Simple Summary
Representatives of genus Sarcocystis (Apicomplexa, Sarcocystidae) are parasites of mammals, birds, and reptiles. They are characterized by two-host prey-predator life cycle. Rodents are reservoirs of zoonotic diseases and play a significant role in a spread of pathogens. At present, about 40 Sarcocystis species are known to form sarcocysts in muscles and brain of rodents. Most of Sarcocystis spp. in these hosts have been characterized by morphological methods and life cycle investigations. In the present study a new Sarcocystis species, S. myodes is described in skeletal muscles of the bank voles (Clethrionomys glareolus) from Lithuania using morphological, molecular, and phylogenetic analysis. Based on five genetic loci, S. myodes was most closely related to Sarcocystis spp. using predatory mammals as their definitive hosts. The analysis of previous studies indicates that Sarcocystis spp. diversity in voles is not fully revealed. Furthermore, some Sarcocystis spp. formerly detected in voles are important pathogens. Therefore, further molecular examinations are needed for the revision of Sarcocystis spp. in these hosts.
Abstract
Numerous rodent species have been broadly examined for Sarcocystis parasites. Nevertheless, recent investigations on Sarcocystis spp. in voles are lacking. As many as 45 bank voles (Clethrionomys glareolus) captured in several locations in Lithuania were examined in the present study. Based on morphological, genetic, and phylogenetic results, sarcocysts detected in one bank vole were described as Sarcocystis myodes n. sp. Using light microscopy analysis, the observed sarcocysts were ribbon-shaped, 6000–3000 × 70–220 µm in size. Sarcocysts were characterized by a relatively thin (about 1 μm) and apparently smooth cyst wall. The lancet-shaped bradyzoites were 9.6–12.0 × 3.1–4.6 μm in size. By transmission electron microscopy, the sarcocyst wall was up to 1 μm thick, parasitophorous vacuolar membrane had small knob-like blebs. Based on 18S rDNA, 28S rDNA, cox1, rpoB, and ITS1 loci, S. myodes showed highest similarity with S. ratti from the black rat (Rattus rattus). According to phylogenetic placement, S. myodes was most closely related to Sarcocystis spp. that employ predatory mammals as their definitive hosts. Morphologically, sarcocysts of S. myodes have similar features to those of S. cernae, S. dirumpens, and S. montanaensis described in voles, however, they use birds of prey or snakes as their definitive hosts.
1. Introduction
Sarcocystis parasites belonging to the order Apicomplexa and Sarcocystidae family are worldwide distributed protozoan parasites, having two hosts in their life cycle. More than 200 species of Sarcocystis species have been described in reptiles, birds, and mammals. The intermediate host becomes infected by ingesting sporocyst-contaminated food or water, while the definitive host gets infected by eating muscular or neural tissues containing mature sarcocysts [1].
Rodentia is the most abundant group of mammals [2]. These animals are important components of the food chain [2,3,4,5] and play a significant role in the spread of pathogens due to their widespread distribution [6,7]. Currently, approximately 40 Sarcocystis species are known to infect rodents [1]. The majority of these species have been described on the basis of morphological and life cycle studies [1]. Only 10 of them, S. cymruensis, S. muris, S. ratti, S. dispersa, S. glareoli, S. microti, S. atheridis, S. singaporensis, S. zamani and S. zuoi have been molecularly characterized [8,9,10,11,12,13,14,15]. It should be noted that mice and rats were examined for Sarcocystis infection most thoroughly [1].
The bank vole Clethrionomys (Myodes) glareolus is one of the most common vole species in forests, shrubby habitats, fruit orchards, and commensal habitats of Lithuania [16,17,18,19]. At present, five species of Sarcocystis are known in bank voles. Three of them are described in the muscles of European voles (S. clethrionomyelaphis, S. dirumpens, and S. muriviperae) and are transmitted via snakes [20,21,22], while two species, S. glareoli and S. microti (previously assigned to genus Frenkelia), are described in the brain of rodents and use buzzards (Buteo spp.) as their definitive hosts [10,23,24]. Among these species, S. glareoli and S. microti have been characterized in complete 28S rDNA (~3280 bp) and partial 18S rDNA (~1630 bp). In the present paper, we describe a new species of Sarcocystis detected in the bank vole from Lithuania by microscopical and DNA sequence analysis.
2. Materials and Methods
2.1. Collection of Samples
We investigated 45 bank voles, trapped in 2018 at three great cormorant colonies in Lithuania, located in western (n = 4), southern (n = 8), and eastern (n = 33) parts of the country (Figure 1). Only adults were selected for the analysis because of their increased risk of infection. A portion of skeletal muscles were stored at −20 °C to examine for Sarcocystis infection.
Figure 1.
Investigation sites in Lithuania. The shore of the Curonian Lagoon in Western Lithuania (WL), the bank shore of the Nemunas River in Southern Lithuania (SL), the Peninsula of Lukštas Lake in Eastern Lithuania (EL).
2.2. Morphological Examination
To detect sarcocysts, fragments of muscle tissue (~0.5–1 g) were stained with methylene blue solution as described by Prakas et al. [15] and studied under light microscopy (LM). Morphological analysis of the observed sarcocysts was performed in fresh-squashed Sarcocystis-positive muscle samples. Sarcocysts were isolated with fine preparation needles. The excised sarcocysts were morphologically characterized on the basis of the form and size of cysts and bradyzoites released from the cyst, as well as the structure of the cyst wall. Three sarcocysts from fresh muscle preparations of the only one infected individual of the bank vole were isolated, and stored for further DNA extraction in sterile 1.5 mL microcentrifuge tubes with 96% ethanol. Sarcocyst wall ultrastructure was examined by transmission electron microscopy (TEM) using the previously described method [15].
2.3. DNA Extraction and PCR
Total DNA extraction procedure was conducted by a commercial kit “GeneJet Genomic DNA Purification Kit” (Thermo Fisher Scientific, Vilnius, Lithuania). The isolated sarcocysts were characterized molecularly in five genetic markers, three nuclear loci (18S rDNA, 28S rDNA, and ITS1), one mitochondrial gene (cox1), and one apicoplastic gene (rpoB). The primer pairs SarAF/SarBR and SarCF/SarDR (for nearly complete 18S rDNA), KL-P1F/KL-P2R (for partial 28S rDNA), P-ITSF/P-ITSR (for complete ITS1 region), SF1/SR5 (for partial cox1), and RPObF/RPObR (for partial rpoB) were used for amplification [25,26,27]. DreamTaq PCR Master Mix (Thermo Fisher Scientific, Vilnius, Lithuania) was used to perform PCR in the final 25 μL volume according to the manufacturer’s instructions. The PCR cycling conditions were as follows: an initial hot start for 5 min at 95 °C, followed by 35 cycles of denaturation for 45 s at 94 °C, annealing for 60 s at 50–58 °C depending on the primer pair, elongation for 70 s at 72 °C, and final extension for 7 min at 72 °C [28].
2.4. Sequence Analysis
The amplified PCR fragments were evaluated visually using 1% agarose gel electrophoresis. PCR products of 18S rDNA, 28S rDNA, and rpoB regions were purified with the help of ExoI and FastAP (Thermo Fisher Scientific Baltics, Vilnius, Lithuania) and sequenced directly using the same forward and reverse primers as for PCR. Sequencing reactions were carried out according to the manufacturer’s instructions using the Big-Dye® Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher Scientific, Vilnius, Lithuania) and the 3500 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). The 18S rDNA gene region sequences were obtained by amplification and sequencing with two pairs of primers, with the resulting sequences manually edited and merged into a single fragment. Samples amplified by means of the primers intended for cox1 and ITS1 regions gave unspecific products, thus before sequencing they were extracted from the gel and purified using commercial kit “GeneJET Gel Extraction Kit” (Thermo Fisher Scientific, Vilnius, Lithuania). In the current study, obtained sequences were analyzed using online Nucleotide BLAST program (http://blast.ncbi.nlm.nih.gov/, accessed on 17 January 2022). Conducting phylogenetic analysis, the sequences gained in the present study were compared with the very similar sequences of numerous Sarcocystis species. For this purpose, the sequence alignments were generated with the MUSCLE algorithm available in MEGAX software [29]. Phylogenetic trees were obtained using Bayesian method implemented in TOPALi software [30]. Toxoplasma gondii was set as the outgroup for the examined Sarcocystis species in all phylogenetic analyses.
3. Results
3.1. Prevalence and Morphological Description of Sarcocysts
Using methylene-blue staining, sarcocysts were detected in one muscle sample of one bank vole out of 45 voles examined (2.2% prevalence; 95% CI (confidence interval) = 0.4–11.6%). The infected individual (N26), a female from the bank of the Nemunas River in South Lithuania (Figure 1), was found to have 36 sarcocysts in 0.5 g of muscle.
Three sarcocysts, morphologically varying in size, were isolated from the infected muscle sample. All sarcocysts had a smooth cyst wall with no visible protrusions (Figure 2a) and further molecular analysis showed that they belonged to the same species. Sarcocysts were ribbon-shaped and measured 1970 × 150 µm (600–3000 × 70–220 µm; n = 5). The cyst wall was thin (~1 μm) and smooth. The septa were clearly visible. They divided sarcocysts into chambers containing numerous lancet-shaped 10.9 × 3.9 (9.6–12.0 × 3.1–4.6; n = 12) μm bradyzoites (Figure 2b).
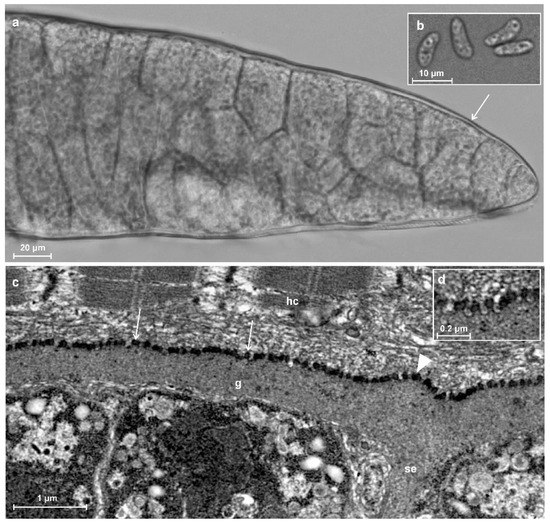
Figure 2.
Morphological features of sarcocyst of Sarcocystis myodes isolated from skeletal muscles of the bank vole (Clethrionomys glareolus) from Lithuania. (a,b) LM analysis. Fresh muscle-squashed preparations. (a) Fragment of sarcocyst. Note a relatively thin and apparently smooth cyst wall (arrow). (b) Lancet-shaped bradyzoites. (c,d) TEM analysis. (c) A fragment of a straight cyst wall with a slight wave near septa (arrowhead); the parasitophorous vacuolar membrane has knob-like blebs (arrows). (d) Enlarged view on bleb-like structure on the sarcocyst wall; note muscular host cell (hc), septa (se), and ground substance (g).
The TEM analysis showed that cyst wall was up to 1 μm in thickness. Parasitophorous vacuolar membrane had small knob-like blebs and was slightly wavy where the ground substance layer passed inside the cyst as septa (Figure 2c,d). The ground substance was smooth. The cyst wall ultrastructure was similar to type 1a described by Dubey et al. [1].
3.2. Molecular Characterization and Phylogeny
Three sarcocysts were characterized at five genetic loci and the molecular data obtained indicated that all three isolates belong to the same species. The resulting sequences were identical to each other at four loci (18S rDNA, 28S rDNA, cox1, and rpoB), and two different sequences with 5 SNPs were obtained in the ITS1 region. The obtained sequences had highest similarity values to those of S. ratti described in muscles of the black rat (Rattus rattus), except when comparing rpoB sequences (Table 1). Based on rpoB, the analyzed sequences were most similar to those of S. fulicae from the Eurasian coot (Fulica atra), S. cornixi from hooded crow (Corvus cornix), S. caninum from dog, and S. arctica from red fox (Vulpes vulpes). It should be noted that rpoB sequences have not been determined for S. ratti yet. The greatest differences with the closely related Sarcocystis species were detected in the ITS1 region (>20%), moderate differences were obtained in rpoB and 28S rDNA (~2–4%), while cox1 and 18S rDNA sequences of Sarcocystis sp. from the bank vole differed by less than 1% as compared with those of S. ratti. Except for S. ratti, the ITS1 sequences of S. myodes were highly distinct from and did not significantly match those of other Sarcocystis species available in GenBank. As a result, no additional molecular analysis of ITS1 sequences was performed.

Table 1.
Molecular characteristics of Sarcocystis myodes from bank vole.
Four different molecular loci were used to build phylogenetic trees. The Hasegawa–Kishino–Yano (HKY) model was used to construct phylogenetic trees for 18S rDNA, 28S rDNA, and rpoB, while the generalized time-reversible (GTR) model was chosen as the evolutionary model for cox1 tree construction. Currently, of the Sarcocystis species whose intermediate hosts are rodents, the most readily available are the 18S rDNA sequences. The final 18S rDNA sequence alignment used 35 sequences and 1652 aligned nucleotide positions. The phylogenetic analysis based on 28S rDNA sequences included 32 sequences and 1347 aligned nucleotide positions. For cox1 and rpoB phylogenetic analysis, 24 sequences with 976 aligned nucleotide positions and 19 sequences with 694 aligned nucleotide positions were used, respectively. In the phylogenetic trees constructed using 18S rDNA, 28S rDNA, and cox1 sequences (Figure 3a–c), Sarcocystis sp. from the bank vole was a sister taxon to S. ratti and showed a close relationship with S. cymruensis and S. muris using the brown rat (Rattus norvegicus) and the house mice (Mus musculus) as their intermediate hosts, respectively [14,31]. Two of these species, S. cymruensis and S. muris, employ predatory mammals as their definitive hosts [14,31,32], while the final hosts of S. ratti are unknown. Based on 18S rDNA, Sarcocystis spp. employing rodents as intermediate hosts and snakes as definitive hosts were placed into one cluster, while the phylogenetic position of the other Sarcocystis spp. that form sarcocysts in the muscles of rodents and employ birds or mammals as definitive hosts was not clear. In the phylograms of 28S rDNA and cox1 sequences, the Sarcocystis sp. from the bank vole did not form a single clade with S. glareoli, S. jamaicensis, S. microti, and S. strixi characterized by rodent-bird (intermediate-definitive host) life cycle [23,33,34]. The Sarcocystis sp. from the bank vole formed a separate branch in the phylogram constructed using rpoB sequences (Figure 3d). However, it should be taken into account that the rpoB sequences of S. ratti, S. cymruensis, and S. muris, most closely related to the Sarcocystis sp. from the bank vole, are not available in the GenBank.
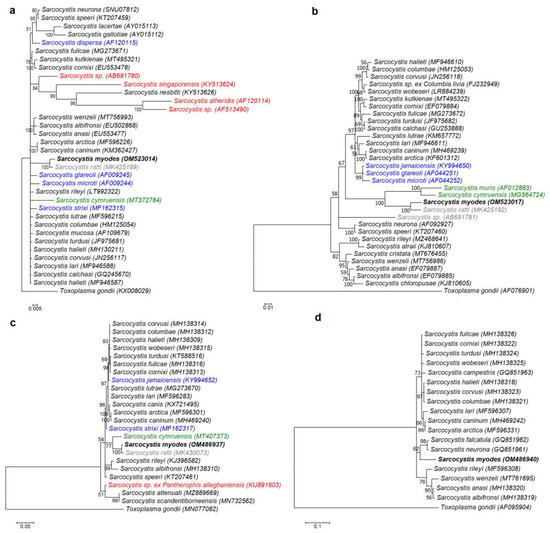
Figure 3.
Phylogenetic trees of selected Sarcocystis species. The trees constructed based on (a) 18S rDNA sequences, (b) 28S rDNA sequences, (c) cox1 sequences, and (d) rpoB sequences. The posterior probability support values are indicated next to branches. All color-coded Sarcocystis species are found in muscles of rodents. Sarcocystis species that are known to be distributed by birds are marked in blue, species that are distributed by snakes are marked in red, those transmitted by predatory mammals are in green, and species whose definitive hosts are not yet known are in a grey color.
3.3. Description of Sarcocystis myodes n. sp.
The Sarcocystis parasite discovered in the present study was morphologically distinct from the three species known to inhabit the musculature of bank voles, S. clethrionomyelaphis, S. dirumpens, and S. muriviperae. Sarcocysts detected in the bank vole from Lithuania were morphologically similar to those of S. glareoli and S. microti, however, the latter two species are confined to the brain. Additionally, reliable genetic differences were distinguished when comparing S. glareoli and S. microti with the species examined in the present study (2.3–2.4% in 18S rDNA and 6.6–7.1% in 28S rDNA). Other Sarcocystis species detected in rodents of the Cricetidae family that are morphologically similar to the species observed in the bank vole in this study are transmitted by birds or snakes. Phylogenetic analysis of the present work shows that Sarcocystis sp. from the Lithuanian bank vole does not group with Sarcocystis species such as S. dispersa, S. glareoli, S. jamaicensis, S. microti, and S. strixi that use birds as definitive hosts [23,33,34,35] or such species as S. singaporensis and S. atheridis that use snakes as definitive hosts [36,37]. When comparing the morphology of the Sarcocystis sp. from bank vole observed in the present study with that of the most closely related species, S. ratti, both parasites have a very similar sarcocyst size, shape, and cyst wall structure, but the species differ in the morphometric parameters of the bradyzoites. It was observed that the bradyzoites of the Sarcocystis sp. from the bank vole (9.6–12.0×3.1–4.6 µm) are longer than those of S. ratti (7.5–9.3 × 3.9–4.8 µm). Taking into account sarcocyst morphology and genetic data obtained, a new species, Sarcocystis myodes, is proposed for an organism found in the muscles of the bank vole form Lithuania.
Taxonomic summary of S. myodes n. sp.
Type intermediate host: The bank vole Clethrionomys (Myodes) glareolus.
Definitive host: Unknown.
Locality: Lithuania.
Morphology of sarcocyst: By LM, sarcocysts were microscopic, ribbon-shaped, 600–3000 × 70–220 µm in size, having a thin (~1 µm) and smooth cyst wall without visible protrusions. Lancet-shaped bradyzoites measured 9.6–12.0 × 3.1–4.6 μm. By TEM, knob-like blebs covered the parasitophorous vacuolar membrane. Type 1a-like.
Specimens deposited: TEM material is available at Laboratory of Molecular Ecology of the Nature Research Centre, Vilnius, Lithuania. All sequences obtained in the study are deposited in NCBI GenBank with accession numbers OM523014–OM523016 (18S rDNA), OM523017–OM523019 (28S rDNA), OM486937–OM486939 (cox1), OM486940–OM486942 (rpoB), and OM523020–OM523022 (ITS1).
Etymology: The Latin name of genus Myodes is used for the species name.
Recorded in URN as urn:lsid:zoobank.org:act:427763E1-B75E-40F7-8CF9-5079C6895459.
4. Discussion
The prevalence of Sarcocystis infection in the bank vole obtained in the present study (2.2%) is similar to that (1.8–14.4%) detected in this host in the previous studies carried out in Lithuania [38,39,40,41]. There is no information in other countries on the prevalence of Sarcocystis muscular infection in naturally infected bank voles. In general, Sarcocystis infection rates in various rodent species from different countries are relatively low/moderate. For instance, the prevalence of Sarcocystis spp. was 0.7% in synanthropic rodents from Spain [42], 11.6% in wild rats (Rattus spp.), 25% in large oriental voles (Eothenomys miletus) in China [43,44], 15.4% in black rats from Latvia [15], 33% in wild rodents from Thailand [45], and 40% in wild rodents from Indonesia [46]. Based on morphological Sarcocystis spp. studies conducted into rodents from Lithuania, it can be concluded that infection prevalence depends on the host species [38,39,40,41]. The lowest infection rates (up to 6%) were previously reported in mice species, the yellow-necked mouse (Apodemus flavicollis), the striped field mouse (Apodemus agrarius), and the domestic mouse. In five examined vole species (European water vole, short-tailed field vole, common vole, tundra vole, and bank vole), Sarcocystis infection prevalence ranged from 1.8% to 20.0%, whereas the highest Sarcocystis infection rates (30.2–52.4%) were detected in rats, the brown rat, and the black rat.
The DNA sequence comparison at five loci revealed significant genetic differences between S. myodes and other Sarcocystis spp., using rodents as their intermediate host. At the 18S rDNA, cox1, 28S rDNA, and ITS1 regions, differences between S. myodes and the most closely related species, S. ratti, were 0.5%, 0.6%, 1.9%, and 22.7–23.0%, respectively. Phylogenetic analysis showed that S. myodes had no close relationship with Sarcocystis spp. employing rodents as their intermediate hosts and birds or snakes as their definitive hosts, and that S. myodes was placed together with S. ratti and S. cymruensis (Figure 3). Rats are intermediate hosts to both S. ratti and S. cymruensis [47]. Domestic cats have been shown to be definitive hosts of S. cymruensis [14], and in some cases, due to cannibalism, rats may serve not only as an intermediate but also as a definitive host of the aforementioned species [43]. Therefore, phylogenetic evidence suggests that predatory mammals are most likely definitive hosts of S. myodes. The studied area abounds in foxes, raccoon dogs, minks, and martens [48], all of which feed on rodents, including the bank vole [16].
To date, three Sarcocystis species have been identified in the muscles of bank voles, and all of them being transmitted by snakes. Sarcocysts of S. clethrionomyelaphis and S. muriviperae have distinct wall structures (about 3–3.5 μm protrusions) that differ from the sarcocysts described in this work, whereas S. dirumpens has an ultrastructure similar to that of S. myodes. The sarcocysts wall of S. dirumpens was thin, corrugated, or folded, and the primary cyst wall was evaginated with knob-like blebs [49]. However, bradyzoites of S. dirumpens were smaller (7.0–9.0 × 1.6–2.0 µm) [20,49] as compared to those of S. myodes (9.6–12.0 × 3.1–4.6 μm). Sarcocysts that were morphologically similar to those of S. myodes were previously reported in bank voles from Lithuania [39]. Based on LM, sarcocysts had thin walls (0.7–1.4 µm) without visible protrusions and relatively large (10.0–14.2 × 2.3–4.7 µm; n = 231) bradyzoites. In the same study, sarcocysts morphologically similar those of S. myodes were also described in short-tailed field vole. Sarcocystis spp. morphologically similar to S. myodes were found in other vole species. Based on the structure of the sarcocyst wall, S. myodes is similar to S. cernae from the common vole and S. montanaensis from the eastern meadow vole (Microtus pennsylvanicus), the long-tailed vole (Microtus longicaudus), and the prairie vole (Microtus achrogaster) [50,51,52]. Transmission experiments revealed that the common kestrel (Falco tinnunculus) was a definitive host of S. cernae [50]. Intermediate hosts of S. montanaensis are prevalent in North America, and it has been demonstrated that this parasite uses a variety of snake species as definitive hosts [52,53]. Bradyzoites of S. cernae were significantly smaller (8–9 × 2–2.5 µm) than those of S. myodes [50], while bradyzoites of S. montanaensis (9.8–12.2 × 2.2–4.3 µm) were similar in size as compared to those detected in the present study [51]. Based on phylogenetic analyses, S. myodes is most closely related to Sarcocystis spp. that are known to employ predatory mammals as their definitive hosts, thus mammals rather than birds and reptiles are suggested definitive hosts of S. myodes. Comparative morphological analysis showed that S. myodes are similar to some Sarcocystis spp. utilizing reptiles or birds as their definitive hosts. Thus, S. myodes is different from other Sarcocystis spp. detected in voles based on morphological, genetic, and phylogenetic examination. The majority of Sarcocystis spp. described in voles are not strictly specific to one intermediate host species [1]. Therefore, it is likely that S. myodes can also be found in several vole species.
The most comprehensive morphological research on Sarcocystis spp. carried out in voles in Lithuania showed the presence of three morphological types of these parasites in the bank vole [39]. In the said study, 46 out of 320 examined bank voles were infected with Sarcocystis. Twenty Sarcocystis spp.-positive animals were chosen for morphological examination using LM. Sarcocystis were differentiated on the basis of the size of sarcocysts, the cyst wall appearance and morphometric parameters of bradyzoites. Sarcocysts similar to those detected in the present study were observed in the majority of tested animals (17/20). It should be pointed out that sarcocysts varied considerably in size (1.1–11.0 × 0.03–0.4 mm). Other two morphological types were characterized by sarcocysts having 3.2–4.2 µm densely packed hair-like protrusions and relatively small bradyzoites (7.0–9.2 × 1.8–2.5 µm) or sarcocysts with 2.7–4.0 µm bird claw-like protrusions and relatively large bradyzoites (11.2–13.0 × 2.5–4.2 µm). Thus, excluding S. myodes, at least two more Sarcocystis species should be present in the muscles of bank voles. In conclusion, many Sarcocystis spp. are described in rodents solely by morphological methods, and molecular revision of the already-named species is required, as the Sarcocystis group found in rodents is large and there is a general lack of recent research into these hosts.
5. Conclusions
From 45 bank voles examined, sarcocysts were discovered in a single specimen. Following investigation, a new species of Sarcocystis is described based on morphological LM and TEM analysis and five molecular loci, 18S rDNA, 28S rDNA, cox1, rpoB, and ITS1 investigation. It is proposed to name the species “S. myodes”. Phylogenetic placement suggests mammals as the definitive hosts of S. myodes. Our findings indicate a lack of Sarcocystis studies in voles, as well as a gap in molecular data when studying Sarcocystis species whose intermediate hosts are rodents.
Author Contributions
Conceptualization, D.B. and E.R.-L.; methodology, E.R.-L., P.P., M.J., L.B., and D.B.; software, P.P.; validation, P.P., L.B. and D.B.; formal analysis, E.R.-L., M.J., V.S. and D.B.; investigation, M.J., E.R.-L., P.P. and V.S.; resources, L.B. and D.B.; writing—original draft preparation, E.R.-L., M.J. and P.P.; writing—review and editing, L.B., V.S. and D.B.; visualization, P.P.; supervision, D.B., P.P. and L.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with Lithuanian (the Republic of Lithuania Law on the Welfare and Protection of Animals No. XI-2271, “Requirements for the Housing, Care and Use of Animals for Scientific and Educational Purposes”, approved by Order No B1-866, 31/10/2012 of the Director of the State Food and Veterinary Service (Paragraph 4 of Article 16) and European legislation (Directive 2010/63/EU) on the protection of animals. Permit for trapping was issued by EPA (No 6, 2018), and approved by the Animal Welfare Committee of the Nature Research Centre, protocol No GGT-7.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors express their gratitude to biomedical technician L. Juknienė from the National Centre of Pathology (Vilnius, Lithuania) for her assistance in performing TEM analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dubey, J.P.; Calero-Bernal, R.; Rosenthal, B.M.; Speer, C.A.; Fayer, R. Sarcocystosis of Animals and Humans, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Wilson, D.E.; Lacher, T.E.; Mittermeier, R.A. Handbook of the Mammals of the World; Lynx Edicions: Barselona, Spain, 2017; Volume 7. [Google Scholar]
- Malecha, A.W.; Antczak, M. Diet of the European polecat Mustela putorius in an agricultural area in Poland. Folia. Zool. 2013, 62, 48–53. [Google Scholar] [CrossRef]
- Grabham, A.A.; Ventress, G.; Hayward, M.W. The diet of denning female European pine martens (Martes martes) in Galloway Forest District, South West Scotland, Great Britain. Mammal. Res. 2019, 64, 87–97. [Google Scholar] [CrossRef]
- Gryz, J.; Krauze-Gryz, D. Changes in the tawny owl Strix aluco diet along an urbanisation gradient. Biologia 2019, 74, 279–285. [Google Scholar] [CrossRef]
- Han, B.A.; Schmidt, J.P.; Bowden, S.E.; Drake, J.M. Rodent reservoirs of future zoonotic diseases. Proc. Natl. Acad. Sci. USA 2015, 112, 7039–7044. [Google Scholar] [CrossRef]
- Rodríguez-Pastor, R.; Escudero, R.; Lambin, X.; Vidal, M.D.; Gil, H.; Jado, I.; Rodríguez-Vargas, M.; Luque-Larena, J.J.; Mougeot, F. Zoonotic pathogens in fluctuating common vole (Microtus arvalis) populations: Occurrence and dynamics. Parasitology 2018, 146, 389–398. [Google Scholar] [CrossRef]
- Votýpka, J.; Hypsa, V.; Jirků, M.; Flegr, J.; Vávra, J.; Lukes, J. Molecular phylogenetic relatedness of Frenkelia spp. (Protozoa, Apicomplexa) to Sarcocystis falcatula Stiles 1893: Is the genus Sarcocystis paraphyletic? J. Eukaryot. Microbiol. 1998, 45, 137–141. [Google Scholar] [CrossRef]
- Dolezel, D.; Koudela, B.; Jirků, M.; Hypsa, V.; Oborník, M.; Votýpka, J.; Modrý, D.; Šlapeta, J.R.; Lukes, J. Phylogenetic analysis of Sarcocystis spp. of mammals and reptiles supports the coevolution of Sarcocystis spp. with their final hosts. Int. J. Parasitol. 1999, 29, 795–798. [Google Scholar] [CrossRef]
- Mugridge, N.B.; Morrison, D.A.; Johnson, A.M.; Luton, K.; Dubey, J.P.; Votýpka, J.; Tenter, A.M. Phylogenetic relationships of the genus Frenkelia: A review of its history and new knowledge gained from comparison of large subunit ribosomal ribonucleic acid gene sequences. Int. J. Parasitol. 1999, 29, 957–972. [Google Scholar] [CrossRef]
- Mugridge, N.B.; Morrison, D.A.; Jäkel, T.; Heckeroth, A.R.; Tenter, A.M.; Johnson, A.M. Effects of sequence alignment and structural domains of ribosomal DNA on phylogeny reconstruction for the protozoan family sarcocystidae. Mol. Biol. Evol. 2000, 17, 1842–1853. [Google Scholar] [CrossRef]
- Šlapeta, J.R.; Kyselová, I.; Richardson, A.O.; Modrý, D.; Lukeš, J. Phylogeny and sequence variability of the Sarcocystis singaporensis Zaman and Colley, (1975) 1976 ssrDNA. Parasitol. Res. 2002, 88, 810–815. [Google Scholar] [CrossRef]
- Hu, J.J.; Meng, Y.; Guo, Y.M.; Liao, J.Y.; Song, J.L. Completion of the life cycle of Sarcocystis zuoi, a parasite from the Norway rat, Rattus norvegicus. J. Parasitol. 2012, 98, 550–553. [Google Scholar] [CrossRef] [PubMed]
- Antunes Murata, F.H.; Cerqueira-Cézar, C.K.; Thompson, P.C.; Tiwari, K.; Mowery, J.D.; Verma, S.K.; Rosenthal, B.M.; Sharma, R.N.; Dubey, J.P. Sarcocystis cymruensis: Discovery in Western Hemisphere in the Brown rat (Rattus norvegicus) from Grenada, West Indies: Redescription, molecular characterization, and transmission to IFN-γ gene knockout mice via sporocysts from experimentally infected domestic cat (Felis catus). Parasitol. Res. 2018, 117, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Prakas, P.; Kirillova, V.; Gavarāne, I.; Grāvele, E.; Butkauskas, D.; Rudaitytė-Lukošienė, E.; Kirjušina, M. Morphological and molecular description of Sarcocystis ratti n. sp. from the black rat (Rattus rattus) in Latvia. Parasitol. Res. 2019, 118, 2689–2694. [Google Scholar] [CrossRef] [PubMed]
- Prūsaitė, J. Lithuanian Fauna. Mammals; Mokslas: Vilnius, Lietuva, 1988. [Google Scholar]
- Balčiauskas, L.; Trakimas, G.; Juškaitis, R.; Ulevičius, A.; Balčiauskienė, L. Atlas of Lithuanian Mammals, Amphibians and Reptiles, 2nd ed.; Akstis: Vilnius, Lithuania, 1999. [Google Scholar]
- Balčiauskas, L.; Čepukienė, A.; Balčiauskienė, L. Small mammal community response to early meadow–forest succession. For. Ecosyst. 2017, 4, 11. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Balčiauskienė, L.; Garbaras, A.; Stirkė, V. Diversity and diet differences of small mammals in commensal habitats. Diversity 2021, 13, 346. [Google Scholar] [CrossRef]
- Häfner, U.; Matuschka, F.R. Life cycle studies on Sarcocystis dirumpens sp. n. with regard to host specificity. Z. Parasitenkd. 1984, 70, 715–720. [Google Scholar] [CrossRef]
- Matuschka, F.R. Sarcocystis clethrionomyelaphis n. sp. from snakes of the genus Elaphe and different voles of the family Arvicolidae. J. Parasitol. 1986, 72, 226–231. [Google Scholar] [CrossRef]
- Matuschka, F.R.; Heydorn, A.O.; Mehlhorn, H.; Abd-Al-Aal, Z.; Diesing, L.; Biehler, A. Experimental transmission of Sarcocystis muriviperae n. sp. to laboratory mice by sporocysts from the Palestinian viper (Vipera palestinae): A light and electron microscope study. Parasitol. Res. 1987, 73, 33–40. [Google Scholar] [CrossRef]
- Upton, S.J.; McKown, R.D. The red-tailed hawk, Buteo jamaicensis, a native definitive host of Frenkelia microti (Apicomplexa) in North America. J. Wildl. Dis. 1992, 28, 85–90. [Google Scholar] [CrossRef]
- Svobodová, M.; Vořišek, P.; Votýpka, J.; Weidinger, K. Heteroxenous coccidia (Apicomplexa: Sarcocystidae) in the populations of their final and intermediate hosts: European buzzard and small mammals. Acta Protozool. 2004, 43, 251–260. [Google Scholar]
- Kutkienė, L.; Prakas, P.; Sruoga, A.; Butkauskas, D. The mallard duck (Anas platyrhynchos) as intermediate host for Sarcocystis wobeseri sp. nov. from the barnacle goose (Branta leucopsis). Parasitol. Res. 2010, 107, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Wendte, J.M.; Miller, M.A.; Nandra, A.K.; Peat, S.M.; Crosbie, P.R.; Conrad, P.A.; Grigg, M.E. Limited genetic diversity among Sarcocystis neurona strains infecting southern sea otters precludes distinction between marine and terrestrial isolates. Vet. Parasitol. 2010, 169, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Gjerde, B. Phylogenetic relationships among Sarcocystis species in cervids, cattle and sheep inferred from the mitochondrial cytochrome c oxidase subunit I gene. Int. J. Parasitol. 2013, 43, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Prakas, P.; Butkauskas, D.; Švažas, S.; Stanevičius, V. Morphological and genetic characterisation of Sarcocystis halieti from the great cormorant (Phalacrocorax carbo). Parasitol. Res. 2018, 117, 3663–3667. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Milne, I.; Wright, F.; Rowe, G.; Marshall, D.; Husmeier, D.; McGuire, G. TOPALi: Software for automatic identification of recombinant sequences within DNA multiple alignments. Bioinformatics 2004, 20, 1806–1807. [Google Scholar] [CrossRef]
- Ruiz, A.; Frenkel, J.K. Recognition of cyclic transmission of Sarcocystis muris by cats. J. Infect. Dis. 1976, 133, 409–418. [Google Scholar] [CrossRef]
- Rommel, M. The ferret (Putorius putorius furo), an additional final host of Sarcocystis muris. Z. Parasitenkd. 1979, 58, 187–188. (In German) [Google Scholar] [CrossRef]
- Krampitz, H.E.; Rommel, M.; Geisel, O.; Kaiser, E. Beiträge zum Lebenszyklus der Frenkelien II. Die ungeschlechtliche Entwicklung von Frenkelia clethrionomyobuteonis in der Rötelmaus. Z. Parasitenkd. 1976, 51, 7–14. [Google Scholar] [CrossRef]
- Verma, S.K.; von Dohlen, A.R.; Mowery, J.D.; Scott, D.; Rosenthal, B.M.; Dubey, J.P.; Lindsay, D.S. Sarcocystis jamaicensis n. sp., from Red-Tailed Hawks (Buteo jamaicensis) definitive host and IFN-γ gene knockout mice as experimental intermediate host. J. Parasitol. 2017, 103, 555–564. [Google Scholar] [CrossRef]
- Černá, Z.; Kolárová, I.; Sulc, P. Contribution to the problem of cyst-producing coccidians. Folia Parasitol. 1978, 25, 9–16. [Google Scholar]
- Paperna, I.; Martelli, P. Fine structural development of microgamonts of Sarcocystis singaporensis in Python reticulatus. Parasitol. Res. 2000, 86, 1022–1025. [Google Scholar] [CrossRef] [PubMed]
- Šlapeta, J.R.; Modrý, D.; Koudela, B. Sarcocystis atheridis sp. nov., a new sarcosporidian coccidium from Nitsche’s bush viper, Atheris nitschei Tornier, 1902, from Uganda. Parasitol. Res. 1999, 85, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Arnastauskienė, T.; Grikienienė, J. Infection of small mammals with sarcosporidians in the South-Eastern Baltic region. Ecology 1993, 2, 47–56. (In Russian) [Google Scholar]
- Grikienienė, J.; Mažeikytė, R. Investigation of sarcosporidians (Sarcocystis) of small mammals in Kamasta landscape reserve and its surroundings. Acta Zool. Litu. 2000, 10, 55–68. [Google Scholar] [CrossRef]
- Grikienienė, J.; Malakauskas, M.; Mažeikytė, R.; Balčiauskas, L.; Senutaitė, J. Muscle parasites (Sarcocystis, Trichinella, Alaria) of wild mammals in Lithuania. Theriol. Litu. 2001, 1, 29–46. [Google Scholar]
- Grikienienė, J. Investigations into endoparasites of small mammals in the environs of Lake Drūkšiai. Acta Zool. Litu. 2005, 15, 109–114. [Google Scholar] [CrossRef]
- Fernández-Escobar, M.; Millán, J.; Chirife, A.D.; Ortega-Mora, L.M.; Calero-Bernal, R. Molecular survey for cyst-forming coccidia (Toxoplasma gondii, Neospora caninum, Sarcocystis spp.) in Mediterranean periurban micromammals. Parasitol. Res. 2020, 119, 2679–2686. [Google Scholar] [CrossRef]
- Hu, J.J.; Liao, J.Y.; Meng, Y.; Guo, Y.M.; Chen, X.W.; Zuo, Y.X. Identification of Sarcocystis cymruensis in wild Rattus flavipectus and Rattus norvegicus from Peoples Republic of China and its transmission to rats and cats. J. Parasitol. 2011, 97, 421–424. [Google Scholar] [CrossRef]
- Hu, J.J.; Liu, Q.; Yang, Y.F.; Esch, G.W.; Guo, Y.M.; Zou, F.C. Sarcocystis eothenomysi n. sp. (Apicomplexa: Sarcocystidae) from the large oriental vole Eothenomys miletus (Thomas) (Cricetidae: Microtinae) from Anning, China. Syst. Parasitol. 2014, 89, 73–81. [Google Scholar] [CrossRef]
- Jäkel, T.; Khoprasert, Y.; Sorger, I.; Kliemt, D.; Seehabutr, V.; Suasa-ard, K.; Hongnark, S. Sarcosporidiasis in rodents from Thailand. J. Wildl. Dis. 1997, 33, 860–867. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, P.J.; Watts, C.H.; Dixon, B.R. Ultrastructure of Sarcocystis spp. (Protozoa: Apicomplexa) in rodents from North Sulawesi and West Java, Indonesia. J. Wildl. Dis. 1987, 23, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Guo, Y.; Ma, C.; Deng, S.; Hu, J.; Zhang, Y. Redescription and molecular characterization of sarcocysts of Sarcocystis cymruensis from Norway rats (Rattus norvegicus) and Sarcocystis ratti from black rats (R. rattus) in China. Parasitol. Res. 2020, 119, 3785–3791. [Google Scholar] [CrossRef] [PubMed]
- Jasiulionis, M.; Balčiauskas, L. Seasonal and daily activity patterns of mammals in the colony of great cormorants. Mammalia 2021, 85, 439–447. [Google Scholar] [CrossRef]
- Häfner, U.; Frank, W. Morphological studies on the muscle cysts of Sarcocystis dirumpens (Hoare 1933) Häfner and Matuschka 1984 in several host species revealing endopolygeny in metrocytes. Z. Parasitenkd. 1986, 72, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Černá, Z.; Loučková, M. Microtus arvalis as the intermediate host of a coccidian from the kestrel (Falco tinnunculus). Folia Parasitol. 1976, 23, 110. [Google Scholar]
- Dubey, J.P. Sarcocystis montanaensis and S. microti sp. n. from the meadow vole (Microtus pennsylvanicus). Proc. Helminthol. Soc. Wash. 1983, 50, 318–324. [Google Scholar]
- Lindsay, D.S.; Upton, S.J.; Blagburn, B.L.; Toivio-Kinnucan, M.; McAllister, C.T.; Trauth, S.E. Sporocysts isolated from the southern copperhead (Agkistrodon contortrix contortrix) produce Sarcocystis montanaensis-like sarcocysts in prairie voles (Microtus ochrogaster). J. Wildl. Dis. 1991, 27, 148–152. [Google Scholar] [CrossRef][Green Version]
- Lindsay, D.S.; Upton, S.J.; Biagburn, B.L.; Toivio-Kinnucan, M.; Dubey, J.P.; McAllister, C.T.; Trauths, S.E. Demonstration that Sarcocystis montanaensis has a Speckled kingsnake-Prairie vole life cycle. J. Helminthol. Soc. Wash. 1992, 59, 9–15. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).