Effect of Motility Factors D-Penicillamine, Hypotaurine and Epinephrine on the Performance of Spermatozoa from Five Hamster Species
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animals and Sperm Collection
2.3. Sperm Motility, Viability and Acrosomal Integrity
2.4. Sperm Velocity and Trajectory
2.5. Sperm ATP Content
2.6. Data Analysis
2.6.1. Principal Component Analyses for Sperm Velocity Parameters
2.6.2. Statistical Analyses
3. Results
3.1. Relative Testes Size and Sperm Numbers
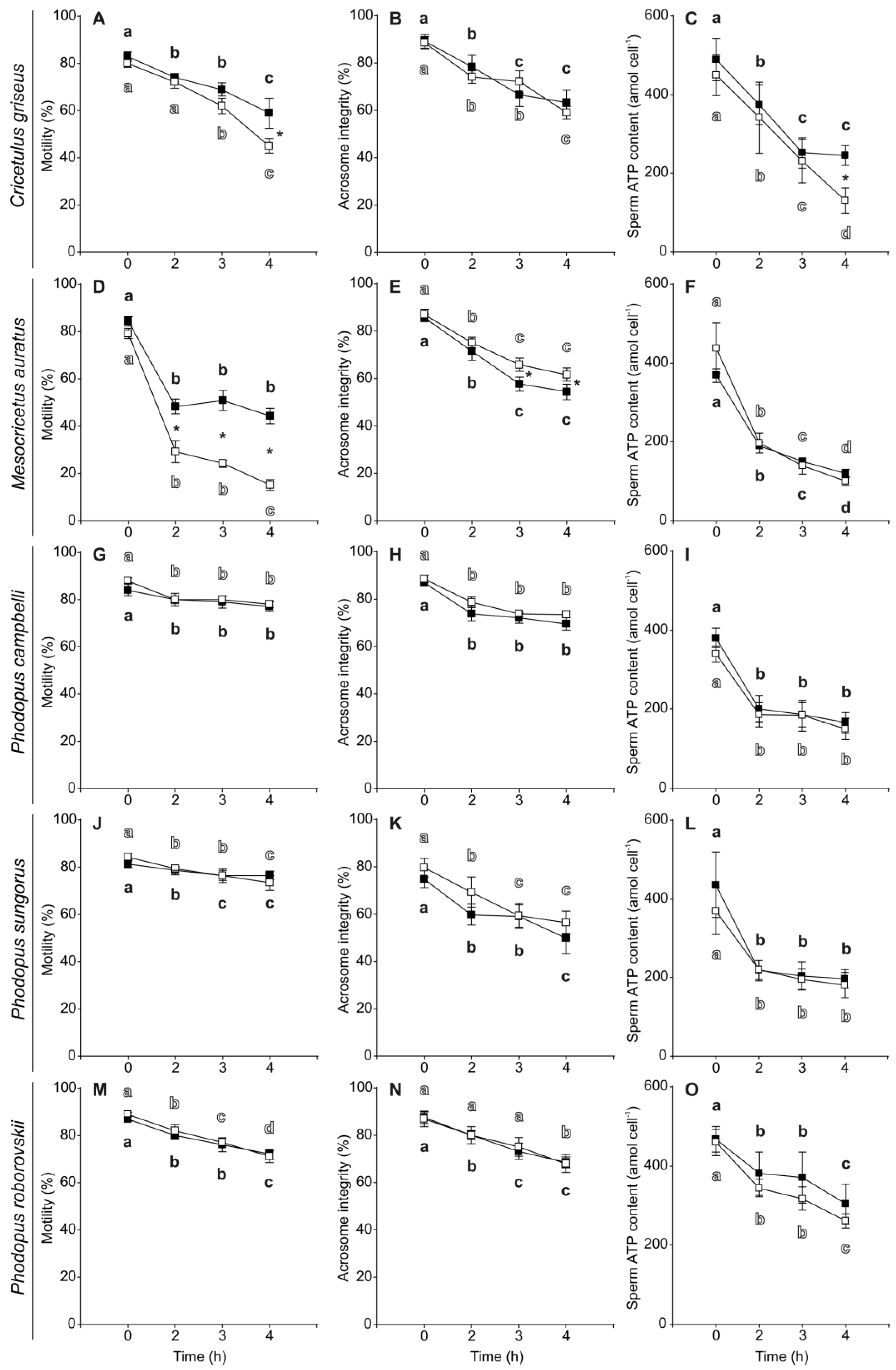
3.2. Sperm Motility, Viability, Acrosome Integrity and ATP Content
3.3. Sperm Velocity and Trajectory
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roldan, E.R.S. Male fertility overview. In Encyclopedia of Reproduction, 2nd ed.; Skinner, M.K., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 1, pp. 408–415. [Google Scholar]
- Roldan, E.R.S. Assessments of sperm quality integrating morphology, swimming patterns, bioenergetics and cell signalling. Theriogenology 2020, 150, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Martinez, H. Laboratory semen assessment and prediction of fertility: Still utopia? Reprod. Domest. Anim. 2003, 38, 312–318. [Google Scholar] [CrossRef]
- Björndahl, L.; Mortimer, D.; Barratt, C.L.R.; Castilla, J.A.; Roelof, M.; Kvist, U.; Álvarez, J.G.; Haugen, T.B. A Practical Guide to Basic Laboratory Andrology; Cambridge University Press: Cambridge, UK, 2010; p. 336. [Google Scholar]
- Chenoweth, P.J.; Lorton, P.S. Animal Andrology: Theories and Applications; CABI: Wallingford, UK, 2014; p. 584. [Google Scholar]
- Srivastava, N.; Pande, M. Protocols in Semen Biology (Comparing Assays); Springer: Singapore, 2017; p. 288. [Google Scholar]
- Oehninger, S.; Ombelet, W. Limits of current male fertility testing. Fertil. Steril. 2019, 111, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Mortimer, D.; Kovacs, D. Male and Sperm Factors that Maximize IVF Success; Cambridge University Press: Cambridge, UK, 2020; p. 244. [Google Scholar]
- Turner, K.A.; Rambhatla, A.; Schon, S.; Agarwal, A.; Krawetz, S.A.; Dupree, J.M.; Avidor-Reiss, T. Male infertility is a women’s health issue-research and clinical evaluation of male infertility is needed. Cells 2020, 9, 990. [Google Scholar] [CrossRef] [Green Version]
- van der Horst, G. Status of sperm functionality assessment in wildlife species: From fish to primates. Animals 2021, 11, 1491. [Google Scholar] [CrossRef]
- Malo, A.F.; Garde, J.J.; Soler, A.J.; Garcia, A.J.; Gomendio, M.; Roldan, E.R.S. Male fertility in natural populations of red deer is determined by sperm velocity and the proportion of normal spermatozoa. Biol. Reprod. 2005, 72, 822–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaniz, J.L.; Silvestre, M.A.; Santolaria, P.; Soler, C. CASA-Mot in mammals: An update. Reprod. Fertil. Dev. 2018, 30, 799–809. [Google Scholar] [CrossRef]
- van der Horst, G. Computer aided sperm analysis (CASA) in domestic animals: Current status, three D tracking and flagellar analysis. Anim. Reprod. Sci. 2020, 220, 106350. [Google Scholar] [CrossRef]
- Buffone, M.G. Sperm Acrosome Biogenesis and Function during Fertilization; Springer: New York, NY, USA, 2016; p. 172. [Google Scholar]
- Suarez, S.S.; Pacey, A.A. Sperm transport in the female reproductive tract. Hum. Reprod. Update 2006, 12, 23–37. [Google Scholar] [CrossRef] [Green Version]
- Hirohashi, N. Site of mammalian sperm acrosome reaction. Adv. Anat. Embryol. Cell Biol. 2016, 220, 145–158. [Google Scholar] [CrossRef]
- La Spina, F.A.; Puga Molina, L.C.; Romarowski, A.; Vitale, A.M.; Falzone, T.L.; Krapf, D.; Hirohashi, N.; Buffone, M.G. Mouse sperm begin to undergo acrosomal exocytosis in the upper isthmus of the oviduct. Dev. Biol. 2016, 411, 172–182. [Google Scholar] [CrossRef]
- Guidobaldi, H.A.; Hirohashi, N.; Cubilla, M.; Buffone, M.G.; Giojalas, L.C. An intact acrosome is required for the chemotactic response to progesterone in mouse spermatozoa. Mol. Reprod. Dev. 2017, 84, 310–315. [Google Scholar] [CrossRef]
- Hirohashi, N.; Yanagimachi, R. Sperm acrosome reaction: Its site and role in fertilization. Biol. Reprod. 2018, 99, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Balestrini, P.A.; Jablonski, M.; Schiavi-Ehrenhaus, L.J.; Marin-Briggiler, C.I.; Sanchez-Cardenas, C.; Darszon, A.; Krapf, D.; Buffone, M.G. Seeing is believing: Current methods to observe sperm acrosomal exocytosis in real time. Mol. Reprod. Dev. 2020, 87, 1188–1198. [Google Scholar] [CrossRef] [PubMed]
- Tourmente, M.; Varea-Sanchez, M.; Roldan, E.R.S. Faster and more efficient swimming: Energy consumption of murine spermatozoa under sperm competitiondagger. Biol. Reprod. 2019, 100, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Tourmente, M.; Villar-Moya, P.; Varea-Sanchez, M.; Luque-Larena, J.J.; Rial, E.; Roldan, E.R.S. Performance of rodent spermatozoa over time is enhanced by increased ATP concentrations: The role of sperm competition. Biol. Reprod. 2015, 93, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tourmente, M.; Roldan, E.R.S. Mass-specific metabolic rate influences sperm performance through energy production in mammals. PLoS ONE 2015, 10, e0138185. [Google Scholar] [CrossRef] [Green Version]
- Tourmente, M.; Rowe, M.; Gonzalez-Barroso, M.M.; Rial, E.; Gomendio, M.; Roldan, E.R.S. Postcopulatory sexual selection increases ATP content in rodent spermatozoa. Evolution 2013, 67, 1838–1846. [Google Scholar] [CrossRef] [Green Version]
- Sansegundo, E.; Tourmente, M.; Roldan, E.R.S. Energy metabolism and hyperactivation of spermatozoa from three mouse species under capacitating conditions. Cells 2022, 11, 220. [Google Scholar] [CrossRef] [PubMed]
- Tvrda, E.; Benko, F.; Slanina, T.; du Plessis, S.S. The role of selected natural biomolecules in sperm production and functionality. Molecules 2021, 26, 5196. [Google Scholar] [CrossRef]
- Gervasi, M.G.; Visconti, P.E. Chang’s meaning of capacitation: A molecular perspective. Mol. Reprod. Dev. 2016, 83, 860–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, M.; Ishikawa, A. Room temperature storage of mouse epididymal spermatozoa: Exploration of factors affecting sperm survival. Theriogenology 2004, 61, 1455–1469. [Google Scholar] [CrossRef] [PubMed]
- Contri, A.; Gloria, A.; Robbe, D.; Valorz, C.; Wegher, L.; Carluccio, A. Kinematic study on the effect of pH on bull sperm function. Anim. Reprod. Sci. 2013, 136, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, L.; Li, J.; Li, H.; Hong, Z.; Xie, M.; Chen, S.; Yao, B. The semen pH affects sperm motility and capacitation. PLoS ONE 2015, 10, e0132974. [Google Scholar] [CrossRef] [Green Version]
- Perez-Cerezales, S.; Lopez-Cardona, A.P.; Gutierrez-Adan, A. Progesterone effects on mouse sperm kinetics in conditions of viscosity. Reproduction 2016, 151, 501–507. [Google Scholar] [CrossRef] [Green Version]
- Mata-Martinez, E.; Darszon, A.; Trevino, C.L. pH-dependent Ca(+2) oscillations prevent untimely acrosome reaction in human sperm. Biochem. Biophys. Res. Commun. 2018, 497, 146–152. [Google Scholar] [CrossRef]
- Giojalas, L.C.; Guidobaldi, H.A. Getting to and away from the egg, an interplay between several sperm transport mechanisms and a complex oviduct physiology. Mol. Cell. Endocrinol. 2020, 518, 110954. [Google Scholar] [CrossRef]
- Ruiz-Diaz, S.; Luongo, C.; Fuentes-Albero, M.C.; Abril-Sanchez, S.; Sanchez-Calabuig, M.J.; Barros-Garcia, C.; De la Fe, C.; Garcia-Galan, A.; Ros-Santaella, J.L.; Pintus, E.; et al. Effect of temperature and cell concentration on dolphin (Tursiops truncatus) spermatozoa quality evaluated at different days of refrigeration. Anim. Reprod. Sci. 2020, 212, 106248. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, B.; Fu, Q.; Wu, J.; Liu, R. A fully integrated biomimetic microfluidic device for evaluation of sperm response to thermotaxis and chemotaxis. Lab Chip 2021, 21, 310–318. [Google Scholar] [CrossRef]
- Yanagimachi, R.; Chang, M.C. In vitro fertilization of golden hamster ova. J. Exp. Zool. 1964, 156, 361–375. [Google Scholar] [CrossRef]
- Roldan, E.R.S.; Yanagimachi, R. Cross-fertilization between Syrian and Chinese hamsters. J. Exp. Zool. 1989, 250, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Bavister, B.D. A consistently successful procedure for in vitro fertilization of golden hamster eggs. Gamete Res. 1989, 23, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Bavister, B.D.; Chen, A.F.; Fu, P.C. Catecholamine requirement for hamster sperm motility in vitro. J. Reprod. Fertil. 1979, 56, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Meizel, S.; Working, P.K. Further evidence suggesting the hormonal stimulation of hamster sperm acrosome reactionsby catecholamines in vitro. Biol. Reprod. 1980, 22, 211–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornett, L.E.; Meizel, S. Stimulation of in vitro activation and the acrosome reaction of hamster spermatozoa by catecholamines. Proc. Natl. Acad. Sci. USA 1978, 75, 4954–4958. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, J.G.; Storey, B.T. Spontaneous lipid peroxidation in rabbit and mouse epididymal spermatozoa: Dependence of rate on temperature and oxygen concentration. Biol. Reprod. 1985, 32, 342–351. [Google Scholar] [CrossRef] [Green Version]
- Meizel, S.; Lui, C.W.; Working, P.K.; Mrsny, R.J. Taurine and Hypotaurine: Their effects on motility, capacitation and the acrosome reaction of hamster sperm in vitro and their presence in sperm and reproductive tract fluids of several mammals. Dev. Growth Differ. 1980, 22, 483–494. [Google Scholar] [CrossRef]
- Fujinoki, M. Regulation and disruption of hamster sperm hyperactivation by progesterone, 17beta-estradiol and diethylstilbestrol. Reprod. Med. Biol. 2014, 13, 143–152. [Google Scholar] [CrossRef]
- Fujinoki, M.; Takei, G.L. gamma-Aminobutyric acid suppresses enhancement of hamster sperm hyperactivation by 5-hydroxytryptamine. J. Reprod. Dev. 2017, 63, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, C.; Fujinoki, M.; Kitazawa, M.; Obayashi, S. Serotonergic signals enhanced hamster sperm hyperactivation. J. Reprod. Dev. 2021, 67, 241–250. [Google Scholar] [CrossRef]
- Brogan, P.T.; Beitsma, M.; Henning, H.; Gadella, B.M.; Stout, T.A. Liquid storage of equine semen: Assessing the effect of d-penicillamine on longevity of ejaculated and epididymal stallion sperm. Anim. Reprod. Sci. 2015, 159, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Leahy, T.; Rickard, J.P.; Aitken, R.J.; de Graaf, S.P. Penicillamine prevents ram sperm agglutination in media that support capacitation. Reproduction 2016, 151, 167–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, M.C.; Yu, A.; Moawad, A.R.; O’Flaherty, C. Peroxiredoxin 6 regulates the phosphoinositide 3-kinase/AKT pathway to maintain human sperm viability. Mol. Hum. Reprod. 2019, 25, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Uribe, P.; Merino, J.; Bravo, A.; Zambrano, F.; Schulz, M.; Villegas, J.V.; Sanchez, R. Antioxidant effects of penicillamine against in vitro-induced oxidative stress in human spermatozoa. Andrologia 2020, 52, e13553. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.C.; Nolan, J.P.; Hammerstedt, R.H.; Bavister, B.D. Role of zinc during hamster sperm capacitation. Biol. Reprod. 1994, 51, 1238–1247. [Google Scholar] [CrossRef]
- Ruiz-Diaz, S.; Oseguera-Lopez, I.; De La Cuesta-Diaz, D.; Garcia-Lopez, B.; Serres, C.; Sanchez-Calabuig, M.J.; Gutierrez-Adan, A.; Perez-Cerezales, S. The presence of D-penicillamine during the in vitro capacitation of stallion spermatozoa prolongs hyperactive-like motility and allows for sperm selection by thermotaxis. Animals 2020, 10, 1467. [Google Scholar] [CrossRef]
- Cornwall, G.A.; Smyth, T.B.; Vindivich, D.; Harter, C.; Robinson, J.; Chang, T.S. Induction and enhancement of progressive motility in hamster caput epididymal spermatozoa. Biol. Reprod. 1986, 35, 1065–1074. [Google Scholar] [CrossRef] [Green Version]
- Andrews, J.C.; Bavister, B.D. Capacitation of hamster spermatozoa with the divalent cation chelators D-penicilamine, L-histidine, and L-cysteine in a protein-free culture medium. Gamete Res. 1989, 23, 159–170. [Google Scholar] [CrossRef]
- Kang, S.S.; Koyama, K.; Huang, W.; Yang, Y.; Yanagawa, Y.; Takahashi, Y.; Nagano, M. Addition of D-penicillamine, hypotaurine, and epinephrine (PHE) mixture to IVF medium maintains motility and longevity of bovine sperm and enhances stable production of blastocysts in vitro. J. Reprod. Dev. 2015, 61, 99–105. [Google Scholar] [CrossRef] [Green Version]
- Boatman, D.E.; Bavister, B.D.; Cruz, E. Addition of hypotaurine can reactivate immotile golden hamster spermatozoa. J. Androl. 1990, 11, 67–72. [Google Scholar]
- Hirose, M.; Ogura, A. The golden (Syrian) hamster as a model for the study of reproductive biology: Past, present, and future. Reprod. Med. Biol. 2019, 18, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Yanagimachi, R.; Kamiguchi, Y.; Sugawara, S.; Mikamo, K. Gametes and fertilization in the Chinese hamster. Gamete Res. 1983, 8, 97–117. [Google Scholar] [CrossRef]
- Tateno, H.; Kamiguchi, Y. In vitro fertilisation of Chinese hamster oocytes by spermatozoa that have undergone ionophore A23187-induced acrosome reaction, and their subsequent development into blastocysts. Zygote 1996, 4, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Tateno, H.; Tamura-Nakano, M.; Kusakabe, H.; Hirohashi, N.; Kawano, N.; Yanagimachi, R. Sperm acrosome status before and during fertilization in the Chinese hamster (Cricetulus griseus), and observation of oviductal vesicles and globules. Mol. Reprod. Dev. 2021, 88, 793–804. [Google Scholar] [CrossRef]
- Parkening, T.A. In vitro fertilization of Siberian hamster oocytes. J. Exp. Zool. 1990, 254, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Raicu, P.; Bratosin, S. Interspecific reciprocal hybrids between Mesocricetus auratus and M. newtoni. Genet Res. 1968, 11, 113–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raicu, P.; Ionescu-Varo, M.; Duma, D. Interspecific crosses between the Rumanian and Syrian hamster. Cytogenetic and histological studies. J. Hered. 1969, 60, 149–152. [Google Scholar] [CrossRef]
- Raicu, P.; Ionescu-Varo, M.; Nicolaescu, M.; Kirillova, M. Interspecific hybrids between Romanian and Kurdistan hamsters. Genetica 1972, 43, 223–230. [Google Scholar] [CrossRef]
- Raicu, P.; Nicolaescu, M.; Kirillova, M. The interspecific hybrids between Kurdistan hamster (Mesocricetus brandti) and golden hamster (Mesocricetus auratus). Rev. Roum. Biol. 1973, 18, 451–455. [Google Scholar]
- Todd, N.B.; Nixon, C.W.; Mulvaney, D.A.; Connelly, M.E. Karyotypes of Mesocricetus brandti and hybridization within the genus. J. Hered. 1972, 63, 73–77. [Google Scholar] [CrossRef]
- Ishishita, S.; Matsuda, Y. Interspecific hybrids of dwarf hamsters and Phasianidae birds as animal models for studying the genetic and developmental basis of hybrid incompatibility. Genes Genet. Syst. 2016, 91, 63–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gureeva, A.V.; Feoktistova, N.Y.; Matveevsky, S.N.; Kolomiets, O.L.; Surov, A.V. Speciation of Eversmann and Mongolian hamsters (Allocricetulus, Cricetinae): Experimental hybridization. Biol. Bull. 2017, 43, 736–742. [Google Scholar] [CrossRef]
- Cummins, J.M.; Woodall, P.F. On mammalian sperm dimensions. J. Reprod. Fertil. 1985, 75, 153–175. [Google Scholar] [CrossRef] [PubMed]
- Tourmente, M.; Gomendio, M.; Roldan, E.R.S. Sperm competition and the evolution of sperm design in mammals. BMC Evol. Biol. 2011, 11, 12. [Google Scholar] [CrossRef] [Green Version]
- Teves, M.E.; Roldan, E.R.S. Sperm bauplan and function and underlying processes of sperm formation and selection. Physiol. Rev. 2022, 102, 7–60. [Google Scholar] [CrossRef]
- Lebedev, V.S.; Bannikova, A.A.; Neumann, K.; Ushakova, M.V.; Ivanova, N.V.; Surov, A.V. Molecular phylogenetics and taxonomy of dwarf hamsters Cricetulus Milne-Edwards, 1867 (Cricetidae, Rodentia): Description of a new genus and reinstatement of another. Zootaxa 2018, 4387, 331–349. [Google Scholar] [CrossRef] [PubMed]
- Romanenko, S.A.; Volobouev, V.T.; Perelman, P.L.; Lebedev, V.S.; Serdukova, N.A.; Trifonov, V.A.; Biltueva, L.S.; Nie, W.; O’Brien, P.C.; Bulatova, N.; et al. Karyotype evolution and phylogenetic relationships of hamsters (Cricetidae, Muroidea, Rodentia) inferred from chromosomal painting and banding comparison. Chromosome Res. 2007, 15, 283–297. [Google Scholar] [CrossRef]
- Romanenko, S.A.; Lebedev, V.S.; Bannikova, A.A.; Pavlova, S.V.; Serdyukova, N.A.; Feoktistova, N.Y.; Jiapeng, Q.; Yuehua, S.; Surov, A.V.; Graphodatsky, A.S. Karyotypic and molecular evidence supports the endemic Tibetan hamsters as a separate divergent lineage of Cricetinae. Sci. Rep. 2021, 11, 10557. [Google Scholar] [CrossRef]
- Kenagy, G.J.; Trombulak, S.C. Size and function of mammalian testes in relation to body size. J. Mammal. 1986, 67, 1–22. [Google Scholar] [CrossRef]
- Shi, Q.X.; Roldan, E.R.S. Bicarbonate/CO2 is not required for zona pellucida- or progesterone-induced acrosomal exocytosis of mouse spermatozoa but is essential for capacitation. Biol. Reprod. 1995, 52, 540–546. [Google Scholar] [CrossRef]
- Gomez Montoto, L.; Magana, C.; Tourmente, M.; Martin-Coello, J.; Crespo, C.; Luque-Larena, J.J.; Gomendio, M.; Roldan, E.R.S. Sperm competition, sperm numbers and sperm quality in muroid rodents. PLoS ONE 2011, 6, e18173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez Montoto, L.; Varea Sanchez, M.; Tourmente, M.; Martin-Coello, J.; Luque-Larena, J.J.; Gomendio, M.; Roldan, E.R.S. Sperm competition differentially affects swimming velocity and size of spermatozoa from closely related muroid rodents: Head first. Reproduction 2011, 142, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Di Rienzo, J.A.; Guzmán, A.W.; Casanoves, F. A multiple comparisons method based on the distribution of the root node distance of a binary tree. J. Agric. Biol. Environ. Stat. 2002, 7, 129–142. [Google Scholar] [CrossRef]
- Tourmente, M.; Villar-Moya, P.; Rial, E.; Roldan, E.R.S. Differences in ATP generation via glycolysis and oxidative phosphorylation and relationships with sperm motility in mouse species. J. Biol. Chem. 2015, 290, 20613–20626. [Google Scholar] [CrossRef] [Green Version]
- Bavister, B.D. The effect of variations in culture conditions on the motility of hamster spermatozoa. J. Reprod. Fertil. 1974, 38, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Bavister, B.D.; Yanagimachi, R. The effects of sperm extracts and energy sources on the motility and acrosome reaction of hamster spermatozoa in vitro. Biol. Reprod. 1977, 16, 228–237. [Google Scholar] [CrossRef]
- Leibfried, M.L.; Bavister, B.D. Effects of epinephrine and hypotaurine on in-vitro fertilization in the golden hamster. J. Reprod. Fertil. 1982, 66, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, J.G.; Storey, B.T. Taurine, hypotaurine, epinephrine and albumin inhibit lipid peroxidation in rabbit spermatozoa and protect against loss of motility. Biol. Reprod. 1983, 29, 548–555. [Google Scholar] [CrossRef] [Green Version]
- Mrsny, R.J.; Meizel, S. Inhibition of hamster sperm Na+, K+-ATPase activity by taurine and hypotaurine. Life Sci. 1985, 36, 271–275. [Google Scholar] [CrossRef]
- Hexum, T.D. The effect of catecholamines on transport (Na,K) adenosine triphosphatase. Biochem. Pharmacol. 1977, 26, 1221–1227. [Google Scholar] [CrossRef]
- Fagan, J.B.; Racker, E. Reversible inhibition of (Na+, K+) ATPase by Mg2+, adenosine triphosphate, and K+. Biochemistry 1977, 16, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Pickworth, S.; Chang, M.C. Fertilization of Chinese hamster eggs in vitro. J. Reprod. Fertil. 1969, 19, 371–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
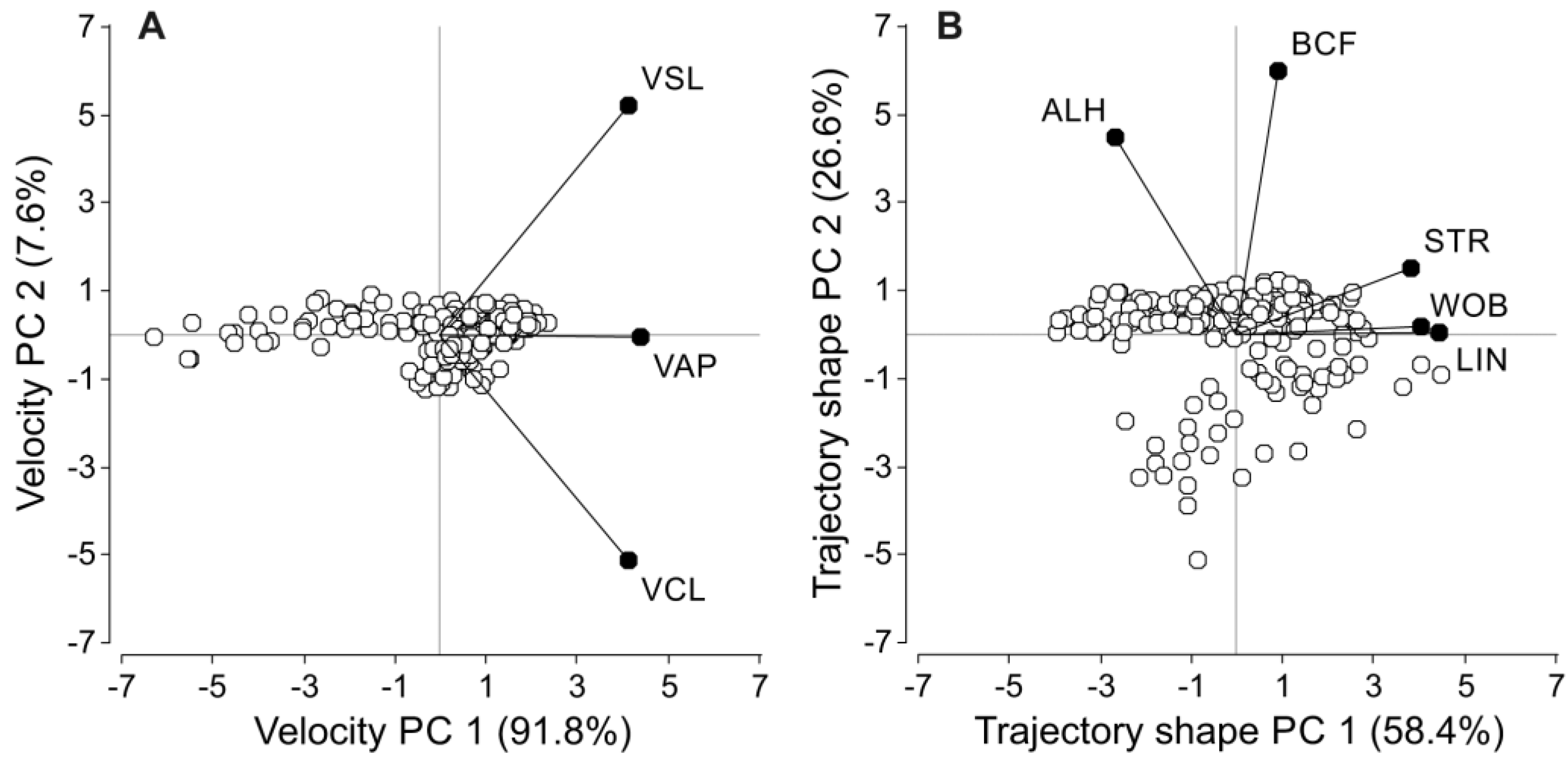

| Variables | Factor Loadings | Factor Correlation | ||
|---|---|---|---|---|
| PC1 | PC2 | PC1 | PC2 | |
| Sperm velocity principal components: | ||||
| Curvilinear velocity | 0.5667 | −0.7023 | 0.9405 | −0.3345 |
| Straight-line velocity | 0.5658 | 0.7118 | 0.9390 | 0.3390 |
| Average path velocity | 0.5989 | −0.0079 | 0.9938 | −0.0038 |
| Sperm trajectory shape principal components | ||||
| Linearity | 0.5800 | 0.0041 | 0.9914 | 0.0047 |
| Straightness | 0.5008 | 0.1996 | 0.8560 | 0.2302 |
| Wobble coefficient | 0.5261 | 0.0217 | 0.8992 | 0.0251 |
| Amplitude of lateral head displacement | −0.3498 | 0.5869 | −0.5980 | 0.6767 |
| Beat-cross frequency | 0.1167 | 0.7844 | 0.1995 | 0.9045 |
| Species | Body Mass (g) | Testes Mass (g) | RTS | Sperm Numbers (×106) |
|---|---|---|---|---|
| Cricetulus griseus | 33.72 ± 0.38 | 1.78 ± 0.04 | 3.83 ± 0.11 | 88.00 ± 07.65 |
| Mesocricetus auratus | 125.00 ± 1.63 | 3.50 ± 0.12 | 2.75 ± 0.11 | 585.88 ± 36.81 |
| Phodopus campbelli | 48.55 ± 3.90 | 2.01 ± 0.08 | 3.30 ± 0.21 | 317.82 ± 25.12 |
| Phodopus sungorus | 46.82 ± 1.25 | 0.94 ± 0.06 | 1.58 ± 0.13 | 160.76 ± 27.07 |
| Phodopus roborovskii | 25.72 ± 1.15 | 1.06 ± 0.04 | 2.82 ± 0.12 | 175.64 ± 26.59 |
| Species | Variable | Control | PHE | ||
|---|---|---|---|---|---|
| Mean | SE | Mean | SE | ||
| Cricetulus griseus | Motility (%) | 80.00 | 1.58 | 83.00 | 2.00 |
| Acrosome Integrity (%) | 88.60 | 2.62 | 89.20 | 2.92 | |
| Viability (%) | 94.00 | 1.41 | 95.60 | 1.29 | |
| ATP content (amol cell−1) | 449.34 | 51.76 | 489.47 | 53.91 | |
| Mesocricetus auratus | Motility (%) | 79.17 | 2.01 | 84.17 | 2.01 |
| Acrosome Integrity (%) | 86.83 | 2.21 | 85.50 | 1.61 | |
| Viability (%) | 98.67 | 0.42 | 97.83 | 0.75 | |
| ATP content (amol cell−1) | 437.68 | 63.39 | 368.09 | 16.23 | |
| Phodopus campbelli | Motility (%) | 88.00 | 1.22 | 84.00 | 2.45 |
| Acrosome Integrity (%) | 88.60 | 1.69 | 86.86 | 1.09 | |
| Viability (%) | 99.40 | 0.40 | 99.40 | 0.40 | |
| ATP content (amol cell−1) | 339.83 | 20.64 | 379.83 | 24.23 | |
| Phodopus sungorus | Motility (%) | 84.29 | 1.70 | 81.43 | 1.80 |
| Acrosome Integrity (%) | 79.71 | 3.89 | 74.86 | 3.74 | |
| Viability (%) | 98.43 | 0.48 | 98.29 | 0.36 | |
| ATP content (amol cell−1) | 369.20 | 59.19 | 435.80 | 83.28 | |
| Phodopus roborovskii | Motility (%) | 89.00 | 1.00 | 87.00 | 1.22 |
| Acrosome Integrity (%) | 86.80 | 3.14 | 87.40 | 2.62 | |
| Viability (%) | 96.40 | 0.93 | 96.40 | 1.69 | |
| ATP content (amol cell−1) | 459.57 | 33.29 | 467.05 | 32.20 | |
| Species | Variable | Time | Treatment | Interaction | |||
|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | ||
| Cricetulus griseus | Motility (%) | 34.27 | <0.001 | 9.14 | 0.005 | 1.34 | 0.280 |
| Acrosome Integrity (%) | 44.62 | <0.001 | 0.33 | 0.570 | 1.54 | 0.225 | |
| Viability (%) | 0.61 | 0.617 | 34.45 | <0.001 | 2.53 | 0.078 | |
| ATP content (amol cell−1) | 35.39 | <0.001 | 15.47 | 0.001 | 4.01 | 0.007 | |
| Mesocricetus auratus | Motility (%) | 254.11 | <0.001 | 110.40 | <0.001 | 6.98 | 0.001 |
| Acrosome Integrity (%) | 86.67 | <0.001 | 6.66 | 0.014 | 0.24 | 0.870 | |
| Viability (%) | 2.10 | 0.118 | 6.04 | 0.019 | 2.00 | 0.132 | |
| ATP content (amol cell−1) | 120.43 | <0.001 | 0.83 | 0.368 | 1.94 | 0.141 | |
| Phodopus campbelli | Motility (%) | 16.81 | <0.001 | 2.44 | 0.129 | 1.06 | 0.381 |
| Acrosome Integrity (%) | 29.84 | <0.001 | 5.17 | 0.085 | 0.22 | 0.880 | |
| Viability (%) | 1.69 | 0.192 | 1.13 | 0.298 | 1.97 | 0.142 | |
| ATP content (amol cell−1) | 43.92 | <0.001 | 1.89 | 0.180 | 0.09 | 0.966 | |
| Phodopus sungorus | Motility (%) | 13.17 | <0.001 | 0.08 | 0.775 | 1.49 | 0.231 |
| Acrosome Integrity (%) | 24.93 | <0.001 | 1.54 | 0.261 | 0.92 | 0.442 | |
| Viability (%) | 0.42 | 0.743 | 0.98 | 0.327 | 0.55 | 0.648 | |
| ATP content (amol cell−1) | 37.52 | <0.001 | 1.34 | 0.253 | 0.46 | 0.709 | |
| Phodopus roborovskii | Motility (%) | 38.87 | <0.001 | 1.06 | 0.312 | 0.44 | 0.727 |
| Acrosome Integrity (%) | 18.66 | <0.001 | 0.03 | 0.868 | 0.08 | 0.972 | |
| Viability (%) | 0.44 | 0.726 | 0.07 | 0.790 | 0.21 | 0.888 | |
| ATP content (amol cell−1) | 14.43 | <0.001 | 1.46 | 0.237 | 0.12 | 0.950 | |
| Species | Variable | Control | PHE | ||
|---|---|---|---|---|---|
| Mean | SE | Mean | SE | ||
| Mesocricetus auratus | VCL (µm s−1) | 114.71 | 2.40 | 123.45 | 3.99 |
| VSL (µm s−1) | 54.66 | 2.33 | 56.76 | 2.27 | |
| VAP (µm s−1) | 76.64 | 2.02 | 82.22 | 2.26 | |
| LIN (VSL/VCL) | 0.47 | 0.02 | 0.46 | 0.02 | |
| STR (VSL/VAP) | 0.67 | 0.02 | 0.66 | 0.01 | |
| WOB (VAP/VCL) | 0.67 | 0.01 | 0.68 | 0.01 | |
| ALH (µm) | 5.17 | 0.11 | 5.67 | 0.22 | |
| BCF (Hz) | 6.32 | 0.18 | 6.27 | 0.18 | |
| Phodopus campbelli | VCL (µm s−1) | 162.79 | 2.25 | 162.80 | 2.01 |
| VSL (µm s−1) | 75.25 | 1.27 | 71.62 | 1.93 | |
| VAP (µm s−1) | 102.05 | 1.48 | 98.86 | 1.33 | |
| LIN (VSL/VCL) | 0.45 | 0.01 | 0.43 | 0.01 | |
| STR (VSL/VAP) | 0.70 | 0.01 | 0.69 | 0.01 | |
| WOB (VAP/VCL) | 0.62 | 0.01 | 0.61 | 0.01 | |
| ALH (µm) | 6.48 | 0.12 | 6.40 | 0.12 | |
| BCF (Hz) | 9.83 | 0.15 | 9.70 | 0.12 | |
| Phodopus sungorus | VCL (µm s−1) | 152.02 | 2.24 | 157.70 | 2.29 |
| VSL (µm s−1) | 68.81 | 2.36 | 69.10 | 2.19 | |
| VAP (µm s−1) | 92.19 | 1.91 | 92.81 | 2.13 | |
| LIN (VSL/VCL) | 0.44 | 0.01 | 0.42 | 0.01 | |
| STR (VSL/VAP) | 0.69 | 0.01 | 0.69 | 0.01 | |
| WOB (VAP/VCL) | 0.60 | 0.01 | 0.58 | 0.01 | |
| ALH (µm) | 6.34 | 0.11 | 6.56 | 0.11 | |
| BCF (Hz) | 8.70 | 0.34 | 9.21 | 0.28 | |
| Phodopus roborovskii | VCL (µm s−1) | 152.85 | 2.91 | 146.54 | 1.01 |
| VSL (µm s−1) | 62.13 | 1.85 | 56.36 | 2.42 | |
| VAP (µm s−1) | 87.26 | 1.68 | 80.80 | 1.50 | |
| LIN (VSL/VCL) | 0.40 | 0.01 | 0.38 | 0.02 | |
| STR (VSL/VAP) | 0.68 | 0.01 | 0.67 | 0.02 | |
| WOB (VAP/VCL) | 0.57 | 0.01 | 0.55 | 0.01 | |
| ALH (µm) | 6.96 | 0.12 | 7.14 | 0.11 | |
| BCF (Hz) | 8.40 | 0.35 | 7.53 | 0.48 | |
| Species | Variable | Time | Treatment | Interaction | |||
|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | ||
| Mesocricetus auratus | VCL (µm s−1) | 28.78 | <0.001 | 67.30 | <0.001 | 3.54 | 0.024 |
| VSL (µm s−1) | 14.62 | <0.001 | 46.25 | <0.001 | 4.43 | 0.011 | |
| VAP (µm s−1) | 29.51 | <0.001 | 50.80 | <0.001 | 3.26 | 0.031 | |
| LIN | 2.75 | 0.056 | 17.82 | <0.001 | 2.83 | 0.074 | |
| STR | 1.04 | 0.384 | 19.68 | <0.001 | 3.80 | 0.017 | |
| WOB | 15.50 | <0.001 | 10.48 | 0.002 | 1.28 | 0.295 | |
| ALH (µm) | 0.68 | 0.573 | 26.39 | <0.001 | 1.10 | 0.361 | |
| BCF (Hz) | 101.82 | <0.001 | 93.48 | <0.001 | 15.10 | <0.001 | |
| VPC1 | 25.98 | <0.001 | 59.27 | <0.001 | 4.09 | 0.014 | |
| TPC1 | 5.12 | 0.004 | 9.61 | 0.004 | 2.45 | 0.077 | |
| TPC2 | 7.08 | 0.001 | 73.25 | <0.001 | 5.73 | 0.003 | |
| Phodopus campbelli | VCL (µm s−1) | 12.90 | <0.001 | 0.22 | 0.644 | 0.52 | 0.669 |
| VSL (µm s−1) | 99.74 | <0.001 | 4.53 | 0.042 | 0.09 | 0.964 | |
| VAP (µm s−1) | 83.46 | <0.001 | 1.57 | 0.221 | 0.24 | 0.867 | |
| LIN | 61.32 | <0.001 | 4.98 | 0.034 | 0.52 | 0.673 | |
| STR | 17.99 | <0.001 | 1.51 | 0.230 | 0.29 | 0.832 | |
| WOB | 196.44 | <0.001 | 14.20 | 0.001 | 1.11 | 0.363 | |
| ALH (µm) | 60.78 | <0.001 | 7.96 | 0.009 | 2.29 | 0.100 | |
| BCF (Hz) | 43.82 | <0.001 | 2.29 | 0.141 | 0.70 | 0.559 | |
| VPC1 | 98.05 | <0.001 | 2.15 | 0.154 | 0.15 | 0.931 | |
| TPC1 | 70.65 | <0.001 | 6.23 | 0.019 | 0.62 | 0.608 | |
| TPC2 | 7.02 | 0.001 | 0.10 | 0.751 | 0.25 | 0.862 | |
| Phodopus sungorus | VCL (µm s−1) | 8.19 | <0.001 | 3.47 | 0.069 | 0.92 | 0.441 |
| VSL (µm s−1) | 7.41 | <0.001 | 2.46 | 0.124 | 0.63 | 0.603 | |
| VAP (µm s−1) | 9.70 | <0.001 | 0.76 | 0.387 | 0.63 | 0.600 | |
| LIN | 2.75 | 0.055 | 6.27 | 0.016 | 0.32 | 0.814 | |
| STR | 1.88 | 0.148 | 2.75 | 0.105 | 0.59 | 0.626 | |
| WOB | 2.95 | 0.043 | 6.38 | 0.015 | 0.27 | 0.846 | |
| ALH (µm) | 1.02 | 0.394 | 5.36 | 0.060 | 0.09 | 0.966 | |
| BCF (Hz) | 1.85 | 0.153 | 0.02 | 0.899 | 1.11 | 0.355 | |
| VPC1 | 9.79 | <0.001 | 0.49 | 0.488 | 0.81 | 0.497 | |
| TPC1 | 2.24 | 0.098 | 3.55 | 0.108 | 0.26 | 0.854 | |
| TPC2 | 5.19 | 0.004 | 3.15 | 0.083 | 1.34 | 0.274 | |
| Phodopus roborovskii | VCL (µm s−1) | 17.20 | <0.001 | 4.75 | 0.038 | 0.22 | 0.879 |
| VSL (µm s−1) | 2.61 | 0.071 | 8 | 0.009 | 0.60 | 0.620 | |
| VAP (µm s−1) | 0.89 | 0.460 | 9.34 | 0.005 | 0.42 | 0.739 | |
| LIN | 21.86 | <0.001 | 2.99 | 0.095 | 0.29 | 0.833 | |
| STR | 9.20 | <0.001 | 0.15 | 0.702 | 0.28 | 0.839 | |
| WOB | 20.12 | <0.001 | 4.99 | 0.035 | 0.22 | 0.884 | |
| ALH (µm) | 30.78 | <0.001 | 0.48 | 0.493 | 0.17 | 0.914 | |
| BCF (Hz) | 4.76 | 0.008 | 2.81 | 0.105 | 1.82 | 0.166 | |
| VPC1 | 0.86 | 0.426 | 8.65 | 0.006 | 0.49 | 0.694 | |
| TPC1 | 22.57 | <0.001 | 2.33 | 0.138 | 0.27 | 0.843 | |
| TPC2 | 11.21 | <0.001 | 0.56 | 0.461 | 0.65 | 0.588 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tourmente, M.; Sanchez-Rodriguez, A.; Roldan, E.R.S. Effect of Motility Factors D-Penicillamine, Hypotaurine and Epinephrine on the Performance of Spermatozoa from Five Hamster Species. Biology 2022, 11, 526. https://doi.org/10.3390/biology11040526
Tourmente M, Sanchez-Rodriguez A, Roldan ERS. Effect of Motility Factors D-Penicillamine, Hypotaurine and Epinephrine on the Performance of Spermatozoa from Five Hamster Species. Biology. 2022; 11(4):526. https://doi.org/10.3390/biology11040526
Chicago/Turabian StyleTourmente, Maximiliano, Ana Sanchez-Rodriguez, and Eduardo R. S. Roldan. 2022. "Effect of Motility Factors D-Penicillamine, Hypotaurine and Epinephrine on the Performance of Spermatozoa from Five Hamster Species" Biology 11, no. 4: 526. https://doi.org/10.3390/biology11040526
APA StyleTourmente, M., Sanchez-Rodriguez, A., & Roldan, E. R. S. (2022). Effect of Motility Factors D-Penicillamine, Hypotaurine and Epinephrine on the Performance of Spermatozoa from Five Hamster Species. Biology, 11(4), 526. https://doi.org/10.3390/biology11040526








