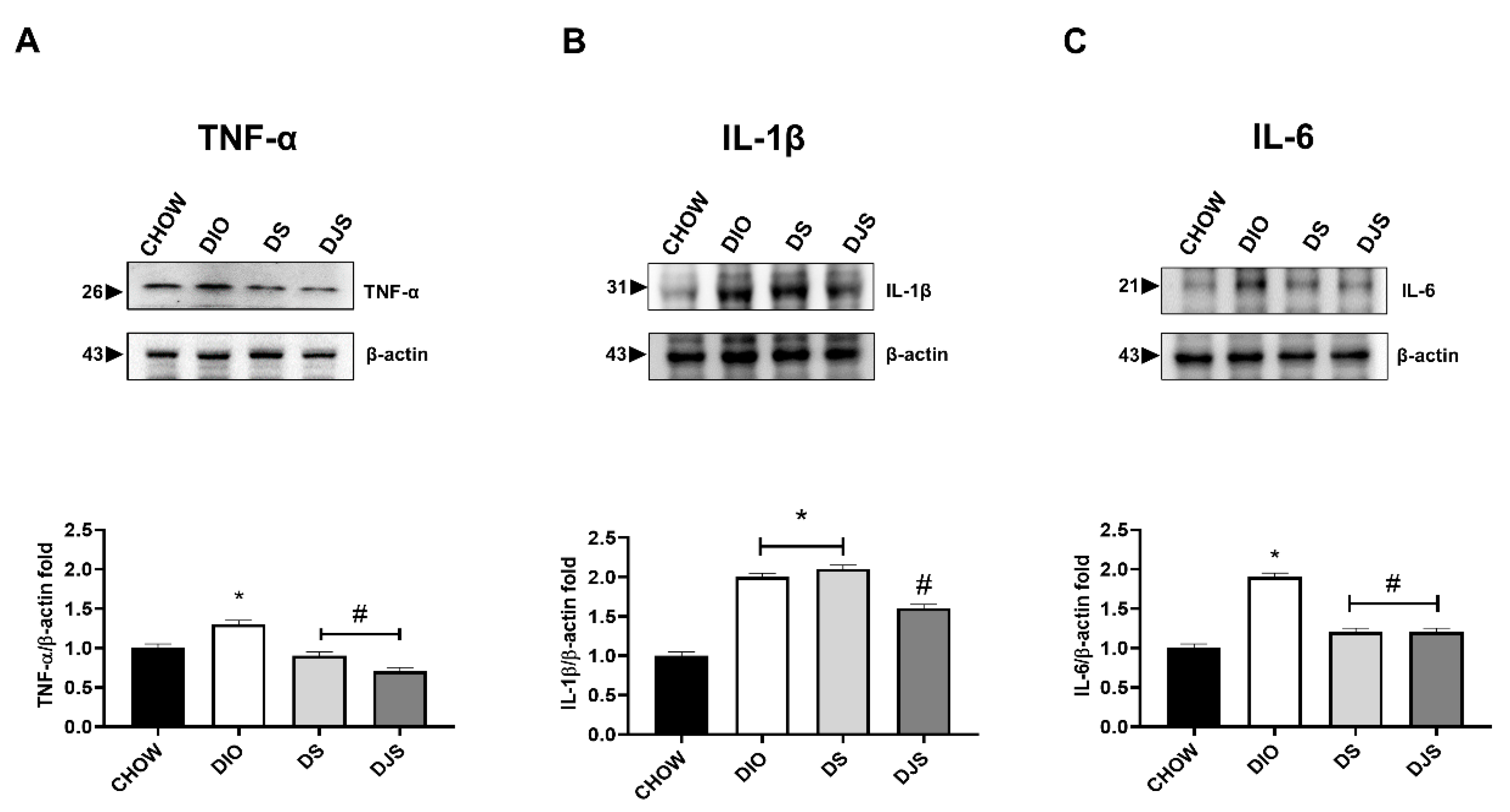
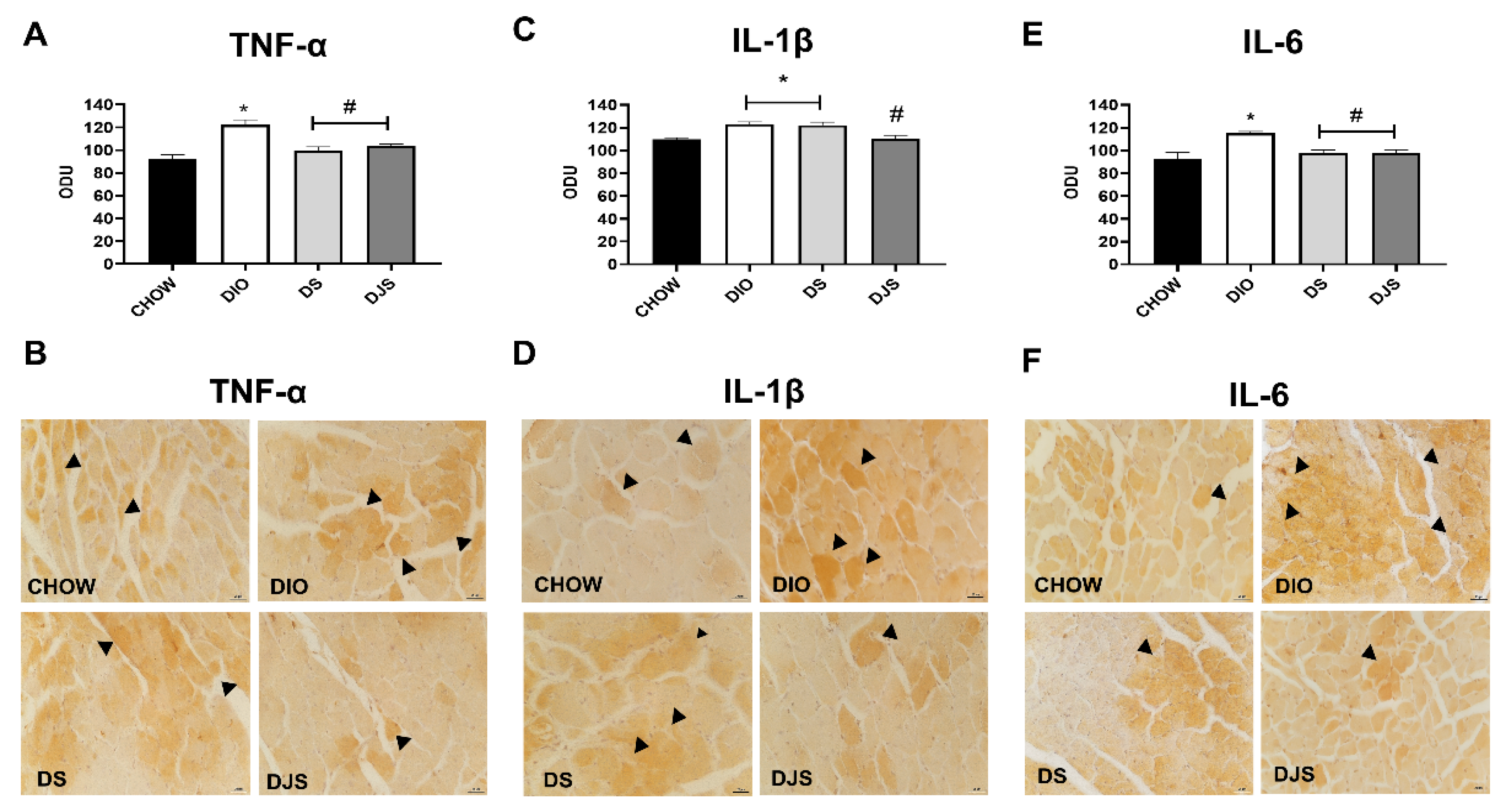
3.3.2. Cytokines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ortega, F.B.; Lavie, C.J.; Blair, S.N. Obesity and Cardiovascular Disease. Circ. Res. 2016, 118, 1752–1770. [Google Scholar] [CrossRef] [PubMed]
- Koliaki, C.; Liatis, S.; Kokkinos, A. Obesity and cardiovascular disease: Revisiting an old relationship. Metabolism 2019, 92, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Battineni, G.; Sagaro, G.G.; Chintalapudi, N.; Amenta, F.; Tomassoni, D.; Tayebati, S.K. Impact of Obesity-Induced Inflammation on Cardiovascular Diseases (CVD). Int. J. Mol. Sci. 2021, 22, 4798. [Google Scholar] [CrossRef] [PubMed]
- Odrowaz-Sypniewska, G. Markers of pro-inflammatory and pro-thrombotic state in the diagnosis of metabolic syndrome. Adv. Med. Sci. 2007, 52, 246–250. [Google Scholar]
- Santilli, F.; Guagnano, M.T.; Vazzana, N.; La Barba, S.; Davi, G. Oxidative stress drivers and modulators in obesity and cardiovascular disease: From biomarkers to therapeutic approach. Curr. Med. Chem. 2015, 22, 582–595. [Google Scholar] [CrossRef]
- Patel, C.; Ghanim, H.; Ravishankar, S.; Sia, C.L.; Viswanathan, P.; Mohanty, P.; Dandona, P. Prolonged reactive oxygen species generation and nuclear factor-kappaB activation after a high-fat, high-carbohydrate meal in the obese. J. Clin. Endocrinol. Metab. 2007, 92, 4476–4479. [Google Scholar] [CrossRef]
- Makki, K.; Froguel, P.; Wolowczuk, I. Adipose tissue in obesity-related inflammation and insulin resistance: Cells, cytokines, and chemokines. ISRN Inflamm. 2013, 2013, 139239. [Google Scholar] [CrossRef]
- Jung, U.J.; Choi, M.S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef]
- Bondia-Pons, I.; Ryan, L.; Martinez, J.A. Oxidative stress and inflammation interactions in human obesity. J. Physiol. Biochem. 2012, 68, 701–711. [Google Scholar] [CrossRef]
- Di Domenico, M.; Pinto, F.; Quagliuolo, L.; Contaldo, M.; Settembre, G.; Romano, A.; Coppola, M.; Ferati, K.; Bexheti-Ferati, A.; Sciarra, A.; et al. The Role of Oxidative Stress and Hormones in Controlling Obesity. Front. Endocrinol. (Lausanne) 2019, 10, 540. [Google Scholar] [CrossRef]
- Roy, P.; Tomassoni, D.; Traini, E.; Martinelli, I.; Micioni Di Bonaventura, M.V.; Cifani, C.; Amenta, F.; Tayebati, S.K. Natural Antioxidant Application on Fat Accumulation: Preclinical Evidence. Antioxidants 2021, 10, 858. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.S.; Adkins, Y.; Laugero, K.D. A Review of the Health Benefits of Cherries. Nutrients 2018, 10, 368. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C. Anthocyanins in cardiovascular disease. Adv. Nutr. 2011, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef]
- Nöthlings, U.; Schulze, M.B.; Weikert, C.; Boeing, H.; van der Schouw, Y.T.; Bamia, C.; Benetou, V.; Lagiou, P.; Krogh, V.; Beulens, J.W.; et al. Intake of vegetables, legumes, and fruit, and risk for all-cause, cardiovascular, and cancer mortality in a European diabetic population. J. Nutr. 2008, 138, 775–781. [Google Scholar] [CrossRef]
- Mozos, I.; Flangea, C.; Vlad, D.C.; Gug, C.; Mozos, C.; Stoian, D.; Luca, C.T.; Horbańczuk, J.O.; Horbańczuk, O.K.; Atanasov, A.G. Effects of Anthocyanins on Vascular Health. BioMolecules 2021, 11, 811. [Google Scholar] [CrossRef]
- Reis, J.F.; Monteiro, V.V.; de Souza Gomes, R.; do Carmo, M.M.; da Costa, G.V.; Ribera, P.C.; Monteiro, M.C. Action mechanism and cardiovascular effect of anthocyanins: A systematic review of animal and human studies. J. Transl. Med. 2016, 14, 315. [Google Scholar] [CrossRef]
- Mazza, G.J. Anthocyanins and heart health. Ann. Ist. Super. Sanita 2007, 43, 369–374. [Google Scholar]
- Tsuda, T.; Horio, F.; Osawa, T. The role of anthocyanins as an antioxidant under oxidative stress in rats. Biofactors 2000, 13, 133–139. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries, and lingonberries. J. Agric. Food Chem. 2003, 51, 502–509. [Google Scholar] [CrossRef]
- Youdim, K.A.; McDonald, J.; Kalt, W.; Joseph, J.A. Potential role of dietary flavonoids in reducing microvascular endothelium vulnerability to oxidative and inflammatory insults. J. Nutr. Biochem. 2002, 13, 282–288. [Google Scholar] [CrossRef]
- Wang, J.; Mazza, G. Inhibitory effects of anthocyanins and other phenolic compounds on nitric oxide production in LPS/IFN- activated RAW 264.7 macrophages. J. Agric. Food Chem. 2002, 50, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.W.; Gong, C.C.; Song, H.F.; Cui, Y.Y. Effects of anthocyanins on the prevention and treatment of cancer. Br. J. Pharmacol. 2017, 174, 1226–1243. [Google Scholar] [CrossRef] [PubMed]
- Katsube, N.; Iwashita, K.; Tsushida, T.; Yamaki, K.; Kobori, M. Induction of apoptosis in cancer cells by Bilberry (Vaccinium myrtillus) and the anthocyanins. J. Agric. Food Chem. 2003, 51, 68–75. [Google Scholar] [CrossRef]
- Galli, R.L.; Shukitt-Hale, B.; Youdim, K.A.; Joseph, J.A. Fruit polyphenolics and brain aging: Nutritional interventions targeting age-related neuronal and behavioral deficits. Ann. N. Y. Acad. Sci. 2002, 959, 128–132. [Google Scholar] [CrossRef]
- Micioni Di Bonaventura, M.V.; Martinelli, I.; Moruzzi, M.; Micioni Di Bonaventura, E.; Giusepponi, M.E.; Polidori, C.; Lupidi, G.; Tayebati, S.K.; Amenta, F.; Cifani, C.; et al. Brain alterations in high fat diet induced obesity: Effects of tart cherry seeds and juice. Nutrients 2020, 12, 623. [Google Scholar] [CrossRef]
- Andriambeloson, E.; Magnier, C.; Haan-Archipoff, G.; Lobstein, A.; Anton, R.; Beretz, A.; Stoclet, J.C.; Andriantsitohaina, R. Natural dietary polyphenolic compounds cause endothelium-dependent vasorelaxation in rat thoracic aorta. J. Nutr. 1998, 128, 2324–2333. [Google Scholar] [CrossRef]
- Martin, S.; Giannone, G.; Andriantsitohaina, R.; Martinez, M.C. Delphinidin, an active compound of red wine, inhibits endothelial cell apoptosis via nitric oxide pathway and regulation of calcium homeostasis. Br. J. Pharmacol. 2003, 139, 1095–1102. [Google Scholar] [CrossRef]
- Demrow, H.S.; Slane, P.R.; Folts, J.D. Administration of wine and grape juice inhibits in vivo platelet activity and thrombosis in stenosed canine coronary arteries. Circulation 1995, 91, 1182–1188. [Google Scholar] [CrossRef]
- Renaud, S.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- do Rosario, V.A.; Chang, C.; Spencer, J.; Alahakone, T.; Roodenrys, S.; Francois, M.; Weston-Green, K.; Hölzel, N.; Nichols, D.S.; Kent, K.; et al. Anthocyanins attenuate vascular and inflammatory responses to a high fat high energy meal challenge in overweight older adults: A cross-over, randomized, double-blind clinical trial. Clin. Nutr. 2021, 40, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Cocci, P.; Moruzzi, M.; Martinelli, I.; Maggi, F.; Micioni Di Bonaventura, M.V.; Cifani, C.; Mosconi, G.; Tayebati, S.K.; Damiano, S.; Lupidi, G.; et al. Tart cherry (Prunus cerasus L.) dietary supplement modulates visceral adipose tissue CB1 mRNA levels along with other adipogenesis-related genes in rat models of diet-induced obesity. Eur. J. Nutr. 2021, 60, 2695–2707. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, I.; Micioni Di Bonaventura, M.V.; Moruzzi, M.; Amantini, C.; Maggi, F.; Gabrielli, M.G.; Fruganti, A.; Marchegiani, A.; Dini, F.; Marini, C.; et al. Effects of Prunus cerasus L. seeds and juice on liver steatosis in an animal model of diet-induced obesity. Nutrients 2020, 12, 1308. [Google Scholar] [CrossRef] [PubMed]
- Moruzzi, M.; Klöting, N.; Blüher, M.; Martinelli, I.; Tayebati, S.K.; Gabrielli, M.G.; Roy, P.; Micioni Di Bonaventura, M.V.; Cifani, C.; Lupidi, G.; et al. Tart Cherry Juice and Seeds Affect Pro-Inflammatory Markers in Visceral Adipose Tissue of High-Fat Diet Obese Rats. Molecules 2021, 26, 1403. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Martinelli, I.; Moruzzi, M.; Maggi, F.; Amantini, C.; Di Bonaventura, M.V.M.; Cifani, C.; Amenta, F.; Tayebati, S.K.; Tomassoni, D. Ion channels alterations in the forebrain of high-fat diet fed rats. Eur. J. Histochem. 2021, 65, 3305. [Google Scholar] [CrossRef]
- Martinelli, I.; Tayebati, S.K.; Roy, P.; Micioni Di Bonaventura, M.V.; Moruzzi, M.; Cifani, C.; Amenta, F.; Tomassoni, D. Obesity-Related Brain Cholinergic System Impairment in High-Fat-Diet-Fed Rats. Nutrients 2022, 14, 1243. [Google Scholar] [CrossRef]
- Qin, F.; Siwik, D.A.; Pimentel, D.R.; Morgan, R.J.; Biolo, A.; Tu, V.H.; Kang, Y.J.; Cohen, R.A.; Colucci, W.S. Cytosolic H2O2 mediates hypertrophy, apoptosis, and decreased SERCA activity in mice with chronic hemodynamic overload. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1453–H1463. [Google Scholar] [CrossRef][Green Version]
- Martinelli, I.; Tomassoni, D.; Moruzzi, M.; Roy, P.; Cifani, C.; Amenta, F.; Tayebati, S.K. Cardiovascular Changes Related to Metabolic Syndrome: Evidence in Obese Zucker Rats. Int. J. Mol. Sci. 2020, 21, 2035. [Google Scholar] [CrossRef]
- Tak, P.P.; Firestein, G.S. NF-kappaB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef]
- Uosaki, H.; Fukushima, H.; Takeuchi, A.; Matsuoka, S.; Nakatsuji, N.; Yamanaka, S.; Yamashita, J.K. Efficient and scalable purification of cardiomyocytes from human embryonic and induced pluripotent stem cells by VCAM1 surface expression. PLoS ONE 2011, 6, e23657. [Google Scholar] [CrossRef]
- Mukaddam-Daher, S.; Menaouar, A.; El-Ayoubi, R.; Gutkowska, J.; Jankowski, M.; Velliquette, R.A.; Ernsberger, P. Cardiac effects of moxonidine in spontaneously hypertensive obese rats. Ann. N Y Acad. Sci. 2003, 1009, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Conti, M.; Renaud, I.M.; Poirier, B.; Michel, O.; Belair, M.F.; Mandet, C.; Bruneval, P.; Myara, I.; Chevalier, J. High levels of myocardial antioxidant defense in aging nondiabetic normotensive Zucker obese rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R793–R800. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.F.; Zenebe, W.J.; Strange, T.B. Cardiovascular function in a rat model of diet-induced obesity. Hypertension 2006, 48, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Cavalera, M.; Wang, J.; Frangogiannis, N.G. Obesity, metabolic dysfunction, and cardiac fibrosis: Pathophysiological pathways, molecular mechanisms, and therapeutic opportunities. Transl. Res. 2014, 164, 323–335. [Google Scholar] [CrossRef]
- Calligaris, S.D.; Lecanda, M.; Solis, F.; Ezquer, M.; Gutiérrez, J.; Brandan, E.; Leiva, A.; Sobrevia, L.; Conget, P. Mice long-term high-fat diet feeding recapitulates human cardiovascular alterations: An animal model to study the early phases of diabetic cardiomyopathy. PLoS ONE 2013, 8, e60931. [Google Scholar] [CrossRef] [PubMed]
- Stolina, M.; Luo, X.; Dwyer, D.; Han, C.Y.; Chen, R.; Zhang, Y.; Xiong, Y.; Chen, Y.; Yin, J.; Shkumatov, A.; et al. The evolving systemic biomarker milieu in obese ZSF1 rat model of human cardiometabolic syndrome: Characterization of the model and cardioprotective effect of GDF15. PLoS ONE 2020, 15, e0231234. [Google Scholar] [CrossRef]
- Aimo, A.; Castiglione, V.; Borrelli, C.; Saccaro, L.F.; Franzini, M.; Masi, S.; Emdin, M.; Giannoni, A. Oxidative stress and inflammation in the evolution of heart failure: From pathophysiology to therapeutic strategies. Eur. J. Prev. Cardiol. 2020, 27, 494–510. [Google Scholar] [CrossRef]
- Jovanovic, A.; Sudar-Milovanovic, E.; Obradovic, M.; Pitt, S.J.; Stewart, A.J.; Zafirovic, S.; Stanimirovic, J.; Radak, D.; Isenovic, E.R. Influence of a High-Fat Diet on Cardiac iNOS in Female Rats. Curr. Vasc. Pharmacol. 2017, 15, 491–500. [Google Scholar] [CrossRef]
- Carlsen, H.; Haugen, F.; Zadelaar, S.; Kleemann, R.; Kooistra, T.; Drevon, C.A.; Blomhoff, R. Diet-induced obesity increases NF-kappaB signaling in reporter mice. Genes Nutr. 2009, 4, 215–222. [Google Scholar] [CrossRef]
- Mussbacher, M.; Salzmann, M.; Brostjan, C.; Hoesel, B.; Schoergenhofer, C.; Datler, H.; Hohensinner, P.; Basílio, J.; Petzelbauer, P.; Assinger, A.; et al. Cell Type-Specific Roles of NF-κB Linking Inflammation and Thrombosis. Front. Immunol. 2019, 10, 85. [Google Scholar] [CrossRef]
- Lee, S.; Park, Y.; Zhang, C. Exercise Training Prevents Coronary Endothelial Dysfunction in Type 2 Diabetic Mice. Am. J. Biomed. Sci. 2011, 3, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Park, Y.; Picchi, A.; Potter, B.J. Maturation-induces endothelial dysfunction via vascular inflammation in diabetic mice. Basic Res. Cardiol. 2008, 103, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Vieira, R.A.L.; de Freitas, R.N.; Volp, A.C. Adhesion molecules and chemokines; relation to anthropometric, body composition, biochemical and dietary variables. Nutr. Hosp. 2014, 30, 223–236. [Google Scholar] [CrossRef]
- Couillard, C.; Ruel, G.; Archer, W.R.; Pomerleau, S.; Bergeron, J.; Couture, P.; Lamarche, B.; Bergeron, N. Circulating levels of oxidative stress markers and endothelial adhesion molecules in men with abdominal obesity. J. Clin. Endocrinol. Metab. 2005, 90, 6454–6459. [Google Scholar] [CrossRef]
- El-Baz, F.K.; Aly, H.F.; Abd-Alla, H.I. The ameliorating effect of carotenoid rich fraction extracted from Dunaliella salina microalga against inflammation-associated cardiac dysfunction in obese rats. Toxicol. Rep. 2019, 7, 118–124. [Google Scholar] [CrossRef]
- Mulhem, A.; Moulla, Y.; Klöting, N.; Ebert, T.; Tönjes, A.; Fasshauer, M.; Dietrich, A.; Schön, M.R.; Stumvoll, M.; Richter, V.; et al. Circulating cell adhesion molecules in metabolically healthy obesity. Int. J. Obes. (Lond) 2021, 45, 331–336. [Google Scholar] [CrossRef]
- Zanni, M.V.; Stanley, T.L.; Makimura, H.; Chen, C.Y.; Grinspoon, S.K. Effects of TNF-alpha antagonism on E-selectin in obese subjects with metabolic dysregulation. Clin. Endocrinol. (Oxf) 2010, 73, 48–54. [Google Scholar] [CrossRef]
- Porres, J.M.; Constantino, J.; Kapravelou, G.; Lopez-Chaves, C.; Galisteo, M.; Aranda, P.; López-Jurado, M.; Martínez, R. The combined treatment with lentil protein hydrolysate and a mixed training protocol is an efficient lifestyle intervention to manage cardiovascular and renal alterations in obese Zucker rats. Eur. J. Nutr. 2020, 59, 3473–3490. [Google Scholar] [CrossRef]
- Wang, H.T.; Liu, C.F.; Tsai, T.H.; Chen, Y.L.; Chang, H.W.; Tsai, C.Y.; Leu, S.; Zhen, Y.Y.; Chai, H.T.; Chung, S.Y.; et al. Effect of obesity reduction on preservation of heart function and attenuation of left ventricular remodeling, oxidative stress and inflammation in obese mice. J. Transl. Med. 2012, 10, 145. [Google Scholar] [CrossRef]
- Piccolella, S.; Fiorentino, A.; Pacifico, S.; D’Abrosca, B.; Uzzo, P.; Monaco, P. Antioxidant properties of sour cherries (Prunus cerasus L.): Role of colorless phytochemicals from the methanolic extract of ripe fruits. J. Agric. Food Chem. 2008, 56, 1928–1935. [Google Scholar] [CrossRef]
- Seymour, E.M.; Singer, A.A.; Kirakosyan, A.; Urcuyo-Llanes, D.E.; Kaufman, P.B.; Bolling, S.F. Altered hyperlipidemia, hepatic steatosis, and hepatic peroxisome proliferator-activated receptors in rats with intake of tart cherry. J. Med. Food 2008, 11, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Seymour, E.M.; Lewis, S.K.; Urcuyo-Llanes, D.E.; Tanone, I.I.; Kirakosyan, A.; Kaufman, P.B.; Bolling, S.F. Regular tart cherry intake alters abdominal adiposity, adipose gene transcription, and inflammation in obesity-prone rats fed a high fat diet. J. Med. Food 2009, 12, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Jayarathne, S.; Stull, A.J.; Miranda, A.; Scoggin, S.; Claycombe-Larson, K.; Kim, J.H.; Moustaid-Moussa, N. Tart Cherry Reduces Inflammation in Adipose Tissue of Zucker Fatty Rats and Cultured 3T3-L1 Adipocytes. Nutrients 2018, 10, 1576. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.R.; Gochenaur, K. Direct vasoactive and vasoprotective properties of anthocyanin-rich extracts. J. Appl. Physiol. 2006, 100, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Kalea, A.Z.; Clark, K.; Schuschke, D.A.; Klimis-Zacas, D.J. Vascular reactivity is affected by dietary consumption of wild blueberries in the Sprague-Dawley rat. J. Med. Food 2009, 12, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Keane, K.M.; George, T.W.; Constantinou, C.L.; Brown, M.A.; Clifford, T.; Howatson, G. Effects of Montmorency tart cherry (Prunus cerasus L.) consumption on vascular function in men with early hypertension. Am. J. Clin. Nutr. 2016, 103, 1531–1539. [Google Scholar] [CrossRef]
- Chai, S.C.; Davis, K.; Zhang, Z.; Zha, L.; Kirschner, K.F. Effects of Tart Cherry Juice on Biomarkers of Inflammation and Oxidative Stress in Older Adults. Nutrients 2019, 11, 228. [Google Scholar] [CrossRef]
- Carrillo, C.; Cavia Mdel, M.; Alonso-Torre, S. Role of oleic acid in immune system; mechanism of action; a review. Nutr. Hosp. 2012, 27, 978–990. [Google Scholar] [CrossRef]
- Vassiliou, E.K.; Gonzalez, A.; Garcia, C.; Tadros, J.H.; Chakraborty, G.; Toney, J.H. Oleic acid and peanut oil high in oleic acid reverse the inhibitory effect of insulin production of the inflammatory cytokine TNF-alpha both in vitro and in vivo systems. Lipids Health Dis. 2009, 8, 25. [Google Scholar] [CrossRef]
- Perdomo, L.; Beneit, N.; Otero, Y.F.; Escribano, Ó.; Díaz-Castroverde, S.; Gómez-Hernández, A.; Benito, M. Protective role of oleic acid against cardiovascular insulin resistance and in the early and late cellular atherosclerotic process. Cardiovasc. Diabetol. 2015, 14, 75. [Google Scholar] [CrossRef]
- Bak, I.; Lekli, I.; Juhasz, B.; Nagy, N.; Varga, E.; Varadi, J.; Gesztelyi, R.; Szabo, G.; Szendrei, L.; Bacskay, I.; et al. Cardioprotective mechanisms of Prunus cerasus (sour cherry) seed extract against ischemia-reperfusion-induced damage in isolated rat hearts. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1329–H1336. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, M.; Mali, V.R.; Pan, G.; Xu, J.; Yang, X.P.; Thandavarayan, R.A.; Palaniyandi, S.S. Increased 4-hydroxy-2-nonenal-induced proteasome dysfunction is correlated with cardiac damage in streptozotocin-injected rats with isoproterenol infusion. Cell Biochem. Funct. 2016, 34, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.J.; Crockett, E.K.; Chongwatpol, P.; Graef, J.L.; Clarke, S.L.; Rendina-Ruedy, E.; Lucas, E.A. Montmorency tart cherry protects against age-related bone loss in female C57BL/6 mice and demonstrates some anabolic effects. Eur. J. Nutr. 2019, 58, 3035–3046. [Google Scholar] [CrossRef] [PubMed]
- Traustadóttir, T.; Davies, S.S.; Stock, A.A.; Su, Y.; Heward, C.B.; Roberts, L.J., 2nd; Harman, S.M. Tart cherry juice decreases oxidative stress in healthy older men and women. J. Nutr. 2009, 139, 1896–1900. [Google Scholar] [CrossRef] [PubMed]
- Mulabagal, V.; Lang, G.A.; DeWitt, D.L.; Dalavoy, S.S.; Nair, M.G. Anthocyanin content, lipid peroxidation and cyclooxygenase enzyme inhibitory activities of sweet and sour cherries. J. Agric. Food Chem. 2009, 57, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Sarić, A.; Sobocanec, S.; Balog, T.; Kusić, B.; Sverko, V.; Dragović-Uzelac, V.; Levaj, B.; Cosić, Z.; Macak Safranko, Z.; Marotti, T. Improved antioxidant and anti-inflammatory potential in mice consuming sour cherry juice (Prunus Cerasus cv. Maraska). Plant Foods Hum. Nutr. 2009, 64, 231–237. [Google Scholar] [CrossRef]
- Ou, B.; Bosak, K.N.; Brickner, P.R.; Iezzoni, D.G.; Seymour, E.M. Processed tart cherry products—Comparative phytochemical content, in vitro antioxidant capacity and in vitro anti-inflammatory activity. J. Food Sci. 2012, 77, H105–H112. [Google Scholar] [CrossRef]
- Schumacher, H.R.; Pullman-Mooar, S.; Gupta, S.R.; Dinnella, J.E.; Kim, R.; McHugh, M.P. Randomized double-blind crossover study of the efficacy of a tart cherry juice blend in treatment of osteoarthritis (OA) of the knee. Osteoarthr. Cartil. 2013, 21, 1035–1041. [Google Scholar] [CrossRef]
- Martin, K.R.; Burrell, L.; Bopp, J. Authentic tart cherry juice reduces markers of inflammation in overweight and obese subjects: A randomized, crossover pilot study. Food Funct. 2018, 9, 5290–5300. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinelli, I.; Tomassoni, D.; Bellitto, V.; Roy, P.; Micioni Di Bonaventura, M.V.; Amenta, F.; Amantini, C.; Cifani, C.; Tayebati, S.K. Anti-Inflammatory and Antioxidant Properties of Tart Cherry Consumption in the Heart of Obese Rats. Biology 2022, 11, 646. https://doi.org/10.3390/biology11050646
Martinelli I, Tomassoni D, Bellitto V, Roy P, Micioni Di Bonaventura MV, Amenta F, Amantini C, Cifani C, Tayebati SK. Anti-Inflammatory and Antioxidant Properties of Tart Cherry Consumption in the Heart of Obese Rats. Biology. 2022; 11(5):646. https://doi.org/10.3390/biology11050646
Chicago/Turabian StyleMartinelli, Ilenia, Daniele Tomassoni, Vincenzo Bellitto, Proshanta Roy, Maria Vittoria Micioni Di Bonaventura, Francesco Amenta, Consuelo Amantini, Carlo Cifani, and Seyed Khosrow Tayebati. 2022. "Anti-Inflammatory and Antioxidant Properties of Tart Cherry Consumption in the Heart of Obese Rats" Biology 11, no. 5: 646. https://doi.org/10.3390/biology11050646
APA StyleMartinelli, I., Tomassoni, D., Bellitto, V., Roy, P., Micioni Di Bonaventura, M. V., Amenta, F., Amantini, C., Cifani, C., & Tayebati, S. K. (2022). Anti-Inflammatory and Antioxidant Properties of Tart Cherry Consumption in the Heart of Obese Rats. Biology, 11(5), 646. https://doi.org/10.3390/biology11050646









