A Reappraisal of Polyploidy Events in Grasses (Poaceae) in a Rapidly Changing World
Abstract
:Simple Summary
Abstract
1. Introduction
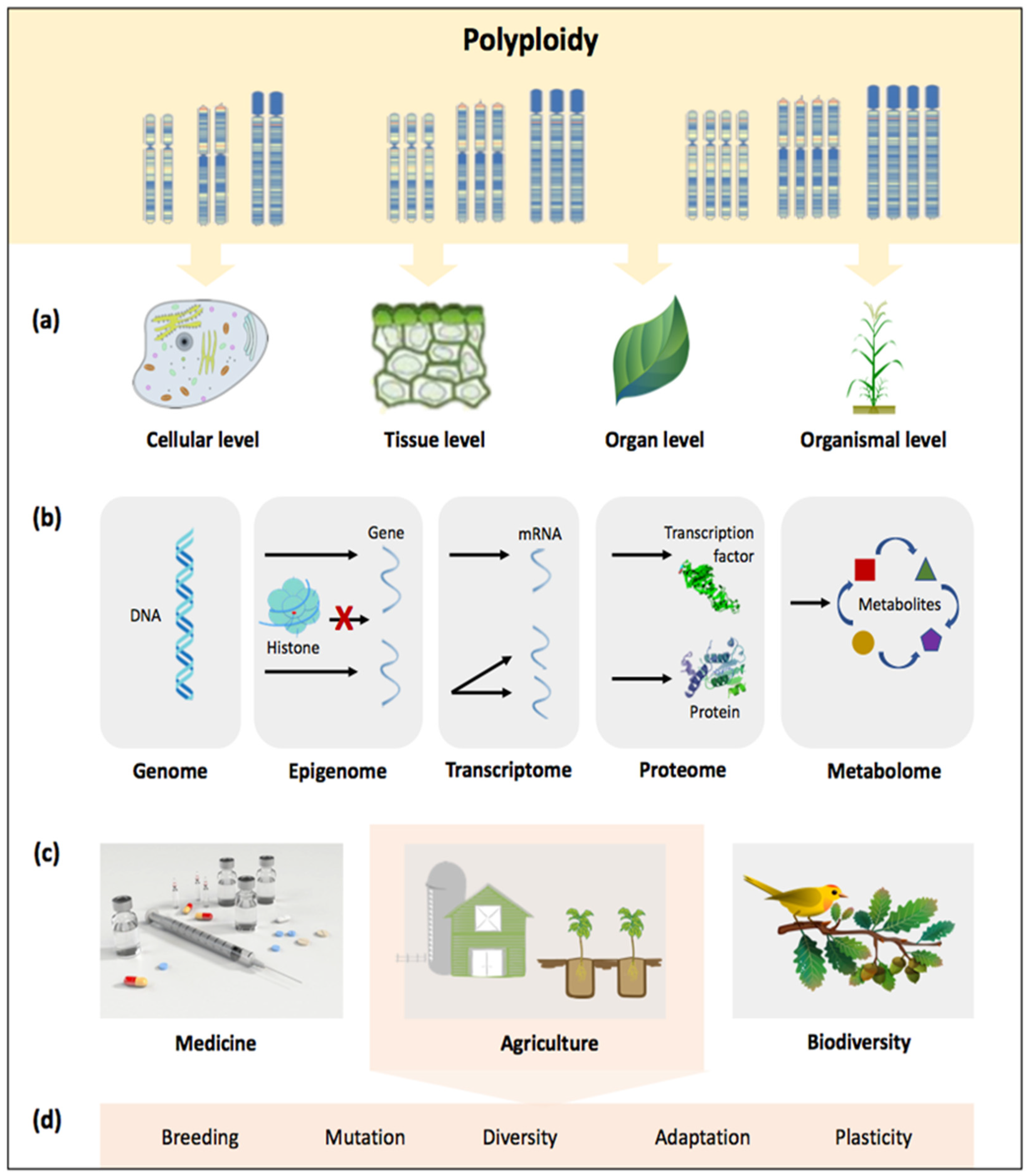
2. Impacts of Polyploidy at a Glance: The Case of Polyploid Grasses
2.1. Diversity and Evolutionary Dynamics
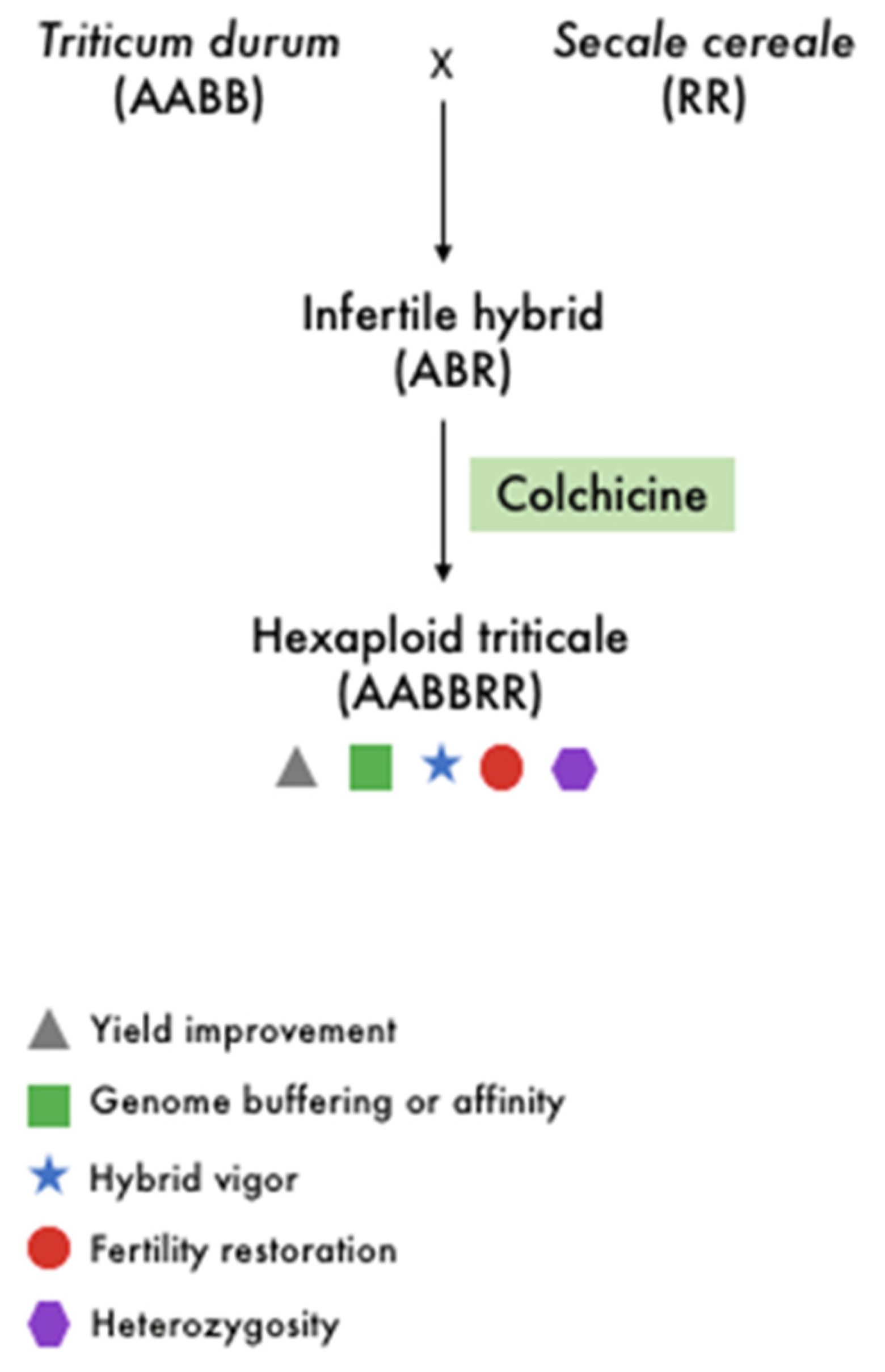
2.2. Crop Improvement
3. Dissecting the Polyploid Potential of Current and Future Crops in the Genomic Era
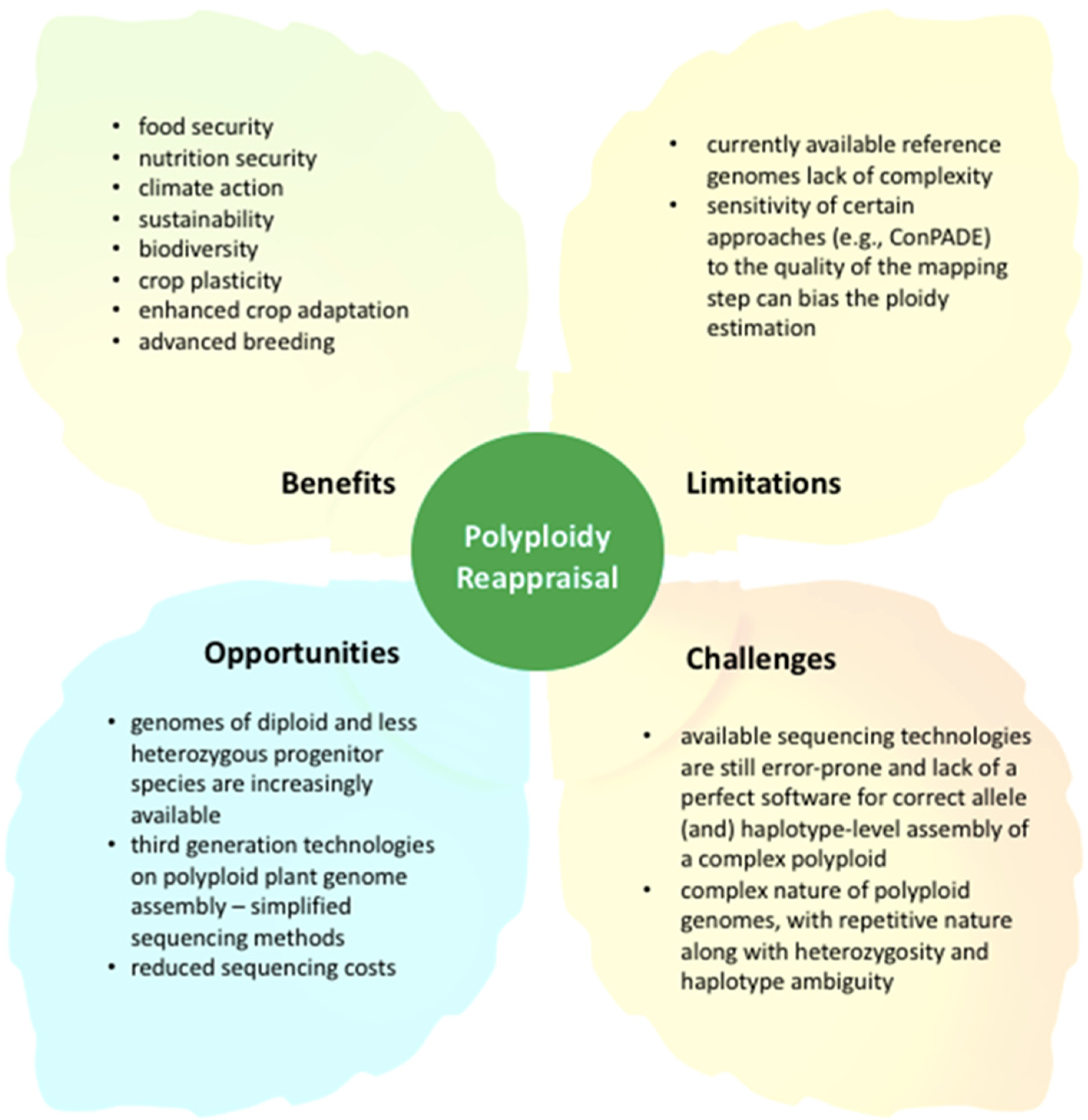
4. Reappraising Polyploidy for Crop Improvement in the 21st Century: The Road Ahead
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Research Council (US). 4, Cretaceous-Tertiary (K/T) Mass Extinction: Effect of Global Change on Calcareous Microplankton. In Panel on Effects of Past Global Change on Life. Effects of Past Global Change on Life; National Academies Press (US): Washington, DC, USA, 1995; pp. 72–93. [Google Scholar]
- Chiarenza, A.A.; Farnsworth, A.; Mannion, P.D.; Lunt, D.J.; Valdes, P.J.; Morgan, J.V.; Allison, P.A. Asteroid impact, not volcanism, caused the end-Cretaceous dinosaur extinction. Proc. Natl. Acad. Sci. USA 2020, 117, 17084–17093. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.R.; Jaramillo, C.; de la Parra, F.; Caballero-Rodríguez, D.; Herrera, F.; Wing, S.; Turner, B.L.; D’Apolito, C.; Romero-Baez, M.; Narvaez, P. Extinction at the end-Cretaceous and the origin of modern tropical rainforests. Science 2021, 372, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, J.A.; Maere, S.; Van De Peer, Y. Plants with double genomes might have had a better chance to survive the Cretaceous–Tertiary extinction event. Proc. Natl. Acad. Sci. USA 2009, 106, 5737–5742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van de Peer, Y.; Ashman, T.-L.; Soltis, P.S.; Soltis, D.E. Polyploidy: An evolutionary and ecological force in stressful times. Plant Cell 2021, 33, 11–26. [Google Scholar] [CrossRef]
- Comai, L. The advantages and disadvantages of being polyploid. Nat. Rev. Genet. 2005, 6, 836–846. [Google Scholar] [CrossRef]
- Morgan, C.; Zhang, H.; Henry, C.E.; Franklin, F.C.H.; Bomblies, K. Derived alleles of two axis proteins affect meiotic traits in autotetraploid Arabidopsis arenosa. Proc. Natl. Acad. Sci. USA 2020, 117, 8980–8988. [Google Scholar] [CrossRef] [Green Version]
- Van de Peer, Y.; Mizrachi, E.; Marchal, K. The evolutionary significance of polyploidy. Nat. Rev. Genet. 2017, 18, 411–424. [Google Scholar] [CrossRef]
- Soltis, P.S.; Marchant, D.B.; Van de Peer, Y.; Soltis, D.E. Polyploidy and genome evolution in plants. Curr. Opin. Genet. Dev. 2015, 35, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Cai, L.; Xi, Z.; Amorim, A.M.; Sugumaran, M.; Rest, J.S.; Liu, L.; Davis, C.C. Widespread ancient whole-genome duplications in Malpighiales coincide with Eocene global climatic upheaval. New Phytol. 2019, 221, 565–576. [Google Scholar] [CrossRef] [Green Version]
- Koenen, E.J.; Ojeda, D.I.; Steeves, R.; Migliore, J.; Bakker, F.T.; Wieringa, J.J.; Kidner, C.; Hardy, O.J.; Pennington, R.T.; Bruneau, A. Large-scale genomic sequence data resolve the deepest divergences in the legume phylogeny and support a near-simultaneous evolutionary origin of all six subfamilies. New Phytol. 2020, 225, 1355–1369. [Google Scholar] [CrossRef] [Green Version]
- Cheng, A.; Raai, M.N.; Zain, N.A.M.; Massawe, F.; Singh, A.; Wan-Mohtar, W.A.A.Q.I. In search of alternative proteins: Unlocking the potential of underutilized tropical legumes. Food Secur. 2019, 11, 1205–1215. [Google Scholar] [CrossRef]
- Sethuraman, G.; Mohd Zain, N.A.; Yusoff, S.; Ng, Y.M.; Baisakh, N.; Cheng, A. Revamping Ecosystem Services through Agroecology—The Case of Cereals. Agriculture 2021, 11, 204. [Google Scholar] [CrossRef]
- Godden, G.T.; Kinser, T.J.; Soltis, P.S.; Soltis, D.E. Phylotranscriptomic analyses reveal asymmetrical gene duplication dynamics and signatures of ancient polyploidy in mints. Genome Biol. Evol. 2019, 11, 3393–3408. [Google Scholar] [CrossRef]
- Leebens-Mack, J.H.; Barker, M.S.; Carpenter, E.J.; Deyholos, M.K.; Gitzendanner, M.A.; Graham, S.W.; Grosse, I.; Li, Z.; Melkonian, M.; Mirarab, S. One thousand plant transcriptomes and the phylogenomics of green plants. Nature 2019, 574, 679–685. [Google Scholar] [CrossRef] [Green Version]
- Renny-Byfield, S.; Wendel, J.F. Doubling down on genomes: Polyploidy and crop plants. Am. J. Bot. 2014, 101, 1711–1725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walden, N.; German, D.A.; Wolf, E.M.; Kiefer, M.; Rigault, P.; Huang, X.-C.; Kiefer, C.; Schmickl, R.; Franzke, A.; Neuffer, B. Nested whole-genome duplications coincide with diversification and high morphological disparity in Brassicaceae. Nat. Commun. 2020, 11, 3795. [Google Scholar] [CrossRef]
- Mohd Hanafiah, N.; Mispan, M.S.; Lim, P.E.; Baisakh, N.; Cheng, A. The 21st century agriculture: When rice research draws attention to climate variability and how weedy rice and underutilized grains come in handy. Plants 2020, 9, 365. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Wang, J.; Lin, W.; Li, S.; Li, H.; Zhou, J.; Ni, P.; Dong, W.; Hu, S.; Zeng, C. The genomes of Oryza sativa: A history of duplications. PLoS Biol. 2005, 3, e38. [Google Scholar] [CrossRef] [Green Version]
- Massawe, F.; Mayes, S.; Cheng, A. Crop diversity: An unexploited treasure trove for food security. Trends Plant Sci. 2016, 21, 365–368. [Google Scholar] [CrossRef]
- Fox, D.T.; Soltis, D.E.; Soltis, P.S.; Ashman, T.-L.; Van de Peer, Y. Polyploidy: A biological force from cells to ecosystems. Trends Cell. Biol. 2020, 30, 688–694. [Google Scholar] [CrossRef]
- Wendel, J.; Doyle, J. Plant diversity and evolution: Genotypic and phenotypic variation in higher plants. In Polyploidy and Evolution in Plants; Henry, R.J., Ed.; CABI Publishing: Wallington, UK, 2005; pp. 97–117. [Google Scholar]
- Chen, Z.J. Molecular mechanisms of polyploidy and hybrid vigor. Trends Plant Sci. 2010, 15, 57–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyriakidou, M.; Tai, H.H.; Anglin, N.L.; Ellis, D.; Strömvik, M.V. Current strategies of polyploid plant genome sequence assembly. Front. Plant. Sci. 2018, 9, 1660. [Google Scholar] [CrossRef] [PubMed]
- McClintock, B. The significance of responses of the genome to challenge. Science 1984, 226, 792–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, K.L.; Wendel, J.F. Polyploidy and genome evolution in plants. Curr. Opin. Plant Biol. 2005, 8, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Scholes, D.R.; Paige, K.N. Plasticity in ploidy: A generalized response to stress. Trends Plant Sci. 2015, 20, 165–175. [Google Scholar] [CrossRef]
- Levin, D.A. Polyploidy and novelty in flowering plants. Am. Nat. 1983, 122, 1–25. [Google Scholar] [CrossRef]
- Doyle, J.J.; Coate, J.E. Polyploidy, the nucleotype, and novelty: The impact of genome doubling on the biology of the cell. Int. J. Plant Sci. 2019, 180, 1–52. [Google Scholar] [CrossRef]
- Koenen, E.J.; Ojeda, D.I.; Bakker, F.T.; Wieringa, J.J.; Kidner, C.; Hardy, O.J.; Pennington, R.T.; Herendeen, P.S.; Bruneau, A.; Hughes, C.E. The origin of the legumes is a complex paleopolyploid phylogenomic tangle closely associated with the Cretaceous–Paleogene (K–Pg) mass extinction event. Syst. Biol. 2021, 70, 508–526. [Google Scholar] [CrossRef]
- Conover, J.L.; Karimi, N.; Stenz, N.; Ané, C.; Grover, C.E.; Skema, C.; Tate, J.A.; Wolff, K.; Logan, S.A.; Wendel, J.F. A Malvaceae mystery: A mallow maelstrom of genome multiplications and maybe misleading methods? J. Integr. Plant Biol. 2019, 61, 12–31. [Google Scholar] [CrossRef]
- Meyer, R.S.; Choi, J.Y.; Sanches, M.; Plessis, A.; Flowers, J.M.; Amas, J.; Dorph, K.; Barretto, A.; Gross, B.; Fuller, D.Q. Domestication history and geographical adaptation inferred from a SNP map of African rice. Nat. Genet. 2016, 48, 1083–1088. [Google Scholar] [CrossRef]
- Woodhouse, M.; Hufford, M. Parallelism and convergence in post-domestication adaptation in cereal grasses. Philos. Trans. R. Soc. B. 2019, 374, 20180245. [Google Scholar] [CrossRef] [PubMed]
- Bennetzin, J.L.; Freeling, M. Grasses as a single genetic system: Genome composition, collinearity and compatibility. Trends Genet. 1993, 9, 259–261. [Google Scholar] [CrossRef]
- Paterson, A.; Bowers, J.; Chapman, B. Ancient polyploidization predating divergence of the cereals, and its consequences for comparative genomics. Proc. Natl. Acad. Sci. USA 2004, 101, 9903–9908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozkan, H.; Levy, A.A.; Feldman, M. Allopolyploidy-induced rapid genome evolution in the wheat (Aegilops–Triticum) group. Plant Cell 2001, 13, 1735–1747. [Google Scholar]
- Chantret, N.; Salse, J.; Sabot, F.; Rahman, S.; Bellec, A.; Laubin, B.; Dubois, I.; Dossat, C.; Sourdille, P.; Joudrier, P. Molecular basis of evolutionary events that shaped the hardness locus in diploid and polyploid wheat species (Triticum and Aegilops). Plant Cell 2005, 17, 1033–1045. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Belcram, H.; Gornicki, P.; Charles, M.; Just, J.; Huneau, C.; Magdelenat, G.; Couloux, A.; Samain, S.; Gill, B.S. Duplication and partitioning in evolution and function of homoeologous Q loci governing domestication characters in polyploid wheat. Proc. Natl. Acad. Sci. USA 2011, 108, 18737–18742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Yang, Z.; Zhao, L.; Sun, F.; Liu, B. A newly formed hexaploid wheat exhibits immediate higher tolerance to nitrogen-deficiency than its parental lines. BMC Plant Biol. 2018, 18, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spor, A.; Roucou, A.; Mounier, A.; Bru, D.; Breuil, M.-C.; Fort, F.; Vile, D.; Roumet, P.; Philippot, L.; Violle, C. Domestication-driven changes in plant traits associated with changes in the assembly of the rhizosphere microbiota in tetraploid wheat. Science 2020, 10, 12234. [Google Scholar] [CrossRef] [PubMed]
- Di Vittori, V.; Gioia, T.; Rodriguez, M.; Bellucci, E.; Bitocchi, E.; Nanni, L.; Attene, G.; Rau, D.; Papa, R. Convergent evolution of the seed shattering trait. Genes 2019, 10, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giraud, D.; Lima, O.; Rousseau-Gueutin, M.; Salmon, A.; Ainouche, M. Gene and transposable element expression evolution following recent and past polyploidy events in Spartina (Poaceae). Front. Genet. 2021, 12, 589160. [Google Scholar] [CrossRef]
- Rutland, C.A.; Hall, N.D.; McElroy, J.S. The impact of polyploidization on the evolution of weed species: Historical understanding and current limitations. Front. Agron. 2021, 3, 5. [Google Scholar] [CrossRef]
- Nieto Feliner, G.; Casacuberta, J.; Wendel, J.F. Genomics of evolutionary novelty in hybrids and polyploids. Front. Genet. 2020, 11, 792. [Google Scholar] [CrossRef] [PubMed]
- Fort, A.; Ryder, P.; McKeown, P.C.; Wijnen, C.; Aarts, M.G.; Sulpice, R.; Spillane, C. Disaggregating polyploidy, parental genome dosage and hybridity contributions to heterosis in Arabidopsis thaliana. New Phytol. 2016, 209, 590–599. [Google Scholar] [CrossRef] [Green Version]
- Weckwerth, W.; Ghatak, A.; Bellaire, A.; Chaturvedi, P.; Varshney, R.K. PANOMICS meets germplasm. Plant Biotechnol. J. 2020, 18, 1507–1525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labroo, M.R.; Studer, A.J.; Rutkoski, J.E. Heterosis and hybrid crop breeding: A multidisciplinary review. Front. Genet. 2021, 12, 234. [Google Scholar] [CrossRef] [PubMed]
- Karbstein, K.; Rahmsdorf, E.; Tomasello, S.; Hodač, L.; Hörandl, E. Moving beyond assumptions: Polyploidy and environmental effects explain a geographical parthenogenesis scenario in European plants. Mol. Ecol. 2021, 30, 2659–2675. [Google Scholar] [CrossRef] [PubMed]
- Blakeslee, A.F. Dédoublement du nombre de chromosomes chez les plantes par traitement chimique. Comptes rendus des séances de l’Académie des Sciences 1937, 205, 476. [Google Scholar]
- Hegarty, M.; Coate, J.; Sherman-Broyles, S.; Abbott, R.; Hiscock, S.; Doyle, J. Lessons from natural and artificial polyploids in higher plants. Cytogenet. Genome Res. 2013, 140, 204–225. [Google Scholar] [CrossRef]
- Mason, A.S.; Wendel, J.F. Homoeologous Exchanges, Segmental Allopolyploidy, and Polyploid Genome Evolution. Front. Genet. 2020, 11, 1014. [Google Scholar] [CrossRef]
- Baduel, P.; Bray, S.; Vallejo-Marin, M.; Kolář, F.; Yant, L. The “Polyploid Hop”: Shifting Challenges and Opportunities Over the Evolutionary Lifespan of Genome Duplications. Front. Ecol. Evol. 2018, 6, 117. [Google Scholar] [CrossRef] [Green Version]
- Silkova, O.G.; Ivanova, Y.N.; Loginova, D.B.; Solovey, L.A.; Sycheva, E.A.; Dubovets, N.I. Karyotype Reorganization in Wheat–Rye Hybrids Obtained via Unreduced Gametes: Is There a Limit to the Chromosome Number in Triticale? Plants 2021, 10, 2052. [Google Scholar] [CrossRef] [PubMed]
- Koide, Y.; Kuniyoshi, D.; Kishima, Y. Fertile tetraploids: New resources for future rice breeding? Front. Plant Sci. 2020, 11, 1231. [Google Scholar] [CrossRef] [PubMed]
- Thirugnanasambandam, P.P.; Hoang, N.V.; Henry, R.J. The challenge of analyzing the sugarcane genome. Front. Plant Sci. 2018, 9, 616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manechini, J.R.V.; da Silva Santos, P.H.; Romanel, E.; dos Santos Brito, M.; Scarpari, M.S.; Jackson, S.; Pinto, L.R.; Vicentini, R. Transcriptomic Analysis of Changes in Gene Expression During Flowering Induction in Sugarcane Under Controlled Photoperiodic Conditions. Front. Plant Sci. 2021, 12, 635784. [Google Scholar] [CrossRef]
- Choulet, F.; Wicker, T.; Rustenholz, C.; Paux, E.; Salse, J.; Leroy, P.; Schlub, S.; Le Paslier, M.-C.; Magdelenat, G.; Gonthier, C. Megabase level sequencing reveals contrasted organization and evolution patterns of the wheat gene and transposable element spaces. Plant Cell 2010, 22, 1686–1701. [Google Scholar] [CrossRef] [Green Version]
- IWGSC; Appels, R.; Eversole, K.; Stein, N.; Feuillet, C.; Keller, B.; Rogers, J.; Pozniak, C.J.; Choulet, F.; Distelfeld, A. Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 2018, 361, eaar7191. [Google Scholar] [CrossRef] [Green Version]
- Cannarozzi, G.; Plaza-Wüthrich, S.; Esfeld, K.; Larti, S.; Wilson, Y.S.; Girma, D.; de Castro, E.; Chanyalew, S.; Blösch, R.; Farinelli, L. Genome and transcriptome sequencing identifies breeding targets in the orphan crop tef (Eragrostis tef). BMC Genom. 2014, 15, 581. [Google Scholar] [CrossRef] [Green Version]
- Hittalmani, S.; Mahesh, H.; Shirke, M.D.; Biradar, H.; Uday, G.; Aruna, Y.; Lohithaswa, H.; Mohanrao, A. Genome and transcriptome sequence of finger millet (Eleusine coracana (L.) Gaertn.) provides insights into drought tolerance and nutraceutical properties. BMC Genom. 2017, 18, 465. [Google Scholar] [CrossRef]
- Mondal, T.K.; Rawal, H.C.; Chowrasia, S.; Varshney, D.; Panda, A.K.; Mazumdar, A.; Kaur, H.; Gaikwad, K.; Sharma, T.R.; Singh, N.K. Draft genome sequence of first monocot-halophytic species Oryza coarctata reveals stress-specific genes. Science 2018, 8, 13698. [Google Scholar] [CrossRef]
- Zou, C.; Li, L.; Miki, D.; Li, D.; Tang, Q.; Xiao, L.; Rajput, S.; Deng, P.; Peng, L.; Jia, W. The genome of broomcorn millet. Nat. Commun. 2019, 10, 436. [Google Scholar] [CrossRef] [Green Version]
- Jiao, W.-B.; Schneeberger, K. The impact of third generation genomic technologies on plant genome assembly. Curr. Opin. Plant Biol. 2017, 36, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Garsmeur, O.; Droc, G.; Antonise, R.; Grimwood, J.; Potier, B.; Aitken, K.; Jenkins, J.; Martin, G.; Charron, C.; Hervouet, C. A mosaic monoploid reference sequence for the highly complex genome of sugarcane. Nat. Commun. 2018, 9, 2638. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, D.E.; Ho, Y.S.; Lightfoot, D.J.; Schmöckel, S.M.; Li, B.; Borm, T.J.; Ohyanagi, H.; Mineta, K.; Michell, C.T.; Saber, N. The genome of Chenopodium quinoa. Nature 2017, 542, 307–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Zhang, X.; Tang, H.; Zhang, Q.; Hua, X.; Ma, X.; Zhu, F.; Jones, T.; Zhu, X.; Bowers, J. Allele-defined genome of the autopolyploid sugarcane Saccharum spontaneum L. Nat. Genet. 2018, 50, 1565–1573. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Wang, X.; Cheng, F. Plant polyploidy: Origin, evolution, and its influence on crop domestication. Hortic. Plant J. 2019, 5, 231–239. [Google Scholar] [CrossRef]
- Salman-Minkov, A.; Sabath, N.; Mayrose, I. Whole-genome duplication as a key factor in crop domestication. Nat. Plants 2016, 2, 16115. [Google Scholar] [CrossRef]
- Renny-Byfield, S.; Rodgers-Melnick, E.; Ross-Ibarra, J. Gene fractionation and function in the ancient subgenomes of maize. Mol. Biol. Evol. 2017, 34, 1825–1832. [Google Scholar] [CrossRef]
- VanBuren, R.; Man Wai, C.; Wang, X.; Pardo, J.; Yocca, A.E.; Wang, H.; Chaluvadi, S.R.; Han, G.; Bryant, D.; Edger, P.P. Exceptional subgenome stability and functional divergence in the allotetraploid Ethiopian cereal teff. Nat. Commun. 2020, 11, 884. [Google Scholar] [CrossRef] [Green Version]
- Wojciechowski, M.F. Tracing the history of chromosome evolution in legumes using genomics. New Phytol. 2019, 223, 1693–1695. [Google Scholar] [CrossRef] [Green Version]
- Cannon, S.B.; Ilut, D.; Farmer, A.D.; Maki, S.L.; May, G.D.; Singer, S.R.; Doyle, J.J. Polyploidy did not predate the evolution of nodulation in all legumes. PLoS ONE 2010, 5, e11630. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zhang, R.; Jiang, K.-W.; Qi, J.; Hu, Y.; Guo, J.; Zhu, R.; Zhang, T.; Egan, A.N.; Yi, T.-S. Nuclear phylotranscriptomics and phylogenomics support numerous polyploidization events and hypotheses for the evolution of rhizobial nitrogen-fixing symbiosis in Fabaceae. Mol. Plant 2021, 14, 748–773. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, D.N. Polyploidy in crop improvement and evolution. In Plant Biology Biotechnology; Springer: New Delhi, India, 2015; pp. 619–638. [Google Scholar]
- Schoenfelder, K.P.; Fox, D.T. The expanding implications of polyploidy. J. Cell. Biol. 2015, 209, 485. [Google Scholar] [CrossRef] [PubMed]
- Paige, K.N. Overcompensation, environmental stress, and the role of endoreduplication. Am. J. Bot. 2018, 105, 1105–1108. [Google Scholar] [CrossRef] [PubMed]
- Forrester, N.J.; Ashman, T.-L. The direct effects of plant polyploidy on the legume–rhizobia mutualism. Ann. Bot. 2018, 121, 209–220. [Google Scholar] [CrossRef] [Green Version]
- Pacey, E.K.; Maherali, H.; Husband, B.C. The influence of experimentally induced polyploidy on the relationships between endopolyploidy and plant function in Arabidopsis thaliana. Ecol. Evol. 2020, 10, 198–216. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Garcia, L.; Lunadei, L.; Barreiro, P.; Robla, I. A review of wireless sensor technologies and applications in agriculture and food industry: State of the art and current trends. Sensors 2009, 9, 4728–4750. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Yu, Y.; Sun, J.; Cao, Q.; Tang, Z.; Liu, M.; Xu, T.; Ma, D.; Li, Z.; Sun, J. Root-zone-specific sensitivity of K+-and Ca2+-permeable channels to H2O2 determines ion homeostasis in salinized diploid and hexaploid Ipomoea trifida. J. Exp. Bot. 2019, 70, 1389–1405. [Google Scholar] [CrossRef] [Green Version]
- Otto, S.P. The evolutionary consequences of polyploidy. Cell 2007, 131, 452–462. [Google Scholar] [CrossRef] [Green Version]
- Spoelhof, J.P.; Soltis, P.S.; Soltis, D.E. Pure polyploidy: Closing the gaps in autopolyploid research. J. Syst. Evol. 2017, 55, 340–352. [Google Scholar] [CrossRef] [Green Version]
| Year | Crop | Genome Size (Mb) | Ploidy Level | Ploidy | Propagation | References |
|---|---|---|---|---|---|---|
| 2010 | Wheat (Triticum aestivum) | ~15,345 | Allohexaploid | 6x = 42 | Selfing | [57] |
| 2014 | Wild rice (Oryza minuta) | ~450 | Tetraploid | 4x = 48 | Selfing | Oryza Comparative Sequencing Project |
| 2014 | Teff (Eragrostis tef) | ~607 | Allotetraploid | 4x = 40 | Selfing | [59] |
| 2017 | Finger millet (Eleusine coracana) | ~1196 | Allotetraploid | 4x = 36 | Selfing | [60] |
| Wild emmer wheat (Triticum dicoccoides) | ~10,495 | Tetraploid | 4x = 28 | Selfing | WEWseq Consortium | |
| 2018 | Wild rice (Oryza coarctata) | ~665 | Tetraploid | 4x = 48 | Clonal | [61] |
| 2019 | Broomcorn millet (Panicum miliaceum) | ~848 | Allotetraploid | 4x = 36 | Selfing | [62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, A.; Mohd Hanafiah, N.; Harikrishna, J.A.; Eem, L.P.; Baisakh, N.; Mispan, M.S. A Reappraisal of Polyploidy Events in Grasses (Poaceae) in a Rapidly Changing World. Biology 2022, 11, 636. https://doi.org/10.3390/biology11050636
Cheng A, Mohd Hanafiah N, Harikrishna JA, Eem LP, Baisakh N, Mispan MS. A Reappraisal of Polyploidy Events in Grasses (Poaceae) in a Rapidly Changing World. Biology. 2022; 11(5):636. https://doi.org/10.3390/biology11050636
Chicago/Turabian StyleCheng, Acga, Noraikim Mohd Hanafiah, Jennifer Ann Harikrishna, Lim Phaik Eem, Niranjan Baisakh, and Muhamad Shakirin Mispan. 2022. "A Reappraisal of Polyploidy Events in Grasses (Poaceae) in a Rapidly Changing World" Biology 11, no. 5: 636. https://doi.org/10.3390/biology11050636
APA StyleCheng, A., Mohd Hanafiah, N., Harikrishna, J. A., Eem, L. P., Baisakh, N., & Mispan, M. S. (2022). A Reappraisal of Polyploidy Events in Grasses (Poaceae) in a Rapidly Changing World. Biology, 11(5), 636. https://doi.org/10.3390/biology11050636






