Understanding the Ovarian Interrelationship with Low Antral Follicle Counts (AFC) in the In Vivo Bos indicus Cow Model: Unilateral and Bilateral Main AFC as Possible Biomarkers of Ovarian Response to Hormonal Synchronisation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vivo Bovine Model
2.2. Determination of Ovarian AFC and Animal Group
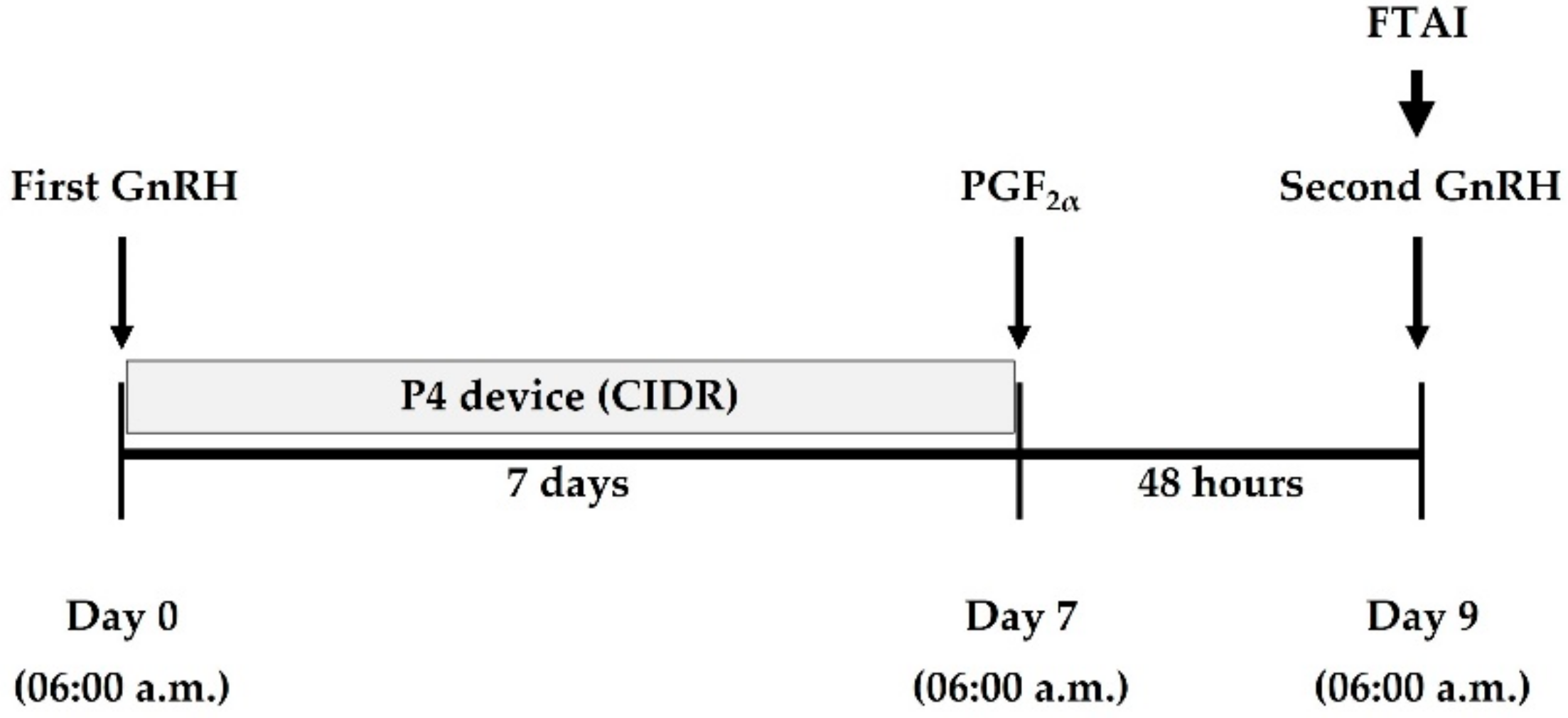
2.3. Hormonal Protocol for Ovarian Stimulation and Determination of Ovarian Response
2.4. Pregnancy Diagnosis
2.5. Analysis of Data
3. Results
3.1. Factors Relating to a Positive Ovarian Response in Bos indicus Cow Model
3.2. The Effect of Ovarian Interrelationship on AFC, DF, and Fertility in All Cows
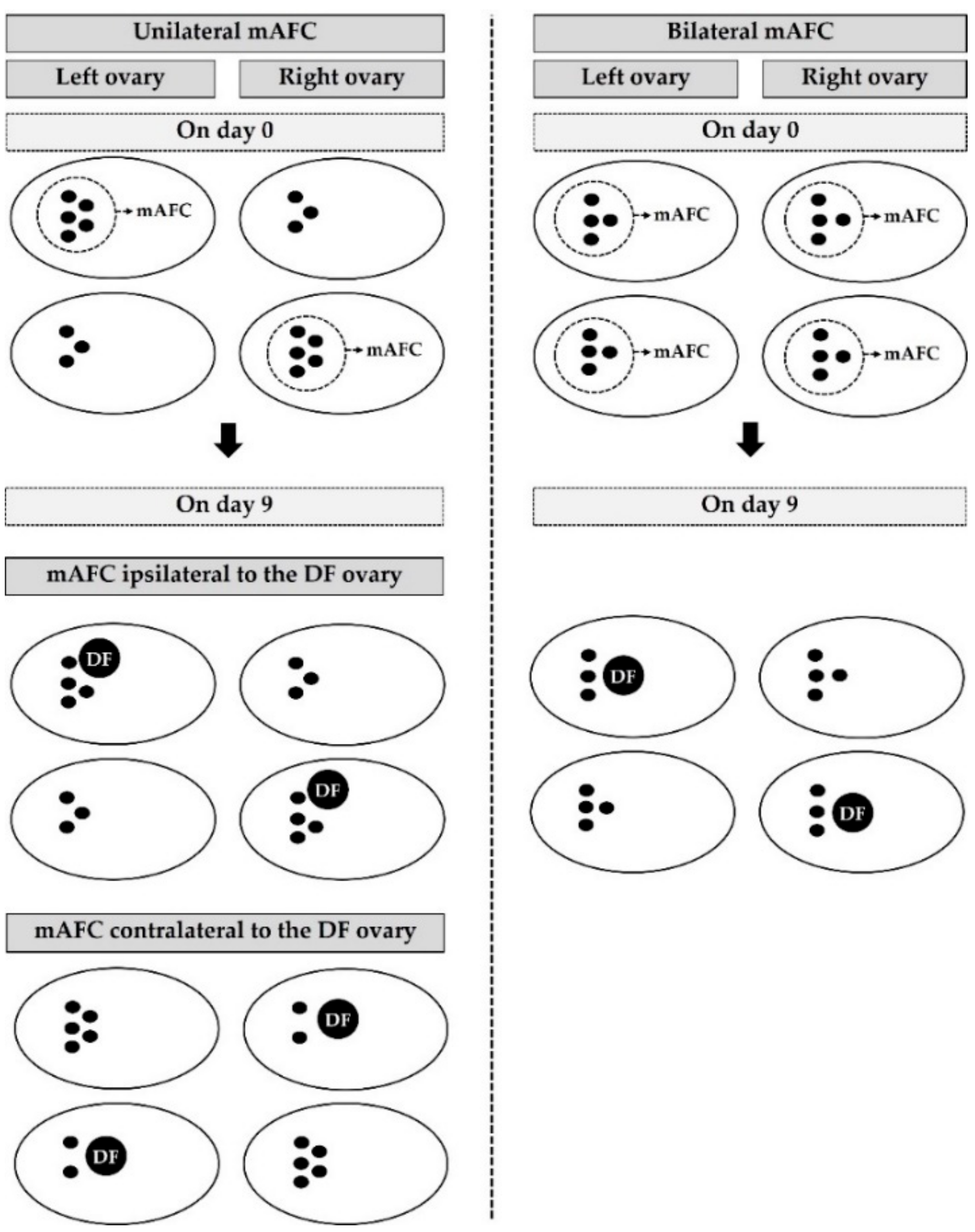
3.2.1. The Impact of Unilateral and Bilateral mAFC on Number and Diameter of AFC, Ovarian Response, and Pregnancy Outcome
3.2.2. The Impact of the Specific Side of the mAFC and the DF Ovaries on Antral Follicle Parameters, Ovarian Response, and Pregnancy Rates in Unilateral mAFC Cows
3.2.3. The Impact of Location of Ovarian Structures and Side of the Ovary on Number and Diameter of AFC, Ovarian Response, and Pregnancy Rates in Bilateral mAFC Cows
3.3. The Effect of Ovarian Interrelationship on AFC, DF, and Fertility in Primiparous and Multiparous Cows
3.3.1. Primiparous Cows
3.3.2. Multiparous Cows
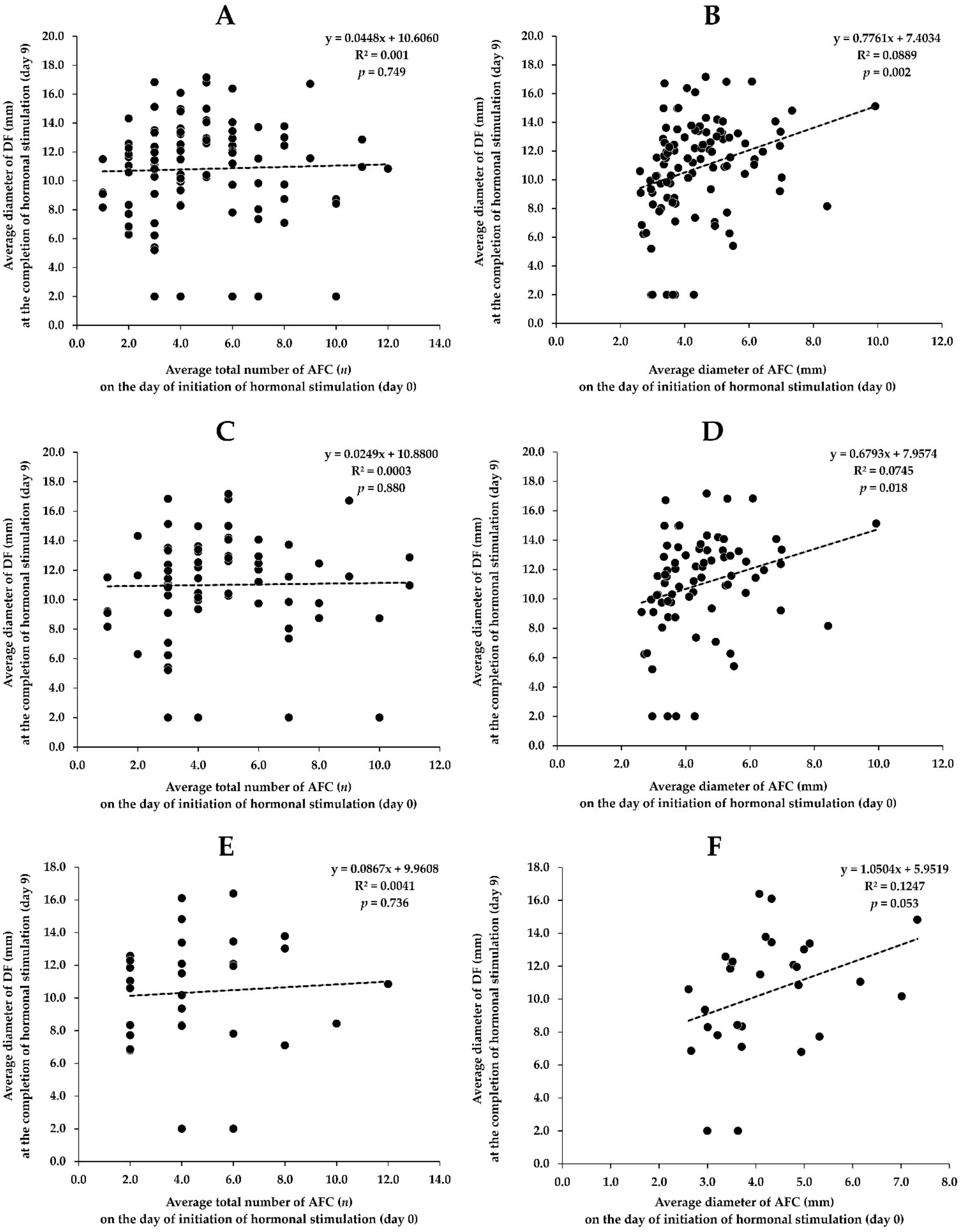
3.4. Association between AFC on the Day of Initiation of Hormonal Stimulation (Day 0) and DF on the Day of the FTAI (Day 9)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brenner, C.A.; Adler, R.R.; Rappolee, D.A.; Pedersen, R.A.; Werb, Z. Gene expression of metalloproteinases and their inhibitors in preimplantation mouse embryos. Genes Dev. 1988, 4, 348–359. [Google Scholar]
- Arias, A.; Richter, A.; Anadon, N.; Glasby, C.J. Reproductive biology of the alien korean bait-worm, Perinereis vancaurica tetradentata (Annelida: Nereididae), from the mar menor lagoon (Western mediterranean); Ecological Impacts of Biological Invasions. In Proceedings of the 7th European Conference on Biological Invasions, Pontevedra, Spain, 12–14 September 2012; p. 207. [Google Scholar]
- ViviD, D.; Bentley, G.E. Seasonal reproduction in vertebrates: Melatonin synthesis, binding, and functionality using tinbergen’s four questions. Molecules 2018, 23, 652. [Google Scholar] [CrossRef]
- Cooke, P.S.; Mesa, A.M.; Sirohi, V.K.; Levin, E.R. Role of nuclear and membrane estrogen signaling pathways in the male and female reproductive tract. Differentiation 2021, 118, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Bearden, H.J.; Fuquay, J.W.; Willard, S.T. Applied Animal Reproduction, 6th ed.; Pearson: Hoboken, NJ, USA, 2004; p. 456. [Google Scholar]
- Comizzoli, P.; Holt, W.V. Breakthroughs and new horizons in reproductive biology of rare and endangered animal species. Biol. Reprod. 2019, 101, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Hamernik, D.L. Farm animals are important biomedical models. Anim. Front. 2019, 9, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Bertocchi, M.; Rigillo, A.; Elmi, A.; Ventrella, D.; Aniballi, C.; Scorpio, D.G.; Scozzoli, M.; Bettini, G.; Forni, M.; Bacci, M.L. preliminary assessment of the mucosal toxicity of tea tree (Melaleuca alternifolia) and rosemary (Rosmarinus officinalis) essential oils on novel porcine uterus models. Int. J. Mol. Sci. 2020, 21, 3350. [Google Scholar] [CrossRef] [PubMed]
- Ginther, O.J.; Siddiqui, M.A.; Baldrighi, J.M.; Hoffman, M.M. Effect of intraovarian proximity between dominant follicle and corpus luteum on dimensions and blood flow of each structure in heifers. Theriogenology 2014, 82, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.S. Spatial relationships of ovarian follicles and luteal structures in dairy cows subjected to ovulation synchronization: Progesterone and risks for luteolysis, ovulation, and pregnancy. J. Dairy Sci. 2019, 102, 5686–5698. [Google Scholar] [CrossRef]
- Yama, P.; Moonmanee, T.; Osathanunkul, M.; Jitjumnong, J.; Karaphuak, W. Locational relationship between Corpus Luteum and ovulatory follicle on ovaries alters follicular dynamics and progesterone concentrations of Thai indigenous beef cows exhibiting two follicular waves. Anim. Prod. Sci. 2018, 59, 2161–2168. [Google Scholar] [CrossRef]
- Cushman, R.A.; Allan, M.F.; Kuehn, L.A.; Snelling, W.M.; Cupp, A.S.; Freetly, H.C. Evaluation of antral follicle count and ovarian morphology in crossbred beef cows: Investigation of influence of stage of the estrous cycle, age, and birth weight. J. Anim. Sci. 2009, 87, 1971–1980. [Google Scholar] [CrossRef]
- Manik, R.S.; Singla, S.K.; Palta, P.; Madan, M.L. Ovarian follicular populations prior to and during superovulation in cattle: Relationship with superovulatory response. Asian-Australas. J. Anim. Sci. 1998, 11, 486–490. [Google Scholar] [CrossRef]
- Ireland, J.L.; Scheetz, D.; Jimenez-Krassel, F.; Themmen, A.P.; Ward, F.; Lonergan, P.; Smith, G.W.; Perez, G.I.; Evans, A.C.; Ireland, J.J. Antral follicle count reliably predicts number of morphologically healthy oocytes and follicles in ovaries of young adult cattle. Biol. Reprod. 2008, 79, 1219–1225. [Google Scholar] [CrossRef]
- Ribadu, A.Y.; Nakao, T. Bovine reproductive ultrasonography: A review. J. Reprod. Dev. 1999, 45, 13–28. [Google Scholar] [CrossRef]
- Morotti, F.; Zangirolamo, A.F.; da Silva, N.C.; da Silva, C.B.; Rosa, C.O.; Seneda, M.M. Antral follicle count in cattle: Advantages, challenges, and controversy. Anim. Reprod. 2017, 14, 414–420. [Google Scholar] [CrossRef]
- Traversari, J.; Aepli, H.; Knutti, B.; Lüttgenau, J.; Bruckmaier, R.M.; Bollwein, H. Relationships between antral follicle count, blood serum concentration of anti-müllerian hormone and fertility in mares. Schweiz. Arch. Tierheilkd. 2019, 161, 627–638. [Google Scholar] [CrossRef]
- Khan, H.L.; Bhatti, S.; Suhail, S.; Gul, R.; Awais, A.; Hamayun, H.; Enver, F.; Abbas, S.; Hassan, Z.; Nisar, R.; et al. Antral follicle count (AFC) and serum anti-müllerian hormone (AMH) are the predictors of natural fecundability have similar trends irrespective of fertility status and menstrual characteristics among fertile and infertile women below the age of 40 years. Reprod. Biol. Endocrinol. 2019, 17, 20. [Google Scholar] [CrossRef]
- Mutlu, M.F.; Erdem, M.; Erdem, A.; Yildiz, S.; Mutlu, I.; Arisoy, O.; Oktem, M. Antral follicle count determines poor ovarian response better than anti-müllerian hormone but age is the only predictor for live birth in in vitro fertilization cycles. J. Assist. Reprod. Genet. 2013, 30, 657–665. [Google Scholar] [CrossRef]
- Rosa, C.O.; Marinho, L.S.R.; Da Rosa, P.R.A.; De Cesaro, M.P.; Lunardelli, P.A.; Santos, K.C.S.; Basso, A.C.; Bordignon, V.; Seneda, M.M. Molecular characteristics of granulosa and cumulus cells and oocyte competence in nelore cows with low and high numbers of antral follicles. Reprod. Domest. Anim. 2018, 53, 921–929. [Google Scholar] [CrossRef]
- Martinez, M.F.; Sanderson, N.; Quirke, L.D.; Lawrence, S.B.; Juengel, J.L. Association between antral follicle count and reproductive measures in New Zealand lactating dairy cows maintained in a pasture-based production system. Theriogenology 2016, 85, 466–475. [Google Scholar] [CrossRef]
- Santos, G.M.G.D.; Silva-Santos, K.C.; Barreiros, T.R.R.; Morotti, F.; Sanches, B.V.; de Moraes, F.L.Z.; Blaschi, W.; Seneda, M.M. High numbers of antral follicles are positively associated with in vitro embryo production but not the conception rate for ftai in nelore cattle. Anim. Reprod. Sci. 2016, 165, 17–21. [Google Scholar] [CrossRef]
- Mossa, F.; Walsh, S.W.; Butler, S.T.; Berry, D.P.; Carter, F.; Lonergan, P.; Smith, G.W.; Ireland, J.J.; Evans, A.C. Low numbers of ovarian follicles ≥3 mm in diameter are associated with low fertility in dairy cows. J. Dairy Sci. 2012, 95, 2355–2361. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.D.; Galligan, D.T.; Thomsen, N. Principal descriptor of body condition score in holstein cows. J. Dairy Sci. 1994, 77, 2695–2703. [Google Scholar] [CrossRef]
- Morotti, F.; Moretti, R.; Dos Santos, G.M.G.; Silva-Santos, K.C.; Cerqueira, P.H.R.; Seneda, M.M. Ovarian follicular dynamics and conception rate in Bos indicus cows with different antral follicle counts subjected to timed artificial insemination. Anim. Reprod. Sci. 2018, 188, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Atkins, J.A.; Smith, M.F.; Wells, K.J.; Geary, T.W. Factors affecting preovulatory follicle diameter and ovulation rate after gonadotropin-releasing hormone in postpartum beef cows. Part I: Cycling cows. J. Anim. Sci. 2010, 88, 2300–2310. [Google Scholar] [CrossRef]
- Edwards, S.A.A.; Atkinson, P.C.; Satake, N.; Boe-Hansen, G.; McGowan, M.R. Ovarian dynamics in response to two modified intravaginal progesterone releasing device and oestradiol benzoate based ovulation synchronisation protocols designed for use in brahman heifers. Anim. Reprod. Sci. 2014, 148, 18–25. [Google Scholar] [CrossRef]
- Monniaux, D.; Clément, F.; Dalbiès-Tran, R.; Estienne, A.; Fabre, S.; Mansanet, C.; Monget, P. The ovarian reserve of primordial follicles and the dynamic reserve of antral growing follicles: What is the link? Biol. Reprod. 2014, 90, 85. [Google Scholar] [CrossRef]
- Ginther, O.J.; Hoffman, M.M. Interactions of side (left and right ovary) with the number of follicles per ovary and with the intraovarian relationships between dominant follicle and corpus luteum in heifers. Theriogenology 2016, 86, 907–913. [Google Scholar] [CrossRef]
- Burns, D.S.; Jimenez-Krassel, F.; Ireland, J.L.; Knight, P.G.; Ireland, J.J. Numbers of antral follicles during follicular waves in cattle: Evidence for high variation among animals, very high repeatability in individuals, and an inverse association with serum follicle-stimulating hormone concentrations. Biol. Reprod. 2005, 73, 54–62. [Google Scholar] [CrossRef]
- Rico, C.; Fabre, S.; Médigue, C.; di Clemente, N.; Clément, F.; Bontoux, M.; Touzé, J.L.; Dupont, M.; Briant, E.; Rémy, B.; et al. Anti-mullerian hormone is an endocrine marker of ovarian gonadotropin-responsive follicles and can help to predict superovulatory responses in the cow. Biol. Reprod. 2009, 80, 50–59. [Google Scholar] [CrossRef]
- Mossa, F.; Jimenez-Krassel, F.; Scheetz, D.; Weber-Nielsen, M.; Evans, A.C.O.; Ireland, J.J. Anti-müllerian hormone (AMH) and fertility management in agricultural species. Reproduction 2017, 154, R1–R11. [Google Scholar] [CrossRef]
- Hatzirodos, N.; Irving-Rodgers, H.F.; Hummitzsch, K.; Harland, M.L.; Morris, S.E.; Rodgers, R.J. Transcriptome profiling of granulosa cells of bovine ovarian follicles during growth from small to large antral sizes. BMC Genom. 2014, 15, 24. [Google Scholar] [CrossRef]
- Robinson, R.S.; Woad, K.J.; Hammond, A.J.; Laird, M.; Hunter, M.G.; Mann, G.E. Angiogenesis and vascular function in the ovary. Reproduction 2009, 138, 869–881. [Google Scholar] [CrossRef]
- Devesa, J.; Caicedo, D. The role of growth hormone on ovarian functioning and ovarian angiogenesis. Front. Endocrinol. 2019, 10, 450. [Google Scholar] [CrossRef]
- Järvelä, I.Y.; Sladkevicius, P.; Kelly, S.; Ojha, K.; Campbell, S.; Nargund, G. Comparison of follicular vascularization in normal versus polycystic ovaries during in vitro fertilization as measured using 3-dimensional power doppler ultrasonography. Fertil. Steril. 2004, 82, 1358–1363. [Google Scholar] [CrossRef]
- Moonmanee, T.; Navanukraw, C.; Aiumlamai, S.; Jarukamjorn, K.; Thammasiri, J.; Redmer, D.A. Quantitative vascularity of antral follicle in Bos indicus using factor VIII immunolocalization. Livest. Sci. 2012, 150, 128–134. [Google Scholar] [CrossRef]
- Moonmanee, T.; Navanukraw, C.; Uriyapongson, S.; Kraisoon, A.; Aiumlamai, S.; Guntaprom, S.; Rittirod, T.; Borowicz, P.P.; Redmer, D.A. Relationships among vasculature, mitotic activity, and endothelial nitric oxide synthase (eNOS) in bovine antral follicles of the first follicular wave. Domest. Anim. Endocrinol. 2013, 45, 11–21. [Google Scholar] [CrossRef]
- Yama, P.; Yadmak, C.; Sangkate, M.; Jitjumnong, J.; U-krit, W.; Promsao, N.; Montha, N.; Sudwan, P.; Mektrirat, R.; Panatuk, J.; et al. In vivo follicular and uterine arterial indices as an indicator of successful hormonal stimulation for inactive ovaries in repeat-breeder crossbred dairy cows using a short-term progesterone-based programme. Animals 2022, 12, 292. [Google Scholar] [CrossRef]
- Bancsi, L.F.; Broekmans, F.J.; Eijkemans, M.J.; de Jong, F.H.; Habbema, J.D.; Velde, E.R. Predictors of poor ovarian response in in vitro fertilization: A prospective study comparing basal markers of ovarian reserve. Fertil. Steril. 2002, 77, 328–336. [Google Scholar] [CrossRef]
- Broekmans, F.J.; de Ziegler, D.; Howles, C.M.; Gougeon, A.; Trew, G.; Olivennes, F. The antral follicle count: Practical recommendations for better standardization. Fertil. Steril. 2010, 94, 1044–1051. [Google Scholar] [CrossRef]
- Karamishabankareh, H.; Hajarian, H.; Shahsavari, M.; Moradinejad, R. In vivo and in vitro study of the function of the left and right bovine ovaries. Theriogenology 2015, 84, 724–731. [Google Scholar] [CrossRef]
- Fukuda, M.; Fukuda, K.; Andersen, C.Y.; Byskov, A.G. Right-sided ovulation favours pregnancy more than left-sided ovulation. Hum. Reprod. 2000, 15, 1921–1926. [Google Scholar] [CrossRef] [PubMed]
- Quail, L.K.; Mund, M.E.; Neuendorff, D.A.; d’Orey Branco, R.A.; Banta, J.P.; Welsh, T.H.; Randel, R.D. Relationships between antral follicle numbers and postpartum interval in multiparous brahman cows. J. Anim. Sci. 2018, 96, 15. [Google Scholar] [CrossRef]
- Ginther, O.J. Intraovarianism. Local mechanisms that affect follicle and luteal dynamics in heifers and women. Biol. Reprod. 2020, 102, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Alves, K.A.; Alves, B.G.; Gastal, G.D.; de Tarso, S.G.; Gastal, M.O.; Figueiredo, J.R.; Gambarini, M.L.; Gastal, E.L. The mare model to study the effects of ovarian dynamics on preantral follicle features. PLoS ONE 2016, 22, e0149693. [Google Scholar] [CrossRef]
- Korsholm, A.S.; Hvidman, H.W.; Bentzen, J.G.; Andersen, A.N.; Petersen, K.B. Left-right differences in ovarian volume and antral follicle count in 1423 women of reproductive age. Gynecol. Endocrinol. 2017, 33, 320–323. [Google Scholar] [CrossRef]
- Järvelä, I.; Nuojua-Huttunen, S.; Martikainen, H. Ovulation side and cycle fecundity a retrospective analysis of frozen/thawed embryo transfer cycles. Hum. Reprod. 2000, 15, 1247–1249. [Google Scholar] [CrossRef][Green Version]
- Lan, K.C.; Huang, F.J.; Lin, Y.C.; Kung, F.T.; Lan, T.H.; Chang, S.Y. Significantly superior response in the right ovary compared with the left ovary after stimulation with follicle-stimulating hormone in a pituitary down-regulation regimen. Fertil. Steril. 2010, 93, 2269–2273. [Google Scholar] [CrossRef]
- Thomson, A.J.; Gazvani, M.R.; Wood, S.J.; Meacock, S.C.; Lewis-Jones, D.I.; Kingsland, C.R. Comparison of ovarian response in right and left ovaries in ivf patients. Hum. Reprod. 2001, 16, 1694–1697. [Google Scholar] [CrossRef]
- De Lima, M.A.; Morotti, F.; Bayeux, B.M.; de Rezende, R.G.; Botigelli, R.C.; De Bem, T.H.C.; Fontes, P.K.; Nogueira, M.F.G.; Meirelles, F.V.; Baruselli, P.S.; et al. Ovarian follicular dynamics, progesterone concentrations, pregnancy rates and transcriptional patterns in Bos indicus females with a high or low antral follicle count. Sci. Rep. 2020, 10, 19557. [Google Scholar] [CrossRef]
- Fricke, P.M.; al-Hassan, M.J.; Roberts, A.J.; Reynolds, L.P.; Redmer, D.A.; Ford, J.J. Effect of gonadotropin treatment on size, number, and cell proliferation of antral follicles in cows. Domest. Anim. Endocrinol. 1997, 14, 171–180. [Google Scholar] [CrossRef]
- Khattab, S.; Mohsen, I.A.; Foutouh, I.A.; Ramadan, A.; Moaz, M. Synchronization of antral follicles: A step further towards a friendly IVF program. Middle East Fertil. Soc. J. 2007, 12, 31–34. [Google Scholar]
- Cakmak, H.; Tran, N.D.; Zamah, A.M.; Cedars, M.I.; Rosen, M.P. A novel “Delayed start” protocol with gonadotropin-releasing hormone antagonist improves outcomes in poor responders. Fertil. Steril. 2014, 101, 1308–1314. [Google Scholar] [CrossRef][Green Version]
| Variable | Responsive Cows (n) | Nonresponsive Cows (n) | Responsive Rate (%, n/n) | OR | 95% CI | p-Value |
|---|---|---|---|---|---|---|
| Ovarian interrelationship (Ref = Bilateral mAFC) | ||||||
| Bilateral mAFC 1 | 19 | 11 | 63.3 (19/30) | |||
| Unilateral mAFC 2 | 61 | 13 | 82.4 (61/74) | 2.717 | 1.061–6.953 | 0.037 |
| Parity (Ref = Primiparous) | ||||||
| Primiparous | 20 | 9 | 69.0 (20/29) | |||
| Multiparous | 60 | 15 | 80.0 (60/75) | 1.800 | 0.685–4.732 | 0.233 |
| BCS (Ref = <2.5) | ||||||
| <2.5 | 10 | 5 | 66.7 (10/15) | |||
| 2.5–3.0 | 62 | 18 | 77.5 (62/80) | 1.722 | 0.523–5.671 | 0.371 |
| >3.0 | 8 | 1 | 94.7 (8/9) | 4.000 | 0.409–39.120 | 0.233 |
| Age (Ref = <72 months) | ||||||
| <72 months | 41 | 16 | 71.9 (41/57) | |||
| 72–96 months | 35 | 7 | 83.3 (35/42) | 1.951 | 0.724–5.260 | 0.186 |
| >96 months | 4 | 1 | 80.0 (4/5) | 1.561 | 0.161–15.089 | 0.700 |
| Items | Ovarian Interrelationships (n = 104) | p-Value | |
|---|---|---|---|
| Unilateral mAFC 1 | Bilateral mAFC 2 | ||
| Total beef cows (n) | 74 | 30 | - |
| On day 0 3 | |||
| Frequency relationship (%, n/n) | 71.2 (74/104) | 28.8 (30/104) | 0.001 |
| Mean number of AFC from left to right ovaries (n) | 4.6 ± 0.27 | 4.7 ± 0.47 | 0.974 |
| Mean diameter of AFC from left to right ovaries (mm) | 4.5 ± 0.16 | 4.2 ± 0.21 | 0.303 |
| On day 9 4 | |||
| Beef cows DF > 8.5 mm in diameter (%, n/n) | 82.4 (61/74) | 63.3 (19/30) | 0.037 |
| Mean diameter of DF (mm) | 11.0 ± 0.40 | 10.4 ± 0.63 | 0.412 |
| On day 32 5 | |||
| Pregnancy rate (%, n/n) | 35.1 (26/74) | 30.0 (9/30) | 0.617 |
| Items | Unilateral mAFC (n = 74) 1 | |||||
|---|---|---|---|---|---|---|
| mAFC Ipsilateral to the DF Ovary | mAFC Contralateral to the DF Ovary | |||||
| mAFC on the Left Ovary | mAFC on the Right Ovary | Total | mAFC on the Left Ovary | mAFC on the Right Ovary | Total | |
| Total beef cows (n) | 13 | 31 | 44 | 14 | 16 | 30 |
| On day 0 2 | ||||||
| Frequency relationship (%, n/n) | 29.5 (13/44) b | 70.5 (31/44) a | 59.5 (44/74) A | 46.7 (14/30) | 53.3 (16/30) | 40.5 (30/74) B |
| Mean number of AFC from left to right ovaries (n) | 4.6 ± 0.60 | 4.7 ± 0.42 | 4.7 ± 0.35 | 4.6 ± 0.45 | 4.9 ± 0.70 | 4.6 ± 0.43 |
| Mean diameter of AFC from left to right ovaries (mm) | 4.3 ± 0.29 | 4.3 ± 0.26 | 4.3 ± 0.20 | 4.4 ± 0.21 | 5.0 ± 0.42 | 4.7 ± 0.25 |
| On day 9 3 | ||||||
| Beef cows DF > 8.5 mm in diameter (%, n/n) | 27.3 (12/44) b | 56.8 (25/44) a | 50.0 (37/74) A | 36.7 (11/30) | 43.3 (13/30) | 32.4 (24/74) B |
| Mean diameter of DF (mm) | 11.1 ± 0.62 | 11.0 ± 0.63 | 11.0 ± 0.48 | 11.2 ± 1.03 | 10.8 ± 0.89 | 10.9 ± 0.68 |
| On day 32 4 | ||||||
| Pregnancy rate (%, n/n) | 9.1 (4/44) b | 25.0 (11/44) a | 20.3 (15/74) | 13.3 (4/30) | 23.3 (7/30) | 14.9 (11/74) |
| Items | Bilateral mAFC (n = 30) 1 | p-Value | |
|---|---|---|---|
| mAFC and DF on the Left Ovary | mAFC and DF on the Right Ovary | ||
| Total beef cows (n) | 15 | 15 | - |
| On day 0 2 | |||
| Frequency relationship (%, n/n) | 50.0 (15/30) | 50.0 (15/30) | 1.000 |
| Mean number of AFC from left to right ovaries (n) | 4.1 ± 0.44 | 5.2 ± 0.80 | 0.267 |
| Mean diameter of AFC from left to right ovaries (mm) | 4.0 ± 0.27 | 4.4 ± 0.32 | 0.326 |
| On day 9 3 | |||
| Beef cows DF > 8.5 mm in diameter (%, n/n) | 53.3 (8/15) | 73.3 (11/15) | 0.264 |
| Mean diameter of DF (mm) | 9.4 ± 1.02 | 11.4 ± 0.63 | 0.116 |
| On day 32 4 | |||
| Pregnancy rate (%, n/n) | 26.7 (4/15) | 33.3 (5/15) | 0.695 |
| Items | Ovarian Interrelationships (n = 29) | p-Value | |
|---|---|---|---|
| Unilateral mAFC 1 | Bilateral mAFC 2 | ||
| Primiparous beef cows (n) | 20 | 9 | - |
| On day 0 3 | |||
| Frequency relationship (%, n/n) | 69.0 (20/29) | 31.0 (9/29) | 0.004 |
| Mean number of AFC from left to right ovaries (n) | 5.8 ± 0.55 | 4.2 ± 0.49 | 0.059 |
| Mean diameter of AFC from left to right ovaries (mm) | 4.2 ± 0.20 | 4.3 ± 0.41 | 0.867 |
| On day 9 4 | |||
| Primiparous cows DF > 8.5 mm in diameter (%, n/n) | 75.0 (15/20) | 55.6 (5/9) | 0.304 |
| Mean diameter of DF (mm) | 10.8 ± 0.89 | 9.2 ± 1.14 | 0.305 |
| On day 32 5 | |||
| Pregnancy rate (%, n/n) | 50.0 (10/20) | 33.3 (3/9) | 0.412 |
| Items | Unilateral mAFC (n = 20) 1 | |||||
|---|---|---|---|---|---|---|
| mAFC Ipsilateral to the DF Ovary | mAFC Contralateral to the DF Ovary | |||||
| mAFC on the Left Ovary | mAFC on the Right Ovary | Total | mAFC on the Left Ovary | mAFC on the Right Ovary | Total | |
| Primiparous beef cows (n) | 4 | 9 | 13 | 5 | 2 | 7 |
| On day 0 2 | ||||||
| Frequency relationship (%, n/n) | 30.8 (4/13) | 69.2 (9/13) | 65.0 (13/20) | 71.4 (5/7) | 28.6 (2/7) | 35.0 (7/20) |
| Mean number of AFC from left to right ovaries (n) | 6.8 ± 0.89 | 6.0 ± 0.93 | 6.2 ± 0.71 | 5.0 ± 0.80 | 4.5 ± 1.77 | 4.9 ± 0.77 |
| Mean diameter of AFC from left to right ovaries (mm) | 4.1 ± 0.38 | 4.4 ± 0.30 | 4.3 ± 0.23 | 4.2 ± 0.36 | 4.0 ± 0.82 | 4.1 ± 0.36 |
| On day 9 3 | ||||||
| Primiparous cows DF > 8.5 mm in diameter (%, n/n) | 30.8 (4/13) | 53.8 (7/13) | 55.0 (11/20) A | 42.9 (3/7) | 14.3 (1/7) | 20.0 (4/20) B |
| Mean diameter of DF (mm) | 12.6 ± 1.31 | 10.5 ± 1.19 | 10.5 ± 1.17 | 10.2 ± 2.31 | 10.0 ± 2.63 | 11.3 ± 1.33 |
| On day 32 4 | ||||||
| Pregnancy rate (%, n/n) | 23.1 (3/13) | 30.8 (4/13) | 35.0 (7/20) | 28.6 (2/7) | 14.3 (1/7) | 15.0 (3/20) |
| Items | Bilateral mAFC (n = 9) 1 | p-Value | |
|---|---|---|---|
| mAFC and DF on the Left Ovary | mAFC and DF on the Right Ovary | ||
| Primiparous beef cows (n) | 7 | 2 | - |
| On day 0 2 | |||
| Frequency relationship (%, n/n) | 77.8 (7/9) | 22.2 (2/9) | 0.022 |
| Mean number of AFC from left to right ovaries (n) | 3.7 ± 0.48 | 6.0 ± 0.00 | 0.005 |
| Mean diameter of AFC from left to right ovaries (mm) | 4.4 ± 0.50 | 4.0 ± 0.55 | 0.708 |
| On day 9 3 | |||
| Primiparous cows DF > 8.5 mm in diameter (%, n/n) | 57.1 (4/7) | 50.0 (1/2) | 0.866 |
| Mean diameter of DF (mm) | 9.0 ± 1.39 | 9.9 ± 1.51 | 0.747 |
| On day 32 4 | |||
| Pregnancy rate (%, n/n) | 28.6 (2/7) | 50.0 (1/2) | 0.593 |
| Items | Ovarian Interrelationships (n = 75) | p-Value | |
|---|---|---|---|
| Unilateral mAFC 1 | Bilateral mAFC 2 | ||
| Multiparous beef cows (n) | 54 | 21 | - |
| On day 0 3 | |||
| Frequency relationship (%, n/n) | 72.0 (54/75) | 28.0 (21/75) | 0.001 |
| Mean number of AFC from left to right ovaries (n) | 4.2 ± 0.29 | 4.9 ± 0.63 | 0.389 |
| Mean diameter of AFC from left to right ovaries (mm) | 4.6 ± 0.20 | 3.8 ± 0.18 | 0.190 |
| On day 9 4 | |||
| Multiparous cows DF > 8.5 mm in diameter (%, n/n) | 85.2 (46/54) | 66.7 (14/21) | 0.074 |
| Mean diameter of DF (mm) | 11.1 ± 0.43 | 9.7 ± 0.90 | 0.825 |
| On day 32 5 | |||
| Pregnancy rate (%, n/n) | 29.6 (16/54) | 28.6 (6/21) | 0.928 |
| Items | Unilateral mAFC (n = 54) 1 | |||||
|---|---|---|---|---|---|---|
| mAFC Ipsilateral to the DF Ovary | mAFC Contralateral to the DF Ovary | |||||
| mAFC on the Left Ovary | mAFC on the Right Ovary | Total | mAFC on the Left Ovary | mAFC on the Right Ovary | Total | |
| Multiparous beef cows (n) | 9 | 22 | 31 | 9 | 14 | 23 |
| On day 0 2 | ||||||
| Frequency relationship (%, n/n) | 29.0 (9/31) b | 71.0 (22/31) a | 57.4 (31/54) | 39.1 (9/23) | 60.9 (14/23) | 42.6 (23/54) |
| Mean number of AFC from left to right ovaries (n) | 3.7 ± 0.52 | 4.1 ± 0.41 | 4.0 ± 0.33 | 4.3 ± 0.52 | 4.8 ± 0.81 | 4.7 ± 0.76 |
| Mean diameter of AFC from left to right ovaries (mm) | 4.4 ± 0.39 | 4.3 ± 0.34 | 4.3 ± 0.27 | 4.5 ± 0.26 | 5.2 ± 0.45 | 4.9 ± 0.30 |
| On day 9 3 | ||||||
| Multiparous cows DF > 8.5 mm in diameter (%, n/n) | 25.8 (8/31) b | 58.1 (18/31) a | 48.1 (26/54) | 34.8 (8/23) | 52.2 (12/23) | 37.0 (20/54) |
| Mean diameter of DF (mm) | 10.5 ± 0.56 | 11.2 ± 0.74 | 10.9 ± 0.58 | 11.7 ± 0.92 | 10.9 ± 0.94 | 11.4 ± 0.64 |
| On day 32 4 | ||||||
| Pregnancy rate (%, n/n) | 3.2 (1/31) b | 22.6 (7/31) a | 14.8 (8/54) | 8.7 (2/23) | 26.1 (6/23) | 14.8 (8/54) |
| Items | Bilateral mAFC (n = 21) 1 | p-Value | |
|---|---|---|---|
| mAFC and DF on the Left Ovary | mAFC and DF on the Right Ovary | ||
| Multiparous beef cows (n) | 8 | 13 | - |
| On day 0 2 | |||
| Frequency relationship (%, n/n) | 38.1 (8/21) | 61.9 (13/21) | 0.127 |
| Mean number of AFC from left to right ovaries (n) | 4.5 ± 0.68 | 5.1 ± 0.91 | 0.636 |
| Mean diameter of AFC from left to right ovaries (mm) | 3.6 ± 0.16 | 4.5 ± 0.35 | 0.046 |
| On day 9 3 | |||
| Multiparous cows DF > 8.5 mm in diameter (%, n/n) | 50.0 (4/8) | 76.9 (10/13) | 0.215 |
| Mean diameter of DF (mm) | 9.7 ± 1.46 | 11.6 ± 0.67 | 0.176 |
| On day 32 4 | |||
| Pregnancy rate (%, n/n) | 25.0 (2/8) | 30.8 (4/13) | 0.782 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
U-krit, W.; Wadsungnoen, S.; Yama, P.; Jitjumnong, J.; Sangkate, M.; Promsao, N.; Montha, N.; Sudwan, P.; Mektrirat, R.; Panatuk, J.; et al. Understanding the Ovarian Interrelationship with Low Antral Follicle Counts (AFC) in the In Vivo Bos indicus Cow Model: Unilateral and Bilateral Main AFC as Possible Biomarkers of Ovarian Response to Hormonal Synchronisation. Biology 2022, 11, 523. https://doi.org/10.3390/biology11040523
U-krit W, Wadsungnoen S, Yama P, Jitjumnong J, Sangkate M, Promsao N, Montha N, Sudwan P, Mektrirat R, Panatuk J, et al. Understanding the Ovarian Interrelationship with Low Antral Follicle Counts (AFC) in the In Vivo Bos indicus Cow Model: Unilateral and Bilateral Main AFC as Possible Biomarkers of Ovarian Response to Hormonal Synchronisation. Biology. 2022; 11(4):523. https://doi.org/10.3390/biology11040523
Chicago/Turabian StyleU-krit, Warittha, Surasak Wadsungnoen, Punnawut Yama, Jakree Jitjumnong, Molarat Sangkate, Nalinthip Promsao, Napatsorn Montha, Paiwan Sudwan, Raktham Mektrirat, Julakorn Panatuk, and et al. 2022. "Understanding the Ovarian Interrelationship with Low Antral Follicle Counts (AFC) in the In Vivo Bos indicus Cow Model: Unilateral and Bilateral Main AFC as Possible Biomarkers of Ovarian Response to Hormonal Synchronisation" Biology 11, no. 4: 523. https://doi.org/10.3390/biology11040523
APA StyleU-krit, W., Wadsungnoen, S., Yama, P., Jitjumnong, J., Sangkate, M., Promsao, N., Montha, N., Sudwan, P., Mektrirat, R., Panatuk, J., Inyawilert, W., Intawicha, P., Tang, P.-C., & Moonmanee, T. (2022). Understanding the Ovarian Interrelationship with Low Antral Follicle Counts (AFC) in the In Vivo Bos indicus Cow Model: Unilateral and Bilateral Main AFC as Possible Biomarkers of Ovarian Response to Hormonal Synchronisation. Biology, 11(4), 523. https://doi.org/10.3390/biology11040523






