Trophic Niche Breadth of Falconidae Species Predicts Biomic Specialisation but Not Range Size
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
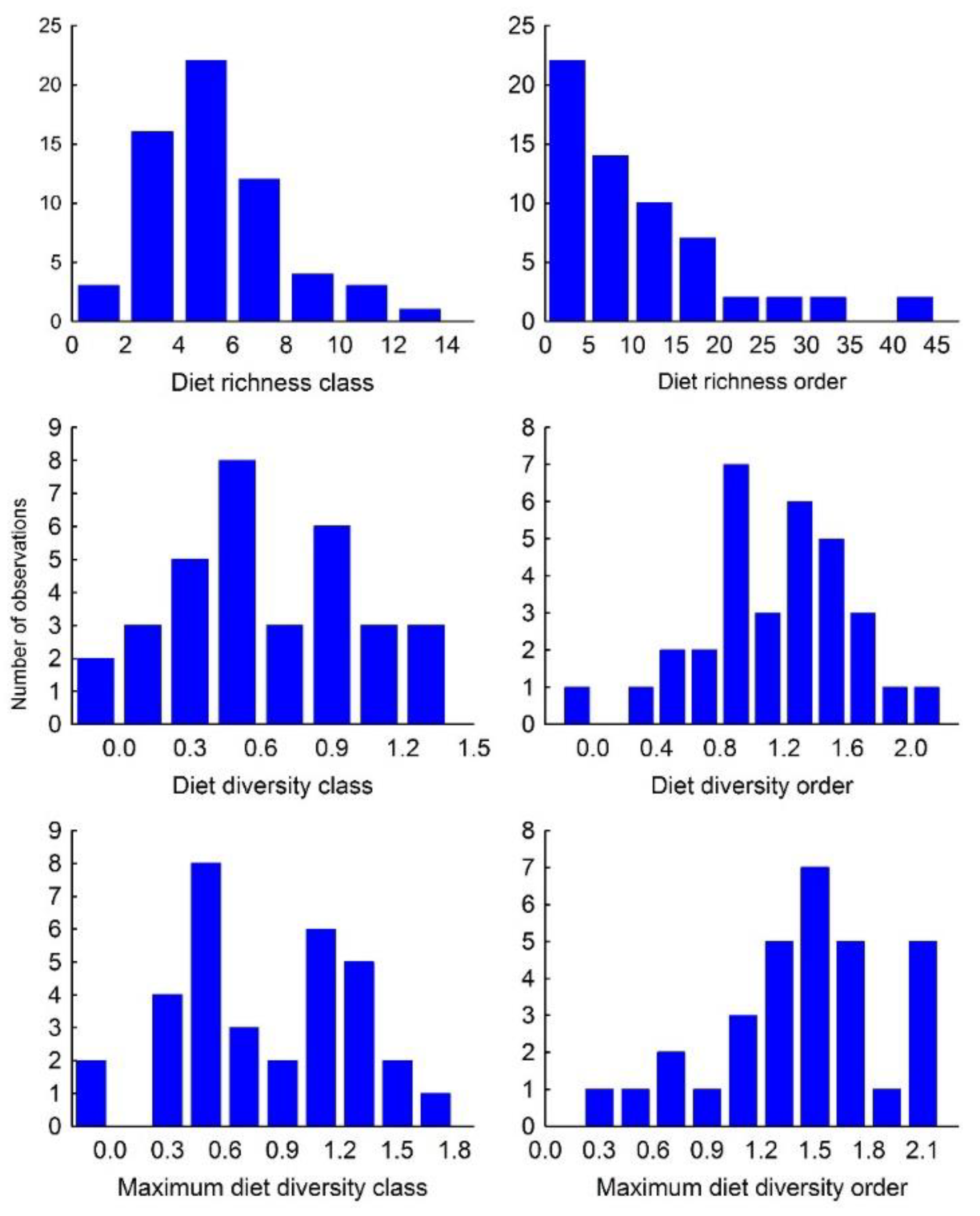
2.1. Trophic Niche Breadth
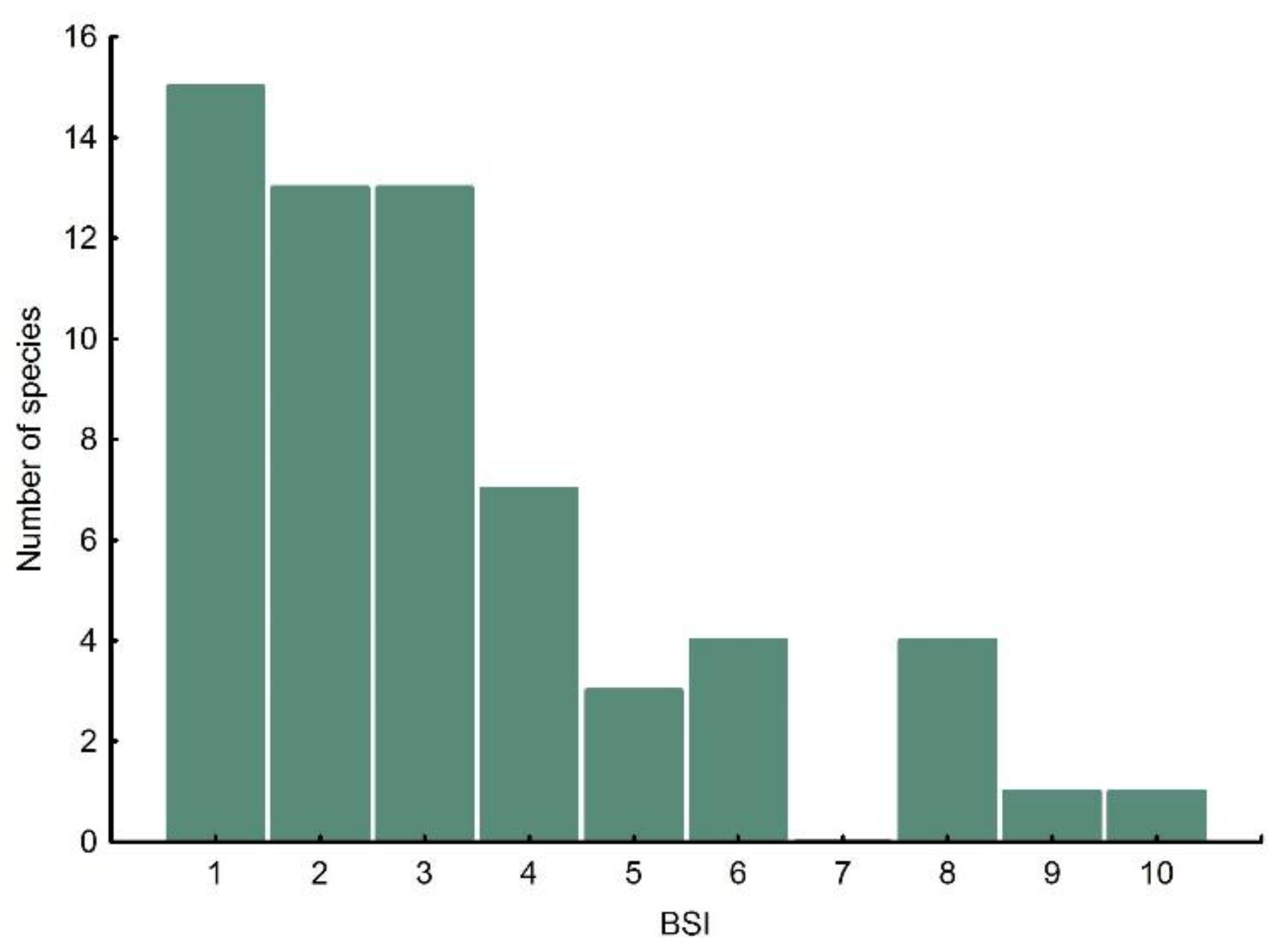
2.2. Geographical Range Size and Biomic Specialisation
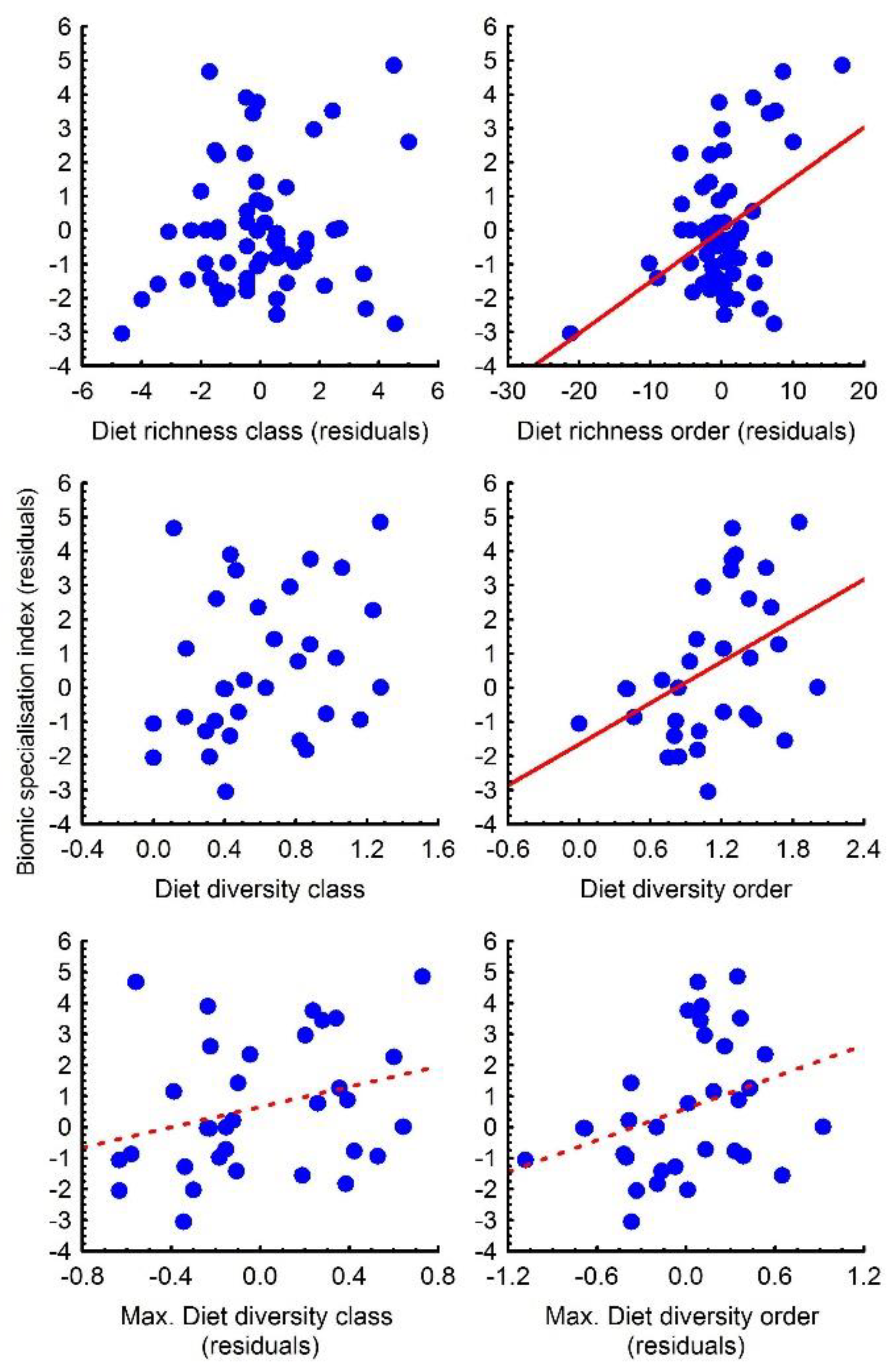
2.3. Statistical Procedures
3. Results
3.1. Diet Richness and Diversity
3.2. Geographical Range Size and Biomic Specialisation Index
3.3. Relationships among Diet, Geographical Range and Biomic Specialisation
4. Discussion
4.1. Trophic Niche Breadth
4.2. Range Size
4.3. Biomic Specialisation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species | DR | DDo | DDc |
|---|---|---|---|
| Caracara plancus | 27 | 7 | 7 |
| Daptrius albogularis | 1 | - | - |
| Daptrius americanus | 2 | - | - |
| Daptrius ater | 4 | - | - |
| Daptrius australis | 3 | 1 | 2 |
| Daptrius carunculatus | 4 | 1 | 1 |
| Daptrius chimachima | 7 | - | - |
| Daptrius chimango | 10 | 2 | 2 |
| Daptrius megalopterus | 5 | 4 | 4 |
| Falco alopex | 1 | - | - |
| Falco amurensis | 5 | 1 | 1 |
| Falco araea | 2 | 2 | 2 |
| Falco ardosiaceus | 1 | - | - |
| Falco berigora | 11 | 5 | 5 |
| Falco biarmicus | 12 | 5 | 5 |
| Falco cenchroides | 3 | - | 1 |
| Falco cherrug | 3 | - | - |
| Falco chicquera | 2 | 1 | 1 |
| Falco columbarius | 10 | 5 | 5 |
| Falco concolor | 6 | 2 | 2 |
| Falco cuvierii | 1 | - | - |
| Falco deiroleucus | 2 | 1 | 1 |
| Falco dickinsoni | 1 | - | - |
| Falco eleonorae | 10 | 1 | 1 |
| Falco fasciinucha | 1 | - | - |
| Falco femoralis | 14 | 7 | 8 |
| Falco hypoleucos | 1 | - | - |
| Falco jugger | 1 | - | - |
| Falco longipennis | 3 | 1 | 1 |
| Falco mexicanus | 11 | 15 | 15 |
| Falco moluccensis | 1 | - | - |
| Falco naumanni | 30 | 21 | 21 |
| Falco newtoni | 4 | 3 | 3 |
| Falco novaeseelandiae | 4 | 1 | 1 |
| Falco peregrinus | 53 | 18 | 18 |
| Falco punctatus | 2 | 1 | 1 |
| Falco rufigularis | 6 | 2 | 3 |
| Falco rupicoloides | 1 | - | - |
| Falco rusticolus | 52 | 8 | 8 |
| Falco severus | 1 | - | - |
| Falco sparverius | 28 | 12 | 12 |
| Falco subbuteo | 10 | 2 | 2 |
| Falco subniger | 2 | 1 | 1 |
| Falco tinnunculus | 68 | 26 | 31 |
| Falco vespertinus | 7 | 1 | 1 |
| Falco zoniventris | 2 | 1 | 1 |
| Herpetotheres cachinnans | 9 | 2 | 2 |
| Micrastur buckleyi | 1 | - | - |
| Micrastur gilvicollis | 1 | - | - |
| Micrastur mirandollei | 1 | - | - |
| Micrastur plumbeus | 1 | - | - |
| Micrastur ruficolis | 6 | - | - |
| Micrastur semitorquatus | 4 | 1 | 1 |
| Microhierax caerulescens | 1 | - | - |
| Microhierax erythrogenys | 1 | - | - |
| Microhierax fringillarius | 1 | - | - |
| Microhierax latifrons | 1 | - | - |
| Microhierax melanoleucos | 1 | - | - |
| Neohierax insignis | 1 | - | - |
| Polihierax semitorquatus | 1 | - | - |
| Spiziapteryx circumcincta | 1 | - | - |
Appendix B
| Species | BSI | DRc | DRo | DDc | DDo | MDDc | MDDo | Range Size | MS |
|---|---|---|---|---|---|---|---|---|---|
| Caracara plancus | 9 | 13 | 43 | 1.276 | 1.853 | 1.721 | 2.009 | 21,370.2 | 0 |
| Daptrius albogularis | 3 | 4 | 8 | - | - | - | - | 1001.6 | 0 |
| Daptrius americanus | 3 | 4 | 3 | - | - | - | - | 15,495.8 | 0 |
| Daptrius ater | 2 | 8 | 10 | - | - | - | - | 8596.5 | 0 |
| Daptrius australis | 1 | 7 | 11 | 0.971 | 1.415 | 1.185 | 1.415 | 297.3 | 0 |
| Daptrius carunculatus | 1 | 7 | 12 | 1.161 | 1.471 | 1.161 | 1.471 | 403.6 | 0 |
| Daptrius chimachima | 4 | 9 | 11 | - | - | - | - | 16,213.8 | 0 |
| Daptrius chimango | 6 | 12 | 28 | 0.354 | 1.430 | 0.538 | 1.582 | 5568.2 | 1 |
| Daptrius megalopterus | 1 | 5 | 9 | 0.858 | 0.997 | 1.275 | 1.329 | 1975.9 | 0 |
| Falco alopex | 3 | 5 | 4 | - | - | - | - | 5408.9 | 1 |
| Falco amurensis | 3 | 7 | 11 | 0.478 | 1.216 | 0.478 | 1.216 | 9821.4 | 1 |
| Falco araea | 1 | 5 | 7 | 0.883 | 1.289 | 1.000 | 1.337 | 0.9 | 0 |
| Falco ardosiaceus | 1 | 9 | 11 | - | - | - | - | 10,655.5 | 0 |
| Falco berigora | 5 | 8 | 16 | 0.881 | 1.684 | 1.287 | 2.012 | 10,226.8 | 0 |
| Falco biarmicus | 8 | 7 | 26 | 0.463 | 1.279 | 1.210 | 1.677 | 44,527.4 | 0 |
| Falco cenchroides | 6 | 5 | 4 | 1.235 | - | 1.235 | - | 10,210.3 | 1 |
| Falco cherrug | 4 | 6 | 8 | - | - | - | - | 27,292.0 | 1 |
| Falco chicquera | 3 | 5 | 6 | 0.000 | 0.000 | 0.000 | 0.000 | 18,207.1 | 0 |
| Falco columbarius | 4 | 7 | 24 | 0.176 | 0.462 | 0.351 | 1.161 | 77,981.6 | 1 |
| Falco concolor | 1 | 5 | 15 | 0.316 | 0.840 | 0.462 | 1.333 | 2836.5 | 1 |
| Falco cuvierii | 2 | 3 | 2 | - | - | - | - | 10,447.2 | 0 |
| Falco deiroleucus | 4 | 2 | 5 | 0.404 | 0.404 | 0.404 | 0.404 | 17,721.3 | 0 |
| Falco dickinsoni | 1 | 8 | 9 | - | - | - | - | 4757.2 | 0 |
| Falco eleonorae | 1 | 3 | 20 | 0.000 | 0.749 | 0.000 | 0.749 | 2956.8 | 1 |
| Falco fasciinucha | 5 | 3 | 2 | - | - | - | - | 1807.5 | 0 |
| Falco femoralis | 8 | 7 | 25 | 0.433 | 1.321 | 0.779 | 1.767 | 19,687.2 | 1 |
| Falco hypoleucos | 1 | 5 | 4 | - | - | - | - | 6636.1 | 1 |
| Falco jugger | 3 | 5 | 6 | - | - | - | - | 3185.6 | 0 |
| Falco longipennis | 6 | 4 | 10 | 0.587 | 1.617 | 0.587 | 1.617 | 8770.1 | 1 |
| Falco mexicanus | 5 | 7 | 17 | 0.679 | 0.990 | 1.025 | 1.450 | 7708.4 | 1 |
| Falco moluccensis | 2 | 5 | 4 | - | - | - | - | 769.5 | 0 |
| Falco naumanni | 3 | 7 | 18 | 0.430 | 0.802 | 1.077 | 1.720 | 34,622.5 | 1 |
| Falco newtoni | 3 | 6 | 6 | 0.812 | 0.933 | 1.096 | 1.459 | 681.5 | 0 |
| Falco novaeseelandiae | 2 | 6 | 11 | 0.511 | 0.699 | 0.511 | 0.699 | 304.4 | 1 |
| Falco peregrinus | 10 | 8 | 41 | 0.115 | 1.291 | 0.599 | 1.934 | 176,850.1 | 1 |
| Falco punctatus | 1 | 5 | 5 | 0.395 | 0.395 | 0.395 | 0.395 | 78.3 | 0 |
| Falco rufigularis | 4 | 4 | 12 | 0.630 | 0.837 | 0.684 | 1.124 | 16,560.7 | 0 |
| Falco rupicoloides | 3 | 6 | 5 | - | - | - | - | 4376.9 | 0 |
| Falco rusticolus | 2 | 5 | 11 | 0.407 | 1.085 | 0.669 | 1.325 | 108,698.0 | 1 |
| Falco severus | 2 | 4 | 3 | - | - | - | - | 5729.1 | 1 |
| Falco sparverius | 8 | 11 | 34 | 1.060 | 1.574 | 1.427 | 2.146 | 38,937.9 | 1 |
| Falco subbuteo | 6 | 5 | 19 | 0.185 | 1.216 | 0.374 | 1.508 | 76,163.1 | 1 |
| Falco subniger | 2 | 6 | 12 | 0.823 | 1.734 | 0.823 | 1.734 | 7374.6 | 1 |
| Falco tinnunculus | 8 | 12 | 35 | 0.768 | 1.042 | 1.453 | 2.045 | 106,347.0 | 1 |
| Falco vespertinus | 3 | 10 | 17 | 0.294 | 1.013 | 0.294 | 1.013 | 27,295.3 | 1 |
| Falco zoniventris | 3 | 5 | 7 | 1.026 | 1.440 | 1.026 | 1.440 | 564.1 | 0 |
| Herpetotheres cachinnans | 3 | 5 | 7 | 0.346 | 0.815 | 0.580 | 0.921 | 15,675.1 | 0 |
| Micrastur buckleyi | 1 | 2 | 2 | - | - | - | - | 1048.1 | 0 |
| Micrastur gilvicollis | 2 | 4 | 4 | - | - | - | - | 7871.2 | 0 |
| Micrastur mirandollei | 2 | 1 | 1 | - | - | - | - | 7916.0 | 0 |
| Micrastur plumbeus | 1 | 3 | 2 | - | - | - | - | 82.6 | 0 |
| Micrastur ruficollis | 4 | 9 | 17 | - | - | - | - | 15,059.9 | 0 |
References
- Soberón, J. Grinnellian and Eltonian niches and geographic distributions of species. Ecol. Lett. 2007, 10, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Bolnick, D.I.; Fitzpatrick, B.M. Sympatric speciation: Models and empirical evidence. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 459–487. [Google Scholar] [CrossRef]
- Belmaker, J.; Sekercioglu, C.H.; Jetz, W. Global patterns of specialization and coexistence in bird assemblages. J. Biogeogr. 2011, 39, 193–203. [Google Scholar] [CrossRef]
- Poisot, T.; Bever, J.D.; Nemri, A.; Thrall, P.H.; Hochberg, M.E. A conceptual framework for the evolution of ecological specialisation. Ecol. Lett. 2011, 14, 841–851. [Google Scholar] [CrossRef] [Green Version]
- Futuyma, D.J.; Moreno, G. The evolution of ecological specialization. Annu. Rev. Ecol. Syst. 1988, 19, 207–233. [Google Scholar] [CrossRef]
- Araújo, M.S.; Bolnick, D.I.; Layman, C.A. The ecological causes of individual specialisation. Ecol. Lett. 2011, 14, 948–958. [Google Scholar] [CrossRef]
- Levins, R. Theory of fitness in a heterogeneous environment. I. The fitness set and adaptive function. Am. Nat. 1962, 96, 361–373. [Google Scholar] [CrossRef]
- Svanbäck, R.; Bolnick, D.I. Intraspecific competition drives increased resource use diversity within a natural population. Proc. R. Soc. B 2007, 274, 839–844. [Google Scholar] [CrossRef]
- Woo, K.J.; Elliott, K.H.; Davidson, M.; Gaston, A.J.; Davoren, G.K. Individual specialization in diet by a generalist marine predator reflects specialization in foraging behaviour. J. Anim. Ecol. 2008, 77, 1082–1091. [Google Scholar] [CrossRef]
- Richmond, C.E.; Breitburg, D.L.; Rose, K.A. The role of environmental generalist species in ecosystem function. Ecol. Model. 2005, 188, 279–295. [Google Scholar] [CrossRef]
- Clavel, J.; Julliard, R.; Devictor, V. Worldwide decline of specialist species: Toward a global functional homogenization? Front. Ecol. Environ. 2011, 9, 222–228. [Google Scholar] [CrossRef] [Green Version]
- Stevens, G.S. The latitudinal gradient in geographical range: How so many species coexist in the tropics. Am. Nat. 1989, 133, 240–256. [Google Scholar] [CrossRef]
- Warren, M.S.; Hill, J.K.; Thomas, J.A.; Asher, J.; Fox, R.; Huntley, B.; Royk, D.B.; Telferk, M.G.; Jeffcoate, S.; Hardingk, P.; et al. Rapid responses of British butterflies to opposing forces of climate and habitat change. Nature 2001, 414, 65–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacArthur, R.H.; Wilson, E.O. The Theory of Island Biogeography; Princeton University Press: Princeton, NJ, USA, 1967. [Google Scholar]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- MacArthur, R.H.; MacArthur, J.W. On bird species diversity. Ecology 1961, 42, 594–598. [Google Scholar] [CrossRef]
- Jiang, M.; Felzer, B.S.; Nielsen, U.N.; Medlyn, B.E. Biome-specific climatic space defined by temperature and precipitation predictability. Glob. Ecol. Biogeogr. 2017, 26, 1270–1282. [Google Scholar] [CrossRef]
- Pianka, E.R. Latitudinal gradients in species diversity: A review of concepts. Am. Nat. 1966, 100, 33–46. [Google Scholar] [CrossRef]
- Hernández Fernández, M.; Vrba, E.S. Rapoport effect and biomic specialization in African mammals: Revisiting the climatic variability hypothesis. J. Biogeogr. 2005, 32, 903–918. [Google Scholar] [CrossRef]
- Hernández Fernández, M.; Vrba, E.S. Body size, biomic specialization and range size of African large mammals. J. Biogeogr. 2005, 32, 1243–1256. [Google Scholar] [CrossRef]
- MacArthur, R.; Levins, R. The limiting similarity, convergence, and divergence of coexisting species. Am. Nat. 1967, 101, 377–385. [Google Scholar] [CrossRef]
- McDonald, J.F.; Ayala, F.J. Genetic response to environmental heterogeneity. Nature 1974, 250, 572–574. [Google Scholar] [CrossRef] [PubMed]
- Roughgarden, J. Niche width: Biogeographic patterns among Anolis lizard populations. Am. Nat. 1974, 108, 429–442. [Google Scholar] [CrossRef]
- Janz, N.; Nylin, S. The oscillation hypothesis of host plant-range and speciation. In Specialization, Speciation, and Radiation: The Evolutionary Biology of Herbivorous Insects; Tilmon, K.J., Ed.; University of California Press: Berkeley, CA, USA, 2008; pp. 203–215. [Google Scholar]
- Hardy, N.B.; Kaczvinsky, C.; Bird, G.; Normark, B.B. What we don’t know about diet-breadth evolution in herbivorous insects. Annu. Rev. Ecol. Evol. Syst. 2020, 51, 103–122. [Google Scholar] [CrossRef]
- Beaudoin, C.P.; Tonn, W.M.; Prepas, E.E.; Wassenaar, L.I. Individual specialization and trophic adaptability of northern pike (Esox lucius): An isotope and dietary analysis. Oecologia 1999, 120, 386–396. [Google Scholar] [CrossRef]
- Harcourt, A.H. Latitude and latitudinal extent: A global analysis of the Rapoport effect in a tropical mammalian taxon: Primates. J. Biogeogr. 2000, 27, 1169–1182. [Google Scholar] [CrossRef]
- Brown, J.H. On the relationship between abundance and distribution of species. Am. Nat. 1984, 124, 255–279. [Google Scholar] [CrossRef]
- Slove, J.; Janz, N. The relationship between diet breadth and geographic range size in the butterfly subfamily Nymphalinae—A study of global scale. PLoS ONE 2011, 6, e16057. [Google Scholar] [CrossRef] [Green Version]
- Slatyer, R.A.; Hirst, M.; Sexton, J.P. Niche breadth predicts geographical range size: A general ecological pattern. Ecol. Lett. 2013, 16, 1104–1114. [Google Scholar] [CrossRef]
- Williams, Y.M.; Williams, S.E.; Alford, R.A.; Waycott, M.; Johnson, C.N. Niche breadth and geographical range: Ecological compensation for geographical rarity in rainforest frogs. Biol. Lett. 2006, 2, 532–535. [Google Scholar] [CrossRef] [Green Version]
- Gaston, K.; Blackburn, T. Pattern and Process in Macroecology; Blackwell Science Ltd.: Oxford, UK, 2000. [Google Scholar]
- Blanco, G.; Laiolo, P.; Fargallo, J.A. Linking environmental stress, feeding-shifts and the ‘island syndrome’: A nutritional challenge hypothesis. Popul. Ecol. 2014, 56, 203–216. [Google Scholar] [CrossRef]
- Ilse, L.M.; Hellgren, E.C. Resource partitioning in sympatric populations of collared peccaries and feral hogs in southern Texas. J. Mammal. 1995, 76, 784–799. [Google Scholar] [CrossRef]
- Quevedo, M.; Svanbäck, R.; Eklöv, P. Intrapopulation niche partitioning in a generalist predator limits food web connectivity. Ecology 2009, 90, 2263–2274. [Google Scholar] [CrossRef] [PubMed]
- Abbas, F.; Morellet, N.; Hewison, A.J.; Merlet, J.; Cargnelutti, B.; Lourtet, B.; Angibault, J.-M.; Daufresne, T.; Aulagnier, S.; Verheyden, H. Landscape fragmentation generates spatial variation of diet composition and quality in a generalist herbivore. Oecologia 2011, 167, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Navarro-López, J.; Fargallo, J.A. Trophic niche in a raptor species: The relationship between diet diversity, habitat diversity and territory quality. PLoS ONE 2015, 10, e0128855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eeley, H.A.; Foley, R.A. Species richness, species range size and ecological specialisation among African primates: Geographical patterns and conservation implications. Biodivers. Conserv. 1999, 8, 1033–1056. [Google Scholar] [CrossRef]
- del Hoyo, J.; Elliott, A.; Sargatal, J.; Cabot, J. Handbook of the Birds of the World. Vol. 2. New World Vultures to Guinea Fowl; Lynx Edicions: Barcelona, Spain, 1994. [Google Scholar]
- Fuchs, J.; Johnson, J.A.; Mindell, D.P. Rapid diversification of falcons (Aves: Falconidae) due to expansion of open habitats in the Late Miocene. Mol. Phylogenetics Evol. 2015, 82 Pt A, 166–182. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST-Palaeontological statistics. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Valdez, U. Caracterización y Comparación de las dietas de Guacabó Herpetotheres cachinans y Guaraguau Polyborus plancus en el Coto de Caza El Angolo y Alrededores. Bachelor’s Thesis, Universidad Nacional Agraria La Molina, Lima, Peru, 1996. [Google Scholar]
- McDonald, P.G.; Olsen, P.D.; Baker-Gabb, D.J. Territory fidelity, reproductive success and prey choice in the brown falcon, Falco berigora: A flexible bet-hedger? Aust. J. Zool. 2003, 51, 399–414. [Google Scholar] [CrossRef]
- Donadio, E.; Bolgeri, M.J.; Wurstten, A. First quantitative data on the diet of the Mountain Caracara (Phalcoboenus megalopterus). J. Rap. Res. 2007, 41, 328–330. [Google Scholar] [CrossRef]
- Zhou, Y.B.; Newman, C.; Xu, W.T.; Buesching, C.D.; Zalewski, A.; Kaneko, Y.; Macdonald, D.W.; Xie, Z.Q. Biogeographical variation in the diet of Holarctic martens (genus Martes, Mammalia: Carnivora: Mustelidae): Adaptive foraging in generalists. J. Biogeogr. 2011, 38, 137–147. [Google Scholar] [CrossRef]
- Schwenk, K. Feeding: Form, Function and Evolution in Tetrapod Vertebrates; Academic Press: San Diego, CA, USA, 2000. [Google Scholar]
- Rasband, W. ImageJ; US National Institutes of Health: Bethesda, MD, USA, 1997.
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.; Kreft, H. Terminology and quantification of environmental heterogeneity in species-richness research. Biol. Rev. 2015, 90, 815–836. [Google Scholar] [CrossRef] [PubMed]
- Walter, H. Vegetationszonen und Klima; Eugen Ulmer: Stuttgart, Germany, 1970. [Google Scholar]
- Cantalapiedra, J.L.; Hernández Fernández, M.; Morales, J. Biomic specialization and speciation rates in ruminants (Cetartiodactyla, Mammalia): A test of the resource-use hypothesis at the global scale. PLoS ONE 2011, 6, e28749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández Fernández, M. Bioclimatic discriminant capacity of terrestrial mammal faunas. Glob. Ecol. Biogeogr. 2001, 10, 189–204. [Google Scholar] [CrossRef]
- Moreno-Bofarull, A.; Arias Royo, A.; Hernández Fernández, M.; Ortiz-Jaureguizar, E.; Morales, J. Influence of continental history on the ecological specialization and macroevolutionary processes in the mammalian assemblage of South America: Differences between small and large mammals. BMC Evol. Biol. 2008, 8, 97. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Smith, G.M. Analysing Ecological Data; Springer: New York, NY, USA, 2007. [Google Scholar]
- Molina-Venegas, R.; Rodríguez, M.Á. Revisiting phylogenetic signal; strong or negligible impacts of polytomies and branch length information? BMC Evol. Biol. 2017, 17, 53. [Google Scholar] [CrossRef] [Green Version]
- Orme, D. The Caper Package: Comparative Analysis of Phylogenetics and Evolution in R, R package version 5; 2013; Available online: http://caper.r-forge.r-project.org/ (accessed on 18 March 2022).
- Colwell, R.K.; Futuyma, D.J. On the Measurement of Niche Breadth and Overlap. Ecology 1971, 52, 567–576. [Google Scholar] [CrossRef]
- Hillebrand, H.; Cardinale, B.J. Consumer effects decline with prey diversity. Ecol. Lett. 2004, 7, 192–201. [Google Scholar] [CrossRef]
- Chazdon, R.L.; Chao, A.; Colwell, R.K.; Lin, S.-Y.; Norden, N.; Letcher, S.G.; Clark, D.B.; Finegan, B.; Arroyo, J.P. A novel statistical method for classifying habitat generalists and specialists. Ecology 2011, 92, 1332–1343. [Google Scholar] [CrossRef]
- Jorge, L.R.; Prado, P.I.; Almeida-Neto, M.; Lewinsohn, T.M. An integrated framework to improve the concept of resource specialisation. Ecol. Lett. 2014, 17, 1341–1350. [Google Scholar] [CrossRef]
- Junker, R.R.; Blüthgen, N.; Brehm, T.; Binkenstein, J.; Paulus, J.; Martin Schaeferm, H.; Stang, M. Specialization on traits as basis for the niche-breadth of flower visitors and as structuring mechanism of ecological networks. Funct. Ecol. 2013, 27, 329–341. [Google Scholar] [CrossRef]
- Eldredge, N.; Cracraft, J. Phylogenetic Patterns and the Evolutionary Process: Method and Theory in Comparative Biology; Columbia University Press: New York, NY, USA, 1980.
- Vrba, E.S. Ecology in relation to speciation rates: Some case histories of Miocene-Recent mammal clades. Evol. Ecol. 1987, 1, 283–300. [Google Scholar] [CrossRef]
| Effect | R2 | λ | Estimate | SE | t | F | p | n (d.f.) |
|---|---|---|---|---|---|---|---|---|
| dDRc | 0.02 | <0.001 | 0.023 | 0.15 | 0.16 | 0.02 | 0.876 | 61 (2,59) |
| dDRo | 0.02 | <0.001 | 0.015 | 0.06 | 0.28 | 0.08 | 0.781 | 61 (2,59) |
| DDc | 0.07 | <0.001 | −2.084 | 1.295 | −1.609 | 2.59 | 0.118 | 33 (2,31) |
| DDo | 0.01 | <0.001 | 0.041 | 1.118 | 0.036 | 0.01 | 0.971 | 32 (2,30) |
| dMDDc | 0.04 | <0.001 | −1.858 | 1.23 | −1.51 | 2.28 | 0.141 | 33 (2,31) |
| dMDDo | 0.03 | <0.001 | 0.076 | 1.20 | 0.06 | 0.01 | 0.951 | 32 (2,30) |
| Effect | R2 | λ | Estimate | SE | t | F | p | n (d.f.) |
|---|---|---|---|---|---|---|---|---|
| dDRc | 0.01 | <0.001 | 0.039 | 0.01 | 0.88 | 0.76 | 0.385 | 61 (2,59) |
| dDRc + Range size | 0.36 | <0.001 | 0.035 | 0.03 | 0.97 | 17.57 | 0.334 | 61 (3,58) |
| dDRo | 0.12 | <0.001 | 0.161 | 0.05 | 3.04 | 9.24 | 0.004 | 61 (2,59) |
| dDRo + Range size | 0.41 | <0.001 | 0.152 | 0.04 | 3.52 | 22.23 | <0.001 | 61 (3,58) |
| DDc | 0.02 | <0.001 | 0.412 | 1.29 | 0.32 | 0.10 | 0.751 | 33 (2,31) |
| DDc + Range size | 0.35 | <0.001 | 1.701 | 1.07 | 1.59 | 9.46 | 0.123 | 33 (3,30) |
| DDo | 0.09 | <0.001 | 2.036 | 0.99 | 2.04 | 4.16 | 0.050 | 32 (2,30) |
| DDo + Range size | 0.42 | <0.001 | 2.013 | 0.80 | 2.25 | 12.12 | 0.018 | 32 (3,29) |
| dMDDc | 0.02 | <0.001 | 0.602 | 1.22 | 0.49 | 0.25 | 0.625 | 33 (2,31) |
| dMDDc + Range size | 0.39 | <0.001 | 1.753 | 0.99 | 1.75 | 9.88 | 0.089 | 33 (3,30) |
| dMDDo | 0.05 | <0.001 | 1.746 | 1.09 | 1.59 | 2.53 | 0.122 | 32 (2,30) |
| dMDDo + Range size | 0.37 | <0.001 | 1.704 | 0.89 | 1.91 | 10.09 | 0.066 | 32 (3,29) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fargallo, J.A.; Navarro-López, J.; Cantalapiedra, J.L.; Pelegrin, J.S.; Hernández Fernández, M. Trophic Niche Breadth of Falconidae Species Predicts Biomic Specialisation but Not Range Size. Biology 2022, 11, 522. https://doi.org/10.3390/biology11040522
Fargallo JA, Navarro-López J, Cantalapiedra JL, Pelegrin JS, Hernández Fernández M. Trophic Niche Breadth of Falconidae Species Predicts Biomic Specialisation but Not Range Size. Biology. 2022; 11(4):522. https://doi.org/10.3390/biology11040522
Chicago/Turabian StyleFargallo, Juan A., Juan Navarro-López, Juan L. Cantalapiedra, Jonathan S. Pelegrin, and Manuel Hernández Fernández. 2022. "Trophic Niche Breadth of Falconidae Species Predicts Biomic Specialisation but Not Range Size" Biology 11, no. 4: 522. https://doi.org/10.3390/biology11040522
APA StyleFargallo, J. A., Navarro-López, J., Cantalapiedra, J. L., Pelegrin, J. S., & Hernández Fernández, M. (2022). Trophic Niche Breadth of Falconidae Species Predicts Biomic Specialisation but Not Range Size. Biology, 11(4), 522. https://doi.org/10.3390/biology11040522








