Diversity Patterns of Macrofungi in Xerothermic Grasslands from the Nida Basin (Małopolska Upland, Southern Poland): A Case Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
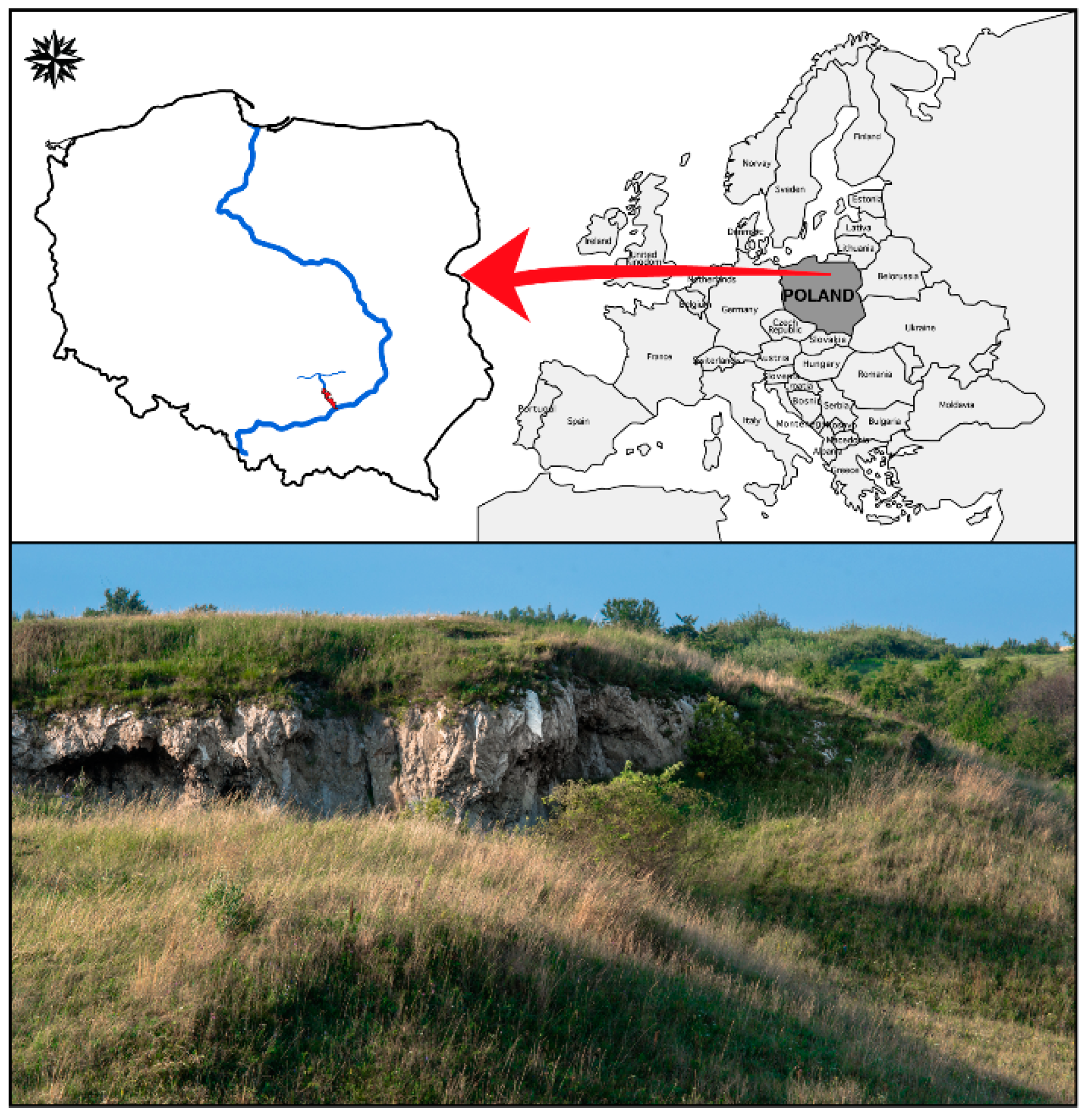
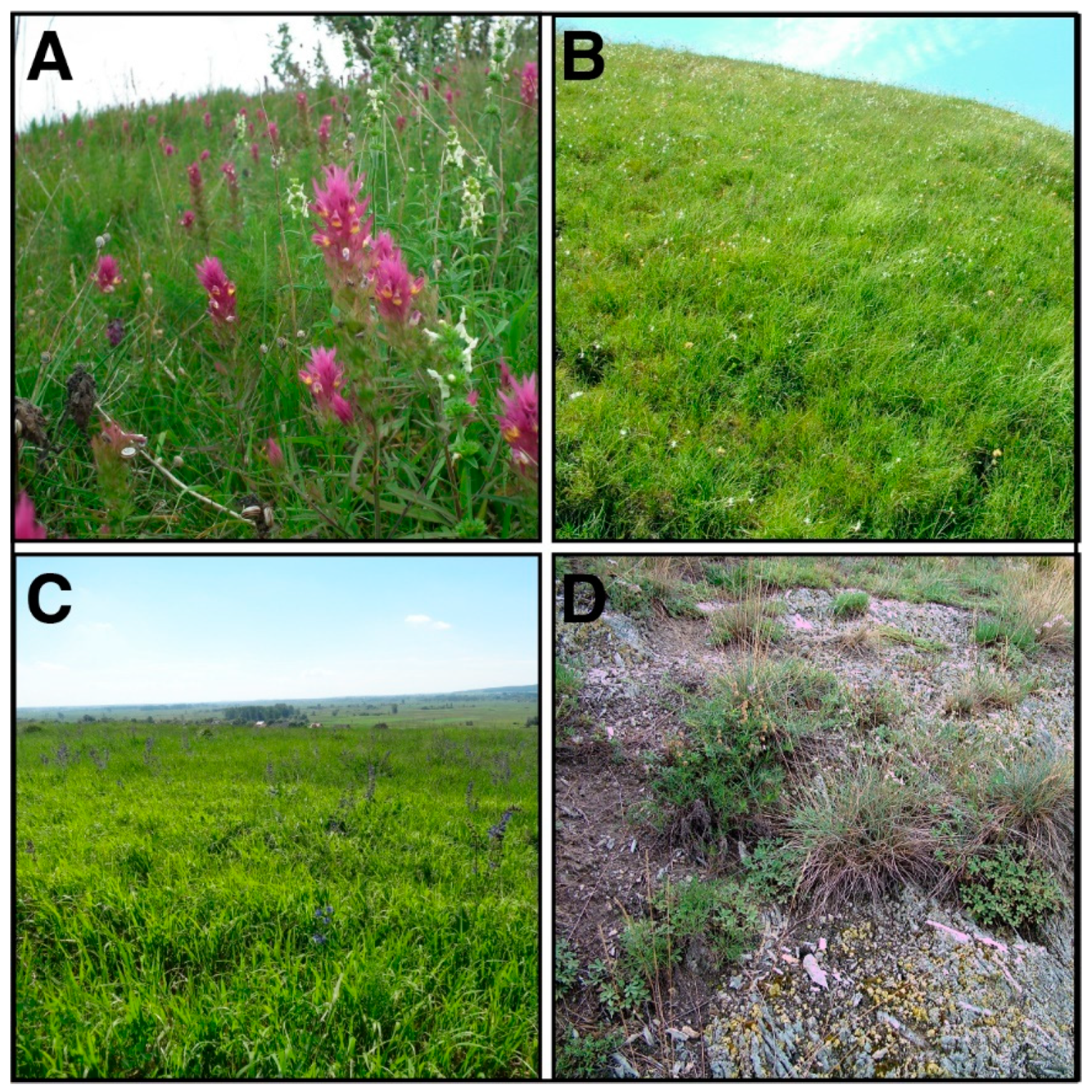
2.1. Study Area
2.2. Sampling and Identification of Macrofungi
2.3. Statistical Analyses
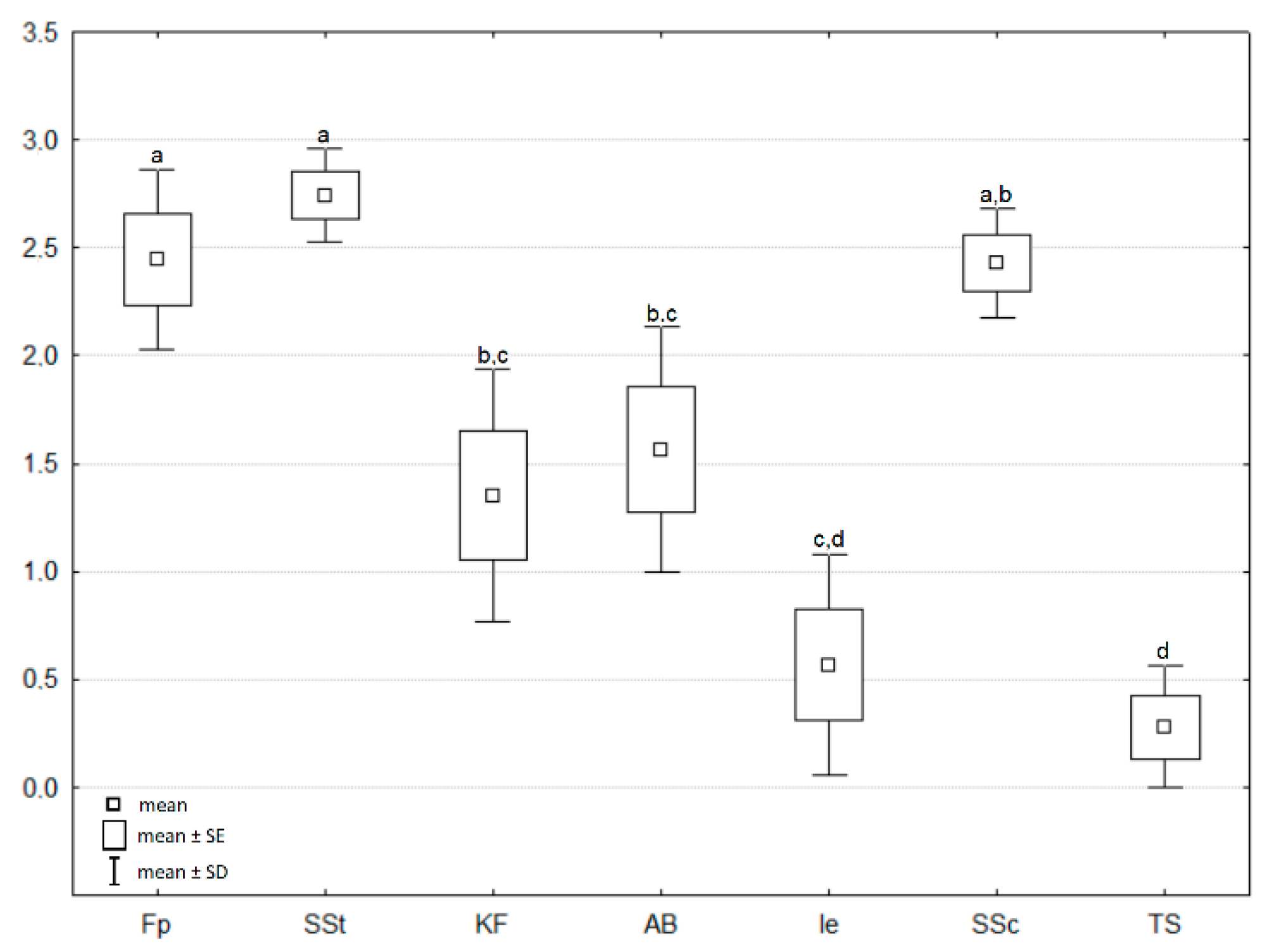
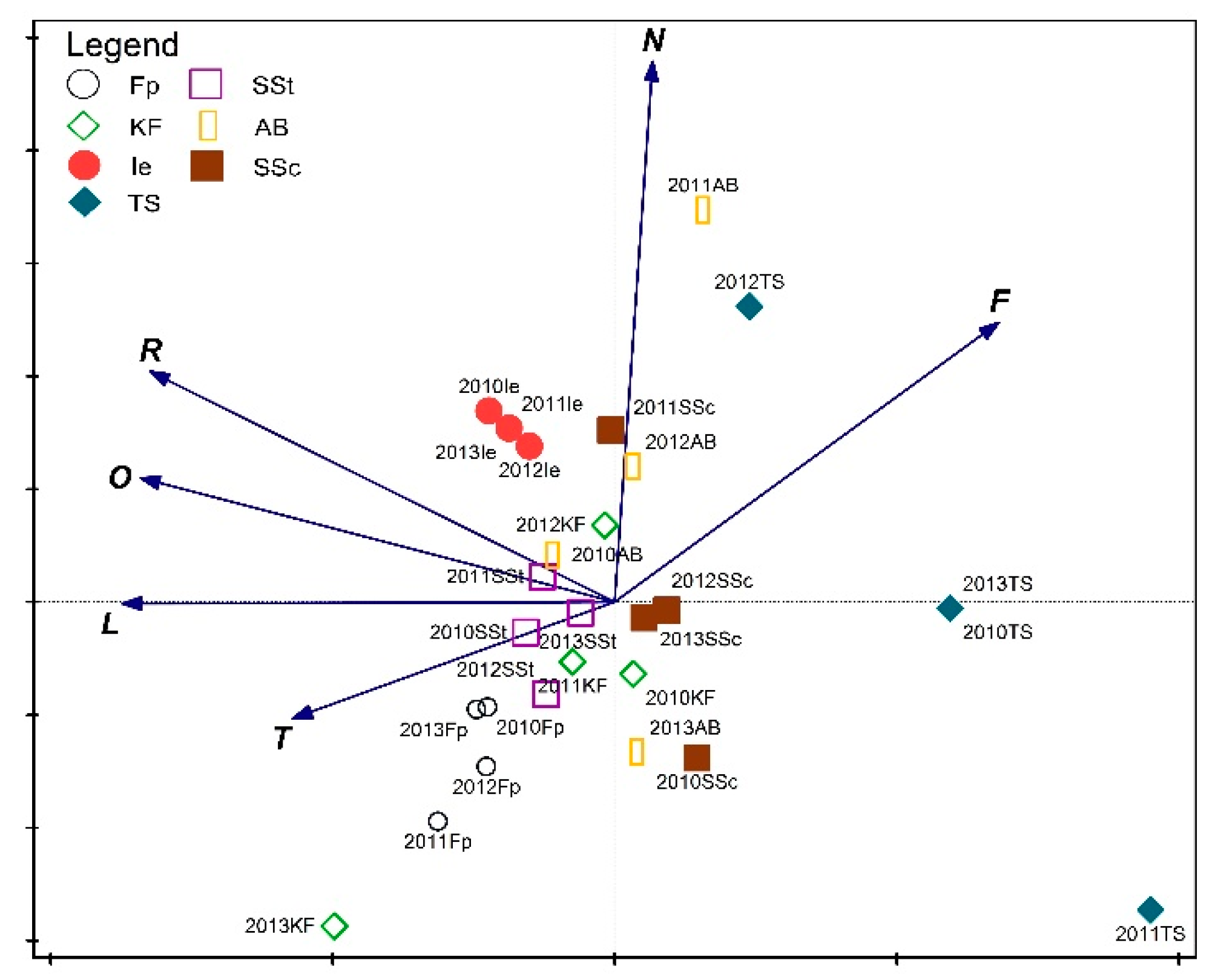
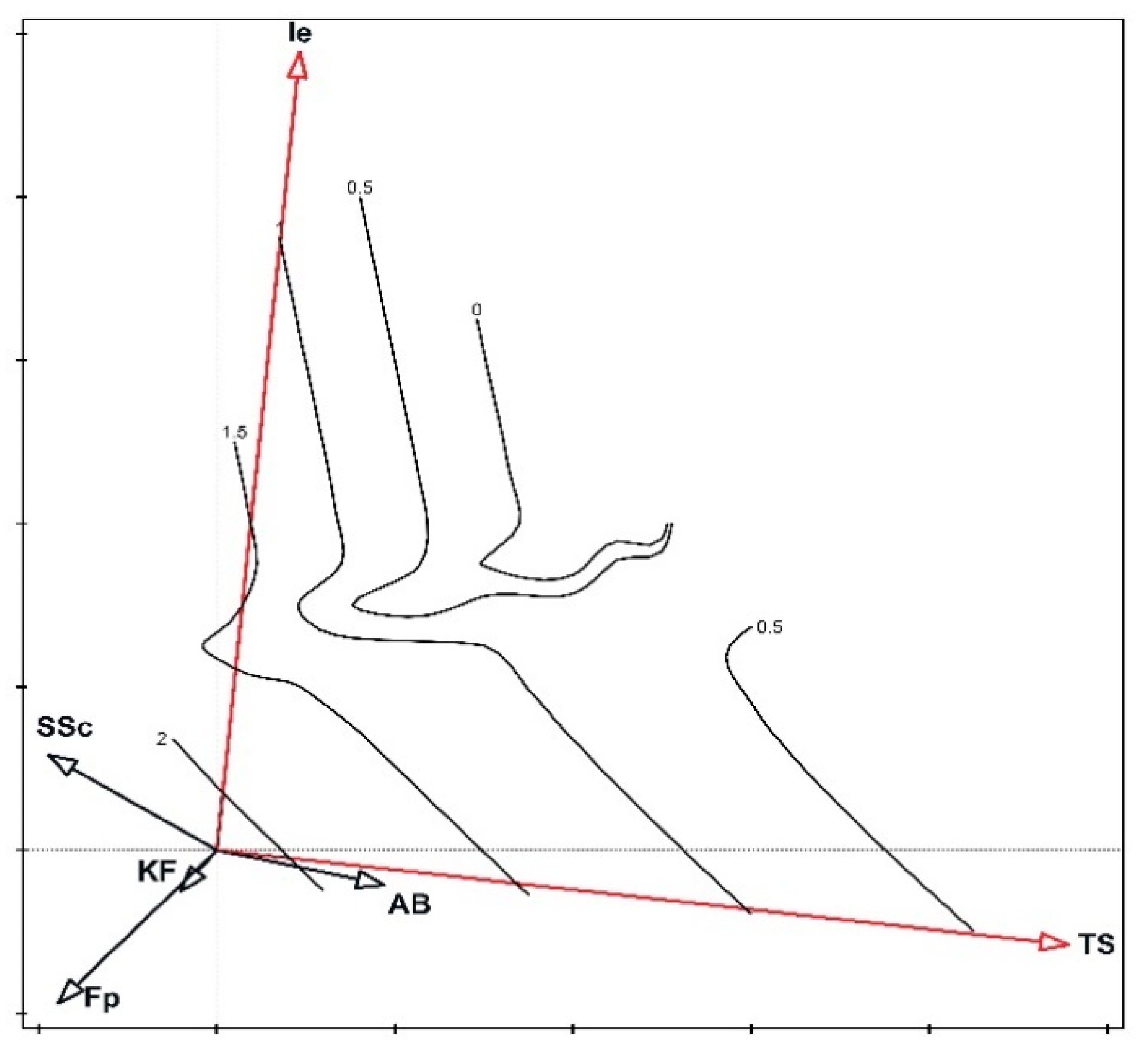
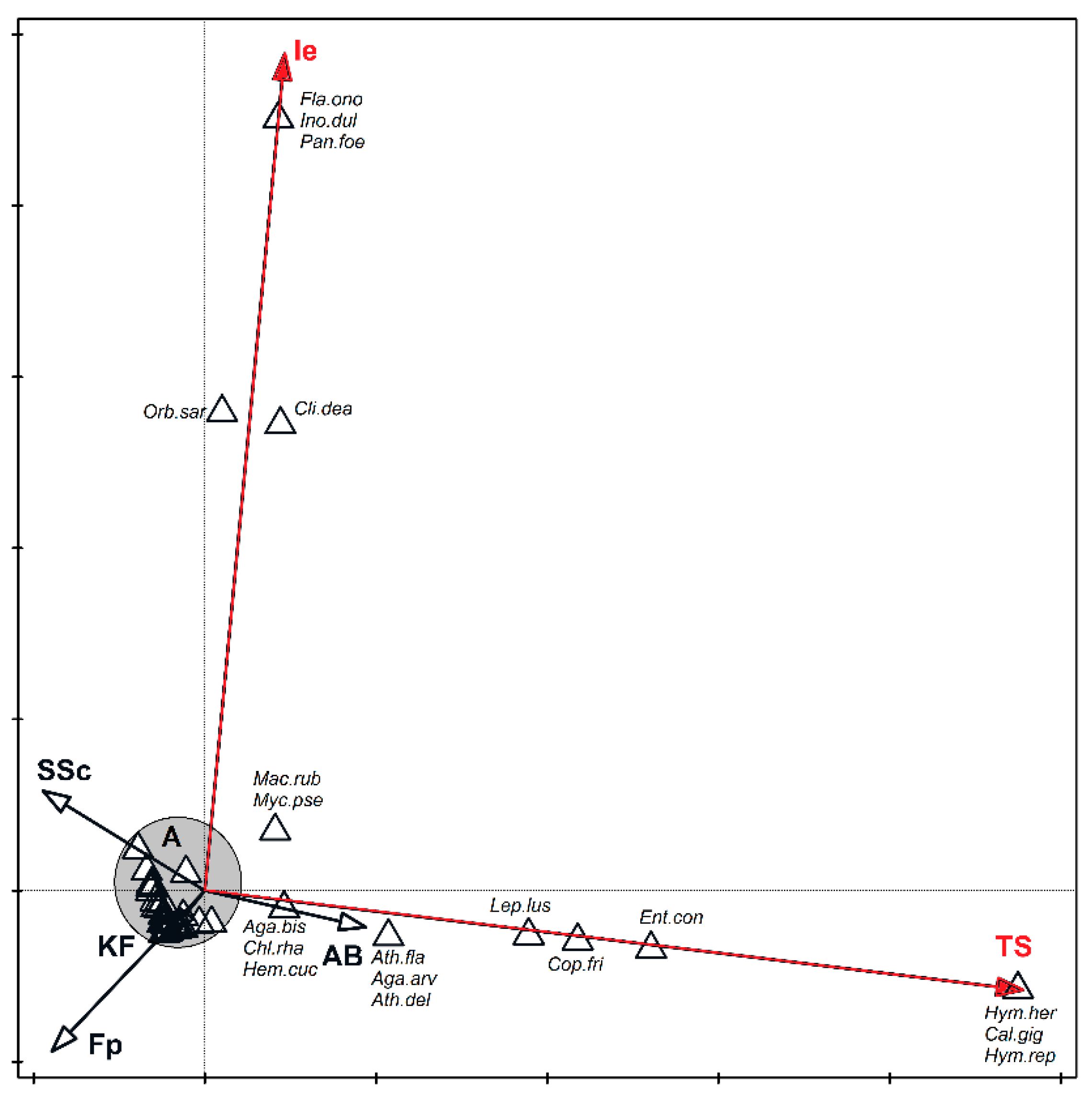
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- López-Quintero, C.A.; Straastsma, G.; Franco-Molano, A.E.; Boekhout, T. Macrofungal diversity in Colombian Amazon forests varies with regions and regimes of disturbance. Biodivers. Conserv. 2012, 21, 2221–2243. [Google Scholar] [CrossRef]
- Hawksworth, D.L. Global species number of fungi: Are tropical studies and molecular approaches contributing to a more robust estimate? Biodivers. Conserv. 2012, 21, 2425–2433. [Google Scholar] [CrossRef]
- Management of Natura 2000 Habitats* Semi-Natural Dry Grasslands (Festuco-Brometalia) 6210. Available online: https://ec.europa.eu/environment/nature/natura2000/management/habitats/pdf/6210_Seminatural_dry_grasslands.pdf (accessed on 1 February 2022).
- European Communities (Ed.) Council Directive 92/43/EEC of 21 May 1992 on the Conservation of Natural Habitats and of Wild Fauna and Flora. 2009. Available online: http://ec.europa.eu/environment/nature/legislation/habitatsdirective/index.en.htm (accessed on 10 January 2022).
- Gheza, G.; Assini, S.; Lelli, C.; Marini, L.; Mayrhofer, H.; Nascimbene, J. Biodiversity and conservation of terricolous lichens and bryophytes in continental lowlands of northern Italy: The role of different dry habitat types. Biodivers. Conserv. 2020, 29, 3533–3550. [Google Scholar] [CrossRef]
- Ceynowa-Giełdon, M.; Adamska, E.; Kamiński, D. The importance of habitat islands in the preservation of relict xerothermic and calcicolous epigeic lichens based on the example of the “Ostnicowe Parowy Gruczna” nature reserve (N Poland). Ecol. Quest. 2017, 25, 19–26. [Google Scholar] [CrossRef][Green Version]
- Podgórska, M.; Łazarski, G. Impact of secondary succession in the xerothermic grassland on the population of the eastern pasque flower (Pulsatilla patens)—Preliminary studies. Sustainability 2021, 13, 12575. [Google Scholar] [CrossRef]
- Richert, A.; Olewnik-Kruszkowska, E.; Adamska, E.; Tarach, I. Enzymatic degradation of bacteriostatic polylactide composites. Int. Biodeterior. Biodegrad. 2019, 142, 103–108. [Google Scholar] [CrossRef]
- McLaughlin, D.J.; Spatafora, J.W. The Mycota. Systematics and Evolution; Part A, VII; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–461. [Google Scholar]
- Egli, S. Mycorrhizal mushroom diversity and productivity—An indicator of forest health. Ann. For. Sci. 2011, 68, 81–88. [Google Scholar] [CrossRef]
- Tedersoo, L.; May, T.W.; Smith, M.E. Ectomycorrhizal lifestyle in fungi: Global diversity, distribution, and evolution of phylogenetic lineages. Mycorrhiza 2010, 20, 217–263. [Google Scholar] [CrossRef]
- Dassen, S.; van der Putten, W.H.; De Deyn, G.B. Severance of arbuscular mycorrhizal fungal mycelial networks in restoration grasslands enhances seedling biomass. New Phytol. 2021, 232, 753–761. [Google Scholar] [CrossRef]
- Ruiz-Almenara, C.; Gandana, E.; Gomez-Hernandez, M. Macrofungal species between intensive mushroom harvesting and non-harvesting areas in Oaxaca, Mexico. PeerJ 2019, 7, e8325. [Google Scholar] [CrossRef]
- Lacheva, M. Larger fungi—Indicator species for xerothermic grasslands of protected area “Sheep Hills” Thracian Lowland (Bulgaria). Trakia J. Sci. 2015, 1, 12–17. [Google Scholar] [CrossRef]
- Bodeker, I.T.M.; Lindahl, B.D.; Olson, A.; Clemmensen, K.E. Mycorrhizal and saprotrophic fungal guilds compete for the same organic substrates but affect decomposition differently. Funct. Ecol. 2016, 30, 1967–1978. [Google Scholar] [CrossRef]
- Jonsell, M.; Nordlander, G. Insects in polypore fungi as indicator species: A comparison between forest sites differing in amounts and contribuity of dead wood. For. Ecol. Manag. 2002, 157, 101–118. [Google Scholar] [CrossRef]
- Bonet, J.A.; de-Miguel, S.; Martinez de Aragón, J.; Pukkala, T.; Palahi, M. Immediate effect of thinning on the yield of Lactarius group delicious in Pinus pinaster forest in North eastern Spain. For. Ecol. Manag. 2012, 265, 211–217. [Google Scholar] [CrossRef]
- Mróz, W.; Bąba, W. Murawy kserotermiczne 6210. In Monitoring Siedlisk Przyrodniczych. Przewodnik Metodyczny; Mróz, W., Ed.; GIOŚ: Warszawa, Poland, 2000; pp. 119–129. [Google Scholar]
- Ignatavičius, G.; Sinkevičius, S.; Ložyté, A. Effects of grassland management on plant communities. Ekologija 2013, 59, 99–110. [Google Scholar] [CrossRef]
- Barańska, K.; Jermaczek, A. Poradnik Utrzymania i Ochrony Siedlisk Przyrodniczych 6210—Murawy Kserotermiczne; Wyd. Klubu Przyrodników: Lubuskie, Poland, 2009; pp. 1–201. [Google Scholar]
- Simmel, J.; Bässler, C.; Poschlod, P. Ellenberg indicator values for macromycetes—A methodological approach and first applications. Fungal Ecol. 2017, 27, 202–212. [Google Scholar] [CrossRef]
- Ciarkowska, K.; Miechówka, A. Gypsic rendzinas of Nida Basin (southern Poland): A review. Soil Sci. Annu. 2018, 2, 101–108. [Google Scholar] [CrossRef]
- Żmudzka, E. Wieloletnie zmiany zasobów termicznych w okresie wegetacyjnym i aktywnego wzrostu roślin w Polsce. Woda Sr. Obsz. Chronione 2012, 3, 399–408. [Google Scholar]
- Michalik, S. Ecological Characterization of the Xerothermic and Montane Vascular Flora of the Ojców National Park; PWN: Warszawa, Poland, 1979; pp. 1–156. [Google Scholar]
- Bąba, W. The species composition and dynamics in well-preserved and restored calcareous xerothermic grasslands (South Poland). Biologia 2004, 59, 447–456. [Google Scholar]
- Kałucka, I. Grzyby w Sukcesji Wtórnej na Gruntach Porolnych w Sąsiedztwie Puszczy Białowieskiej. Ph.D. Thesis, Department of Algology and Fungi, University of Lodz, Łódź, Poland, 1999; pp. 1–258. [Google Scholar]
- Larsson, E.; Orstadius, L. Fourteen coprophilous species of Psathyrella identified in the Nordic countries using morphology and nuclear rDNA sequence data. Mycol. Res. 2008, 112, 1165–1185. [Google Scholar] [CrossRef]
- Braun-Blanquet, J. Pflanzensoziologie, Grundzüge der Vegetationskunde [Plant Sociology, Basics of Vegetation Science], 3rd ed.; Springer: Berlin/Heidelberg, Germany, 1964; pp. 1–134. [Google Scholar]
- Mirek, Z.; Piękoś-Mirkowa, H.; Zając, A.; Zając, M. Flowering Plants and Pteridophytes of Poland. A Checklist. Biodiversity of Poland; W. Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2002; pp. 1–441. [Google Scholar]
- Matuszkiewicz, W. Przewodnik do Oznaczania Zbiorowisk Roślinnych Polski; PWN: Warszawa, Poland, 2013; pp. 1–540. [Google Scholar]
- Index Fungorum. 2022. Available online: http://www.indexfungorum.org/NAMES/IndexFungorumPublicationsListing.asp (accessed on 1 February 2022).
- Wijayawardene, N.N.; Hyde, K.D.; Lumbsch, H.T.; Liu, J.K.; Maharachchikumbura, S.S.N.; Ekanayaka, A.H.; Tian, Q.; Phookamsak, R. Outline of Ascomycota: 2017. Fungal Divers. 2017, 88, 167–263. [Google Scholar] [CrossRef]
- Hawksworth, D.L. The fungal dimension of biodiversity: Magnitude, significance, and conservation. Mycol. Res. 1991, 6, 641–655. [Google Scholar] [CrossRef]
- Rudnicka-Jezierska, W. Podstawczaki (Basidiomycetes), Purchawkowe (Lycoperdales), Tęgoskórowe (Sclerodermatales), Pałeczkowe (Tulostomatales), Gniazdnicowe (Nidulariales), Sromotnikowe (Phallales), Osiakowe (Podaxales); Institute of Botany PAN: Kraków, Poland, 1991; pp. 1–208. [Google Scholar]
- Wright, J.E. The Genus Tulostoma (Gasteromycetes)—A World Monograph. Bibliotheca Mycologica; J. Cramer: Berlin, Germany; Stuttgart, Germany, 1987; pp. 1–338. [Google Scholar]
- Sarasini, M. Gasteromiceti Epigei; Associazione micologica Bresadola: Trento, Italy, 2005; pp. 1–406. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Paleontol. Electron. 2001, 4, 9. [Google Scholar]
- StatSoft. 2009. Available online: https://www.statsoft.pl/ (accessed on 1 February 2022).
- Ter Braak, C.J.F.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, Version 5.0; Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- Łuszczyński, J.; Łuszczyńska, B.; Tomaszewska, A.; Sobaś, A.; Kostrzewa, M.; Grudzień, K. Flammulina ononidis first record in Poland. Acta Mycol. 2014, 1, 79–85. [Google Scholar] [CrossRef][Green Version]
- Bas, C. Genus Flammulina P. Karst. In Flora Agaricina Neerlandica; Bas, C., Kuyper, T.W., Noordeloos, M.E., Vellinga, E.C., Eds.; Aa Balkema: Rotterdam, The Netherlands, 1995; Volume 3, pp. 170–173. [Google Scholar]
- Ripková, S.; Adamčik, S.; Kučera, V.; Palko, L. Fungi of the Protected Landscape Area of Vihorlat; Institute of Botany SAS: Bratislava, Slovakia, 2007; pp. 1–149. [Google Scholar]
- Guzmán, P.; Gilbertson, R.L.; Nodari, R.; Johnson, W.C.; Temple, S.R.; Mandala, D.; Mkandawire, A.B.C.; Gepts, P. Characterization of variability in the fungus Phaeoisariopsis griseola suggests coevolution with the common bean (Phaseolus vulgaris). Phytopathology 1995, 85, 6000–6007. [Google Scholar] [CrossRef]
- Hu, J.; Zhou, Y.; Geng, J.; Dai, Y.; Ren, H.; Lamour, K. A new dollar spot disease of turfgrass caused by Clarireedia paspali. Mycol. Prog. 2020, 18, 1423–1435. [Google Scholar] [CrossRef]
- Andersson, L.; Alexeeva, N.; Kuznetsova, E. Identification manual of species to be used during survey at stand level. In Survey of Biologically Valuable Forests in North-Western European Russia; Pobeda Publ.: St. Petersburg, Russia, 2009; Volume 2, pp. 1–258. [Google Scholar]
- Amandeep, K.; Atri, N.S.; Munruchi, K. A checklist of coprophilous agarics of India. Curr. Res. Environ. Appl. Mycol. J. Fungal Biol. 2015, 5, 322–348. [Google Scholar] [CrossRef]
- Lima, R.A.; Rother, D.C.; Muler, A.E.; Lepsch, I.F.; Rodrigues, R.R. Bamboo overabundance alters forest structure and dynamics in the Atlantic Forest hotspot. Biodivers. Conserv. 2012, 147, 32–39. [Google Scholar] [CrossRef]
- Matsushima, Y.; Eguchi, F.; Kikukawa, T.; Matsuda, T. Historical overview of psychoactive mushrooms. Inflamm. Regen. 2009, 1, 47–58. [Google Scholar] [CrossRef]
- Ślusarczyk, J.; Adamska, E.; Czerwik-Marcinkowska, J. Fungi and Algae as Sources of Medicinal and Other Biologically Active Compounds: A Review. Nutrients 2021, 13, 3178. [Google Scholar] [CrossRef]
- Andrianova, M.; Logacheva, M.; Seplyarskiy, V.B.; Naumenko, S.A.; Klepikova, A.V.; Gerasimov, E.S.; Bazykin, G.A.; Kondrashov, A.S. Extraordinary genetic diversity in a wood decay mushroom. Mol. Biol. Evol. 2015, 31, 3016–3025. [Google Scholar]
- Coetzee, J.C.; van Wyk, A.E. The genus Calvatia (“Gasteromycetes”, Lycoperdaceae): A review of its ethnomycology and biotechnological potential. Afr. J. Biotechnol. 2009, 8, 6007–6015. [Google Scholar]
- Rimóczi, I. Ecology, cenology, and distribution of the giant puffball (Langermannia gigantea [Batsch ex Pers.] in Hungary. Acta Bot. Hung. 1987, 33, 279–294. [Google Scholar]
- Chmiel, M.A. Checklist of Polish larger Ascomycetes. In Biodiversity of Poland; Mirek, Z., Ed.; W. Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2006; pp. 1–123. [Google Scholar]
- Tomaszewska, A.; Łuszczyński, J.; Lechowicz, Ł.; Chrapek, M. Selected rare and protected macrofungi (Agaricomycetes) as bioindicators of communities of xerothermic vegetation in the Nida Basin. Acta Mycol. 2015, 50, 1–12. [Google Scholar] [CrossRef][Green Version]
- Smith, C.W.; Aptroot, A.; Coppins, B.J.; Fletcher, A.; Gilbert, O.L.; James, P.W.; Wolseley, P.A. The Lichens of Great Britain and Ireland; The British Lichen Society: London, UK, 2009; p. 45. [Google Scholar]
- Fabiszewski, J.; Szczepańska, K. Ecological indicator values of some lichen species noted in Poland. Acta Soc. Bot. Pol. 2010, 79, 305–313. [Google Scholar] [CrossRef]
- Peay, K.G.; Kennedy, P.G.; Talbot, J.M. Dimensions of biodiversity in the Earth mycobiome. Nat. Rev. Microbiol. 2016, 14, 434–447. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Anslan, S.; Bahram, M.; Wurzbacher, C.; Baldrian, P.; Tedersoo, L. Mycobiome diversity: High-throughput sequencing and identification of fungi. Nat. Rev. Microbiol. 2019, 17, 95–109. [Google Scholar] [CrossRef]
- Hay, C.R.J.; Thorn, R.G.; Jacobs, C.R. Taxonomic survey of Agaricomycetes (Fungi: Basidiomycota) in Ontario tallgrass prairies determined by fruiting body and soil rDNA sampling. Can. Field Nat. 2018, 132, 407–424. [Google Scholar] [CrossRef]
- Ovaskainen, O.; Schigiel, D.; Ali-Kovero, H.; Auvinen, P.; Paulin, L.; Norden, B.; Norden, J. Combining high-throughput sequencing with fruit body surveys reveals contrasting life-history strategies in fungi. Microb. Popul. Commun. Ecol. 2013, 7, 1696–1709. [Google Scholar] [CrossRef]
| Class of Insolation | % of Insolation | % of Insolated Surface | Values for Stand Quality |
|---|---|---|---|
| 1 | 76–85 | 6.7 | 1 |
| 2 | 86–95 | 3.3 | 2 |
| 3 | 96–105 | 16.7 | 3 |
| 4 | 106–115 | 23.3 | 4 |
| 5 | 116–125 | 26.7 | 5 |
| 6 | 126–135 | 23.3 | 6 |
| Species | RI Values |
|---|---|
| Mycena pseudopicta | 500 |
| Lycoperdon dermoxantha | 483 |
| Bovista tomentosa | 470 |
| Geastrum striatum | 470 |
| Species | RI Values | Species | RI Values |
|---|---|---|---|
| Cyathus olla | 450 | Gastrosporium simplex | 440 |
| Geastrum campestre | 450 | Tulostoma squamosum | 440 |
| Geastrum minimum | 450 | Disciseda candida | 437 |
| Lycoperdon lividum | 450 | Galerina graminea | 437 |
| Atheniella flavoalba | 446 | Cuphophyllus virgineus | 433 |
| Tulostoma brumale | 446 | Disciseda bovista | 433 |
| Tulostoma melanocyclum | 446 | Marasmius oreades | 420 |
| Crinipellis scabella | 443 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łuszczyński, J.; Adamska, E.; Wojciechowska, A.; Czerwik-Marcinkowska, J. Diversity Patterns of Macrofungi in Xerothermic Grasslands from the Nida Basin (Małopolska Upland, Southern Poland): A Case Study. Biology 2022, 11, 531. https://doi.org/10.3390/biology11040531
Łuszczyński J, Adamska E, Wojciechowska A, Czerwik-Marcinkowska J. Diversity Patterns of Macrofungi in Xerothermic Grasslands from the Nida Basin (Małopolska Upland, Southern Poland): A Case Study. Biology. 2022; 11(4):531. https://doi.org/10.3390/biology11040531
Chicago/Turabian StyleŁuszczyński, Janusz, Edyta Adamska, Anna Wojciechowska, and Joanna Czerwik-Marcinkowska. 2022. "Diversity Patterns of Macrofungi in Xerothermic Grasslands from the Nida Basin (Małopolska Upland, Southern Poland): A Case Study" Biology 11, no. 4: 531. https://doi.org/10.3390/biology11040531
APA StyleŁuszczyński, J., Adamska, E., Wojciechowska, A., & Czerwik-Marcinkowska, J. (2022). Diversity Patterns of Macrofungi in Xerothermic Grasslands from the Nida Basin (Małopolska Upland, Southern Poland): A Case Study. Biology, 11(4), 531. https://doi.org/10.3390/biology11040531







