Simple Summary
Gene engineering techniques are essential for genetic characterizations and metabolic engineering. A stable and robust gene editing method can speed up the explorations of nonmodel microbes which show tremendous potential for a variety of applications. In recent years, base editors have achieved precise point mutation and multiplex gene editing in a wide range of microbes. Without causing double stranded breaks and requiring a donor DNA template, base editors are more available than CRISPR/Cas9 for those species with a low homologous recombination system. Here, we introduce the latest development and applications of base editors in nonmodel microbes. This versatile method is suitable for gene editing from precise point mutation to genome-wide engineering in nonmodel microbes and holds good promise for future development of nonmodel microbes.
Abstract
Nonmodel microbes with unique and diverse metabolisms have become rising stars in synthetic biology; however, the lack of efficient gene engineering techniques still hinders their development. Recently, the use of base editors has emerged as a versatile method for gene engineering in a wide range of organisms including nonmodel microbes. This method is a fusion of impaired CRISPR/Cas9 nuclease and base deaminase, enabling the precise point mutation at the target without inducing homologous recombination. This review updates the latest advancement of base editors in microbes, including the conclusion of all microbes that have been researched by base editors, the introduction of newly developed base editors, and their applications. We provide a list that comprehensively concludes specific applications of BEs in nonmodel microbes, which play important roles in industrial, agricultural, and clinical fields. We also present some microbes in which BEs have not been fully established, in the hope that they are explored further and so that other microbial species can achieve arbitrary base conversions. The current obstacles facing BEs and solutions are put forward. Lastly, the highly efficient BEs and other developed versions for genome-wide reprogramming of cells are discussed, showing great potential for future engineering of nonmodel microbes.
1. Introduction
Microbial species play essential roles in the human world, which are both attractive and hateful to humans. On the one hand, they are applied widely in the agricultural, pharmaceutical, biofuel, and food industries, and so on [1,2]. On the other hand, microbial pathogens cause severe infections with the rising resistance against drugs. Since the yields and productivity of host strains are not economically competitive [3,4], and the production costs are high via industrial fermentation [3,5], it is crucial to achieve highly efficient and economical production. Previous technologies, including repeated cycles of screening for desired phenotypes through physical or chemical mutagenesis, and fermentation optimization, have improved the microbial productivity and reduced costs [6]; however, these methods are still challenging in terms of improving the yields of desired products, due to the limited mutation rates and unwanted genetic alterations. In order to increase the production of the target metabolites, synthetic biology and metabolic engineering are developed to optimize genetic and regulatory processes within cells. One of the most useful tools to learn and modify biosynthetic pathways is gene engineering techniques, which can ultimately alter microbial genotypes and phenotypes by insertions, deletions, and mutations of nucleotides [7]. Model microbes such as Escherichia coli (E. coli) and Saccharomyces cerevisiae (S. cerevisiae) have been researched and used for industrial biochemical production in the long term [8]. Despite their well-characterized metabolisms and availability of a large genetic toolbox for rapid gene modification [9], these model strains are unable to produce all desirable products to satisfy the increasing industrial needs.
In contrast, nonmodel microbes that are derived from a more complex environment and have evolved to utilize cheaper and more environmentally friendly sources of carbon than model microbes, often possess versatile physiology and metabolic capabilities that are important for the future production of biofuel, as well as chemical and novel antibiotics that may ease the problem of multi-drug resistance [8,10]. They are also able to tolerate extreme industrial processing environments, such as low pH, high salt, and high temperatures; therefore, nonmodel microbes have become one of the hot spots in metabolic engineering. However, the lack of facile gene editing tools hinders the genetic characterizations and modifications of nonmodel microbes [11]. Moreover, the understanding and mitigating drug-resistance mechanisms require the gene engineering approaches to aid in targeting and editing pathogenic microbial genomes [12].
The advent of the CRISPR/Cas9 (CRISPR, clustered regularly interspaced short palindromic repeats/Cas9, the dual RNA-guided DNA endonuclease) opens the door to manipulate the genome of microbes that are traditionally difficult to be edited (such as nonmodel bacteria [13,14,15,16], fungi [17], protozoan parasites [18,19], etc.). As shown in Figure 1A, the guide RNA (gRNA) directs Cas9 to the targeted DNA containing the protospacer adjacent motif (PAM, 5′-NGG-3′) to introduce DNA double-stranded breaks (DSBs) [20]. This induces homologous recombination (HR) to replace the DNA sequence with homologous templates. However, the generation of DSBs is reported to be lethal to some bacteria, such as Clostridium cellulolyticum [21] and E. coli [22], and it induces nonhomologous end joining (NHEJ), which results in random insertions or deletions (indels) [23,24]. The lack of a strong HR system in some microbes, such as Mycobacterium tuberculosis (M. tuberculosis) [25] and Yarrowia lipolytica [26], also adds difficulties to CRISPR/Cas9 editing.
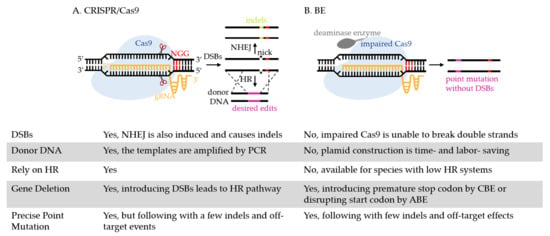
Figure 1.
The process of gene editing using CRISPR/Cas9 and a BE. (A) Directed by gRNA, CRISPR/Cas9 recognizes the PAM site (5′-NGG-3′, highlighted in red) and introduces DSBs at the target. DSBs induces two cellular DNA repair systems, NHEJ and HR. Through NHEJ, a few imprecise indels are created through error-prone DNA repair. Through HR, the target is replaced by homologous donor DNA with desired edits and is therefore introduced with deletions, insertions, or substitutions of nucleotides. (B) The BE is also directed by gRNA to the target. At the target, deaminase enzyme achieves base conversion without causing DSBs and requiring donor DNA. CBE mediates the conversion of a C:G base pair to a T:A, which can replace four codons (CAA, CAG, CGA, TGG) with premature stop codons (TAA, TAG, TGA) to inactivate the gene. ABE mediates the base conversion from an A:T base pair to G:C, which can replace start codon ATG with GTG or ACG to disrupt the initiation of gene translation. Other than point mutation, BEs can achieve multiplex gene editing, in vivo evolution of protein and strain, metabolic engineering, etc., in various microbes, as discussed in detail below.
Recently, a base editor (BE), which was fusing a deaminase enzyme with an impaired CRISPR/Cas nuclease (unable to cleave DNA double strands) together [27], has emerged as an efficient template-free gene editing method. The impaired Cas nuclease generated from Streptococcus pyogenes Cas9 (SpCas9) can either be a catalytically dead Cas9 (dCas9 containing D10A and H840A mutations), that is completely unable to cleave DNA double strands, or a Cas9 nickase (nCas9, retaining D10A mutation) that nicks the nonedited strand [28]. In a gRNA-programmed manner, the fusion protein is recruited to the target without causing DSBs [29], where cytosine deaminase is fused with impaired Cas9 (cytosine base editor, CBE) to convert cytidine (C) to thymidine (T), and adenine deaminase is fused with impaired Cas9 (adenine base editor, ABE) to convert adenosine (A) to guanosine (G). As shown in Figure 1, BEs are more convenient and refined for precise gene editing than CRISPR/Cas9.
2. Advancement of BEs in Microbes
Previous reviews [30,31,32] have concluded the applications of BEs in many microbes, such as model species S. cerevisiae [33,34,35], E. coli [36,37,38], Corynebacterium glutamicum (C. glutamicum) [39,40,41], Aspergillus niger (A. niger) [42], and nonmodel microbes Psedomonas spp. [43], Yarrowia lipolytica (Y. lipolytica) [26], Strptomyces spp. [44,45,46], Clostridium beijerinckii (C. beijerinckii) [47], Rhodobacter sphaeroides (R. sphaeroides) [48], and Shewanella oneidensis (S. oneidensis) [49]. However, since then, BEs have been further extended to new types and applied in new species (especially nonmodel microbes); therefore we construct a universal phylogenetic tree in Figure 2 to directly present all current microbial genera in which BEs have been established so far. The newly developed BEs that have overcome the editing limitations in microbes are further discussed below and listed in Table 1. After that, we focus on the specific applications of BEs in nonmodel microbes. The successful and failed examples of BEs in nonmodel microbes, which have rarely been concluded before, are comprehensively listed in Table 2, providing a friendly guide for future research.
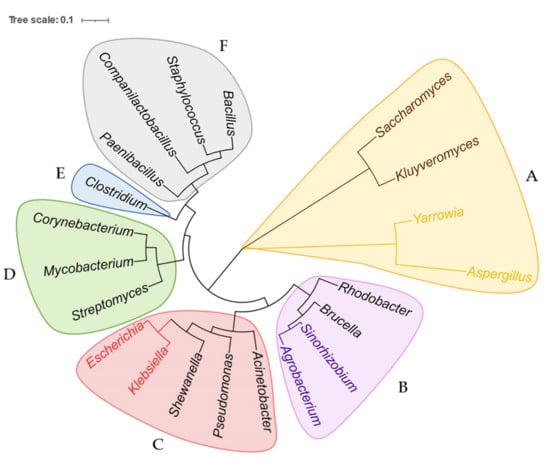
Figure 2.
Phylogenetic tree of all microbes that have been researched by BEs, based on small-subunit rRNA sequences and constructed using the maximum-likelihood estimation. The phylogenetic tree includes 21 genera and six classes which are shadowed in different colors. (A) Microbes belong to the kingdom Fungi, phylum Ascomycota, and class Saccharomycetes. Saccharomyces and Kluyveromyces belong to the same family, Saccharomycetaceae, highlighted in brown. Yarrowia and Aspergillus belong to the same family, Dipodascaceae, highlighted in yellow. (B) Microbes belong to the kingdom Bacteria, phylum Proteobacteria, and class Alphaproteobacteria. Rhodobacter and Brucella belong to the family Rhodobacteraceae and Rhizobiaceae, respectively. Agrobacterium and Sinorhizobium belong to the same family, Brucellaceae, highlighted in purple. (C) Microbes belong to the kingdom Bacteria, phylum Proteobacteria, and class Gammaproteobacteria. Pseudomonas, Acinetobacter, and Shewanella belong to the family Pseudomonadaceae, Moraxellaceae, and Shewanellaceae, respectively. Escherichia and Klebsiella belong to the same family, Enterobacteriaceae, highlighted in pink. (D) Microbes belong to the kingdom Bacteria, phylum Actinobacteria, and class Actinomycetia. Streptomyces, Mycobacterium, and Corynebacterium belong to the family Streptomycetaceae, Corynebacteriaceae, and Mycobacteriaceae, respectively. (E) Genus Clostridium belongs to the kingdom Bacteria, phylum Firmicutes, class Clostridia, and family Clostridiaceae. (F) Microbes belong to kingdom Bacteria, phylum Firmicutes, and class Bacilli. Paenibacillus, Companilactobacillus, Staphylococcus, and Bacillus belong to the family Paenibacillaceae, Lactobacillaceae, Staphylococcaceae, and Bacillaceae, respectively.
2.1. Constructs and Mechanisms of Developed BEs
2.1.1. Latest Development of CBE and ABE in Microbes
The basic constructs and working mechanisms of CBE and ABE are illustrated in Figure 3A,B. CBE in Figure 3A is named Target-AID, fusing Petromyzon marinus CDA1 (PmCDA1) to the C terminus of d/nCas9 (d/nCas9-PmCDA1). The editing window of Target-AID is commonly at the positions −16 to −20 (counting the PAM as positions 1–3), highlighted in dark red in Figure 3. It is important to note that another type of CBE, not shown in Figure 3, fuses rat APOBEC1 (rAPOBEC1) to the N terminus of d/nCas9 (rAPOBEC1-d/nCas9) with the editing window at −13 to −17. In past years, various versions of CBE and ABE have been developed to overcome the editing limitations, such as low editing efficiency, off-target effects, and PAM requirements, by utilizing Cas9 homologs and variants, deaminase homologs and variants, modifying gRNA, and so on. Their characteristics and applications in animals, plants, and bacteria are concluded comprehensively by excellent reviews [27,30,32,50,51,52]. Anzalone and colleagues also introduced a decision tree for choosing different BEs on the basis of several criteria [38]. Recently, several developed BEs have been reported to further address editing limitations in microbes. For example, PmCDA1 was engineered with intensive truncations, and several mutations were inlaid in the middle of nCas9 [53], which not only performed comparable editing efficiency to Target-AID, but also greatly minimized the off-target effect and molecular size in S. cerevisiae. The gRNA structure was engineered with a bubble hairpin that contained a 5′ extended sequence that was complementary to the guide sequence in order to decrease the off-targets of both CBE and ABE in E. coli [54]. In Bacillus subtilis (B. subtilis), CBE has been developed from a primary version dCas9-PmCDA1 with a narrow editing window (−17 to −18) [55] compared with dCas9-PmCDA1-UGI [56], with almost 100% editing efficiency at positions −16 to −20 by adding UGI and replacing the strong promoter Pgrac with a weak one, Pspac. Furthermore, to overcome the low transformation of nCas9-PmCDA1 [36,55,56], which is probably caused by the toxicity of the fusion protein, the PmCDA1-nCas9-UGI was integrated into the genome and performed with approximately 100% efficiency at positions −15 to −20 [57]. The 20 nucleotides (nt) gRNA was also engineered using extensions or adding an artificial stem loop to increase the editing efficiency and to expand the editable window [57]. The development of CBE and ABE to overcome their limitations in microbes are all concluded in Table 1.
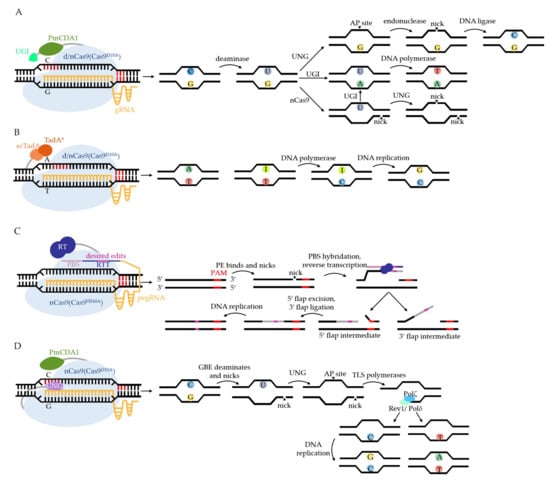
Figure 3.
The basic components and working mechanisms of CRISPR-mediated base editing systems. The common editing windows are highlighted in dark red in the DNA sequence. (A) CBE consists of cytosine deaminase (PmCDA1 in (A)), impaired Cas9, gRNA, and UGI (inhibits uracil DNA glycosylase, abbreviated to UNG, to improve the efficiency of CBE but is not essential). Under the R-loop, CBE deaminates C to U. If UNG removes U, the apurinic/apyrimidinic (AP site) will soon be reversed to C. If UGI is added to CBE, U will be retained at the locus where DNA polymerase will read U as T. The C:G is successfully conversed to T:A. If dCas9 is replaced with nCas9, the nonedited strand will be nicked. Without the inhibition of UGI, U will be removed at the other strand, where AP endonuclease nicks the edited strand and leads to DSBs, bringing indels or cell death. (B) ABE consists of impaired Cas9, gRNA, and ecTadA–TadA* homodimer (ecTadA: wild-type tRNA adenosine deaminase from E. coli, TadA*: evolved ecTadA that can operate on DNA). ABE deaminates A to I at the target, where I is misread as G by DNA polymerase. (C) PE consists of nCas9, reverse transcriptase (RT), and prime editing gRNA (pegRNA). PegRNA comprises two essential parts: PBS (primer binding site) and RTT (reverse transcriptase template). PE nicks the edited strand. The strand then hybridizes with PBS and extends with the copy of RTT by RT so that the mutation is introduced to the strand. The 5′ flap intermediate is removed by flap endonuclease, and the 3′ flap is ligated. The desired edits are consequently introduced into the DNA sequence. (D) GBE consists of nCas9, PmCDA1, and UNG in E. coli. GBE deaminates the C at the edited strand and nicks the other strand. U is removed by UNG, so the target becomes an AP site where TLS polymerases assemble and extend the nicked strand by Polζ. When passing by the locus opposite to the AP site, Rev1 cooperates with C and Polδ with T, which results in the creation of G:C and A:T, respectively, after DNA repair or replication.
C:G and T:A are currently interchangeable through the combination of CBE and ABE, which is able to generate 62 different amino acid substitutions [44]. ABE has been used to disrupt genes by converting start codon ATG to ACG in Streptomyces [45], which is a good supplement for achieving genome-wide targeting with CBE. However, scientists are still looking forward to achieving DNA base transversion (conversion between purine and pyridine) so that any base can be arbitrarily converted to any other. As newly developed gene engineering technologies, prime editors (PE) and glycosylase base editors (GBE) meet the abovementioned need, and supplement the current use of BEs.
2.1.2. Latest Development of PEs in Microbes
The basic construct and working mechanism of a PE are shown in Figure 3C, as reviews [27,31] have also discussed, but there have been few reports of a PE applied in microbes. Recently, PEs have been established as a versatile gene editing tool in E. coli [58]. By optimizing the length of PBS (13~17 nt) and RTT (13 nt), a PE could substitute, insert, and delete chromosome DNA with 6.8%, 12.2%, and 26% efficiency, respectively. However, the introduction of a second gRNA to nick the opposite strand, which further increases the efficiency in mammalian cells [59], led to low transformation, and therefore, compromised the use of PEs in microbes. The dual-editing events also showed low efficiency (<1%). Although PEs achieve highly versatile editing with few byproducts and off-target events, its low efficiency still hinders its broad application in microbes.
2.1.3. Latest Development of GBE in Microbes
nCas9-CBE has been reported to obtain quite a few C to non-T products in S. cerevisiae [33], C. glutamicum [39], R. sphaeroides [48], Klebsiella pneumoniae (K. pneumoniae) [60], and so on. Inspired by the observations above, Kurt’s group [61] and Zhao’s group [62] fused UNG to the C terminus of BE4max (an improved version of CBE with two UGIs and biparticle SV40 NLS) and rAPOBEC1-nCas9, respectively, to amplify the effect on product impurities. The results both showed a great preference for C-to-G conversion in mammalian cells. Kurt and colleagues further optimized the GBE system with the APOBEC1 variant (R33A) to reach 41.7–71.5% editing efficiency [61]. In the meantime, Zhao and colleagues reported that fusing UNG to the N terminus of nCas9-AID (UNG-nCas9-AID), shown in Figure 3D, preferred to convert C to A in E. coli with an average editing specificity of 93.8% ± 4.8%, and an editing efficiency of 87.2% ± 6.9% [62].
The different editing preference of GBE in mammalian cells and E. coli attracted Jiang and colleagues’ attention. They further revealed this mechanism in S. cerevisiae [63], as illustrated in Figure 3D. The essential steps for GBE editing are the AP site formation at the edited strand and the nick at the other strand. Then, the replicative DNA polymerases are recruited to repair the nick, known as translesion synthesis (TLS), which are conserved from bacteria to mammals [64]. By knocking out genes encoding TLS polymerases Polζ, Rev1, Polη, and Polδ, respectively, Polζ was proven to work as an extender, Rev1 was shown to specifically insert C at the opposite locus of the AP site, and Polδ was shown to mainly incorporate T or A, whereas Polη was not involved in this process [63]. The different TLS polymerases working on the AP site led to various base conversions, possibly providing new methods to fuse together polymerases with BEs to accomplish any desired conversions in the future. Recently several base excision repair (BER) proteins including DNA polymerase β, DNA ligase III, and XRCC1, were fused separately with rAPOBEC1-nCas9 to manipulate the BER pathway downstream of AP creation, giving rise to G as the major product [65]. Koblan and colleagues investigated the impact of DNA repair proteins on GBE efficiency by using CIRISPR interference (CRISPRi) screens. They engineered new GBEs with diverse editing profiles by fusing various DNA repair proteins, deaminases, and Cas proteins together [66]. Trained in a library of results at 10,638 genomically integrated target sites, the machine learning models, CGBE-Hive, could predict GBE editing efficiency and purity, as well as bystanders’ editing patters with high accuracy. To overcome the limitations of sequence preference and PAM requirement, Chen and colleagues tested a series of new GBEs and argued that the rational design of deaminase, rather than the addition of BER proteins, improved the editing efficiency [67]. The optimized eAID variant-nCas9 fusion protein improved the target compatibility of the GBE system in the GC context [67]. The spCas9 was further replaced with its variants, SpG Cas9 and SpRY Cas9, to expand the targeting range.

Table 1.
Developed Versions of BEs to Overcome Editing Limitations in Microbes.
Table 1.
Developed Versions of BEs to Overcome Editing Limitations in Microbes.
| BE type | Year | Fusing Enzyme | Cas9 Protein | gRNA | Construct | Improved Editing Activity | Applications | Refs |
|---|---|---|---|---|---|---|---|---|
| To narrow the editing window | ||||||||
| CBE | 2019 | PmCDA1 with a series of C-terminal truncations | nCas9D10A | 20 nt | PmCDA1 variants-nCas9-UGI | Prefer to edit at positions −17 to −18 while retaining editing efficiency | Edit polyC motifs and Can1 to test narrow editing windows of C-terminal truncations such as CDA1Δ190, Δ192, Δ194 in S. cerevisiae | [34] |
| CBE | 2020 | APOBEC3A with a series of C-terminal truncations and mutations | nCas9D10A | 20 nt | APOBEC3A variants-nCas9-UGI | Prefer to edit at positions −15 to −16 with decreased off-target RNA editing | Edit Can1 to test editing activity of APOBEC3A truncations such as Δ182, Δ186 and Δ190 in S. cerevisiae | [35] |
| To expand the editing range | ||||||||
| CBE | 2019 | PmCDA1 | nVQRD10A, nVRERD10A, nxCas9D10A, nCas9-NGD10A | 20 nt | nCas9 variants-PmCDA1 | Recognize the targets at non-NGG PAM with high editing efficiency | Introduce an amino acid transition T311I of LysC to obtain the mutant strain with 1.7 g/L lysine production in C. glutamicum | [40] |
| nCas9D10A | 18–30 nt | nCas9-PmCDA1 | Increase editing efficiency at positions −14 and −15 by using 18 nt gRNA; increase efficiency at position −21 by using 22 and 24 nt gRNA | Edit poly C motifs in the plasmids and chromosomes of C. glutamicum to test the editing window shift by using truncated or extended gRNAs | ||||
| CBE | 2020 | PmCDA1 | nVQRD10A, nVRERD10A, nxCas9D10A, nCas9-NGD10A | 20 nt | PmCDA1 variants-nCas9 variants-UGI | Recognize the targets at non-NGG PAM with a relatively narrow editing window from −17 to −18 | Edit polyC motifs to test the availability of Cas9 variants, editing efficiency, and window in S. cerevisiae | [35] |
| CBE | 2021 | PmCDA1 | dCas9 | 20 nt | dCas9-PmCDA1-UGI | Broaden the editing window from −16 to −20 with 100% efficiency and increase the multiplex gene editing efficiency to 75.5% for quintuple targets by adding UGI | Test five different constructs of CBE in B.subtilis by inactivating GFP and multiple genes | [56] |
| CBE | 2021 | PmCDA1 | nCas9D10A | 20 nt | PmCDA1-nCas9-UGI (integrated into genome) | Broaden the editing window at position −15 to −20 with 97–100% efficiency | In situ mutate Sec-translocase and BceB protein to obtain mutant strains with 3.6-fold transportation efficiency and different sensitivity to bacitracin, respectively, in B. subtilis | [57] |
| 21–26 nt | Increase editing efficiency by using 21 and 22 nt gRNA; expand editing window from −15 to −22 by using 23–26 nt gRNA; | |||||||
| 20 nt with a stem loop at 3‘ end of gRNA | increase the editing efficiency at position −15 from 70% to 87% | |||||||
| To decrease the off-target effect | ||||||||
| CBE | 2020 | rAPOBEC1 variants | dCas9 | 20 nt | rAPOBEC1 variants-dCas9-UGI | Balance efficient, on-target editing with greatly decreased gRNA-independent editing | Develop multiple rapid, cost-effective methods to screen the propensity of different deaminase variants and engineer the YE1 variants that retain high editing activity with minimal gRNA-independent off-target editing | [68] |
| CBE | 2021 | tCDA1EQ (PmCDA1Δ30-150, W122E, W133Q) tCDA1EQ | nCas9D10A | 20 nt | tCDA1EQ-nCas9 | Significant decrease (5–79 fold) gRNA-independent off-target effects with comparable editing efficiency to original CBE | Edit Can1 to test editing activity in S. cerevisiae, evaluate the editing efficiency and window in mammalian cells, and compare them with existing improved CBEs | [53] |
| nCas9 1054aa-tCDA1EQ-1055aa (inlaid architecture) | ||||||||
| CBE | 2021 | rAPOBEC1 | nCas9D10A | 20nt with H12-B3-P5 (a 3 nt bubble positioned from positions 5 to 7 into a 12 nt hairpin) | rAPOBEC1-nCas9-UGI | Significantly decrease off-target editing without sacrificing on-target editing efficiency | Test editing efficiency and gRNA-dependent off-targets in E. coli | [54] |
| ABE | ecTadA-TadA* | 20 nt with H12-B3-P4 | ecTadA-TadA*-nCas9 | |||||
| To achieve DNA base transversion | ||||||||
| PE | 2021 | M-MLV (reverse transcriptase) | nCas9H840A | 20 nt with 13–17 nt PBS and 13 nt RTT | nCas9-M-MLV2 | Substitutions, insertions, and deletions with 6.8%, 12.2% and 26% efficiency, respectively, in chromosome with few bystanders and off-targets | Achieve gene substitutions, deletions (up to 97 bp), insertions (up to 33 bp), and multiplex editing in E. coli | [58] |
| GBE | 2021 | PmCDA1 | nCas9D10A | 20 nt | UNG-nCas9-PmCDA1 | Convert C to A with an average editing efficiency of 87.2% ± 6.9% with no detectable gRNA-dependent off-target | Convert C to A at four loci in lacZ and develop the NBE (any base editing) strategy in E. coli by combining CBE, ABE, and GBE | [62] |
2.2. Recent Applications of BEs in Nonmodel Microbes
BEs have been applied in a wide range of nonmodel microbes. Some BEs perform high editing efficiency, but some of them can barely function in microbes; therefore, we present a detailed list of all constructs and applications of BEs in nonmodel microbes, shown in Table 2. The latest applications that have not been reviewed before are discussed below. It is important to note that the editing activities of BEs described in Table 2 have some differences with the descriptions in previous reviews [30,31]. For example, the efficiency of CBE in Streptomyces [44] is described as 30–100% in Table 2, which is based on the original text, “Overall, the cytidines in the editing window were converted into thymidines with frequencies between 30% and 100%” [44], whereas Wang et al. [30] chose the highest efficiency, 100%, in Streptomyces. The efficiency of ABE in Streptomyces [44] is described as 0–100% in Table 2, based on Figure 2D from the original paper [44], from the lowest efficiency, 0, to the highest efficiency, 100%. Although Jiang et al. [31] chose the result in Supplementary Material Figure S1.B from the original paper [44], which showed the editing efficiency was from 0 to 40% [44]. Similarly, the efficiency in Clostridium beijerinckii is 20–100% in Table 2, whereas it is 40–100% in Jiang et al. [31] The lowest efficiency in Table 2 is based on the result of Figure 5a in the original paper [47], which shows only one mutant is successfully edited from the five picked colonies. Jiang et al. chose the result described in original text; “Nine out of those twenty clones obtained from pCBEclos-cbei1006-g2 which grew on 5-FOA medium were all shown by Sanger sequencing of amplified PCR products, to contained the desired mutations”.

Table 2.
The Characteristics and Applications of BEs in Nonmodel Microbes.
Table 2.
The Characteristics and Applications of BEs in Nonmodel Microbes.
| Species | Major Function | Type | Year | gRNA Promoter | Construct of Fusion Protein | PAM | Editing Window | Editing Efficiency | Multiplex Gene Editing | Applications of BEs | Off-Targets | Refs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Industrially Important Microbes | ||||||||||||
| Kluyveromyces marxianus | Industrial production of various enzymes, chemicals, and macromolecules, as well as the utilization of cell biomass | CBE | 2017 | PSNR52 | PTSNR52 | NGG | −17 to −18 | 12.5–25% | nr | Inactivate Nej1 and Dnl4 to build NHEJ null mutants with an increased efficiency of homologous recombination and to facilitate multiple integration mediated by CRISPR/Cas9 | nr | [69] |
| Psedomonas spp. | An excellent bacterial host to produce polymers, bulk chemicals, drugs, and high-price specialties | CBE | 2018 | Ptrc | PrpsL-rAPOBEC1-nCas9D10A | NGG | −13 to −18 | 100% | nr | Inactivate genes in P. aeruginosa PAO1, P. putida KT2440, P. fluorescens GcM5-1A, and P. syringae DC3000 to test editing window and efficiency | nd in the six similar spacers of the rhlR and rhlB genes | [41] |
| CBE | 2020 | Pj23119 | Pbs/ParaBAD-rAPOBEC1-nCas9D10A | na, none of the selected colonies achieved C-to-T mutations | [70] | |||||||
| CBE | 2020 | Pj23119 | Pbs-rAPOBEC1-eSpCas9ppD10A | |||||||||
| CBE | 2020 | Pj23119 | ParaBAD-rAPOBEC1-eSpCas9ppD10A | NGG | nr | 20% | nr | Edit ttgA to test editing efficiency | nr | |||
| CBE | 2020 | Pj23119 | Pm-rAPOBEC1-eSpCas9ppD10A-UGI | NGG | −13 to −18 | 40–60% | nr | nr | ||||
| CBE | 2020 | Pj23119 | ParaBAD-rAPOBEC1-eSpCas9ppD10A-UGI | NGG | −13 to −18 | 80–100% | 100% for double targets and 35% for triple targets | Inactivate genes in P. putida, P. aeruginosa, P. fluorescens, and P. entomophila to prove CBE general availability; simultaneously edit genes by one-plasmid and two-plasmid system | nd by Sanger sequencing the potential sites predicted by CasOT | |||
| CBE | 2020 | Pj23119 | ParaBAD-rAPOBEC1-eSpCas9ppD10A-NG-UGI | NG | −13 to −18 | 100% | 100% for double targets recognized by eSpCas9pp and eSpCas9-NG in a two-plasmid system | Inactivate pykA and pcaH in one step; mutate G136 in AroF-2 to select a mutant strain with increased PCA titer up to 264.87 mg/L | nr | |||
| CBE | 2020 | Pj23119 | ParaBAD-YE1-eSpCas9ppD10A-UGI | NGG | −14 to −17 | 62.5% | nr | Precisely edit ttgA, which contains multiple cytidines with enhanced editing efficiency from 25% to 62.5% | nr | |||
| Yarrowia lipolytica | GRAS and industrial production of lipase and organic acids | CBE | 2019 | PSCR1’-tRNAGly | PUAS1B8-TEF(136)-nCas9D10A-PmCDA1-UGI | NGG | −14 to −20 | 28% | 6.7% for double targets | Inactivate TRP1, PEX10, HIS3 in ku70Δ strain to test editing efficiency | nr | [26] |
| CBE | 2019 | PSCR1’-tRNAGly | PTEFin-nCas9D10A-PmCDA1-UGI | NGG | −14 to −20 | 94.3 ± 5% | 31% for double targets | nr | ||||
| Streptomyces spp. | Industrial production of bioactive secondary metabolites, such as antifungals, antivirals, antitumorals, anti-hypertensives, and mainly antibiotics, etc. | CBE | 2019 | PermE* | PtipA-rAPOBEC1-nCas9D10A-UGI | NGG | −11 to −17 | 30–100% | 33.3% for triple targets | Substitute amino acids in SCO5087 and SCO5092; inactivate genes of BGCs in nonmodel strain S. griseofuscus; efficiently and simultaneously inactivate two identical copies of kirN | 38–56 by WGS (24–34 meaningful amino acid changes); whereas 29 SNVs in wild-type strain (18 amino acid changes); | [44] |
| ABE | 2019 | PermE* | PtipA-TadA-TadA*-nCas9D10A-UGI | NGG | −12 to −17 | 0–100% | nr | Target SCO5087 and a designed matrix containing NA motifs to test efficiency and preference | 27–33 by WGS (20–21 meaningful amino acid changes) | |||
| CBE | 2019 | PkasO* | PrpsL-rAPOBEC1-dCas9-UGI | NGG | −13 to −17 | 43–70% | 43% for double targets | Edit redD and actl to test C-to-T efficiency with a few C-to-G and C-to-A mutations | nr | [45] | ||
| CBE | 2019 | PkasO* | PrpsL-rAPOBEC1-nCas9D10A-UGI | NGG | −13 to −17 | 100% | 100% for double targets; 60% for quintuple targets | Simultaneously disrupt the genes of polyketide synthase clusters to increase production of avermectin | 3 by Sanger sequencing the sites predicted by CasOT; | |||
| CBE | 2019 | PkasO* | PrpsL-rAPOBEC1-HF-nCas9D10A-UGI | NGG | −13 to −17 | 80% | nr | Edit olm to test off-target events, which was decreased to an undetectable level | nd by Sanger sequencing the sites mentioned above | |||
| ABE | 2019 | PkasO* | PrpsL-TadA-TadA*-dCas9 | na, all selected colonies showed the A/G overlapping peak in sanger sequencing | ||||||||
| ABE | 2019 | PkasO* | PrpsL-TadA-TadA*-nCas9D10A | NGG | −14 to −17 | 100% | nr | Disrupt the initiation of actVB translation by converting ATG start codon to ACG to accumulate actinoperylone | nr | |||
| CBE | 2019 | Pj23119 | PermEp*-dCas9-PmCDA1-UL | na, growth of cells is severely delayed when CBE was overexpressed by the strong constitutive promoter | [46] | |||||||
| CBE | 2019 | Pj23119 | PtipAp-dCas9-PmCDA1-UL | NGG | −16 to 20 | 10–100% | 60% for double targets; 20% for triple targets | Inactivate genes in S. coelicolor and S.rapamycinicus to test editing efficiency and general availability to other strains | 1 by Sanger sequencing the potential sites predicted by Cas-OFFinder | |||
| CBE | 2019 | Pj23119 | PtipAp-nCas9D10A-PmCDA1-UL | NGG | −16 to 20 | 15% | nr | Edit redW with low efficiency from C to T but 85% efficiency for C-to-G mutation | nr | |||
| CBE | 2021 | Pgapdh (EL) | PrpsL(XC)-rAPOBEC1-dCas9-UGI | NGG | −13 to −18 | 1–20% | nr | Edit redN, redD, and act_β-ketoacyl to test editing efficiency | 16.50 ± 8.35 by WGS | [71] | ||
| CBE | 2021 | Pgapdh (EL) | PrpsL(XC)-rAPOBEC1-nCas9D10A-UGI | NGG | −13 to −18 | 3–25% | nr | nr | ||||
| CBE | 2021 | Pgapdh (EL) | PrpsL(XC)-rAPOBEC1-dCas9-UGI with asRNA | NGG | −13 to −18 | 21.2–65.8% | nr | 13.50 ± 3.32 by WGS | ||||
| CBE | 2021 | Pgapdh (EL) | PrpsL(XC)-rAPOBEC1-nCas9D10A-UGI with asRNA | NGG | −13 to −18 | 26.2–79.4% | nr | nr | ||||
| Clostridium beijerinckii | Production of acetone, n-butanol, isopropanol etc. | CBE | 2019 | Pj23119 | Pthl-rAPOBEC1-nCas9D10A-UGI | NGG | −13 to −17 | 20–100% | nr | Edit pyrE, xylR, spo0A, and araR to test efficiency of codon-optimized CBE; inactivate xylR to enhance the xylose fermentation | nr | [47] |
| Clostridium ljungdahlii | Production of acetic acid and ethanol from waste gas | CBE | 2020 | Pj23119 | P2TetO1-dCas9-PmCDA1-UL | NGG | −11 to −19 | 2–55.6% | nr | Inactivate adhE1 and aor2 separately to increase acetate yield as well as lower ethanol production under either heterotrophic or autotrophic conditions | nr | [72] |
| Rhodobacter sphaeroides | Industrial production of CoQ10, isoprenoids, poly-β-hydroxybutyrate, hydrogen | CBE | 2020 | Pj23119 | PLac-dCas9-PmCDA1-UL | NGG | −14 to 20 | 16.7% | nr | Inactivate appA and ppsR to test efficiency with pure C-to-T conversion | nr | [48] |
| CBE | 2020 | Pj23119 | PLac-nCas9D10A-PmCDA1-UL | NGG | 14 to 20 | 10–96.7% | 43% for double targets; 46.7% for triple targets | Inactivate appA, etc., to test C-to-T efficiency with C-to-G and C-to-A byproducts; disrupt ubiF, ubiA, ubiG, and ubiX to reveal their importance in the CoQ10 biosynthetic pathway | nr | |||
| ABE | 2020 | Pj23119 | PLac-TadA-TadA*-dCas9 | NGG | −14 to −16 | 0–5% | nr | Edit appA, ppsR, crtB, and bchG to alter translation level or block translation initiation | nr | |||
| ABE | 2020 | Pj23119 | PLac-TadA-TadA*-nCas9D10A | NGG | −14 to −16 | 0–30% | nr | Edit appA, etc to alter translation level or block translation initiation | nr | |||
| Shewanella oneidensis | Bioelectricity production from biomass wastes | CBE | 2020 | Ptac | PrpsL-rAPOBEC1-nCas9D10A | NGG | −13 to −18 | 33.3–100% | 87.5% for double targets | Target NC motifs to test editing preference; inactivate gfp, blaA, and dmsE to test editing activity; identify key genes in GlcNAc or glucose metabolism to obtain a mutant strain with enhanced degradation efficiencies for organic pollutants | nr | [49] |
| Companilactobacillus crustorum | Production of bacteriocin and 3-phenyllactic acid | CBE | 2021 | P3 | PsppA-rAPOBEC1-nCas9D10A | NGG | −14 to −18 | 75–100% | nr | Edit seven C-rich spacer sequences in a plasmid to test editing window and efficiency | nr | [73] |
| Agriculturally Important Microbes | ||||||||||||
| Paenibacillus polymyxa | Nitrogen fixation, plant growth promotion, soil phosphorus solubilization and production of cxopolysaccharides, hydrolytic enzymes, antibiotics, and cytokinin | CBE | 2021 | Para | Pgrac-nCas9D10A-PmCDA1 | na, no transformant was obtained due to the toxicity of the fusion protein | [56] | |||||
| CBE | 2021 | Para | Pspac-dCas9-PmCDA1-UGI | NGG | −16 to 20 | 100% | 100% for double and triple targets; 83.3% for quadruple targets; 75.5% for quintuple targets | Disrupt genes of five known BGCs to reveal the antimicrobial spectrum of the novel antibiotics in the sixth unknown BGCs | 8.5 SNVs including 4.2 amino acid changes by WGS | |||
| Agrobacterium spp. | Nature’s genetic engineer for diverse species including crops | CBE | 2021 | Pj23119 | PaadA-dCas9-PmCDA1-UGI-LVA | na, no correct clones were obtained in E. coli probably due to the toxicity | [74] | |||||
| CBE | 2021 | Pj23119 | PvirB-dCas9-PmCDA1-UL | NGG | −15 to −19 | 91% | 80% for double targets | Inactivate recA to maintain stability for plant transformation; separately inactivate rolB, rolC, and orf13 to confirm their importance in hair root construction | nr | |||
| Sinorhizobium meliloti | Perform symbiotic nitrogen fixation within leguminous host plants such as alfalfa, an important forage crop | ABE | 2021 | PSigA/PRpoN/Ptyr | PHemA-TadA-TadA*-nCas9D10A | na, failed to mediate the A-to-G transition when gRNA is expressed by promoter SigA, RpoN or tyr | [75] | |||||
| ABE | 2021 | PRpsT | PHemA-TadA-TadA*-nCas9D10A | NGG | −11 to −17 | 60% | nr | Edit nodA to test the editing efficiency | nr | |||
| ABE | 2021 | PRpmJ | PHemA-TadA-TadA*-nCas9D10A | NGG | −11 to −17 | 100% | 90% for triple targets | Edit nodA, nodB, nodC, nifD, nifH, and nifK to test if the promoters can drive the expression of the fusion protein to perform efficient editing | nd by Sanger sequencing the potential sites predicted by Cas-OFFinder | |||
| ABE | 2021 | PRpmJ | PNeo-TadA-TadA*-nCas9D10A | NGG | −11 to −17 | 100% | ||||||
| ABE | 2021 | PRpmJ | PTau-TadA-TadA*-nCas9D10A | NGG | −11 to −17 | 80% | ||||||
| CBE | 2021 | PRpmJ | PHemA-rAPOBEC1-nCas9D10A-UGI | NGG | −13 to −17 | 75% | nr | Inactivate nodA (W7*) to test if the growth of plants inoculated with the mutant strain was retarded | ||||
| CBE | 2021 | PRpmJ | PTau-rAPOBEC1-nCas9D10A-UGI | NGG | −13 to −17 | 100% | nr | |||||
| CBE | 2021 | PRpmJ | PHemA-nCas9D10A-PmCDA1-UGI | NGG | −13 to −20 | 100% | 80% for double targets; 50–70% for triple targets | Edit nodA, etc to test editing efficiency | ||||
| GBE | 2021 | PRpmJ | PHemA-nCas9D10A-PmCDA1-UNG | NGG | −14 to −18 | 33–80% | nr | nr | ||||
| Clinically Important Microbes | ||||||||||||
| Brucella melitensis | The most important agent of human brucellosis | CBE | 2018 | PLlacO-1 | Ptrc-rAPOBEC1-nCas9D10A-UGI-NLS | NGG | −15 | 100% | nr | Inactivate virB10 by targeting three sites with 100% editing efficiency at only one site | nr | [37] |
| Klebsiella pneumoniae | Cause pneumonia, bloodstream infections, wound, or surgical site infections and meningitis; biosynthesize 1,3-propanediol and 2,3-butanediol | CBE | 2018 | Pj23119 | PrpsL-rAPOBEC1-nCas9D10A | NGG | −13 to −18 | 100% | nr | Edit fosA and dhaK to test editing efficiency with a few C-to-A byproducts; inactivate the blaKPC-2 and blaCTX-M-65 to dissect drug-resistance mechanisms | nr | [60] |
| Staphylococcus aureus | Cause infections ranging from skin infections to severe systemic infections | CBE | 2018 | Pcap 1A | PrpsL-rAPOBEC1-nCas9D10A | NGG | −13 to −17 | 100% | nr | Inactivate agrA and cntA to test efficiency | nr | [76] |
| ABE | 2020 | Pcap 1A | PrpsL-ecTadA-TadA*-nCas9D10A | NGG | −13 to −17 | 50–100% | 100% for double targets | Screen key residues of cntBC targeted by 38 gRNAs to obtain 42 mutant strains | nd gRNA-dependent off-target by WGS | [77] | ||
| Acinetobacter baumannii | causing ventilator-associated pneumonia and bloodstream infections, and mortality rates can reach 35% | CBE | 2019 | Pj23119 | Ptac-rAPOBEC1-nCas9D10A | NGG | −13 to −18 | 20–100% | nr | Edit tynA, acel, adeB, cpdA, entE, and oxyR to test editing efficiency and preference of TC motifs; disrupt drug-resistance relevant genes blaOXA-23, blaTEM-1D, and blaADC-25 to dissect drug-resistance mechanisms | nr | [78] |
| Mycobacterium spp. | Causes tuberculosis, getting 10 million infections and 1.45 million deaths in 2018 worldwide | CBE | 2021 | Pj23119 | PtetR-rAPOBEC1-dSt1Cas9 | NNRGAA | nr | 4–15% | nr | Test availability of dSt1Cas9-BE in Mycobacterium with low efficiency for C-to-T but 18–70% efficiency for C-to-G | nr | [79] |
| CBE | 2021 | Pj23119 | PtetR-rAPOBEC1-dSt1Cas9-UGI-UGI | NNRGAA | nr | 12–95% | nr | Inactivate katG to obtain mutant strain with increasing resistance to Isoniazid treatment | nr | |||
| CBE | 2021 | Pj23119 | PtetR-rAPOBEC1-dSt1Cas9evolve-UGI-UGI | NNNNAA | −10 to −14 | 20–95% | 87.5% for both double and triple targets | Inactivate the essential L-leucine biosynthesis genes leuB and lueC; inactivate ctpE to increase bacterium aggregation in the presence of EGTA | nd gRNA-dependent off-target by WGS | |||
| GBE | 2021 | Pj23119 | PtetR-rAPOBEC1-dSt1Cas9-UNG | NNRGAA | nr | 100% | nr | Edit five different loci to test editing efficiency | nr | |||
| GBE | 2021 | Pj23119 | PtetR-rAPOBEC1-dSt1Cas9evolve-UNG | NNNNAA | −13 to −16 | 20–65% | 75% for triple targets | Edit 29 endogenous genomics sites to find only TC motif is available for editing | nr | |||
| CBE | 2021 | Pj23119 | PtetO-rAPOBEC1-dSt1Cas9-UGI | NNAGGAC | nr | 1.2% | nr | Inactivate gfp to test editing efficiency | nr | [80] | ||
| CBE | 2021 | Pj23119 | PtetO-rAPOBEC1-nSt1Cas9-UGI | NNAGGAC | nr | 10.3% | nr | nr | ||||
| CBE | 2021 | Pj23119 | PtetO-rAPOBEC1-nSt1Cas9-UGI with assistant plasmid expressing recX | NNAGGAC | −12 to −18 | 37.5–100% | nr | nr | ||||
| CBE | 2021 | Pj23119 | PtetO-rAPOBEC1-nSt1Cas9-UGI with assistant plasmid expressing recX and nucSE107A | NNAGGAC | nr | 12.5–75% | nr | Inavtivate Rv0582, Rv0627 and Rv2530 to test efficiency; Inactivate katG to build a mutant stain with higher 50% minimum inhibitory concentration than the wild-type strain | nr | |||
nr: not reported, nd: not detected, na: not available, UL: UGI-LVA (protein degradation tag), SNVs: single-nucleotide variants, WGS: whole-genome sequencing, GRAS: generally recognized as safe, BGC: biosynthetic gene cluster. The construct of BEs that failed to work in microbes are marked in red.
2.2.1. Industrially Important Nonmodel Microbes
Kluyveromyces marxianus
As a nonmodel yeast with high growth rate, thermotolerance and a wide sugar assimilation spectrum, Kluyveromyces marxianus (K. marxianus) has great potential for industrial applications to produce various enzymes, chemicals, ethanol, and so on [81]. The high activity of the NHEJ system in K. marxianus hinders the development of CRISPR/Cas9; therefore, an alternative and efficient gene editing method is required to accelerate its genetic and metabolic characterizations. After Target-AID was established in S. cerevisiae [33], it was further applied in K. marxianus, belonging to the same class with S. cerevisiae, to inactivate NHEJ-related genes, Nej1 and Dnl4 [69]. The NHEJ null mutants with enhanced HR events facilitated the markerless integration mediated by CRISPR/Cas9. Although the mutants were successfully obtained, the editing efficiency of CBE was quite low, which can be improved in the future. With the combination of BEs and CRISPR/Cas9, this nonmodel species can be further explored for industrial applications.
Psedomonas putida
Psedomonas is a widespread genus from all over the world, especially found in extreme environments. It is reported to have strong potential for detoxifying environmental pollutants [82] and producing industrial bioactive compounds and pharmaceutical proteins. Psedomonas putida (P. putida) was used to produce monoterpene folic acid, fatty alcohols, rhamnolipids (Rs), and polyhydroxyalkanoates (PHAs) [83]. P. aeruginosa and P. fluorescens were reported to be the best-characterized phenazine producers as well [83]. After CBE, rAPOBEC1-nCas9, was proven to be able to inactivate genes in the Psedomonas species [43], and researchers were committed to improving its editing activity. Sun and colleagues [70] established a developed CBE, fusing rAPOBEC1 and UGI with an enhanced specificity nCas9 variant (eSpCas9ppD10A, containing mutations K848A/K1003A/R1060A) to fulfill almost 100% editing efficiency with no detectable gRNA-dependent off-target event in P. putida KT2440, P. aeruginosa PAO1, P. fluorescens Pf-5, and P. entomophila L48. Furthermore, similar to previous studies [35,40], using Cas9 variants to extend editable sites in the genome, eSpCas9ppD10A was engineered with additional mutations (L1111R, D1135V, G1218R, E1219F, A1322R, R1335A, T1337R), named eSpCas9ppD10A-NG, recognizing the NG PAM site in P. putida with 100% editing efficiency. rAPOBEC1 was also replaced with YE1[68] to narrow the editing window to nearly 2 nt in case unwanted conversions were created at other cytidine sites and led to low editing efficiency at the target [70]. Variants of deaminase enzymes can also be applied to other microbes to achieve precise conversion when the editing window comprises multiple cytidines.
Streptomyces lividans 66
Streptomyces, as a well-known antibiotic producer, plays an important role in producing more than two-thirds of medically and agriculturally important secondary metabolites, such as polyketides and nonribosomal peptides [84]. However, the production of many microbial drugs is very low in original strains since most BGCs are not or poorly expressed under traditional laboratory conditions. The solutions can be knocking out the competitive pathways, replacing negative promoters with strong ones, overexpressing positive regulatory genes [85], etc., which are all manipulated by efficient gene engineering techniques. Primary works have made great developments with CBE and ABE in Streptomyces to achieve single gene inactivation, multiplex gene editing, and reduction of off-target effects by fusing the high fidelity Cas9 variant [44,45,46]. Recently, Zhang and colleagues [71] developed an antisense RNA (asRNA) interference-enhanced CBE to increase editing efficiency in Streptomyces lividans 66 (S. lividans 66) since rAPOBEC1-d/nCas9-UGI in S. lividans 66 demonstrated a much lower editing efficiency than that in Streptomyces colicolor, shown in Table 2. They verified that the deletion of ung1 in S. lividans could significantly improve the editing efficiency without toxicity. Considering that the permanent inactivation of ung might be detrimental for industrial applications, they added an asRNA with CBE to transiently downregulate UNG which could become a normal level after plasmid curing. This design showed an efficiency enhancement of approximately 2.8 to 65.8 times compared with the original CBE and was more controllable than gene deletion. This construct can additionally be applied in other species, especially for those with a low editing efficiency of CBE.
Clostridium ljungdahlii
Clostridium, as a predominant cluster of commensal bacteria in the human gut, has an important place in biofuel and chemical production thanks to its unique capability of utilizing virtually all biomass-derived carbohydrates and waste gases [86]. Although the CRISPR/Cas9 technique has made great advancements in producing precise and fast gene editing in these species [87], limitations still exist such as the low transformation of bulky plasmids comprising large donor templates and low HR efficiency in some species [88]. Previously, Li and colleagues first established a CBE system, rAPOBEC1-nCas9-UGI, in C.beijerinckii [47]. The success of CBE working in C. beijerincki brought hope to its application in other species, such as C. ljungdahlii, which is popular for converting inorganic one-carbon (C1) gases into industrially important products, such as acetate and butanol [89], but is reported to be very inefficient and not robust for foreign DNA transformation [90].
In 2020, the carbon flux of ethanol production in C. ljungdahlii was reprogrammed by CBE to improve acetate production [72]. Researchers applied a relatively mild construct dCas9-PmCDA1-UL, rather than rAPOBEC1-nCas9-UGI, to lower the expression of CBE in C. ljungdahlii. By using a relatively loose linker (121 amino acids), CBE was able to edit within positions −2 to −19 of the protospacer and it exhibited the highest efficiency between positions −16 to −19 in C. ljungdahlii. Position −20 was not observed as being edited despite 20 nt and 22 nt gRNA being used, which could be investigated later with more targets or longer gRNAs. The genes involved in ethanol production were inactivated step by step to engineer strains with higher acetate yields, which could be accelerated by multiplex gene editing in the future. Although C. ljungdahlii is an A-T rich bacterium, it still comprises 99.83% editable sites for CBE to introduce missense mutations, nonsense mutations, or premature stop codons, estimated by a genome-scale algorithm [72]
Companilactobacillus crustorum
Companilactobacillus crustorum (C. crustorum, formerly named Lactobacillus crustorum), a newly isolated lactic acid bacterium from koumiss, is regarded as a novel probiotic agent because multiple components of the antimicrobial peptide transport system were discovered in it [91], such as 3-phenyllactic acid (PLA), which is a broad-spectrum antimicrobial compound that is widely used in the food and textile industries [92]. Unfortunately, C. crustorum has been poorly studied due to the lack of an efficient genetic method. Wang and colleagues recently established the CRISPR/Cas9 and CBE system in C. crustorum to identify the role of 12 bacteriocin-encoding genes [73]. rAPOBEC1-nCas9 was available to edit the C-rich spacer sequences in plasmid with almost 100% editing efficiency at positions −14 to −18. Multiplex gene editing using BEs can be further applied in chromosomes to investigate probiotic gene clusters and to characterize the unknown metabolic pathways in C. crustorum and others in the Lactobacillus family.
2.2.2. Agriculturally Important Nonmodel Microbes
Paenibacillus polymyxa
Paenibacillus polymyxa (P. polymyxa, formerly known as Bacillus polymyxa) is an agriculturally important microbe as it can directly promote crop growth via nitrogen fixation and phosphate solubilization [93]. It naturally produces a large number of valuable compounds like exopolysaccharides (EPS), 2,3-butanediol, antibiotics, and antimicrobial peptides such as polymyxin and fusaricidin [94]. Since the traditional gene editing methods had a low efficiency and required the integration of a selection marker, the discoveries of the unknown characteristics and physiochemical properties of P. polymyxa need a robust and efficient gene editing method.
Recently, after editing activities of different CBEs were compared with B. subtilis [56], dCas9-PmCDA1-UGI, which has the best editing efficiency, was also applied in P. polymyxa. There are six BGCs in the P. polymyxa genome and five of them are known to produce antibiotics [56]. After the five known BGCs were inactivated by CBE multiplex gene editing, the uncharacterized polyketide produced by the sixth unknown BGC was evaluated. The establishment of CBE in B. subtilis and P. polymyxa would greatly accelerate the discovery of hidden antibiotics and could be further applied in other Baccilus and Paenibacillus species. To the best of our knowledge, fusing Cas9 variants with BEs to recognize more PAM sites has not yet been reported in Baccilus and Paenibacillus species, which can be further explored in order to greatly reduce the number of noneditable genes.
Agrobacterium spp.
Agrobacterium, as a remarkable soil phytopathogen, can achieve a stable transformation using any gene of interest in the plant genome via delivery of transferred (T)-DNA [95]. With the help of a Agrobacterium-mediated transfer, CRISPR/Cas9 and BEs have been successfully established in plants to modify genomes for both biological functional analysis and crop improvement [96,97]. However, there has been a lack of characterization of genetic parts in Agrobacterium and poor exploration of its metabolic and physiological functions [98]. To improve the transformation of Agrobacterium in plants, which was previously reported to be inefficient [99,100], understanding and modifying transformation-related genes in Agrobacterium are also important. CBE has recently been established in two widely used plant transformation strains, A. tumefaciens and A. rhizogenes [74].
When CRISPR/Cas9 was found to be lethal in A. tumefaciens [74], the researchers set their sights on CBE, aiming to avoid the lethality caused by DSBs. The construct of dCas9-PmCDA1-UL, by modulating the length of gRNA from 20 nt to 18 nt, was proven to be available for gene editing with at least 98% efficiency in A. tumefaciens and A. rhizogenes. Interestingly, the CBE that was driven by the constitutive promoter was probably lethal to E. coli since no correct clones were obtained. After switching to the AS-activated promoter virB, the system could be successfully cloned in E. coli and worked well in Agrobacterium with or without AS treatment. The inactivation of rolB and rolC was efficiently achieved to confirm their importance in hairy root development. CBE facilitates the understanding and engineering of nature’s engineer, Agrobacterium. Further applications of BEs can be used in the field of plant-microbe interactions that help deep learning and improvement of agricultural plants and microbes for beneficial use.
Sinorhizobium meliloti
Sinorhizobium meliloti (S. meliloti) is a soil bacterium that can form nitrogen-fixing nodules on the roots of leguminous plants. The varied and rich metabolic capabilities of S. meliloti, such as carbohydrate metabolism, allow it to adapt to very different environmental and nutritional conditions in free-living form or as a plant symbiont [101]. Past studies have reported that S. meliloti can be successfully cultivated using starch industry wastewater as feedstock [102], produce cellulase using waste tobacco as a substrate [103], and can be engineered as a high-yield vitamin B12 strain [104]. It is also important to engineer its high nitrogen fixing capacity for the benefit of plant survival and reducing environmental toxicity.
Recently, single-plasmid CRISPR-mediated base editing tools (CBE, ABE, and GBE) have all been established in S. meliloti [75], which are saving a lot of time and labor compared to traditional tools such as Cre/loxP [105]. After testing a series of promoters for gRNA, the researchers found that an pRpmJ promoter (PRpmJ) performed a better editing activity than other promoters. ABE, TadA-TadA*-nCas9, was driven by PHemA, PNeo, or PTau, all of which performed nearly 100% editing efficiency and showed a preference for TA combinations over others, which is consistent with the specificity of ABE in Streptomyces coelicolor [44]. CBE and GBE were both driven by PHemA because it could drive the fusion proteins with sufficient efficiency without an inducer. With respect to the CBE used in S. meliloti, PmCDA1-CBE exhibited a slightly higher editing efficiency and a wider editing window than rAPOBEC1-CBE, shown in Table 2. After the inactivation of nodA in S. meliloti, the growth of plants inoculated with the mutant strain was retarded under nitrogen limiting conditions, which proved that nodA played an essential role in nitrogen fixation. GBE, nCas9-PmCDA1-UNG was reported to dominantly convert C to A in E. coli [62], but instead preferred to produce C-to-G with 30%~80% efficiency in S. meliloti. The editing specificity of C-to-G was lower than that of C-to-T. There was no detectable off-target event caused by CBE and ABE, both of which could simultaneously edit multiple genes with 50–90% efficiency. Through whole-genome prediction, there was a 96% editable stop site located in the first 80% of the coding region in S. meliloti, showing that BEs have promising potential for high-throughput genome engineering [75].
2.3. Clinically Important Nonmodel Microbes
CBE has been established in many human pathogens, including Brucella melitensis [37], P. aeruginosa [43], K. pneumoniae [60], Staphylococcus aureus (S. aureus) [76], and Acinetobacter baumannii [78]. It is worth mentioning the applications in them because the use of BEs can accelerate the understanding of bacterial physiology and drug-resistant mechanisms to develop novel therapeutic strategies.
Recently, ABE was developed as an effective screening tool in S. aureus to explore the functional residues of CntBC, a staphylopine/metal complex transporter [77]. ABE 7.10 was linked with 38 different spacers to mutate cntBC at various sites separately so that 42 mutant strains were acquired. The growth curves of these strains were tested because the mutations of CntBC residues might relieve the toxicity under a high concentration of cobalt, whereas the overload of cobalt restrained the growth of the wild strain. Through the ABE system, the key residues of CntBC were identified. Multiplex gene editing can be further applied to explore functional genes and new treatments.
M. tuberculosis (Mtb) is also a therapeutic challenge because of its high resistance to antibiotics [106]. New strategies against Mtb infections are urgently needed but are hampered by the unknown genetic backgrounds of the species. The lack of a compatible HR system limits the application of CRISPR/Cas9 in Mtb. Fortunately, BEs have recently made great developments in Mycobacterium to achieve highly desirable editing. Zhang and colleagues reported that catalytically inactive Streptococcus thermoplilus Cas9 (dSt1Cas9) fused with CBE was available to produce C-to-T and C-to-G conversions with 4–15% and 18–70% efficiency, respectively, compared with no detectable base editing by dspCas9- and dLbCpf1- CBE [79]. To overcome the impure editing products, two UGIs and UNGs were separately linked to the C terminus of dStlCas9, yielding dSt1Cas9-CBE, converting C to T with 69–86% efficiency, and dSt1Cas9-GBE, converting C to G with 100% efficiency, respectively. To expand the strict PAM sequence (5′-NNRGAA-3′, R = A/G), wild-type St1Cas9 was replaced with a KQKL variant (St1Cas9evolve) containing multiple substitutions with 5′-NNNNAA-3′ PAM specificity, which can inactivate more than 75% open reading frame (ORF) in mycobacterium species by introducing at least one premature stop codon within the top 75% of the ORF body [79]. Later, Ding and colleagues [80] proved that the inhibition of recA in HR, and nucS in MMR, by expressing RecX and NucSE107A in assistant plasmids, could facilitate CBE efficient editing in Mtb. Further manipulation of DNA polymerases to increase the editing activity of BEs could be applied in other species.
3. Current Obstacles of BEs in Microbes
3.1. Limitation of Editing Activity
As illustrated in Table 2, the multiplex gene editing and off-target effects are still the major problems of BEs. Only a few BEs can simultaneously edit up to five targets in nonmodel microbes [45,56]. Most BEs just achieve double or triple simultaneous editing with low efficiency. One reason might be the technical limitation of creating long arrays of gRNAs. The different efficiencies at various targets are also one of the reasons, because BEs are reported to perform almost 100% editing efficiency at some targets, although they are unable to work at some sites, probably due to the essentiality of genes [44,48,57]. The other serious reason is that as the number of gRNAs increases, they must compete for a dwindling pool of fusion protein, which in turn decreases the efficiency of every gRNA [107]. This problem widely exists in CRISPR technologies and can be possibly solved by using conditional gRNAs, which are selectively triggered as needed in vivo [107].
The off-target effects of BEs also need to be considered and surmounted in the future. The gRNA-dependent off-targets, based on mismatch annealing of gRNAs, can be decreased to an undetectable level by replacing spCas9 with high-fidelity Cas proteins [45], modifications of gRNA structure [54], etc., which are the same strategies used to decrease the off-targets of CRISPR/Cas9 [108]. However, the gRNA-independent off-targets are major and specific problems for BEs. They are thought to be nonspecific and random deamination by the deaminase domain. The gRNA-independent off-targets commonly happen at the transcribed regions because the R-loops formed by the exposure of single-stranded DNA by RNA transcription is a preferred substrate for the deaminases [53]. The on-target activities of BEs still need to be improved in the future. Currently, the structural engineering of deaminases to improve their specificity is one of the solutions, illustrated in Table 1. The expression levels and duration of BEs can also be optimized to mitigate the gRNA-independent off-targets [32]. One-step multiplex editing is recommended, rather than iterative editing, to minimize the off-target effects as the number of targets does not have a large impact on the off-target mutations [56].
3.2. Variability of Base Editing Activity
Although BEs generally possess 5 nt editing windows, narrower or broader editing windows with different editing efficiencies are shown in Table 2. CBE, ABE, and GBE all seem to show preferences for editing at TC or TA motifs with a higher efficiency than other motifs [44,94,99,105], probably due to the deaminase-specific substrate preference. Recent research [109] has tested thousands of target sites and concluded that base editing activity strongly depends on the combination of its position in the target and adjacent base. A preceding T leads to a wider editing window in both CBE and ABE, whereas a preceding G in CBE, and A in ABE, leads to a narrower one, and following T increases C to G editing in CBE. Although the mechanisms behind the variability have not yet been revealed, based on the large target library datasets, the machine learning models, such as FORECasT-BE [109], BE-Hive [110], BE-DICT [111], etc., were trained to predict the editing outcomes of BEs with high accuracy, guiding the applications of current and novel BEs.
3.3. Unavailability of Some BEs in Microbes
As reported in previous studies, some BE constructs are unavailable to some microbes, such as E. coli [36,62], Streptomyces [45,46], B. subtilis [55,56], Psedomonas [70], Agrobacterium [74], and S. meliloti [75]. The toxicity of CRISPR/Cas9 has also been reported [112,113]. Although there is no mechanism to explain the unavailability, some solutions can be put forward. Replacing strong promoters with relatively weak promoters, or adding LVA tag to decrease the expression of fusion protein, worked in some cases. The integration of fusion protein into a genome also efficiently worked [57]. Recently, a platform for in vivo rapid investigation of BE components (fusion proteins and sgRNA) in E. coli (IRI-CCE) was developed to assess the differential-strength, promoters-driven base conversion [114]. IRI-CCE revealed an appropriate amount of BE expression, which was a crucial factor in achieving high editing efficiency [114], and could be further expanded upon in other microbes.
4. Future Scope of BEs in Other Microbes
Thus far, efficient BE working systems have been established in lots of microbes. In some, base pairs could be converted to any of the others using a combination of CBE, ABE, and GBE. The microbes that have not been fully established by BEs are listed in Figure 4 and will hopefully be explored in the future. We also believe that the nonmodel microbes belonging to the same genera of the universal phylogenetic tree in Figure 2, or that are available for CRISPR/Cas9, hold great hope for the future establishment of BEs. The reason why there has been no huge applications of BEs in other microbes is probably due to the novelty of Bes, since they have just been developed in recent years. The toxicity of fusion protein is another reason, but it can probably be solved by replacing nCas9 with dCas9 and decreasing its expression level as mentioned above. The applications of BEs may also be affected by the polyploidy in some species, as multiplex gene editing is not efficient in some microbes; however, BEs still show powerful potential for precise gene editing and genome-wide engineering in other microbes. The primary reasons are as follows.
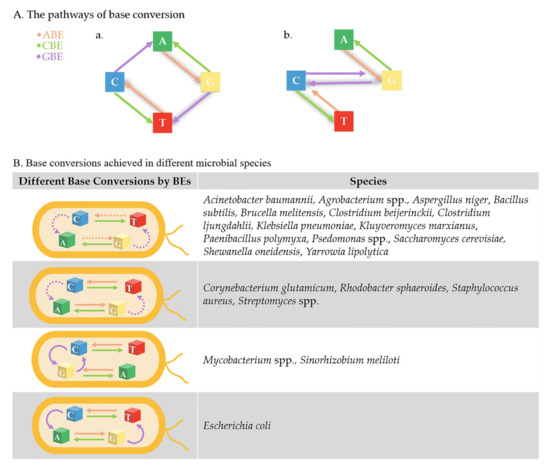
Figure 4.
Current development of BEs in different microbial species. (A) In theory, any base pair could be converted to any of the others, which only takes three steps at most using the combination of ABE, CBE, and GBE. Firstly, C:G and T:A can be converted to each other by CBE and ABE. Secondly, C:G can be converted to G:C or A:T by GBE. (a) If C:G is predominantly converted to A:T by GBE, the most complicated editing step, shaded in grey, is from A:T to C:G, by firstly converting A:T to G:C by ABE, then to T:A by GBE, and finally to C:G by ABE. (b) If C:G is predominantly converted to G:C by GBE, the most complicated conversion, shaded in grey, is from A:T to T:A, firstly converting A:T to G:C by ABE, then to C:G by GBE, and finally converting to T:A by CBE. (B) The establishment of BEs in different microbes are listed in the table. The achieved base conversions are drawn with a solid line, and base conversions that have not been completed in some microbes are drawn with a dotted line.
Firstly, BEs are more applicable than CRISPR/Cas9 for microbes, especially nonmodel microbes. For those species available for CRISPR/Cas9, BEs are very convenient for plasmid construction and transformation. For those unavailable for CRISPR/Cas9, BEs can still achieve editing with high efficiency since the working process does not depend on DSBs and HR [94,105,106]. The CRISPR/Cas9 and BEs can also complement gene editing. For example, they are designed as a double-check system to increase the editing efficiency of BEs to 99% at some sites [38]. The inactivation of NHEJ genes by BEs facilitate the further application of CRISPR/Cas9 [69]. The insertions of heterologous metabolic pathways into a genome require CRISPR/Cas9 and in situ mutagenesis to increase the industrial production need for BEs [115].
Moreover, the availability of a BE can cover almost the whole genome in microbes. For example, only 24 genes accounting for 0.77% of the genome in C. glutamicum [40], and 32 genes in B. subtilis [55], are inaccessible for Cas9 variant-BEs by genome-wide analysis. The mutable and knockout targets for CBE account for 99.8% and 96.6%, respectively, in the genome of R. sphaeroides [48]. The editable targets account for 99.83% in Clostridium ljungdahlii [72], and 96% of editable stop sites are located in the first 80% of the coding region in S. meliloti [75]. For S. aureus, almost 68.8% of the genes in MRSA252 strain, and 70.36% of the genes in the Newman strain, possess editable stop sites by CBE [79], and 75% ORF can be inactivated in M. tuberculosis [79]. Thirdly, BEs can introduce multiplex random mutagenesis in up to five targets in Streptomyces [45] and B. subtilis [57] for metabolic pathway engineering. The engineering of B. subtilis with an enhanced stability to secrete heterologous proteins only takes two rounds of editing to inactivate eight extracellular protease genes [55].
On the one hand, BEs are perfect for precise gene editing, with fewer indels than CRISPR/Cas9. On the other hand, the capability of BEs to produce in situ mutation provides a new method for microbial evolvement. In vivo evolution of proteins was successfully achieved by BEs [57], and was superior to in vitro protein evolution limited by hard heterologous expression. By mutating different sites of genes, the mutant libraries are obtained and can be selected for further analysis. Through this method, mutant stains with improved or decreased performance can be acquired and further used in industrial production and new drug strategies. For example, a B. bacillus mutant with an evolved Bacitracin-resistant protein (BceB) can be constructed as a bacitracin-producing cell factory [57]. In situ mutagenesis of a general transcription factor gene SPT15 gained 36 S. cerevisiae mutant strains with sensitive or enhanced stress tolerance, which can be adaptable to various harsh industrial conditions [116]. The mutant libraries also provide large valuable information for future research. Moreover, CBE-mediated multiplex gene editing was developed into a Base Editor-Targeted and Template-free Expression Regulation (BETTER) method in C. glutamicum and B. subtilis, generating large numbers of genetic combinations of diverse ribosome binding sites, 5′ untranslated regions and promoters to improve xylose catabolism, glycerol catabolism, and lycopene biosynthesis [115]. A BE-mediated in vivo mutagenesis method was further engineered with different systems. Recently, various base deaminases (AID, rAPOBEC1, pmCDA1, and TadA*) were separately fused to T7 RNA polymerase (T7RNAP), which specifically recognized a T7 promoter oriented towards the target sequence, to introduce random mutagenesis as an efficient strategy for continuous in vivo evolution of proteins and metabolic engineering [117,118]. A random base editing (rBE) system was developed by fusing an unspecific single-stranded DNA (ssDNA)-binding protein with rAPOBEC1 in S. cerevisiae to achieve genome-scale mutations, and it finally obtained a yeast cell factory resistant to 9% isobutanol [119]. Since the strategies above can be adapted in various organisms, it is possible to look forward to the further evolution of nonmodel microbes.
5. Conclusions
In summary, BEs have currently performed efficient editing in 21 microbial genera (Figure 2) and a wide range of nonmodel microbes (Table 2), and they have strong potential to be extended into other species due to their great availability. The applications of BEs in nonmodel microbes are not only limited to precise point mutation, such as gene inactivation and amino acid substitution, but also genome-wide engineering, such as in vivo protein and strain evolution. Although the methods have overcome the limitations of CRSIPR/Cas9 in some microbes, their specific obstacles, such as gRNA-independent off-targets, also need to be improved in the future. However, collectively speaking, BEs are expected to be powerful gene engineering tools which speed up the genetic characterizations and metabolic reprogramming in nonmodel microbes.
Author Contributions
M.L., Y.-X.H. and S.G. worked on the design, methodology, and drafting of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Key R&D Program of China (Grant Nos. 2019YFA0904104 and 2021YFC2100500).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kalsoom, M.; Rehman, F.U.; Shafique, T.; Junaid, S.; Khalid, N.; Adnan, M.; Zafar, I.; Abdullah Tariq, M.; Raza, M.A.; Zahra, A.; et al. Biological Importance of Microbes in Agriculture, Food and Pharmaceutical Industry: A Review. Innovare J. Life Sci. 2020, 8, 1–4. [Google Scholar] [CrossRef]
- Peralta-Yahya, P.P.; Keasling, J.D. Advanced Biofuel Production in Microbes. Biotechnol. J. 2010, 5, 147–162. [Google Scholar] [CrossRef] [PubMed]
- da Silva, T.L.; Gouveia, L.; Reis, A. Integrated Microbial Processes for Biofuels and High Value-Added Products: The Way to Improve the Cost Effectiveness of Biofuel Production. Appl. Microbiol. Biotechnol. 2014, 98, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Gronchi, N.; Favaro, L.; Cagnin, L.; Brojanigo, S.; Pizzocchero, V.; Basaglia, M.; Casella, S. Novel Yeast Strains for the Efficient Saccharification and Fermentation of Starchy By-Products to Bioethanol. Energies 2019, 12, 714. [Google Scholar] [CrossRef]
- Alonso, S.; Rendueles, M.; Díaz, M. Microbial Production of Specialty Organic Acids from Renewable and Waste Materials. Crit. Rev. Biotechnol. 2015, 35, 497–513. [Google Scholar] [CrossRef]
- Michael, S.; Diethard, M. Construction of Microbial Cell Factories for Industrial Bioprocesses. J. Chem. Technol. Biotechnol. 2012, 87, 445–450. [Google Scholar] [CrossRef]
- Ko, Y.S.; Kim, J.W.; Lee, J.A.; Han, T.; Kim, G.B.; Park, J.E.; Lee, S.Y. Tools and Strategies of Systems Metabolic Engineering for the Development of Microbial Cell Factories for Chemical Production. Chem. Soc. Rev. 2020, 49, 4615–4636. [Google Scholar] [CrossRef]
- Riley, L.A.; Guss, A.M. Approaches to Genetic Tool Development for Rapid Domestication of Non-Model Microorganisms. Biotechnol. Biofuels 2021, 14, 1–17. [Google Scholar] [CrossRef]
- Fatma, Z.; Schultz, J.C.; Zhao, H. Recent Advances in Domesticating Non-Model Microorganisms. Biotechnol. Prog. 2020, 36, e3008. [Google Scholar] [CrossRef]
- Moore, S.J.; MacDonald, J.T.; Wienecke, S.; Ishwarbhai, A.; Tsipa, A.; Aw, R.; Kylilis, N.; Bell, D.J.; McClymont, D.W.; Jensen, K.; et al. Rapid Acquisition and Model-Based Analysis of Cell-Free Transcription–Translation Reactions from Non-Model Bacteria. Proc. Natl. Acad. Sci. USA 2018, 115, E4340–E4349. [Google Scholar] [CrossRef]
- Yan, Q.; Fong, S.S. Challenges and Advances for Genetic Engineering of Non-Model Bacteria and Uses in Consolidated Bioprocessing. Front. Microbiol. 2017, 8, 2060. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, M.; Moore, R.T.; Rajamani, S.; Rekha, G.P. Bacterial Genome Engineering and Synthetic Biology: Combating Pathogens. BMC Microbiol. 2016, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cobb, R.E.; Wang, Y.; Zhao, H. High-Efficiency Multiplex Genome Editing of Streptomyces Species Using an Engineered CRISPR/Cas System. ACS Synth. Biol. 2015, 4, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Z.T.; Seo, S.O.; Lynn, P.; Lu, T.; Jin, Y.S.; Blaschek, H.P. Bacterial Genome Editing with CRISPR-Cas9: Deletion, Integration, Single Nucleotide Modification, and Desirable “Clean” Mutant Selection in Clostridium Beijerinckii as an Example. ACS Synth. Biol. 2016, 5, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Wang, J.; Qi, Q. Prophage Recombinases-Mediated Genome Engineering in Lactobacillus Plantarum. Microb. Cell Factories 2015, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Muras, A.; Romero, M.; Mayer, C.; Otero, A. Biotechnological Applications of Bacillus Licheniformis. Crit. Rev. Biotechnol. 2021, 41, 609–627. [Google Scholar] [CrossRef]
- Shi, T.; Gao, J.; Wang, W.; Wang, K.; Xu, G.; Huang, H.; Ji, X. CRISPR/Cas9-Based Genome-Editing in the Filamentous Fungus Fusarium Fujikuroi and Its Application in Strain Engineering for Gibberellic Acid Production. ACS Synth. Biol. 2019, 8, 445–454. [Google Scholar] [CrossRef]
- Rico, E.; Jeacock, L.; Kovářová, J.; Horn, D. Inducible High-Efficiency CRISPR-Cas9-Targeted Gene Editing and Precision Base Editing in African Trypanosomes. Sci Rep. 2018, 8, 7960. [Google Scholar] [CrossRef]
- Vergnes, B.; Gazanion, E.; Mariac, C.; Du Manoir, M.; Sollelis, L.; Lopez-Rubio, J.J.; Sterkers, Y.; Bañuls, A.L. A Single Amino Acid Substitution (H451Y) In Leishmania Calcium-Dependent Kinase SCAMK Confers High Tolerance and Resistance to Antimony. J. Antimicrob. Chemother. 2019, 74, 3231–3239. [Google Scholar] [CrossRef]
- LaFountaine, J.S.; Fathe, K.; Smyth, H.D. Delivery and Therapeutic Applications of Gene Editing Technologies ZFNs, TALENs, and CRISPR/Cas9. Int. J. Pharm. 2015, 494, 180–194. [Google Scholar] [CrossRef]
- Xu, T.; Li, Y.; Shi, Z.; Hemme, C.L.; Li, Y.; Zhu, Y.; van Nostrand, J.D.; He, Z.; Zhou, J. Efficient Genome Editing in Clostridium Cellulolyticum via CRISPR-Cas9 Nickase. Appl. Environ. Microbiol. 2015, 81, 4423–4431. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Bikard, D. Consequences of Cas9 Cleavage in the Chromosome of Escherichia Coli. Nucleic Acids Res. 2016, 44, 4243–4251. [Google Scholar] [CrossRef] [PubMed]
- Symington, L.S.; Gautier, J. Double-Strand Break End Resection and Repair Pathway Choice. Annu. Rev. Genet. 2011, 45, 247–271. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.D.; Lander, E.S.; Zhang, F. Development and Applications of CRISPR-Cas9 for Genome Engineering. Cell 2014, 157, 1262–1278. [Google Scholar] [CrossRef]
- van Kessel, J.C.; Hatfull, G.F. Recombineering in Mycobacterium Tuberculosis. Nat. Methods 2007, 4, 147–152. [Google Scholar] [CrossRef]
- Bae, S.J.; Park, B.G.; Kim, B.G.; Hahn, J.S. Multiplex Gene Disruption by Targeted Base Editing of Yarrowia Lipolytica Genome Using Cytidine Deaminase Combined with the CRISPR/Cas9 System. Biotechnol. J. 2020, 15, e1900238. [Google Scholar] [CrossRef]
- Anzalone, A.V.; Koblan, L.W.; Liu, D.R. Genome Editing with CRISPR–Cas Nucleases, Base Editors, Transposases and Prime Editors. Nat. Biotechnol. 2020, 38, 824–844. [Google Scholar] [CrossRef]
- Chen, X.; Janssen, J.M.; Liu, J.; Maggio, I.; ‘t Jong, A.E.; Mikkers, H.M.; Gonçalves, M.A. In Trans Paired Nicking Triggers Seamless Genome Editing without Double-Stranded DNA Cutting. Nat. Commun. 2017, 8, 1–15. [Google Scholar] [CrossRef]
- Jiang, F.; Doudna, J.A. CRISPR–Cas9 Structures and Mechanisms. Annu. Rev. Biophys. 2017, 46, 505–529. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Zheng, P.; Sun, J.; Wang, M. Microbial Base Editing: A Powerful Emerging Technology for Microbial Genome Engineering. Trends Biotechnol. 2021, 39, 165–180. [Google Scholar] [CrossRef]
- Jiang, Z.; Hong, X.; Zhang, S.; Yao, R.; Xiao, Y.I. CRISPR Base Editing and Prime Editing: DSB and Template-Free Editing Systems for Bacteria and Plants. Synth. Syst. Biotechnol. 2020, 5, 277–292. [Google Scholar] [CrossRef]
- Arazoe, T.; Kondo, A.; Nishida, K. Targeted Nucleotide Editing Technologies for Microbial Metabolic Engineering. Biotechnol. J. 2018, 13, e1700596. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Arazoe, T.; Yachie, N.; Banno, S.; Kakimoto, M.; Tabata, M.; Mochizuki, M.; Miyabe, A.; Araki, M.; Hara, K.Y.; et al. Targeted Nucleotide Editing Using Hybrid Prokaryotic and Vertebrate Adaptive Immune Systems. Science 2016, 353, aaf8729. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Zhang, F.; Karcher, D.; Bock, R. Engineering of High-Precision Base Editors for Site-Specific Single Nucleotide Replacement. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Tan, J.; Zhang, F.; Karcher, D.; Bock, R. Expanding the Genome-Targeting Scope and the Site Selectivity of High-Precision Base Editors. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Banno, S.; Nishida, K.; Arazoe, T.; Mitsunobu, H.; Kondo, A. Deaminase-Mediated Multiplex Genome Editing in Escherichia Coli. Nat. Microbiol. 2018, 3, 423–429. [Google Scholar] [CrossRef]
- Zheng, K.; Wang, Y.; Li, N.; Jiang, F.; Wu, C.; Liu, F.; Chen, H.; Liu, Z. Highly Efficient Base Editing in Bacteria Using a Cas9-Cytidine Deaminase Fusion. Commun. Biol. 2018, 1, 32. [Google Scholar] [CrossRef]
- Xin, X.; Li, J.; Zhao, D.; Li, S.; Xie, Q.; Li, Z.; Fan, F.; Bi, C.; Zhang, X. Double-Check Base Editing for Efficient A to G Conversions. ACS Synth. Biol. 2019, 8, 2629–2634. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Liu, J.; Guo, Y.; Fan, L.; Ni, X.; Zheng, X.; Wang, M.; Zheng, P.; Sun, J.; et al. MACBETH: Multiplex Automated Corynebacterium Glutamicum Base Editing Method. Metab. Eng. 2018, 47, 200–210. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Li, J.; Yang, Y.; Ni, X.; Cheng, H.; Huang, T.; Guo, Y.; Ma, H.; Zheng, P.; et al. Expanding Targeting Scope, Editing Window, and Base Transition Capability of Base Editing in Corynebbacterium Glutamicum. Biotechnol. Bioeng. 2019, 116, 3016–3029. [Google Scholar] [CrossRef]
- Huang, H.; Bai, L.; Liu, Y.; Li, J.; Wang, M.; Hua, E. Development and Application of BE3 Cytidine Base Editor in Corynebacterium Glutamicum. Biotechnol. Bull. 2020, 36, 95–101. [Google Scholar] [CrossRef]
- Huang, L.; Dong, H.; Zheng, J.; Wang, B.; Pan, L. Highly Efficient Single Base Editing in Aspergillus Niger with CRISPR/Cas9 Cytidine Deaminase Fusion. Microbiol. Res. 2019, 223, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, Y.; Zhang, Y.; Pi, Y.; Gu, T.; Song, L.; Wang, Y.; Ji, Q. CRISPR/Cas9-Based Genome Editing in Pseudomonas Aeruginosa and Cytidine Deaminase-Mediated Base Editing in Pseudomonas Species. IScience 2018, 6, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Whitford, C.M.; Robertsen, H.L.; Blin, K.; Jørgensen, T.S.; Klitgaard, A.K.; Gren, T.; Jiang, X.; Weber, T.; Lee, S.Y. Highly Efficient DSB-Free Base Editing for Streptomycetes with CRISPR-BEST. Proc. Natl. Acad. Sci. USA 2019, 116, 20366–20375. [Google Scholar] [CrossRef]
- Zhong, Z.; Guo, J.; Deng, L.; Chen, L.; Wang, J.; Li, S.; Xu, W.; Deng, Z.; Sun, Y. Base Editing in Streptomyces with Cas9-Deaminase Fusions. BioRxiv 2019, 630137. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, J.; Zheng, G.; Chen, J.; Sun, C.; Yang, Z.; Zimin, A.A.; Jiang, W.; Deng, Z.; Wang, Z.; et al. Multiplex Genome Editing Using a dCas9-Cytidine Deaminase Fusion in Streptomyces. Sci. China Life Sci. 2020, 63, 1053–1062. [Google Scholar] [CrossRef]
- Li, Q.; Seys, F.M.; Minton, N.P.; Yang, J.; Jiang, Y.; Jiang, W.; Yang, S. CRISPR–Cas9D10A Nickase-Assisted Base Editing in the Solvent Producer Clostridium Beijerinckii. Biotechnol. Bioeng. 2019, 116, 1475–1483. [Google Scholar] [CrossRef]
- Luo, Y.; Ge, M.; Wang, B.; Sun, C.; Wang, J.; Dong, Y.; Xi, J.J. CRISPR/Cas9-Deaminase Enables Robust Base Editing in Rhodobacter Sphaeroides 2.4.1. Microb. Cell Factories 2020, 19, 1–14. [Google Scholar] [CrossRef]
- Cheng, L.; Min, D.; He, R.L.; Cheng, Z.H.; Liu, D.F.; Yu, H.Q. Developing a Base-Editing System to Expand the Carbon Source Utilization Spectra of Shewanella Oneidensis MR-1 for Enhanced Pollutant Degradation. Biotechnol. Bioeng. 2020, 117, 2389–2400. [Google Scholar] [CrossRef]
- Yang, B.; Yang, L.; Chen, J. Development and Application of Base Editors. CRISPR J. 2019, 2, 91–104. [Google Scholar] [CrossRef]
- Park, S.; Beal, P.A. Off-Target Editing by CRISPR-Guided DNA Base Editors. Biochemistry 2019, 58, 3727–3734. [Google Scholar] [CrossRef] [PubMed]
- Eid, A.; Alshareef, S.; Mahfouz, M.M. CRISPR Base Editors: Genome Editing without Double-Stranded Breaks. Biochem. J. 2018, 475, 1955–1964. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Mitsunobu, H.; Yoshioka, S.; Takahisa, S.; Kondo, A.; Nishida, K. Cytosine Base Editing Systems with Minimized Off-Target Effect and Molecular Size. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, Y.; Liu, Q.; Qiu, Y.; Zhong, Z.; Li, K.; Li, W.; Deng, Z.; Sun, Y. Improving the Precision of Base Editing by Bubble Hairpin Single Guide RNA. Mbio 2021, 12, e00342–e00421. [Google Scholar] [CrossRef]
- Yu, S.; Price, M.A.; Wang, Y.; Liu, Y.; Guo, Y.; Ni, X.; Rosser, S.J.; Bi, C.; Wang, M. CRISPR-dCas9 Mediated Cytosine Deaminase Base Editing in Bacillus Subtilis. ACS Synth. Biol. 2020, 9, 1781–1789. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, H.R.; Jeong, D.E.; Choi, S.K. Cytosine Base Editor-Mediated Multiplex Genome Editing to Accelerate Discovery of Novel Antibiotics in Bacillus Subtilis and Paenibacillus Polymyxa. Front. Microbiol. 2021, 12, 691839. [Google Scholar] [CrossRef]
- Hao, W.; Cui, W.; Cheng, Z.; Han, L.; Suo, F.; Liu, Z.; Zhou, L.; Zhou, Z. Development of a Base Editor for Protein Evolution via in Situ Mutation in Vivo. Nucleic Acids Res. 2021, 49, 9594–9605. [Google Scholar] [CrossRef]
- Tong, Y.; Jørgensen, T.S.; Whitford, C.M.; Weber, T.; Lee, S.Y. A Versatile Genetic Engineering Toolkit for E. Coli Based on CRISPR-Prime Editing. Nat. Commun. 2021, 12, 1–11. [Google Scholar] [CrossRef]
- Habib, O.; Habib, G.; Hwang, G.H.; Bae, S. Comprehensive Analysis of Prime Editing Outcomes in Human Embryonic Stem Cells. BioRxiv 2021, 50, 1187–1197. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Chen, W.; Song, L.; Zhang, Y.; Shen, Z.; Yu, F.; Li, M.; Ji, Q. CRISPR-Cas9 and CRISPR-Assisted Cytidine Deaminase Enable Precise and Efficient Genome Editing in Klebsiella Pneumoniae. Appl. Environ. Microbiol. 2018, 84, e01834-18. [Google Scholar] [CrossRef]
- Koblan, L.W.; Doman, J.L.; Wilson, C.; Levy, J.M.; Tay, T.; Newby, G.A.; Maianti, J.P.; Raguram, A.; Liu, D.R. Improving Cytidine and Adenine Base Editors by Expression Optimization and Ancestral Reconstruction. Nat. Biotechnol. 2018, 36, 843–846. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Li, J.; Li, S.; Xin, X.; Hu, M.; Price, M.A.; Rosser, S.J.; Bi, C.; Zhang, X. Glycosylase Base Editors Enable C-to-A and C-to-G Base Changes. Nat. Biotechnol. 2021, 39, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Wang, J.; Zhao, D.; Chen, X.; Pu, S.; Zhang, C.; Li, J.; Li, Y.; Yang, J.; Li, S.; et al. Molecular Mechanism of the Cytosine CRISPR Base Editing Process and the Roles of Translesion DNA Polymerases. ACS Synth. Biol. 2021, 10, 3353–3358. [Google Scholar] [CrossRef]
- Sale, J.E. Translesion DNA Synthesis and Mutagenesis in Eukaryotes. Cold Spring Harb. Perspect. Biol. 2013, 5, a012708. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Park, J.E.; Paa, P.; Rajakumar, P.D.; Prekop, H.T.; Chew, Y.T.; Manivannan, S.N.; Chew, W.L. Programmable C: G to G: C Genome Editing with CRISPR-Cas9-Directed Base Excision Repair Proteins. Nat. Commun. 2021, 12, 1–7. [Google Scholar] [CrossRef]
- Koblan, L.W.; Arbab, M.; Shen, M.W.; Hussmann, J.A.; Anzalone, A.V.; Doman, J.L.; Newby, G.A.; Yang, D.; Mok, B.; Replogle, J.M.; et al. Efficient C•G-to-G•C Base Editors Developed Using CRISPRi Screens, Target-Library Analysis, and Machine Learning. Nat. Biotechnol 2021, 39, 1414–1425. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Z.; Lai, L.; Li, Z. Efficient C-to-G Base Editing with Improved Target Compatibility Using Engineered Deaminase-nCas9 Fusions. CRISPR J. 2022. [Google Scholar] [CrossRef]
- Doman, J.L.; Raguram, A.; Newby, G.A.; Liu, D.R. Evaluation and Minimization of Cas9-Independent Off-Target DNA Editing by Cytosine Base Editors. Nat. Biotechnol. 2020, 38, 620–628. [Google Scholar] [CrossRef]
- Nambu-Nishida, Y.; Nishida, K.; Hasunuma, T.; Kondo, A. Development of a Comprehensive Set of Tools for Genome Engineering in a Cold- And Thermo-Tolerant Kluyveromyces Marxianus Yeast Strain. Sci Rep. 2017, 7, 8993. [Google Scholar] [CrossRef]
- Sun, J.; Lu, L.B.; Liang, T.X.; Yang, L.R.; Wu, J.P. CRISPR-Assisted Multiplex Base Editing System in Pseudomonas Putida KT2440. Front. Bioeng. Biotechnol. 2020, 8, 905. [Google Scholar] [CrossRef]
- Zhang, Y.; Yun, K.; Huang, H.; Tu, R.; Hua, E.; Wang, M. Antisense RNA Interference-Enhanced CRISPR/Cas9 Base Editing Method for Improving Base Editing Efficiency in Streptomyces lividans 66. ACS Synth. Biol. 2021, 10, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.F.; Casini, I.; Schulz, S.; Klask, C.M.; Angenent, L.T.; Molitor, B. Reprogramming Acetogenic Bacteria with CRISPR-Targeted Base Editing via Deamination. ACS Synth. Biol. 2020, 9, 2162–2171. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yi, Y.; Lü, X. CRISPR/Cas9-Based Genome Editing Platform for Companilactobacillus Crustorum to Reveal the Molecular Mechanism of Its Probiotic Properties. J. Agric. Food Chem. 2021, 69, 15279–15289. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.D.; Karimi, M.; Impens, L.; van Lerberge, E.; Coussens, G.; Aesaert, S.; Rombaut, D.; Holtappels, D.; Ibrahim, H.M.M.; Montagu, M.V.; et al. Efficient CRISPR-Mediated Base Editing in Agrobacterium Spp. Proc. Natl. Acad. Sci. USA 2021, 118, e2013338118. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiao, Y.; Wei, X.; Pan, J.; Duanmu, D. Highly Efficient CRISPR-Mediated Base Editing in Sinorhizobium meliloti. Front. Microbiol. 2021, 12, 686008. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.; Zhao, S.; Pi, Y.; Chen, W.; Chen, C.; Liu, Q.; Li, M.; Han, D.; Ji, Q. Highly Efficient Base Editing in Staphylococcus Aureus Using an Engineered CRISPR RNA-Guided Cytidine Deaminase. Chem Sci 2018, 9, 3248–3253. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Wang, Z.; Wu, Z.; Wang, Y.; Tang, N.; Xu, X.; Zhao, S.; Chen, W.; Ji, Q. Programmable Adenine Deamination in Bacteria Using a Cas9–Adenine-Deaminase Fusion. Chem. Sci. 2020, 11, 1657–1664. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Chen, Y.; Hua, X.; Yu, Y.; Ji, Q. A highly Efficient CRISPR-Cas9-Based Genome Engineering Platform in Acinetobacter Baumannii to Understand the H2O2-Sensing Mechanism of OxyR. Cell Chem. Biol. 2019, 26, 1732–1742. [Google Scholar] [CrossRef]
- Ji, Q.; Zhang, H.; Zhang, Y.; Wang, W.; Chen, W.; Zhang, X.; Huang, X.; Chen, W. PAM-Expanded Streptococcus Thermophilus Cas9 C-to-T and C-to-G Base Editors for Programmable Base Editing in Mycobacteria. ChemRxiv 2021. Available online: https://chemrxiv.org/engage/chemrxiv/article-details/6170f1308acf7e6647c5caaa (accessed on 23 March 2022).
- Ding, X.Y.; Li, S.S.; Geng, Y.M.; Yan, M.Y.; Li, G.B.; Zhang, G.L.; Sun, Y.C. Programmable Base Editing in Mycobacterium tuberculosis Using an Engineered CRISPR RNA-Guided Cytidine Deaminase. Front. Genome Ed. 2021, 3, 734436. [Google Scholar] [CrossRef]
- Nurcholis, M.; Lertwattanasakul, N.; Rodrussamee, N.; Kosaka, T.; Murata, M.; Yamada, M. Integration of Comprehensive Data and Biotechnological Tools for Industrial Applications of Kluyveromyces Marxianus. Appl. Microbiol. Biotechnol. 2020, 104, 475–488. [Google Scholar] [CrossRef]
- Wasi, S.; Tabrez, S.; Ahmad, M. Use of Pseudomonas Spp. for the Bioremediation of Environmental Pollutants: A Review. Environ. Monit. Assess. 2013, 185, 8147–8155. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cui, J.; Bilal, M.; Hu, H.; Wang, W.; Zhang, X. Pseudomonas spp. as cell factories (MCFs) for value-added products: From rational design to industrial applications. Crit. Rev. Biotechnol. 2020, 40, 1232–1249. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Hwang, S.; Lee, Y.; Cho, S.; Palsson, B.; Cho, B.K. Synthetic Biology Tools for Novel Secondary Metabolite Discovery in Streptomyces. J. Microbiol. Biotechnol. 2019, 29, 667–686. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, G.; Chen, Y.; Lu, Y. Challenges and Advances in Genome Editing Technologies in Streptomyces. Biomolecules 2020, 10, 734. [Google Scholar] [CrossRef] [PubMed]
- Charubin, K.; Bennett, R.K.; Fast, A.G.; Papoutsakis, E.T. Engineering Clostridium Organisms as Microbial Cell-Factories: Challenges & Opportunities. Metab. Eng. 2018, 50, 173–191. [Google Scholar] [CrossRef]
- Huang, H.; Chai, C.; Li, N.; Rowe, P.; Minton, N.P.; Yang, S.; Jiang, W.; Gu, Y. CRISPR/Cas9-Based Efficient Genome Editing in Clostridium Ljungdahlii, an Autotrophic Gas-Fermenting Bacterium. ACS Synth. Biol. 2016, 5, 1355–1361. [Google Scholar] [CrossRef]
- Joseph, R.C.; Kim, N.M.; Sandoval, N.R. Recent Developments of the Synthetic Biology Toolkit for Clostridium. Front. Microbiol. 2018, 9, 154. [Google Scholar] [CrossRef]
- Humphreys, C.M.; Minton, N.P. Advances in Metabolic Engineering in the Microbial Production of Fuels and Chemicals from C1 Gas. Curr. Opin. Biotechnol. 2018, 50, 174–181. [Google Scholar] [CrossRef]
- Molitor, B.; Kirchner, K.; Henrich, A.W.; Schmitz, S.; Rosenbaum, M.A. Expanding the Molecular Toolkit for the Homoacetogen Clostridium Ljungdahlii. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Yi, L.; Luo, L.; Lü, X. Efficient Exploitation of Multiple Novel Bacteriocins by Combination of Complete Genome and Peptidome. Front. Microbiol. 2018, 9, 1567. [Google Scholar] [CrossRef]
- Chaudhari, S.S.; Gokhale, D.V. Phenyllactic Acid: A Potential Antimicrobial Compound in Lactic Acid Bacteria. J. Bacteriol. Mycol. Open Access 2016, 2, 121–125. [Google Scholar] [CrossRef][Green Version]
- Grady, E.N.; MacDonald, J.; Liu, L.; Richman, A.; Yuan, Z. Current Knowledge and Perspectives of Paenibacillus: A Review. Microb Cell Fact. 2016, 15, 203. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Choi, S.K.; Ryu, C.M.; Park, S.H. Chronicle of a Soil Bacterium: Paenibacillus polymyxa E681 as a Tiny Guardian of Plant and Human Health. Front. Microbiol 2019, 10, 1–16. [Google Scholar] [CrossRef]
- Hwang, H.H.; Yu, M.; Lai, E.M. Agrobacterium-Mediated Plant Transformation: Biology and Applications. Arab. Book 2017, 15. [Google Scholar] [CrossRef]
- Bharat, S.S.; Li, S.; Li, J.; Yan, L.; Xia, L. Base Editing in Plants: Current Status and Challenges. Crop. J. 2020, 8, 384–395. [Google Scholar] [CrossRef]
- Danilo, B.; Perrot, L.; Mara, K.; Botton, E.; Nogué, F.; Mazier, M. Efficient and Transgene-Free Gene Targeting Using Agrobacterium-Mediated Delivery of the CRISPR/Cas9 System in Tomato. Plant Cell Rep. 2019, 38, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.G.; Moore, W.M.; Hummel, N.F.; Pearson, A.N.; Barnum, C.R.; Scheller, H.V.; Shih, P.M. Agrobacterium tumefaciens: A Bacterium Primed for Synthetic Biology. BioDesign Res. 2020, 2020, 1–16. [Google Scholar] [CrossRef]
- Tie, W.; Zhou, F.; Wang, L.; Xie, W.; Chen, H.; Li, X.; Lin, Y. Reasons for Lower Transformation Efficiency in Indica Rice Using Agrobacterium Tumefaciens-Mediated Transformation: Lessons from Transformation Assays and Genome-Wide Expression Profiling. Plant Mol. Biol. 2012, 78, 1–18. [Google Scholar] [CrossRef]
- Opabode, J.T. Agrobacterium-Mediated Transformation of Plants: Emerging Factors That Influence Efficiency. Biotechnol. Mol. Biol. Rev. 2006, 1, 12–20. [Google Scholar] [CrossRef]
- Galardini, M.; Mengoni, A.; Brilli, M.; Pini, F.; Fioravanti, A.; Lucas, S.; Lapidus, A.; Cheng, J.; Goodwin, L.; Pitluck, S.; et al. Exploring the Symbiotic Pangenome of the Nitrogen-Fixing Bacterium Sinorhizobium Meliloti. BMC Genom. 2011, 12, 1–15. [Google Scholar] [CrossRef]
- Rouissi, T.; Mahmoudi, A.; Tyagi, R.D.; Brar, S.K.; Prèvost, D.; Surampalli, R.Y. Optimisation of Spray Drying by Response Surface Methodology for the Production of Sinorhizobium Meliloti Powder Formulation by Using Starch Industry Wastewater. Biosyst. Eng. 2013, 114, 334–343. [Google Scholar] [CrossRef]
- Buntić, A.V.; Milić, M.D.; Stajković-Srbinović, O.S.; Rasulić, N.V.; Delić, D.I.; Mihajlovski, K.R. Cellulase Production by Sinorhizobium Meliloti Strain 224 Using Waste Tobacco as Substrate. Int. J. Environ. Sci. Technol. 2019, 16, 5881–5890. [Google Scholar] [CrossRef]
- Cai, Y.; Xia, M.; Dong, H.; Qian, Y.; Zhang, T.; Zhu, B.; Wu, J.; Zhang, D. Engineering a vitamin B 12 High-Throughput Screening System by Riboswitch Sensor in Sinorhizobium Meliloti. BMC Biotechnol. 2018, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Döhlemann, J.; Brennecke, M.; Becker, A. Cloning-Free Genome Engineering in Sinorhizobium Meliloti Advances Applications of Cre/loxP Site-Specific Recombination. J. Biotechnol. 2016, 233, 160–170. [Google Scholar] [CrossRef]
- Nguyen, L. Antibiotic Resistance Mechanisms in M. Tuberculosis: An Update. Arch. Toxicol. 2016, 90, 1585–1604. [Google Scholar] [CrossRef]
- McCarty, N.S.; Graham, A.E.; Studená, L.; Ladesma-Amaro, R. Multiplexed CRISPR Technologies for Gene Editing and Transcriptional Regulation. Nat. Commun 2020, 11, 1281. [Google Scholar] [CrossRef]
- Naeem, M.; Majeed, S.; Hoque, M.Z.; Ahmad, I. Latest Developed Strategies to Minimize the Off-Target Effects in CRISPR-Cas-Mediated Genome Editing. Cells 2020, 9, 1608. [Google Scholar] [CrossRef]
- Pallaseni, A.; Peets, E.M.; Koeppel, J.; Weller, J.; Crepaldi, L.; Allen, F.; Parts, L. Predicting Base Editing Outcomes Using Position-Specific Sequence Determinants. BioRxiv 2021. [Google Scholar] [CrossRef]
- Arbab, M.; Shen, M.W.; Mok, B.; Wilson, C.; Matuszek, Ż.; Cassa, C.A.; Liu, D.R. Determinants of Base Editing Outcomes from Target Library Analysis and Machine Learning. Cell 2020, 182, 463–480. [Google Scholar] [CrossRef]
- Marquart, K.F.; Allam, A.; Janjuha, S.; Sintsova, A.; Villiger, L.; Frey, N.; Krauthammer, M.; Schwank, G. Predicting Base Editing Outcomes with an Attention-Based Deep Learning Algorithm Trained on High-Throughput Target Library Screens. Nat. Commun. 2021, 12, 1–9. [Google Scholar] [CrossRef]
- Misra, C.S.; Bindal, G.; Sodani, M.; Wadhawan, S.; Kulkarni, S.; Gautam, S.; Mukhopadhyaya, R.; Rath, D. Determination of Cas9/dCas9 Associated Toxicity in Microbes. BioRxiv 2019, 848135. [Google Scholar] [CrossRef]
- Ye, S.; Enghiad, B.; Zhao, H.; Takano, E. Fine-Tuning the Regulation of Cas9 Expression Levels for Efficient CRISPR-Cas9 Mediated Recombination in Streptomyces. J. Ind. Microbiol. Biotechnol. 2020, 47, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Shelake, R.M.; Pramanik, D.; Kim, J.Y. In Vivo Rapid Investigation of CRISPR-Based Base Editing Components in Escherichia coli (IRI-CCE): A Platform for Evaluating Base Editing Tools and Their Components. Int. J. Mol. Sci. 2022, 23, 1145. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cheng, H.; Liu, Y.; Liu, Y.; Wen, X.; Zhang, K.; Ni, X.; Gao, N.; Fan, L.; Zhang, Z.; et al. In-Situ Generation of Large Numbers of Genetic Combinations for Metabolic Reprogramming via CRISPR-Guided Base Editing. Nat. Commun. 2021, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lin, Y.; Guo, Y.; Wu, F.; Zhang, Y.; Qi, X.; Wang, Z.; Wang, Q. Stress Tolerance Enhancement via SPT15 Base Editing in Saccharomyces cerevisiae. Biotechnol. Biofuels 2021, 14, 1–18. [Google Scholar] [CrossRef]
- Álvarez, B.; Mencía, M.; de Lorenzo, V.; Fernández, L.Á. In Vivo Diversification of Target Genomic Sites Using Processive Base Deaminase Fusions Blocked by dCas9. Nat. Commun. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Cravens, A.; Jamil, O.K.; Kong, D.; Sockolosky, J.T.; Smolke, C.D. Polymerase-Guided Base Editing Enables In Vivo Mutagenesis and Rapid Protein Engineering. Nat. Commun. 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Pan, Y.; Xia, S.; Dong, C.; Pan, H.; Cai, J.; Huang, L.; Xu, Z.; Lian, J. Random Base Editing for Genome Evolution in Saccharomyces Cerevisiae. ACS Synth. Biol. 2021, 10, 2440–2446. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).