Alterations of the Sympathoadrenal Axis Related to the Development of Alzheimer’s Disease in the 3xTg Mouse Model
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Genotype Determinations
2.3. Primary Cultures of Mouse Chromaffin Cells
2.4. RNA Extraction and Quantitative Reverse Transcription PCR (RT-qPCR)
2.5. Recording of Ca2+ Channel Currents
2.6. Immunohistochemistry of Adrenal Glands
2.7. Data Representation and Statistical Analysis
3. Results
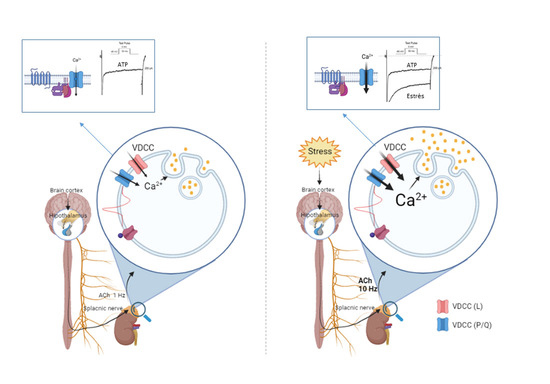
3.1. The Modulation of IBa by ATP in Chromaffin Cells Is Greater in 3xTg than in WT Mice
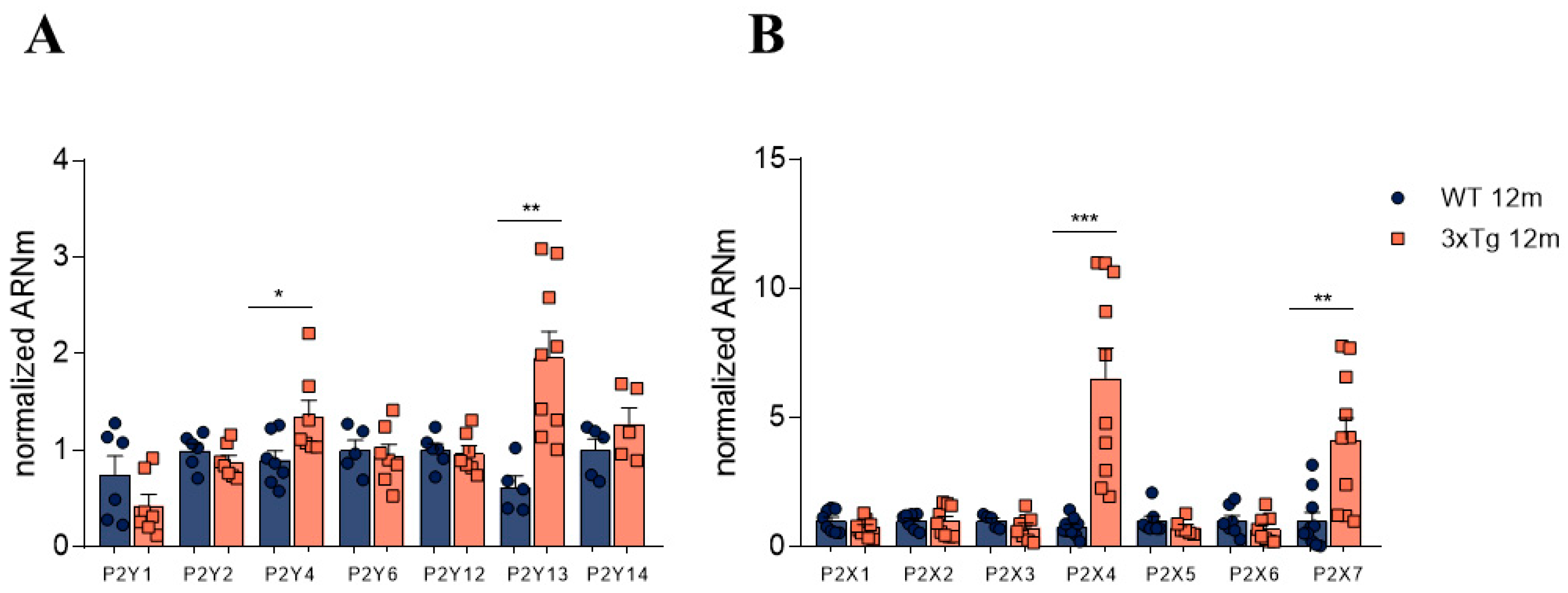
3.2. Altered Relative Expression of Different Purinergic Receptors in Adrenal Medulla
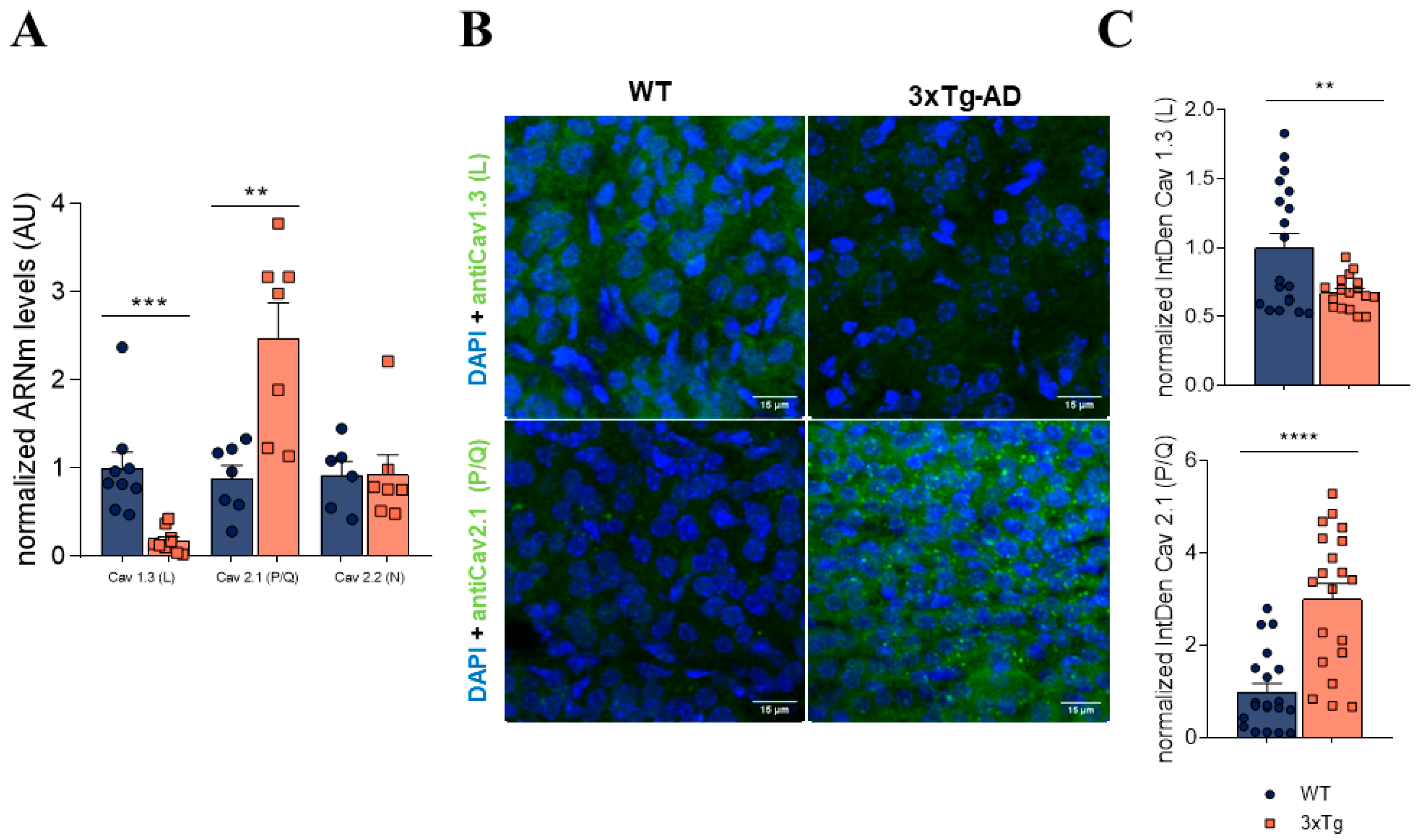
3.3. Increase in P/Q Currents and Decrease in L Currents in 3xTg Mice
3.4. In 3xTg Adrenal Medulla There Is an Increase in P/Q Channels and a Decrease in L Subtype Channel Expression
3.5. Alterations Observed in mRNA Levels of Purinergic Receptors and Calcium Channels Are Found Also in the Hippocampus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hardy, J.; Selkoe, D.J. The Amyloid Hypothesis of Alzheimer’s Disease: Progress and Problems on the Road to Therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Selkoe, D.J. Alzheimer’s Disease Is a Synaptic Failure. Science 2002, 298, 789–791. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Mesulam, M.-M.; Cuello, C.; Khachaturian, A.S.; Vergallo, A.; Farlow, M.R.; Snyder, P.J.; Giacobini, E. Revisiting the cholinergic hypothesis in alzheimer’s disease: Emerging evidence from translational and clinical research. J. Prev. Alzheimer’s Dis. 2018, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bartus, R.T.; Dean, R.L., III; Beer, B.; Lippa, A.S. The cholinergic hypothesis of geriatric memory dysfunction. Science 1982, 217, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Bell, K.F.; Cuello, A.C. Altered synaptic function in Alzheimer’s disease. Eur. J. Pharmacol. 2006, 545, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, S.R.; Ehrhart-Bornstein, M.; Androutsellis-Theotokis, A.; Eisenhofer, G.; Vukicevic, V.; Licinio, J.; Wong, M.L.; Calissano, P.; Nisticò, G.; Preziosi, P.; et al. Chromaffin cells: The peripheral brain. Mol. Psychiatry 2012, 17, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, B.; Beglopoulos, V.; Wines-Samuelson, M.; Zhang, D.; Dragatsis, I.; Südhof, T.C.; Shen, J. Presenilins are essential for regulating neurotransmitter release. Nature 2009, 460, 632–636. [Google Scholar] [CrossRef]
- Larsen, K.E.; Schmitz, Y.; Troyer, M.D.; Mosharov, E.; Dietrich, P.; Quazi, A.Z.; Savalle, M.; Nemani, V.; Chaudhry, F.A.; Edwards, R.H.; et al. Synuclein Overexpression in PC12 and Chromaffin Cells Impairs Catecholamine Release by Interfering with a Late Step in Exocytosis. J. Neurosci. 2006, 26, 11915–11922. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Shen, J.; Zhang, Y.; Li, S.-H.; Li, X.-J.; Li, H. Immunohistochemical Localization of Huntingtin-associated Protein 1 in Endocrine System of the Rat. J. Histochem. Cytochem. 2005, 53, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, K.D.; Duffield, M.D.; Peiris, H.; Phillips, L.; Zanin, M.P.; Teo, E.H.; Zhou, X.-F.; Keating, D.J. Huntingtin-associated protein 1 regulates exocytosis, vesicle docking, readily releasable pool size and fusion pore stability in mouse chromaffin cells. J. Physiol. 2014, 592, 1505–1518. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, K.D.; Lim, Y.; Duffield, M.D.; Chataway, T.; Zhou, X.-F.; Keating, D.J. Huntingtin-associated protein-1 (HAP1) regulates endocytosis and interacts with multiple trafficking-related proteins. Cell. Signal. 2017, 35, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Gallardo, E.; De Pascual, R.; Fernández-Morales, J.-C.; Arranz-Tagarro, J.-A.; Maroto, M.; Nanclares, C.; Gandía, L.; De Diego, A.M.G.; Padín, J.-F.; García, A.G. Depressed excitability and ion currents linked to slow exocytotic fusion pore in chromaffin cells of the SOD1G93A mouse model of amyotrophic lateral sclerosis. Am. J. Physiol. Physiol. 2015, 308, C1–C19. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Diego, A.M.; Lorrio, S.; Calvo-Gallardo, E.; Garcia, A.G. Smaller quantal size and faster kinetics of single exocytotic events in chromaffin cells from the APP/PS1 mouse model of Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2012, 428, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Villanueva, M.; Haynes, C.L.; Seipel, A.T.; Buhler, L.A.; Wightman, R.M. Catecholamine exocytosis is diminished in R6/2 Huntington’s disease model mice. J. Neurochem. 2007, 103, 2102–2110. [Google Scholar] [CrossRef]
- Martínez-Ramírez, C.; Baraibar, A.M.; Nanclares, C.; Méndez-López, I.; Gómez, A.; Muñoz, M.P.; De Diego, A.M.G.; Gandía, L.; Casarejos, M.J.; García, A.G. Altered excitability and exocytosis in chromaffin cells from the R6/1 mouse model of Huntington’s disease is linked to over-expression of mutated huntingtin. J. Neurochem. 2018, 147, 454–476. [Google Scholar] [CrossRef]
- Calorio, C.; Gavello, D.; Guarina, L.; Salio, C.; Sassoè-Pognetto, M.; Riganti, C.; Bianchi, F.T.; Hofer, N.T.; Tuluc, P.; Obermair, G.J.; et al. Impaired chromaffin cell excitability and exocytosis in autistic Timothy syndrome TS2-neo mouse rescued by L-type calcium channel blockers. J. Physiol. 2019, 597, 1705–1733. [Google Scholar] [CrossRef] [PubMed]
- De Diego, A.M.G.; Ortega-Cruz, D.; García, A.G.G. Disruption of Exocytosis in Sympathoadrenal Chromaffin Cells from Mouse Models of Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 1946. [Google Scholar] [CrossRef] [PubMed]
- Cannon, W.B. Organization for physiological homeostasiS. Physiol. Rev. 1929, 9, 399–431. [Google Scholar] [CrossRef]
- Hernández-Guijo, J.-M.; Gandía, L.; Lara, B.; García, A.G. Autocrine/paracrine modulation of calcium channels in bovine chromaffin cells. Pflügers Archiv. Eur. J. Physiol. 1998, 437, 104–113. [Google Scholar] [CrossRef] [PubMed]
- García, A.G.; García-De-Diego, A.M.; Gandia, L.; Borges, R.; Garcia-Sancho, J. Calcium Signaling and Exocytosis in Adrenal Chromaffin Cells. Physiol. Rev. 2006, 86, 1093–1131. [Google Scholar] [CrossRef] [PubMed]
- Gandía, L.; Garcia, A.G.; Morad, M. ATP modulation of calcium channels in chromaffin cells. J. Physiol. 1993, 470, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Albillos, A.; Carbone, E.; Gandia, L.; Garcia, A.G.; Polio, A. Opioid Inhibition of Ca2+ Channel Subtypes in Bovine Chromaffin Cells: Selectivity of Action and Voltage-dependence. Eur. J. Neurosci. 1996, 8, 1561–1570. [Google Scholar] [CrossRef] [PubMed]
- Oddo, S.; Caccamo, A.; Shepherd, J.D.; Murphy, M.P.; Golde, T.E.; Kayed, R.; Metherate, R.; Mattson, M.P.; Akbari, Y.; LaFerla, F.M. Triple-Transgenic Model of Alzheimer’s Disease with Plaques and Tangles: Intracellular Abeta and Synaptic Dysfunction. Neuron 2003, 39, 409–421. [Google Scholar] [CrossRef]
- de Pascual, R.; Miranda-Ferreira, R.; Galvão, K.M.; Lameu, C.; Ulrich, H.; Smaili, S.S.; Jurkiewicz, A.; García, A.G.; Gandía, L. Lower density of L-type and higher density of P/Q-type of calcium channels in chromaffin cells of hypertensive, compared with normotensive rats. Eur. J. Pharmacol. 2013, 706, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Hamill, O.P.; Marty, A.; Neher, E.; Sakmann, B.; Sigworth, F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981, 391, 85–100. [Google Scholar] [CrossRef]
- Fenwick, E.M.; Marty, A.; Neher, E. Sodium and calcium channels in bovine chromaffin cells. J. Physiol. 1982, 331, 599–635. [Google Scholar] [CrossRef]
- Hernández-Guijo, J.M.; Gandia, L.; Cuchillo-Ibañez, I.; Albillos, A.; Novalbos, J.; Gilsanz, F.; Larrañaga, E.; De Pascual, R.; Abad, F.; Garcia, A.G. Altered regulation of calcium channels and exocytosis in single human pheochromocytoma cells. Pflügers Archiv. Eur. J. Physiol. 2000, 440, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Carbone, E.; Borges, R.; Eiden, L.E.; Garcia, A.G.; Hernandez-Cruz, A. Chromaffin Cells of the Adrenal Medulla: Physiology, Pharmacology, and Disease. Compr. Physiol. 2019, 9, 1443–1502. [Google Scholar] [CrossRef]
- Albillos, A.; Neher, E.; Moser, T. R-Type Ca2+ Channels Are Coupled to the Rapid Component of Secretion in Mouse Adrenal Slice Chromaffin Cells. J. Neurosci. 2000, 20, 8323–8330. [Google Scholar] [CrossRef]
- Mahapatra, S.; Calorio, C.; Vandael, D.; Marcantoni, A.; Carabelli, V.; Carbone, E. Calcium channel types contributing to chromaffin cell excitability, exocytosis and endocytosis. Cell Calcium 2012, 51, 321–330. [Google Scholar] [CrossRef]
- Hernández-Guijo, J.M.; de Pascual, R.; García, A.G.; Gandía, L. Separation of calcium channel current components in mouse chromaffin cells superfused with low- and high-barium solutions. Pflügers Archiv. Eur. J. Physiol. 1998, 436, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Lommen, J.; Detken, J.; Harr, K.; von Gall, C.; Ali, A. Analysis of Spatial and Temporal Distribution of Purinergic P2 Receptors in the Mouse Hippocampus. Int. J. Mol. Sci. 2021, 22, 8078. [Google Scholar] [CrossRef] [PubMed]
- Vinet, J.; Sík, A. Expression pattern of voltage-dependent calcium channel subunits in hippocampal inhibitory neurons in mice. Neuroscience 2006, 143, 189–212. [Google Scholar] [CrossRef] [PubMed]
- Albillos, A.; Gandia, L.; Michelena, P.; A Gilabert, J.; Del Valle, M.; Carbone, E.; García, A.G. The mechanism of calcium channel facilitation in bovine chromaffin cells. J. Physiol. 1996, 494, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.; Segura-Chama, P.; Albiñana, E.; Hernández-Cruz, A.; Hernández-Guijo, J.M. Down-Modulation of Ca2+ Channels by Endogenously Released ATP and Opioids: From the Isolated Chromaffin Cell to the Slice of Adrenal Medullae. Cell. Mol. Neurobiol. 2010, 30, 1209–1216. [Google Scholar] [CrossRef]
- Hernández, A.; Segura-Chama, P.; Jiménez, N.; García, A.G.; Hernández-Guijo, J.M.; Hernández-Cruz, A. Modulation by endogenously released ATP and opioids of chromaffin cell calcium channels in mouse adrenal slices. Am. J. Physiol. Physiol. 2011, 300, C610–C623. [Google Scholar] [CrossRef]
- Amar, L.; Servais, A.; Gimenez-Roqueplo, A.-P.; Zinzindohoue, F.; Chatellier, G.; Plouin, P.-F. Year of Diagnosis, Features at Presentation, and Risk of Recurrence in Patients with Pheochromocytoma or Secreting Paraganglioma. J. Clin. Endocrinol. Metab. 2005, 90, 2110–2116. [Google Scholar] [CrossRef]
- Gandía, L.; Mayorgas, I.; Michelena, P.; Cuchillo, I.; de Pascual, R.; Abad, F.; Novalbos, J.M.; Larrañaga, E.; García, A.G. Human adrenal chromaffin cell calcium channels: Drastic current facilitation in cell clusters, but not in isolated cells. Pflügers Archiv—Eur. J. Physiol. 1998, 436, 696–704. [Google Scholar] [CrossRef]
- Herrmann, N.; Lanctôt, K.L.; Khan, L.R. The Role of Norepinephrine in the Behavioral and Psychological Symptoms of Dementia. J. Neuropsychiatry Clin. Neurosci. 2004, 16, 261–276. [Google Scholar] [CrossRef]
- Raskind, M.A.; Peskind, E.R. Neurobiologic bases of noncognitive behavioral problems in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 1994, 8, 54–60. [Google Scholar] [CrossRef]
- Cieślak, M.; Wojtczak, A. Role of purinergic receptors in the Alzheimer’s disease. Purinergic Signal. 2018, 14, 331–344. [Google Scholar] [CrossRef]
- Espada, S.; Ortega, F.; Molina-Jijón, E.; Rojo, A.I.; Pérez-Sen, R.; Pedraza-Chaverri, J.; Miras-Portugal, M.T.; Cuadrado, A. The purinergic P2Y13 receptor activates the Nrf2/HO-1 axis and protects against oxidative stress-induced neuronal death. Free Radic. Biol. Med. 2010, 49, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sen, R.; Queipo, M.J.; Morente, V.; Ortega, F.; Delicado, E.G.; Miras-Portugal, M.T. Neuroprotection Mediated by P2Y 13 Nucleotide Receptors in Neurons. Comput. Struct. Biotechnol. J. 2015, 13, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Zarrinmayeh, H.; Territo, P.R. Purinergic Receptors of the Central Nervous System: Biology, PET Ligands, and Their Applications. Mol. Imaging 2020, 19, 1536012120927609. [Google Scholar] [CrossRef] [PubMed]
- Calzaferri, F.; Ruiz-Ruiz, C.; De Diego, A.M.G.; De Pascual, R.; Méndez-López, I.; Cano-Abad, M.F.; Maneu, V.; Ríos, C.D.L.; Gandía, L.; García, A.G. The purinergic P2X7 receptor as a potential drug target to combat neuroinflammation in neurodegenerative diseases. Med. Res. Rev. 2020, 40, 2427–2465. [Google Scholar] [CrossRef]
- Martin, E.; Amar, M.; Dalle, C.; Youssef, I.; Boucher, C.; Le Duigou, C.; Brückner, M.; Prigent, A.; Sazdovitch, V.; Halle, A.; et al. New role of P2X7 receptor in an Alzheimer’s disease mouse model. Mol. Psychiatry 2019, 24, 108–125. [Google Scholar] [CrossRef] [PubMed]
- McLarnon, J.G.; Ryu, M.J.K.; Walker, D.G.; Choi, B.H.B. Upregulated Expression of Purinergic P2X7Receptor in Alzheimer Disease and Amyloid-β Peptide-Treated Microglia and in Peptide-Injected Rat Hippocampus. J. Neuropathol. Exp. Neurol. 2006, 65, 1090–1097. [Google Scholar] [CrossRef]
- Adinolfi, E.; Giuliani, A.L.; De Marchi, E.; Pegoraro, A.; Orioli, E.; Di Virgilio, F. The P2X7 receptor: A main player in inflammation. Biochem. Pharmacol. 2018, 151, 234–244. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Montero, A.; de Pascual, R.; Sáez-Mas, A.; Colmena, I.; Gandía, L. Alterations of the Sympathoadrenal Axis Related to the Development of Alzheimer’s Disease in the 3xTg Mouse Model. Biology 2022, 11, 511. https://doi.org/10.3390/biology11040511
Muñoz-Montero A, de Pascual R, Sáez-Mas A, Colmena I, Gandía L. Alterations of the Sympathoadrenal Axis Related to the Development of Alzheimer’s Disease in the 3xTg Mouse Model. Biology. 2022; 11(4):511. https://doi.org/10.3390/biology11040511
Chicago/Turabian StyleMuñoz-Montero, Alicia, Ricardo de Pascual, Anabel Sáez-Mas, Inés Colmena, and Luis Gandía. 2022. "Alterations of the Sympathoadrenal Axis Related to the Development of Alzheimer’s Disease in the 3xTg Mouse Model" Biology 11, no. 4: 511. https://doi.org/10.3390/biology11040511
APA StyleMuñoz-Montero, A., de Pascual, R., Sáez-Mas, A., Colmena, I., & Gandía, L. (2022). Alterations of the Sympathoadrenal Axis Related to the Development of Alzheimer’s Disease in the 3xTg Mouse Model. Biology, 11(4), 511. https://doi.org/10.3390/biology11040511