Simple Summary
Sea urchins are key in the trophic cascades of benthic communities. They also possess physiological characteristics of low digestibility. Thus, we explored the relationship between the indicators (digestive enzymes activities and the gut emptying) and molecules (TRPA1 and 5-HT) related to digestive physiology in the sea urchin Strongylocentrotus intermedius. The results in the present study indicate that digestive enzyme activities and gut emptying are correlated with the TRPA1 and 5-HT in the gut of S. intermedius. This novel finding not only is important for understanding the low food digestibility of sea urchins but provides an entry point to further investigate the molecular details of digestive physiology of sea urchins.
Abstract
The energetic link in the benthic community is based on physiological characteristics of the low food absorption efficiency of sea urchins. Low food absorption efficiency of sea urchins is correlated with the activity of digestive enzymes and the duration of food in their gut. Thus, the digestive enzymes activities (pepsin and amylase enzyme activities) and gut emptying are important indicators in assessing nutrient digestion and absorption in sea urchins. In the present study, the relationship between these indicators and molecules related to digestive physiology were quantified in sea urchins. We found (1) an inter-regulatory relationship existed between Transient receptor potential cation channel, subfamily A, member 1 (TRPA1), and serotonin (5-hydroxytryptamine; 5-HT) in the gut of Strongylocentrotus intermedius; (2) digestive enzyme activities were negatively correlated with the TRPA1 and concentration of 5-HT in the gut of S. intermedius; (3) gut emptying rate was positively correlated with TRPA1 and concentration of 5-HT in the gut of S. intermedius. The present study revealed that the digestion and absorption of food are correlated with the TRPA1 and 5-HT in the gut of S. intermedius, which provides valuable information about the digestive physiology of sea urchins. This novel finding is relevant to understanding the low food digestibility of sea urchins. It also provides valuable information to the digestive physiology of sea urchins, which are key to maintaining the stability of food webs in the marine ecosystem.
1. Introduction
Sea urchins are major consumers of macroalgae in shallow waters [1], and play a key trophic role by capturing kelp and making it available to a suite of benthic detritivores [2]. They thus play an important role in regulating kelp forests ecosystem [3]. The low food digestibility (absorption efficiency) of sea urchins [4,5,6] affects their ecological function. Low food digestibility of sea urchins requires large food consumption for the survival and reproduction [7]. Due to the inefficient digestive system of sea urchins [8,9], a substantial portion of urchin feces is relatively unprocessed vegetative materials [10,11]. The feces are important sources of calories and enriched nutrients for nearby consumers [12,13]. Thus, sea urchin feces represent an important energetic in benthic community [9] and play a positive nutritional role in kelp ecosystem [2].
Low food digestibility of sea urchins is correlated with the activity of digestive enzymes [7] and the duration during which the food stays in their gut [14]. Digestive enzymes directly affect the absorption and utilization of nutrients in the sea urchin Strongylocentrotus intermedius, thereby affecting their growth [15,16]. Amylase and pepsin have been extensively studied as representative digestive enzymes of sea urchins and their activities have been used to assess the digestive and absorptive capacities of sea urchins [15,16]. Gastric emptying is a common method used to quantify gastric motility [17], which greatly affects the rate of digestion and absorption of nutrients [18,19,20,21]. Antarctic echinoderms have low-energy diets and require long periods of digestion [7]. They feed slowly to ensure enough gut-passage time for adequate enzymatic breakdown of food, thus providing more energy [7]. Therefore, digestive enzymes activities and gut emptying are appropriate indicators of digestion and absorption capacities of S. intermedius. However, the relationship between these indicators and molecules related to digestive physiology remains largely unknown in sea urchins.
Transient receptor potential cation channel, subfamily A, member 1 (TRPA1), a member of the TRP family, is expressed in sensory neurons and associated with somatosensation [22,23,24]. TRPA1 is abundantly expressed in the gastrointestinal tract of mammals and plays an important role as a molecular receptor in regulating their gastrointestinal functions [25,26]. TRPA1 was also reported to be expressed in the gut of S. intermedius [27]. This suggests that TRPA1 is probably involved in regulating gut function in S. intermedius.
Serotonin (5-hydroxytryptamine; 5-HT) is an important signaling molecule that regulates animal gastrointestinal functions, including digestion and absorption [28,29]. The release of 5-HT coordinates the function of the gastrointestinal tract, which promotes digestion and absorption of nutrients in mammals [28], insects [30], and crustaceans [29]. Further, TRPA1 mediates the release of 5-HT to regulate gastrointestinal functions in rats, including gastric emptying and gastric motility [25,31]. Although the serotonergic nervous system was also reported in sea urchins [32], to our knowledge, 5-HT has not been investigated in digestive physiology of sea urchins. We hypothesized that a correlation exists between the indicators (digestive enzymes activities and the gut emptying) and molecules (TRPA1 and 5-HT) related to digestive physiology in sea urchins.
To test this hypothesis, we ask: (1) whether an inter-regulatory relationship exists between TRPA1 and 5-HT in the gut of S. intermedius; (2) whether a correlation exists between digestive enzyme activities and the reciprocal regulation of TRPA1 and 5-HT in the gut of S. intermedius; and (3) whether a correlation exists between gut emptying and reciprocal regulation of TRPA1 and 5-HT in the gut of S. intermedius.
2. Materials and Methods
2.1. Sea Urchins
Cultural conditions before the experiments: Sea urchins (test diameter: ~35 mm) were obtained from a local aqua-farm and transported to the Key Laboratory of Mariculture and Stock Affairs at Dalian Ocean University (38°52′ N, 121°34′ E). They were maintained at 13–14 °C in the laboratory in tanks (1000 L) at ambient temperature with sand filtered sea water. The temperature of the seawater in the laboratory corresponds to the temperature of the local aqua-farm where the sea urchins were collected. Sea urchins were fed freshly-collected Saccharina japonica and Ulva lactuca ad libitum before the experiments. One third of the seawater was changed every three days, with uneaten food and feces removed.
Experimental culture conditions: S. intermedius (33.22 ± 0.06 mm test diameter; 13.63 ± 0.07 g body weight) were used for experiments 1–4 (more details see the Results section). For experiments 1, 30 individuals were randomly selected from the laboratory tank (1000 L) under the natural culture condition. For experiments 2–4, S. intermedius were randomly selected from the laboratory tank and placed in temperature-controlled tanks (750 × 430 × 430 mm, ~139 L; Huixin Co., Dalian, China). Five tanks were used for each treatment group, and five individuals were placed into an individual cylindrical plastic cage (diameter: 100 mm, mesh size: 8 mm) in each tank (n = 5). Thus, 25 temperature-controlled tanks are in total for experiment 2, and 35 temperature-controlled tanks in total for experiment 3 and 4, respectively. Sea urchins used in experiments 2–3 were fed with fresh macroalgae Undaria pinnatifida. Sea urchins for experiment 4 were not fed for two weeks until the experiments started. We attached a net bag to the outside of each cage to collect the 48-h accumulative feces (Figure 1). The plastic cage and its outer net bag allowed free water exchange in each tank. Experiments 2–4 were carried out under illumination of ~300 lx and at the seawater temperature of 14.8–15.1 °C. The natural photoperiod was 10 h light: 14 h dark.
Figure 1.
Diagram of the feces collection in each temperature-controlled tank.
2.2. Chemicals
HC-030031, a TRPA1 selective antagonist, inhibited TRPA1 activity in vivo and in vitro [33] (Aladdin, Shanghai, China), dimethyl sulfoxide (DMSO) (Aladdin, Shanghai, China), stroke-physiological saline solution for marine animals (2% sodium chloride solution; Leagene, Beijing, China), and 5-HT (Sigma-Aldrich, Shanghai, China), were used in this study. HC-030031 and 5-HT were dissolved in 10% DMSO in distilled water and stroke-physiological saline solution for marine animals (marine saline), respectively. The dosage of chemicals in experiments 2, 3 and 4 were as follows: HC-030031 (2.5 mg/mL, 40 μL/ind); 10% DMSO (40 μL/ind); 5-HT (25 μg/g ind−1, the solution volume depended on the body weight of different individuals) [29]; marine saline (the same solution volume as 5-HT/ind). According to our preliminary experiment, the most effective drug durations were 2–3.5 h (df1 = 2, df2 = 12, F = 35.518, p < 0.001, Figure 2A) for HC-030031 and 0–0.5 h for 5-HT (df1 = 2, df2 = 12, F = 129.133, p < 0.001, Figure 2B) after injection for HC-030031 and 5-HT, respectively. For experiments 2, 3 and 4, all chemicals were injected into the peristomial membrane of S. intermedius [34] by using micro sampling syringes. To avoid cross-contamination, each chemical corresponded to its own individual syringe.
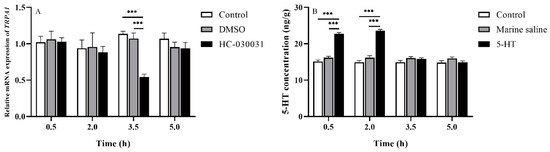
Figure 2.
(A) TRPA1 expression in the gut of Strongylocentrotus intermedius after the HC-030031 injection (n = 5). (B) The 5-HT level in the gut of S. intermedius after the 5-HT injection. The symbol *** means p < 0.001 (n = 5, mean ± s.e.m.).
2.3. Sample Collection for TRPA1 Expression, 5-HT Concentration and Enzyme Activities
For each experiment, we carefully collected gut samples at the same time. For experiment 1, each sea urchin’s gut tissue was divided into three samples. The three samples were stored in separate sterile microcentrifuge tubes for the subsequent analysis of TRPA1 expression, 5-HT concentration, and digestive enzyme activities, respectively. For experiment 2, gut tissues were collected from each S. intermedius with the HC-030031 injection for the subsequent analysis of 5-HT concentration. The gut tissues from each individual with the 5-HT injection were used for the subsequent analysis of TRPA1 expression. For experiment 3, the gut sample of each sea urchin was used for the subsequent analysis of digestive enzyme activities.
The samples for transcriptional analysis of TRPA1 expression and digestive enzyme activities were immediately snap-frozen in liquid nitrogen, and then stored at −80 °C until the extraction of RNA and determination of digestive enzyme activities. The samples for the analysis of 5-HT concentration were immersed in the 0.01 M phosphate buffer solution (PBS, pH = 7.4) and then stored at −20 °C until the determination of 5-HT concentration.
2.4. Total RNA Extraction and cDNA Synthesis
Total RNA was isolated from all samples obtained in the above experiments according to the instruction of RNAprep pure Tissue Kit (Tiangen, Beijing, China). The integrity of RNA was shown by visualization on agarose gel, whose concentration and quantity were evaluated by spectrophotometer (NanoPhotometer, Munich, Germany). The cDNA was synthesized using the PrimeScript™ RT reagent Kit (TaKaRa, Dalian, China) in a 20 μL reaction system with 1000 ng total RNA, 4 μL 5 × PrimeScript™ buffer, 2.5 pmol/μL Oligo dT Primer, 5 pmol/μL Random 6 mers and 1 μL PrimeScript RT Enzyme Mix I. Reactions were incubated at 37 °C for 15 min, followed by a final 5 s denaturation at 85 °C to deactivate the enzyme. All cDNA samples were stored at −20 °C for quantitative real-time PCR.
2.5. Transcriptional Analysis of TRPA1
The expression of TRPA1 in all experiments was analyzed by quantitative real-time PCR (qRT-PCR), which performed in triplicate using the Applied Biosystem 7500 Real-time system (Applied Biosystem, Foster, CA, USA). According to the manufacturer’s instructions, the reaction volume was 20 μL containing 2 μL of 1:5 dilution cDNA, 6 μL Nuclease-free Water, 10 μL TB Green Premix Ex Taq II, 0.4 μL of ROX Reference Dye II (TB Green™ Premix Ex Taq™ II; TaKaRa, Dalian, China), 0.4 μM of each primer (Table 1). The qRT-PCR program included 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s, and 60 °C for 32 s. Amplification products were analyzed by melting curve at the end of each PCR to confirm amplification specificity. In this study, 18S rRNA gene was used as the reference gene [34,35]. The relative expression level of TRPA1 was calculated using the comparative Ct method (2−△△CT method) [36].

Table 1.
PCR primers used in this study [27].
2.6. Analysis of 5-HT Concentration
The gut samples obtained in the above experiments were weighed and then minced to small pieces which were homogenized in 0.01 M phosphate buffer solution (PBS, pH = 7.4) with a glass homogenizer on ice. The volume depended on the weight of the tissue. Nine mL PBS was added in the 1 g gut sample. The homogenates were then centrifuged for 5 min at 5000× g to acquire the supernatant. The supernatant was used for further analysis of 5-HT concentration. The 5-HT concentration was measured by using the 5-Hydroxytryptamine (5-HT) ELISA kit (Mlbio, Shanghai, China). The determination principle, method and unit definition of 5-HT are in accordance with the instructions of the kit. The absorbance of samples at 450 nm was measured using the SpectraMax i3x (Molecular devices, Wals, Austria).
2.7. Analysis of Digestive Enzyme Activities
The samples of the gut obtained in the above experiments were immediately added to a precooled glass blender in an ice bath, after which physiological saline solution (0.85% sodium chloride) was added. The addition ratio is 1 g of sample to 10 mL of 0.85% sodium chloride solution [37]. The homogenate was centrifuged at 4500× g for 40 min at 4 °C. The supernatant was used for the analysis of digestive enzyme activities. The activities of enzymes were measured using the kits of amylase and pepsin (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The determination principle, method and unit definition of pepsin and amylase activities refer to the kit instructions. The samples were measured by a visible spectrophotometer (VIS7200A; Shanghai Techcomp Instrument Ltd., Shanghai, China) at the absorbance of 600 nm for determination of amylase activity and 680 nm for determination of pepsin activity.
2.8. Statistical Analysis
All data were tested for normal distribution and homogeneity of variance using Kolmogorov-Smirnov test and Levene test, respectively. Data from experiment 1 were analyzed using independent sampled t-test. Data from experiments 2, 3 and 4 were analyzed using one-way ANOVA. Fisher’s least significant difference (LSD) multiple comparisons were conducted to compare significant differences among treatments when a significant effect was found. All data are expressed as mean values ± standard error (mean ± s.e.m.). All statistical analysis was performed using SPSS 22.0 statistical software. The level of significance was considered as p < 0.05.
3. Results
3.1. Experiment 1: TRPA1 Is Involved in the Food Digestion of S. intermedius
To investigate whether TRPA1 is involved in the food digestion of S. intermedius, we randomly selected 30 sea urchins from the laboratory tank (1000 L) under the natural culture condition and measured TRPA1 expression level of their guts. Subsequently, we ranked the expression levels of TRPA1 of the 30 sea urchins, and compared the 5 sea urchins with the highest expression levels of TRPA1 (named as group H) with the 5 with the lowest expression levels of TRPA1 (named as group L). Then, we measured the pepsin and amylase enzyme activities of intestinal samples with the corresponding groups H and L. The expression of TRPA1 in the guts of 30 sea urchins was ranked from low to high (from left to right) (n = 30, Figure 3A).
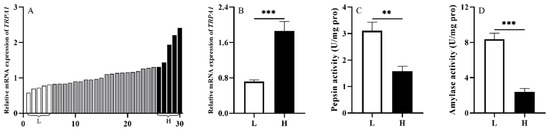
Figure 3.
TRPA1 is involved in food digestion of Strongylocentrotus intermedius. (A) The expression of TRPA1 in the gut of S. intermedius (n = 30). (B) TRPA1 expression of groups L and H (n = 5). (C,D) The activities of pepsin and amylase in the gut of the groups L and H (n = 5). The symbols ** and *** mean p < 0.01 and p < 0.001, respectively (mean ± s.e.m.).
We found that sea urchins of group H exhibited significantly higher expression of TRPA1 than those of group L (df = 8, t = 5.258, p < 0.01, Figure 3B). The pepsin and amylase activities of sea urchins in group L were significantly higher than those in group H (df = 8, t = −4.140, p < 0.01; df = 8, t = −7.745, p < 0.001) (Figure 3C,D). These results showed that TRPA1 is involved in the food digestion of S. intermedius.
3.2. Experiment 2: An Interregulatory Relationship Exists between TRPA1 and 5-HT in the Gut of S. intermedius
The 5-HT concentration of groups H and L was measured accordingly in experiment 1 (n = 5). We found that 5-HT concentration of group H was significantly higher than that of group L (df = 8, t = 5.336, p < 0.001, Figure 4A).
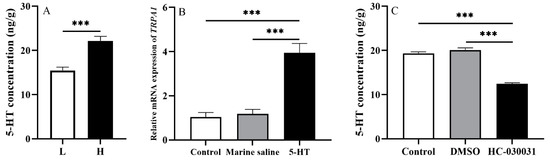
Figure 4.
An inter-regulatory relationship between TRPA1 and 5-HT in the gut of Strongylocentrotus intermedius. (A) The 5-HT level in the gut of groups L and H (n = 5). (B) TRPA1 expression after the 5-HT injection (n = 5). (C) The 5-HT level after the HC-030031 injection (n = 5). The symbol *** means p < 0.001, (mean ± s.e.m.).
To further investigate whether an inter-regulatory mechanism exists in the gut of S. intermedius, we firstly analyzed the 5-HT concentration in the guts of sea urchins injected with HC-030031. The average 5-HT concentration of the 5 individuals was considered as the 5-HT concentration for each temperature-controlled tank (n = 5). Five urchins in each of the 5 tanks were injected with 10% DMSO as the corresponding vehicle control group. Untreated sea urchins (without any chemical administration) in each of 5 tanks were used as the control group (n = 5). The results showed that TRPA1 expression of sea urchins injected with 5-HT was significantly higher than those in the untreated ones and the ones injected with marine saline (df1 = 2, df2 = 12, F = 31.304, both p < 0.001, Figure 4B). No significant difference was found between the sea urchins injected with marine saline and the untreated sea urchins (p > 0.05).
We further analyzed the expression pattern of TRPA1 in the guts of those injected with 5-HT. The average expression of TRPA1 of the 5 individuals was considered as the TRPA1 expression for each temperature-controlled tank (n = 5). Sea urchins were injected with marine saline as the corresponding vehicle control group (n = 5, named as the marine saline group). Untreated sea urchins (without any chemical administration) were used as the control group (n = 5). Sea urchins injected with HC-030031 exhibited significantly lower 5-HT concentration than those injected with DMSO and the untreated sea urchins (df1 = 2, df2 = 12, F = 112.016, both p < 0.001, Figure 4C). However, no significant difference was shown between sea urchins injected with DMSO and the untreated sea urchins (p > 0.05).
From the results in this experiment, we found that an interregulatory relationship existed between TRPA1 and 5-HT in the gut of S. intermedius.
3.3. Experiment 3: Digestive Enzyme Activities Are Negatively Correlated with TRPA1 and Concentration of 5-HT in the Gut of S. intermedius
Amylase and pepsin as representative digestive enzymes of sea urchins and their activities have been extensively used to evaluate the food digestion and absorption of sea urchins [15,16]. To investigate whether a correlation exists between digestive enzyme (amylase and pepsin) activities and the reciprocal regulation of TRPA1 and 5-HT in the gut of S. intermedius, we conducted a set of cascading experiments. The replicate set-up schemes of sea urchins were the same among all treatment groups in this experiment: 5 sea urchins in each of the 5 tanks. The average digestive enzyme activities of the 5 individuals were considered as the digestive enzyme activities for each temperature-controlled tank (n = 5).
First, sea urchins were injected with HC-030031 to inhibit TRPA1 (named as the HC-030031 group). Sea urchins were injected with 10% DMSO as the corresponding vehicle control group (named as the DMSO group). Pepsin and amylase enzyme activities were individually measured for each group 3.5 h after the drug injection. Untreated sea urchins (without any chemical administration) were used as the control group. The HC-030031 group showed significantly higher pepsin and amylase activities than the DMSO and control groups (pepsin: df1 = 2, df2 = 12, F = 16.300, both p < 0.001; amylase: df1 = 2, df2 = 12, F = 274.045, both p < 0.001, Figure 5A). The activities were not significantly different between the DMSO and the control groups (both p > 0.05).
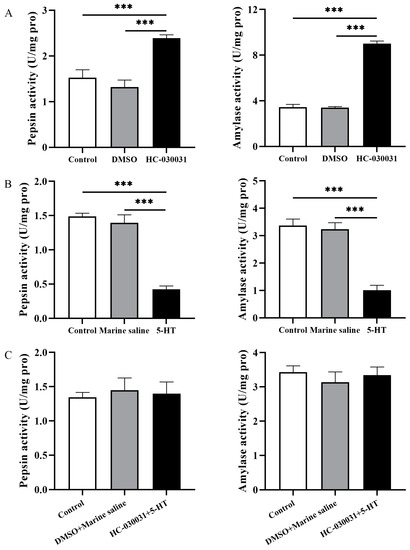
Figure 5.
Digestive enzyme activities are negatively correlated with the reciprocal regulation of TRPA1 and 5-HT in the gut of Strongylocentrotus intermedius. (A) The activities of pepsin and amylase of the control, DMSO, and HC-030031 groups (n = 5). (B) The activities of pepsin and amylase of the control, marine saline, and 5-HT groups (n = 5). (C) The activities of pepsin and amylase of control, DMSO + marine saline, and HC-030031 + 5-HT groups (n = 5). The symbol *** means p < 0.001 (mean ± s.e.m.).
We then measured pepsin and amylase enzyme activities of the sea urchins 0.5 h after the 5-HT injection (named as 5-HT group). Sea urchins were injected with marine saline as the corresponding vehicle control group (named as the marine saline group). Untreated sea urchins (without any chemical administration) were used as the control group. The pepsin and amylase activities of 5-HT group were significantly lower than the control and marine saline groups (pepsin: df1 = 2, df2 = 12, F = 57.499, both p < 0.001; amylase: df1 = 2, df2 = 12, F = 36.704, both p < 0.001, Figure 5B). No significant difference was found between the control and marine saline groups (both p > 0.05).
Finally, sea urchins were injected with HC-030031, and then injected with 5-HT 3 h after the HC-030031 injection. After 3.5 h of the HC-030031 injection and 0.5 h of the 5-HT injection, sea urchins of the experimental group (named as HC-030031 + 5-HT group) were individually measured for their pepsin and amylase enzyme activities. DMSO + marine saline group was established as the vehicle control group accordingly. Untreated sea urchins (without any chemical administration) were used as control group. There were no significant difference of pepsin and amylase activities among the control, DMSO + marine saline, and HC-030031 + 5-HT groups (pepsin: df1 = 2, df2 = 12, F = 0.118, both p > 0.05; amylase: df1 = 2, df2 = 12, F = 0.364, both p > 0.05, Figure 5C).
These results show that digestive enzyme activities are negatively correlated with TRPA1 and concentration of 5-HT in the gut of S. intermedius.
3.4. Experiment 4: Gut Emptying Is Positively Correlated with TRPA1 and Concentration of 5-HT in the Gut of S. intermedius
Considering that gut emptying is another important indicator for food digestion and absorption of sea urchins, we further investigated whether a correlation exists between gut emptying and the reciprocal regulation of TRPA1 and 5-HT in the gut of S. intermedius. At the beginning of the experiment, 1.5 g of U. pinnatifida was fed to each sea urchin among different treatment groups [38] to ensure that they ate all the provided U. pinnatifida within 7 h and that they did not produce feces. The replicate set-up schemes of sea urchins and subsequent injection protocols were same as in the experiment 3. Finally, we removed the net bags containing the feces at the end of the 48-h experiment and put them in the oven at 110 °C (5 h) for drying to constant weight. The dry weight of the feces was measured by an analytical balance (AL204, Mettler-Toledo Instruments Co., Shanghai, China).
The HC-030031 group exhibited significantly lower dry feces weight than other groups (df1 = 2, df2 = 12, F = 9.571, both p < 0.01, Figure 6A). No significant difference was found between the control and DMSO groups (p > 0.05).
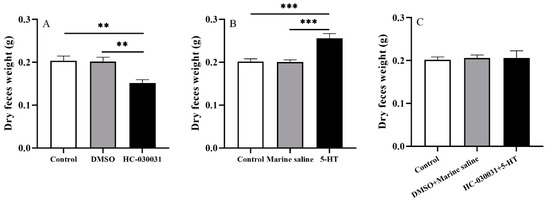
Figure 6.
Gut emptying is positively correlated with the reciprocal regulation of TRPA1 and 5-HT in the gut of Strongylocentrotus intermedius. (A) Dry weight of the 48-h accumulated feces of the control, DMSO, and HC-030031 groups (n = 5). (B) Dry weight of the 48-h accumulated feces of the control, marine saline, and 5-HT groups (n = 5). (C) The dry weight of the 48-h accumulated feces of the control, DMSO + marine saline, and HC-030031 + 5-HT groups (n = 5). The symbols ** and *** mean p < 0.01 and p < 0.001, respectively (mean ± s.e.m.).
The dry weight of the 48-h accumulated feces was significantly higher in the 5-HT group than those in the control and marine saline groups (df1 = 2, df2 = 12, F = 14.773, both p < 0.001, Figure 6B). The dry feces weight showed no significance between the control and marine saline groups (p > 0.05).
There was no significant difference of the 48-h accumulated dry feces weight among control, DMSO + marine saline, and HC-030031 + 5-HT groups (df1 = 2, df2 = 12, F = 0.044, p > 0.05, Figure 6C).
These results show that gut emptying is positively correlated with TRPA1 and concentration of 5-HT in the gut of S. intermedius.
4. Discussion
Sea urchins are considered as “marine shredders” [2]. Their poor digestion and absorption of kelps make this energy source more available to a suite of other benthic detritivores, thus playing a positive role in the marine food webs. To understand the underlying molecular regulatory relationships behind this physiological characteristic, we investigated whether digestive enzyme activities and gut emptying are correlated with the reciprocal regulation of TRPA1 ion channels and 5-HT in the gut of S. intermedius.
4.1. TRPA1 Is Involved in the Digestion of S. intermedius
The food ingested by sea urchins is enclosed in a mucous membrane in the pharynx to form small pellets, which move through the pharynx and oesophagus to the intestinal tract where digestion and nutrient uptake occur [39]. Interestingly, the digestive enzyme activities (both pepsin and amylase) of S. intermedius with the highest expression of TRPA1 was significantly lower than that of S. intermedius with the lowest TRPA1 expression in the present study. This indicates that TRPA1 is involved in the food digestion and absorption of S. intermedius. This conclusion is not consistent with the finding of Fothergill et al. [40] that the TRPA1 channel in the digestive tract is activated by several compounds of mammal feed, thus improving their nutritional digestion efficiency. This disagreement suggests that the role of TRPA1 in digestive physiology is not conserved in the animal kingdom. The present finding suggests that TRPA1 expression varies among individuals under the same natural conditions. Considering that TRPA1 expression in the tube feet of S. intermedius shows a significant response to long-term low water temperature [27], we speculate that the expression pattern of TRPA1 in the gut tissues of S. intermedius is similar to that in tube feet. Individuals with higher TRPA1 expression are more sensitive to cold water temperature, which results in lower digestive enzyme activities and consequently play a negative role in the digestive function of sea urchins. It suggests that sea urchins probably have relatively poor absorption efficiency under the condition of cold water, producing more nutritious feces. This process provides more nutrients to a suite of benthic detritivores [13,41], playing a positive role in the marine food webs.
4.2. An Inter-Regulatory Relationship Exists between TRPA1 and 5-HT in the Gut of S. intermedius
The 5-HT level of S. intermedius with the highest expression of TRPA1 was significantly higher than those with the lowest TRPA1 expression. This indicates a link between TRPA1 and 5-HT in the gut of S. intermedius. To further investigate whether an inter-regulatory mechanism exists in the gut of S. intermedius, we measured the TRPA1 expression and 5-HT level in the gut of S. intermedius after the 5-HT and HC-030031 injection, respectively. As expected, the 5-HT level of sea urchins with TRPA1 inhibition was significantly lower than the control level, and the sea urchins with the 5-HT injection showed significantly higher TRPA1 expression than the control level. This is consistent with the finding in rats (Rattus norvegicus) and mice (Mus musculus) that stimulation of TRPA1 evokes the release of 5-HT from cells and that TRPA1 in the gut was largely inhibited by the 5-HT receptor antagonists [25,31]. All these findings support the idea that an inter-regulatory mechanism exists between TRPA1 and 5-HT in the gut of S. intermedius, which offers an entry point to further investigate the molecular details of digestive physiology of sea urchins. This is important for food web stability in marine ecosystems.
4.3. Digestive Enzyme Activities Are Negatively Correlated with the TRPA1 and Concentration of 5-HT in the Gut of S. intermedius
The absorption and utilization of nutrients by S. intermedius are directly affected by digestive enzymes activities [15,16]. In this experiment, we used pepsin and amylase enzymes activities as two representative indicators to evaluate digestive and absorptive capacities of sea urchins. The inhibition of TRPA1 significantly contributes to higher digestive enzymes activities (pepsin and amylase) in the gut of S. intermedius. The phenomenon implies that digestive enzyme activities are negatively correlated with the TRPA1 expression in the gut of S. intermedius. However, the high expression of TRPA1 in cells of mammals (human, rat and mouse) increases gastric vagal nerve activity (GVNA), and promotes nutrient digestion and absorption [42,43]. This difference indicates that TRPA1 is probably not conservative in regulating digestion in various animals.
We subsequently investigated the relationship between 5-HT and digestive enzymes in the gut of S. intermedius. Sea urchins injected with 5-HT showed significantly lower activities of digestive enzymes. This suggests that 5-HT is involved in the digestive and absorptive capacities of S. intermedius. Similarly, 5-HT also play an important role in the food digestion and absorption of other animals. For example, dyspepsia was associated with the increasing of 5-HT in humans [44]. These findings suggest that the food digestion and absorption of S. intermedius was negatively correlated with 5-HT concentration in the gut.
To investigate whether a correlation exists between digestive enzymes activities and the reciprocal regulation of TRPA1 and 5-HT in the gut of S. intermedius, the HC-030031 + 5-HT experiment was carried out. We found that the positive effects of TRPA1 antagonist on the digestive enzyme activities were abolished after subsequent 5-HT injection. Taken together, digestive enzyme activities are negatively correlated with the TRPA1 and concentration of 5-HT in the gut of S. intermedius, thus negatively affecting their digestion and absorption.
4.4. Gut Emptying Is Positively Correlated with the TRPA1 and Concentration of 5-HT in the Gut of S. intermedius
In addition to digestive enzyme activities, gut emptying is another important indicator for food digestion and absorption of S. intermedius. This is supported by the finding that Antarctic sea urchins have a low-energy diet and require long periods of digestion [7]. This suggests that decreased gastric emptying is beneficial because it improves the absorption and utilization in sea urchins by increasing the time that food stays in the digestive tract [7]. In the present study, TRPA1 inhibition of S. intermedius produced significantly less feces (dry weight) within 48 h, indicating that a positive link exists between gut emptying and TRPA1 in the gut of the S. intermedius. Combined with the findings of this study regarding the role of TRPA1 in digestive enzyme activities, we conclude that the food digestion and absorption of S. intermedius are negatively correlated with TRPA1 expression in the gut. However, high expression of TRPA1 in digestive tract delayed gastric emptying in mice [45], which is contrary to our conclusion. The disagreement indicates that the role of TRPA1 in digestion and absorption is not conservative among animals, which supports the hypothesis that the role of TRPA1 in gastrointestinal motility depends on species [46]. Strongylocentrotus intermedius with 5-HT injection produced significantly more feces (dry weight) within 48 h, which is consistent with the result of 5-HT increased gastric emptying in rats [47]. This phenomenon is consistent with the finding that the 5-HT injection reduced the retention time of food in the gut of the Chinese mitten crab Eriocheir sinensis [29] and humans [48,49].
Further, we investigated whether a correlation exists between gut emptying and the reciprocal regulation of TRPA1 and 5-HT. As expected, the effect of TRPA1 antagonist on the gut emptying was abolished in S. intermedius treated with 5-HT. This result clearly indicates that gut emptying is positively correlated with TRPA1 and concentration of 5-HT in the gut of S. intermedius. It is also consistent with the finding that digestive enzyme activities are negatively correlated with the TRPA1 and concentration of 5-HT in the gut of S. intermedius, thus negatively impacting their digestion and absorption. On the contrary, TRPA1 delays gastric emptying in rats through 5-HT pathways [31]. This indicates that the relationship between gastrointestinal tract emptying and TRPA1 and 5-HT is species dependent.
Based on the results above, we conclude that the digestive enzyme activities and gut emptying are correlated with the reciprocal regulation of TRPA1 and 5-HT in the gut of S. intermedius. We speculate that if the molecules (TRPA1 and 5-HT) related to digestive physiology of sea urchins are affected, the digestive and absorptive functions of sea urchins are consequently altered. For example, TRPA1 is highly expressed in the gut of sea urchins under cold water conditions, resulting in low digestive enzyme activities and fast gut emptying rates. This situation is unhelpful to the digestion and absorption of food nutrients by sea urchins, which reflects poor digestion and absorption capacities of sea urchins. This explains the observation that the food digestion and absorption of sea urchins are worse at low water temperatures than those at high water temperatures [50,51,52]. Ingestion rates and absorption efficiencies were lower in L. variegatus kept at the lower temperatures than those in L. variegatus kept at the higher temperatures [50]. Consistently, when S. franciscanus was fed with either a prepared diet or kelp (N. luetkeana), the feeding rates increased with increased water temperature [51]. Furthermore, there was a significant and linear increase in feed intake of S. droebachiensis with increased temperature, both in summer and winter [52]. Collectively, it suggests that the gut of sea urchins with high TRPA1 expression probably have poorer digestion and absorption capacities under the low water temperature, producing feces with more energy and nutrients, thus providing relatively high-quality food for detritivores [14]. This bonus is critical to the benthic community, contributing to maintaining the stability of food webs in marine ecosystem.
5. Conclusions
The present study revealed three findings as follows: an inter-regulatory relationship exists between TRPA1 and 5-HT in the gut of S. intermedius, digestive enzyme activities are negatively correlated with the TRPA1 and concentration of 5-HT in the gut of S. intermedius, and gut emptying rate is positively correlated with TRPA1 and concentration of 5-HT in the gut of S. intermedius. Digestion and absorption of food are correlated with the reciprocal regulation of TRPA1 and 5-HT in the gut of S. intermedius and provide a baseline to further investigate the molecular details of digestive physiology of sea urchins. Furthermore, this novel finding explains the low food digestibility by sea urchins and provides valuable information to the digestive physiology of sea urchins. These results are key to understand the stability of food webs in the marine ecosystem.
Author Contributions
Conceptualization, J.D., C.Z. and Y.C.; data curation, J.D., Z.L. and H.W.; funding acquisition, Y.C. and C.Z.; investigation, J.D., H.W., Z.L., J.S., X.C., P.D. and M.Y.; methodology, J.D. and C.Z.; project administration, Y.C. and C.Z.; supervision, C.Z.; visualization, J.D., H.W. and J.S.; writing—original draft, J.D. and C.Z.; writing—review and editing, J.D. and C.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (41506177), Key Special Project for Introduced Talents Team of Southern Marine Science and Engineering Guangdong Laboratory (GML2019ZD0402), Liaoning Province “Xingliao Talents Plan” project (XLYC2002107) and Fund of Key Laboratory of South China Sea Fishery Resources Exploitation and Utilization, Ministry of Agriculture and Rural Affairs, China (FREU2020-02).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets of the current study are available from the corresponding author on reasonable request.
Acknowledgments
We thank John Lawrence, Michael Russell and Wei Tang for editorial suggestions.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- Pearse, J. Ecological role of purple sea urchins. Science 2006, 314, 940–941. [Google Scholar] [CrossRef] [PubMed]
- Yorke, C.E.; Page, H.M.; Miller, R.J. Sea urchins mediate the availability of kelp detritus to benthic consumers. Proc. R. Soc. B 2019, 286, 20190846. [Google Scholar] [CrossRef] [PubMed]
- Agatsuma, Y.; Takagi, S.; Inomata, E.; Aoki, M.N. Process of deterioration of a kelp (Ecklonia bicyclis Kjellman) bed as a result of grazing by the sea urchin Mesocentrotus nudus (Agassiz) in shizugawa bay in northeastern Honshu, Japan. J. Appl. Phycol. 2018, 31, 599–605. [Google Scholar] [CrossRef]
- Lawrence, J.M. On the relationships between marine plants and sea urchins. Oceanogr. Mar. Biol. 1975, 13, 213–286. [Google Scholar]
- Lawrence, J.M.; Lawrence, A.L.; Watts, S.A. Feeding, digestion and digestibility in sea urchins. In Sea Urchins: Biology and Ecology, 3rd ed.; Lawrence, J.M., Ed.; Academic Press: San Diego, CA, USA, 2013; pp. 135–154. [Google Scholar] [CrossRef]
- Lawrence, J.M.; Lawrence, A.L.; Watts, S.A. Ingestion, digestion, and digestibility of regular sea urchins. In Sea Urchins: Biology and Ecology, 4th ed.; Lawrence, J.M., Ed.; Academic Press: San Diego, CA, USA, 2020; pp. 165–190. [Google Scholar] [CrossRef]
- Klinger, T.S.; Mcclintock, J.B.; Watts, S.A. Activities of digestive enzymes of polar and subtropical echinoderms. Polar. Biol. 1997, 18, 154–157. [Google Scholar] [CrossRef]
- Larson, B.R.; Vadas, R.L.; Keser, M. Feeding and nutritional ecology of the sea urchin Strongylocentrotus drobachiensis in Maine, USA. Mar. Biol. 1980, 59, 49–62. [Google Scholar] [CrossRef]
- Mamelona, J.; Pelletier, E. Green urchin as a significant source of fecal particulate organic matter within nearshore benthic ecosystems. J. Exp. Mar. Biol. Ecol. 2005, 314, 163–174. [Google Scholar] [CrossRef]
- Peduzzi, P.; Herndl, G. Role of bacteria in decomposition of fecal pellets egested by the epiphyte-grazing gastropod Gibbula umbilicaris. Mar. Biol. 1986, 92, 417–424. [Google Scholar] [CrossRef]
- Yoon, W.D.; Marty, J.C.; Sylvain, D.; Nival, P. Degradation of faecal pellets in Pegea confoederata (Salpidae, Thaliacea) and its implication in the vertical flux of organic matter. J. Exp. Mar. Biol. Ecol. 1996, 203, 147–177. [Google Scholar] [CrossRef]
- Vadas, R. Preferential feeding: An optimization strategy in sea urchins. Ecol. Monogr. 1977, 47, 337–371. [Google Scholar] [CrossRef]
- Sauchyn, L.; Lauzon-Guay, J.; Scheibling, R. Sea urchin fecal production and accumulation in a rocky subtidal ecosystem. Aquat. Biol. 2011, 13, 215–223. [Google Scholar] [CrossRef]
- Dethier, M.N.; Hoins, G.; Kobelt, J.; Lowe, A.T.; Galloway, A.W.E.; Schram, J.B.; Raymore, M.; Duggins, D.O. Feces as food: The nutritional value of urchin feces and implications for benthic food webs. J. Exp. Mar. Biol. Ecol. 2019, 514, 95–102. [Google Scholar] [CrossRef]
- Hou, S.Q.; Zuo, R.T.; Chang, Y.Q.; Li, G.; Song, J.; Zhao, C. Effects of dietary lipid level on growth performance, digestive enzyme activity and anti-oxidative capacity after heat stress in juvenile sea urchin Strongylocentrotus intermedius. J. Dalian Fish. Univ. 2016, 31, 538–543, (In Chinese with an English abstract). [Google Scholar] [CrossRef]
- Onitsuka, T.; Niwa, K.; Unuma, T.; Umezu, Y. Dietary shifts in the juvenile sea urchin Strongylocentrotus intermedius associated with the development of digestive enzymes. Mar. Biol. 2015, 162, 869–880. [Google Scholar] [CrossRef]
- Horowitz, M.; O’Donovan, D.; Jones, K.L.; Feinle, C.; Rayner, C.K.; Samsom, M. Gastric emptying in diabetes: Clinical significance and treatment. Diabet. Med. 2002, 19, 177–194. [Google Scholar] [CrossRef]
- Mahé, S.; Roos, N.; Benamouzig, R.; Davin, L.; Luengo, C.; Gagnon, L.; Gaussergès, N.; Rautureau, J.; Tomé, D. Gastrojejunal kinetics and the digestion of [15N] ß-lactoglobulin and casein in humans: The influence of the nature and quantity of the protein. Am. J. Clin. Nutr. 1996, 63, 546–552. [Google Scholar] [CrossRef]
- Rehner, G.; Heil, M.; Auge, M.; Harzer, G.; Daniel, H. Effect of proteins on availability of zinc. I. Gastrointestinal transit time of casein and whey protein and zinc absorption in weaned rats. Z. Ernährungswiss 1985, 24, 245–255. [Google Scholar] [CrossRef]
- Lawrence, J.M.; McClintock, J. Energy acquisition and allocation by Echinoderms (Echinodermata) in polar seas: Adaptations for success? In Echinoderms through Time; David, B., Guille, A., Feral, J.P., Roux, M., Eds.; Balkema: Rotterdam, The Netherlands, 1994; pp. 39–52. [Google Scholar]
- McClintock, J. Trophic biology of antarctic shallow-water echinoderms. Mar. Ecol. Prog. Ser. 1994, 111, 119–202. [Google Scholar] [CrossRef]
- Damann, N.; Voets, T.; Nilius, B. TRPs in our senses. Curr. Biol. 2008, 18, 880–889. [Google Scholar] [CrossRef]
- Macpherson, L.J.; Dubin, A.E.; Evans, M.J.; Marr, F.; Schultz, P.G.; Cravatt, B.F.; Patapoutian, A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 2007, 445, 541–545. [Google Scholar] [CrossRef]
- Pedersen, S.F.; Owsianik, G.; Nilius, B. TRP channels: An overview. Cell Calcium 2005, 38, 233–252. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, K.; Kawabata-Shoda, E.; Doihara, H.; Kojima, R.; Okada, H.; Mochizuki, S.; Sano, Y.; Inamura, K.; Matsushime, H.; Koizumi, T.; et al. Trpa1 regulates gastrointestinal motility through serotonin release from enterochromaffin cells. Proc. Natl. Acad. Sci. USA 2009, 106, 3408–3413. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Y.; Yu, M.G.; Yu, S.Y. Trp channel functions in the gastrointestinal tract. Semin. Immunopathol. 2015, 38, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.Y.; Yu, Y.S.; Yang, M.F.; Shi, D.T.; Li, Z.Q.; Chi, X.M.; Chang, Y.Q.; Wang, Q.Z.; Zhao, C. TRPA1 expression provides new insights into thermal perception by the sea urchin Strongylocentrotus intermedius. J. Mar. Biol. Assoc. UK 2019, 99, 1825–1829. [Google Scholar] [CrossRef]
- Grundy, D. 5-HT system in the gut: Roles in the regulation of visceral sensitivity and motor functions. Eur. Rev. Med. Pharmacol. 2008, 12, 63–67. [Google Scholar] [CrossRef]
- Li, T. Effect of 5-HT on the Intestinal Motility of Eriocheir sinensis. Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2016. CNKI:CDMD:2.1016.912281. [Google Scholar]
- Harris, M.N.; Shafinaz, M.N.M.; Yaakop, S.; Wahida, O.N. Distribution of serotonin (5-HT) and dopamine (DA) on digestive tract of red palm weevil larva, Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae). Serangga 2016, 21, 39–50. [Google Scholar]
- Doihara, H.; Nozawa, K.; Kawabata-Shoda, E.; Kojima, R.; Yokoyama, T.; Ito, H. Trpa1 agonists delay gastric emptying in rats through serotonergic pathways. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2009, 380, 353–357. [Google Scholar] [CrossRef]
- Carroll, N.K.; Scully, T.K.; Brown, K.M. Serotonin signaling initiates gastrulation in the sea urchin. Dev. Biol. 2010, 344, 451. [Google Scholar] [CrossRef][Green Version]
- Eid, S.R.; Crown, E.D.; Moore, E.L.; Liang, H.A.; Urban, M.O. Hc-030031, a trpa1 selective antagonist, attenuates inflammatory- and neuropathy-induced mechanical hypersensitivity. Mol. Pain 2008, 4, 48. [Google Scholar] [CrossRef]
- Han, L.S.; Ding, J.; Wang, H.; Zuo, R.T.; Quan, Z.J.; Fan, Z.H.; Liu, Q.D.; Chang, Y.Q. Molecular characterization and expression of SiFad1 in sea urchin (Strongylocentrotus intermedius). Gene 2019, 705, 133–141. [Google Scholar] [CrossRef]
- Zhou, Z.C.; Bao, Z.M.; Dong, Y.; Wang, L.M.; He, C.B.; Liu, W.D. MYP gene expressions at transcription level in different stages of gonads of sea urchin Strongylocentrotus intermedius and hybrids. Hereditas 2008, 30, 1453–1458. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Han, Y.; Ren, T.; Jiang, Z.; Wang, F.; Liao, M.; Wang, J. Effects of dietary lysine levels on growth, intestinal digestive enzymes, and coelomic fluid nonspecific immune enzymes of sea cucumber, Apostichopus japonicus, juveniles. J. World Aquac. Soc. 2016, 48, 290–302. [Google Scholar] [CrossRef]
- Chang, Y.Q.; Ding, J.; Song, J.; Yang, W. Biology and Aquaculture of Sea Cucumbers and Sea Urchins; China Ocean Press: Beijing, China, 2004. (In Chinese) [Google Scholar]
- Brothers, C.J.; Pol, W.J.V.D.; Morrow, C.D.; Hakim, J.A.; Koo, H.; McClintock, J.B. Ocean warming alters predicted microbiome functionality in a common sea urchin. Proc. R. Soc. B 2018, 285, 20180340. [Google Scholar] [CrossRef]
- Fothergill, L.J.; Callaghan, B.; Rivera, L.R.; Lieu, T.M.; Poole, D.P.; Cho, H.J.; Bravo, D.M.; Furness, J.B. Effects of food components that activate trpa1 receptors on mucosal ion transport in the mouse intestine. Nutrients 2016, 8, 623. [Google Scholar] [CrossRef]
- Sauchyn, L.; Scheibling, R. Degradation of sea urchin feces in a rocky subtidal ecosystem: Implications for nutrient cycling and energy flow. Aquat. Biol. 2009, 6, 99–108. [Google Scholar] [CrossRef]
- Ohara, K.; Fukuda, T.; Ishida, Y.; Takahashi, C.; Ohya, R.; Katayama, M.; Uchida, K.; Tominaga, M.; Nagai, K. β-Eudesmol, an oxygenized sesquiterpene, stimulates appetite via TRPA1 and the autonomic nervous system. Sci. Rep. 2017, 7, 15785. [Google Scholar] [CrossRef]
- Shen, J.; Niijima, A.; Tanida, M.; Horii, Y.; Maeda, K.; Nagai, K. Olfactory stimulation with scent of lavender oil affects autonomic nerves, lipolysis and appetite in rats. Neurosci. Lett. 2005, 383, 188–193. [Google Scholar] [CrossRef]
- Coleman, N.S.; Foley, S.; Dunlop, S.P.; Wheatcroft, J.; Blackshaw, E.; Perkins, A.C.; Singh, G.; Marsden, C.A.; Holmes, G.K.; Spiller, R.C. Abnormalities of serotonin metabolism and their relation to symptoms in untreated celiac disease. Clin. Gastroenterol. Hepatol. 2006, 4, 874–881. [Google Scholar] [CrossRef]
- Kim, M.J.; Son, H.J.; Song, S.H.; Jung, M.; Kim, Y.; Rhyu, M.R. The TRPA1 agonist, methyl syringate suppresses food intake and gastric emptying. PLoS ONE 2013, 8, e71603. [Google Scholar] [CrossRef]
- Holzer, P. Transient receptor potential (TRP) channels as drug targets for diseases of the digestive system. Pharmacol. Ther. 2011, 131, 142–170. [Google Scholar] [CrossRef] [PubMed]
- Francis, J.; Critchley, D.; Dourish, C.T.; Cooper, S.J. Comparisons between the effects of 5-HT and dl-fenfluramine on food intake and gastric emptying in the rat. Pharmacol. Biochem. Behav. 1995, 50, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Mckinzie, S.; Fox, J.; Foxxorenstein, A.; Burton, D.; Thomforde, G.; Baxter, K.; Zinsmeister, A. Effect of renzapride on transit in constipation-predominant irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 2004, 2, 895–904. [Google Scholar] [CrossRef]
- Degen, L.; Petrig, C.; Studer, D.; Schroller, S.; Beglinger, C. Effect of tegaserod on gut transit in male and female subjects. Neurogastroent. Motil. 2006, 17, 821–826. [Google Scholar] [CrossRef]
- Klinger, T.S.; Hsieh, H.L.; Pangallo, R.A.; Chen, C.P.; Lawrence, J.M. The Effect of Temperature on Feeding, Digestion, and Absorption of Lytechinus Variegatus (Lamarck) (Echinodermata, Echinoidea). Physiol. Zool. 1986, 59, 332–336. [Google Scholar] [CrossRef]
- McBride, S.C.; Pinnix, W.D.; Lawrence, J.M.; Lawrence, A.L.; Mulligan, T.J. The effects of temperature on production of gonads by the sea urchin Strongylocentrotus franciscanus fed natural and prepared diets. J. World Aquacult. Soc. 1997, 28, 357–365. [Google Scholar] [CrossRef]
- Siikavuopio, S.I.; Christainsen, J.S.; Dale, T. Effects of temperature and season on gonad growth and feed intake in the green sea urchin (Strongylocentrotus droebachiensis). Aquaculture 2006, 255, 389–394. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).