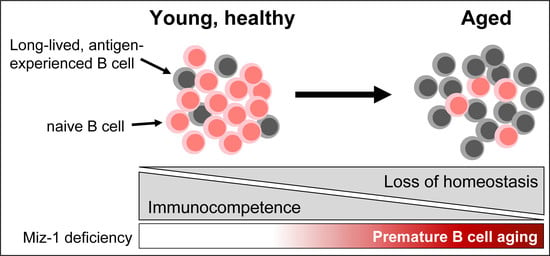
Myc-Interacting Zinc Finger Protein 1 (Miz-1) Is Essential to Maintain Homeostasis and Immunocompetence of the B Cell Lineage
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Flow Cytometry and Antibodies
2.3. B Cell Isolation
2.4. RNA Isolation and Quantitative Real-Time PCR
2.5. Isolation of Intestinal Antibodies
2.6. Enzyme-Linked Immunosorbent Assay
2.7. Statistical Analysis
3. Results
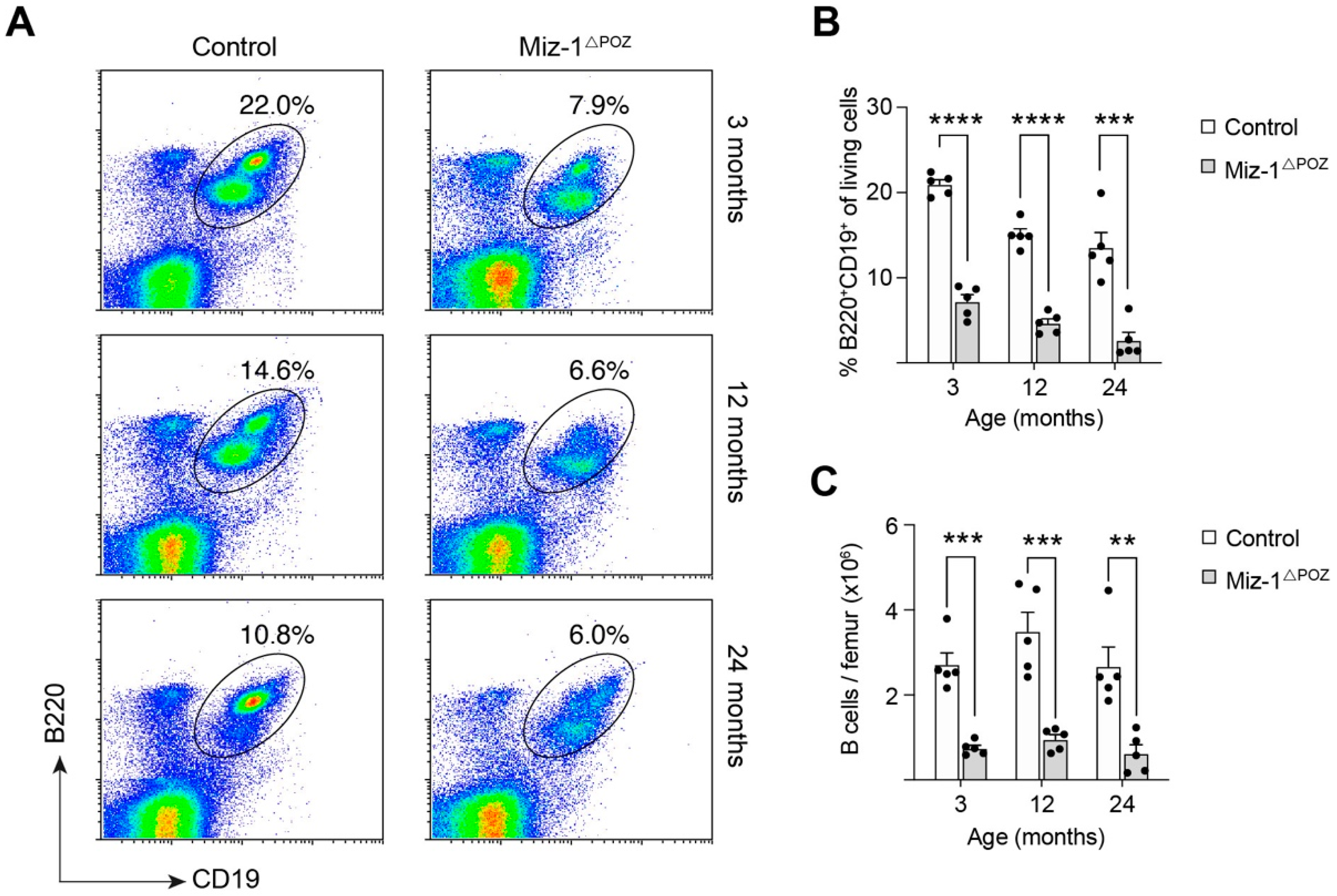
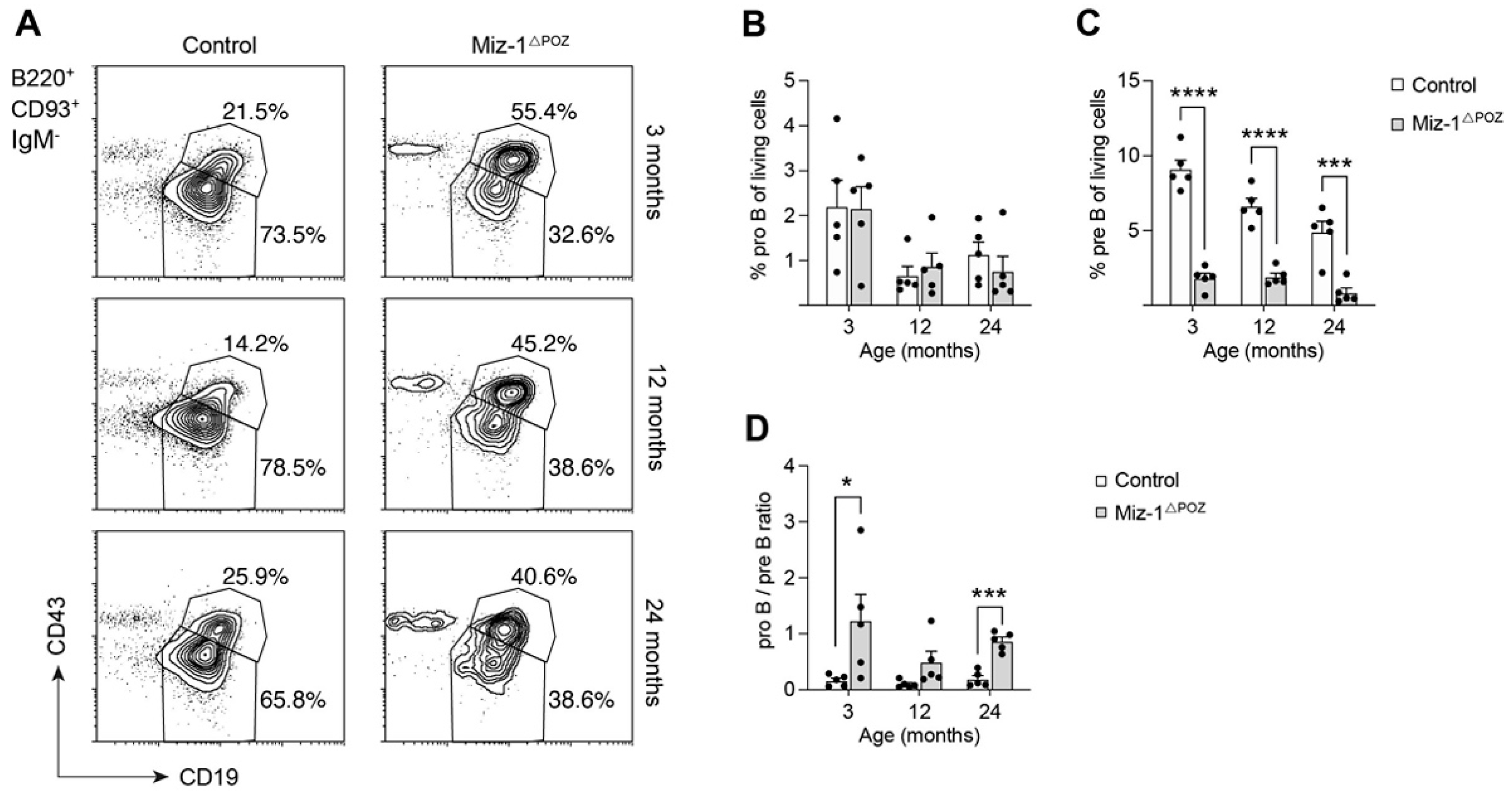
3.1. Deletion of Miz-1 Reduces the B Cell Differentiation Potential in the Bone Marrow
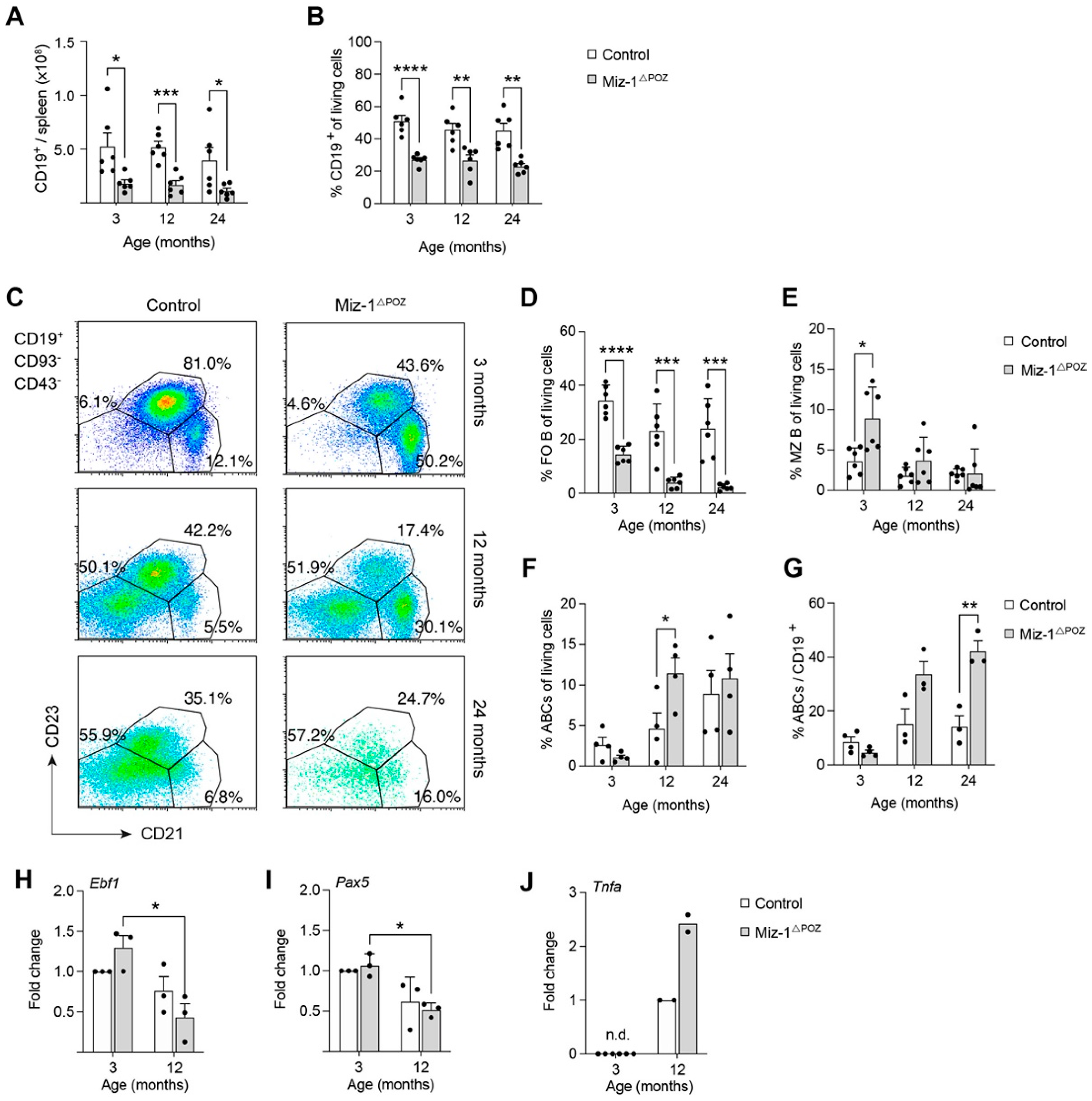
3.2. Miz-1 Is Critical for B Cell Homeostasis and Immunocompetence in the Periphery
3.3. Miz-1 Deficiency Favors Accumulation of Long-Lived B Cell Subsets after Antigen Challenge
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gems, D.; Partridge, L. Genetics of Longevity in Model Organisms: Debates and Paradigm Shifts. Annu. Rev. Physiol. 2013, 75, 621–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijg, J.; Campisi, J. Puzzles, promises and a cure for ageing. Nature 2008, 454, 1065–1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfego, D.; Rodeck, U.; Kriete, A. Global mapping of transcription factor motifs in human aging. PLoS ONE 2018, 13, e0190457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boraschi, D.; del Giudice, G.; Dutel, C.; Ivanoff, B.; Rappuoli, R.; Grubeck-Loebenstein, B. Ageing and immunity: Addressing immune senescence to ensure healthy ageing. Vaccine 2010, 28, 3627–3631. [Google Scholar] [CrossRef]
- Boraschi, D.; Aguado, M.T.; Dutel, C.; Goronzy, J.; Louis, J.; Grubeck-Loebenstein, B.; Rappuoli, R.; Del Giudice, G. The Gracefully Aging Immune System. Sci. Transl. Med. 2013, 5, 185ps8. [Google Scholar] [CrossRef] [Green Version]
- McElhaney, E.J.; Effros, R.B. Immunosenescence: What does it mean to health outcomes in older adults? Curr. Opin. Immunol. 2009, 21, 418–424. [Google Scholar] [CrossRef] [Green Version]
- Goronzy, J.J.; Weyand, C.M. Understanding immunosenescence to improve responses to vaccines. Nat. Immunol. 2013, 14, 428–436. [Google Scholar] [CrossRef] [Green Version]
- Goronzy, J.J.; Weyand, C.M. Immune aging and autoimmunity. Cell. Mol. Life Sci. 2012, 69, 1615–1623. [Google Scholar] [CrossRef] [Green Version]
- Frasca, D.; Blomberg, B.B. Effects of aging on B cell function. Curr. Opin. Immunol. 2009, 21, 425–430. [Google Scholar] [CrossRef] [Green Version]
- Riley, R.L.; Khomtchouk, K.; Blomberg, B.B. Inflammatory immune cells may impair the preBCR checkpoint, reduce new B cell production, and alter the antibody repertoire in old age. Exp. Gerontol. 2018, 105, 87–93. [Google Scholar] [CrossRef]
- Johnson, S.A.; Rozzo, S.J.; Cambier, J. Aging-Dependent Exclusion of Antigen-Inexperienced Cells from the Peripheral B Cell Repertoire. J. Immunol. 2002, 168, 5014–5023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratliff, M.; Alter, S.; Frasca, D.; Blomberg, B.B.; Riley, R.L. In senescence, age-associated B cells secrete TNFα and inhibit survival of B-cell precursors*. Aging Cell 2013, 12, 303–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, K.L.; Wu, Y.-C.; Barnett, Y.; Duggan, O.; Vaughan, R.; Kondeatis, E.; Nilsson, B.-O.; Wikby, A.; Kipling, D.; Dunn-Walters, D.K. B-cell diversity decreases in old age and is correlated with poor health status. Aging Cell 2009, 8, 18–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, C.; Kelsoe, G. Ig VH hypermutation is absent in the germinal centers of aged mice. J. Immunol. 1995, 155, 3377–3384. [Google Scholar]
- Yang, X.; Stedra, J.; Cerny, J. Relative contribution of T and B cells to hypermutation and selection of the antibody repertoire in germinal centers of aged mice. J. Exp. Med. 1996, 183, 959–970. [Google Scholar] [CrossRef] [Green Version]
- Zidi, B.; Vincent-Fabert, C.; Pouyet, L.; Seillier, M.; Vandevelde, A.; N’Guessan, P.; Poplineau, M.; Guittard, G.; Mancini, S.J.C.; Duprez, E.; et al. TP53INP1 deficiency maintains murine B lymphopoiesis in aged bone marrow through redox-controlled IL-7R/STAT5 signaling. Proc. Natl. Acad. Sci. USA 2019, 116, 211–216. [Google Scholar] [CrossRef] [Green Version]
- Stephan, R.P.; Lill-Elghanian, A.D.; Witte, P.L. Development of B cells in aged mice: Decline in the ability of pro-B cells to respond to IL-7 but not to other growth factors. J. Immunol. 1997, 158, 1598–1609. [Google Scholar]
- Miller, J.P.; Allman, D. The Decline in B Lymphopoiesis in Aged Mice Reflects Loss of Very Early B-Lineage Precursors. J. Immunol. 2003, 171, 2326–2330. [Google Scholar] [CrossRef]
- Maryanovich, M.; Zahalka, A.H.; Pierce, H.; Pinho, S.; Nakahara, F.; Asada, N.; Wei, Q.; Wang, X.; Ciero, P.; Xu, J.; et al. Adrenergic nerve degeneration in bone marrow drives aging of the hematopoietic stem cell niche. Nat. Med. 2018, 24, 782–791. [Google Scholar] [CrossRef]
- Stephan, R.P.; Reilly, C.R.; Witte, P.L. Impaired ability of bone marrow stromal cells to support B-lymphopoiesis with age. Blood 1998, 91, 75–88. [Google Scholar] [CrossRef] [Green Version]
- Gonda, H.; Sugai, M.; Nambu, Y.; Katakai, T.; Agata, Y.; Mori, K.J.; Yokota, Y.; Shimizu, A. The Balance Between Pax5 and Id2 Activities Is the Key to AID Gene Expression. J. Exp. Med. 2003, 198, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.; Rother, M.B.; Brusletto, B.S.; Olstad, O.K.; Aass, H.C.D.; Van Zelm, M.C.; Kierulf, P.; Gautvik, K.M. Increased ID2 Levels in Adult Precursor B Cells as Compared with Children Is Associated with Impaired Ig Locus Contraction and Decreased Bone Marrow Output. J. Immunol. 2013, 191, 1210–1219. [Google Scholar] [CrossRef] [Green Version]
- Martin, V.; Wu, Y.-C.B.; Kipling, D.; Dunnwalters, D.K. Ageing of the B-cell repertoire. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landin, A.M.; Frasca, D.; Harrison, P.; Scallan, M.; Riley, R.L.; Blomberg, B.B. E47 retroviral rescue of intrinsic B-cell defects in senescent mice. Aging Cell 2011, 10, 327–337. [Google Scholar] [CrossRef]
- Frasca, D.; Landin, A.M.; Alvarez, J.P.; Blackshear, P.; Riley, R.L.; Blomberg, B.B. Tristetraprolin, a Negative Regulator of mRNA Stability, Is Increased in Old B Cells and Is Involved in the Degradation of E47 mRNA. J. Immunol. 2007, 179, 918–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frasca, D.; Romero, M.; Landin, A.M.; Diaz, A.; Riley, R.L.; Blomberg, B.B. Protein phosphatase 2A (PP2A) is increased in old murine B cells and mediates p38 MAPK/tristetraprolin dephosphorylation and E47 mRNA instability. Mech. Ageing Dev. 2010, 131, 306–314. [Google Scholar] [CrossRef] [Green Version]
- Frasca, D.; Van der Put, E.; Landin, A.M.; Gong, D.; Riley, R.L.; Blomberg, B.B. RNA Stability of the E2A-Encoded Transcription Factor E47 Is Lower in Splenic Activated B Cells from Aged Mice. J. Immunol. 2005, 175, 6633–6644. [Google Scholar] [CrossRef] [Green Version]
- Frasca, D.; van der Put, E.; Riley, R.L.; Blomberg, B.B. Reduced Ig Class Switch in Aged Mice Correlates with Decreased E47 and Activation-Induced Cytidine Deaminase. J. Immunol. 2004, 172, 2155–2162. [Google Scholar] [CrossRef] [Green Version]
- Rubtsov, A.V.; Rubtsova, K.; Fischer, A.; Meehan, R.T.; Gillis, J.Z.; Kappler, J.W.; Marrack, P. Toll-like receptor 7 (TLR7)–driven accumulation of a novel CD11c+ B-cell population is important for the development of autoimmunity. Blood 2011, 118, 1305–1315. [Google Scholar] [CrossRef] [Green Version]
- Manni, M.; Gupta, S.; Ricker, E.; Chinenov, Y.; Park, S.H.; Shi, M.; Pannellini, T.; Jessberger, R.; Ivashkiv, L.B.; Pernis, A.B. Regulation of age-associated B cells by IRF5 in systemic autoimmunity. Nat. Immunol. 2018, 19, 407–419. [Google Scholar] [CrossRef]
- Frasca, D.; Diaz, A.; Romero, M.; Landin, A.M.; Blomberg, B.B. High TNF-α levels in resting B cells negatively correlate with their response. Exp. Gerontol. 2014, 54, 116–122. [Google Scholar] [CrossRef] [Green Version]
- Frasca, D.; Romero, M.; Diaz, A.; Alter-Wolf, S.; Ratliff, M.; Landin, A.M.; Riley, R.L.; Blomberg, B.B. A Molecular Mechanism for TNF-alpha-mediated Downregulation of B Cell Responses. J. Immunol. 2011, 188, 279–286. [Google Scholar] [CrossRef] [Green Version]
- Keren, Z.; Naor, S.; Nussbaum, S.; Golan, K.; Itkin, T.; Sasaki, Y.; Schmidt-Supprian, M.; Lapidot, T.; Melamed, D. B-cell depletion reactivates B lymphopoiesis in the BM and rejuvenates the B lineage in aging. Blood 2011, 117, 3104–3112. [Google Scholar] [CrossRef] [Green Version]
- Guerrettaz, L.M.; Johnson, S.A.; Cambier, J.C. Acquired hematopoietic stem cell defects determine B-cell repertoire changes associated with aging. Proc. Natl. Acad. Sci. USA 2008, 105, 11898–11902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kline, G.H.; Hayden, T.A.; Klinman, N.R. B cell Maintainance in Aged Mice Reflects Both Increased B cell Longevity and Decreased B Cell Generation. J. Immunol. 1999, 162, 3342–3349. [Google Scholar] [PubMed]
- King, A.M.; van Der Put, E.; Blomberg, B.B.; Riley, R.L. Accelerated Notch-Dependent Degradation of E47 Proteins in Aged B Cell Precursors Is Associated with Increased ERK MAPK Activation. J. Immunol. 2007, 178, 3521–3529. [Google Scholar] [CrossRef] [PubMed]
- van Der Put, E.; Frasca, D.; King, A.M.; Blomberg, B.B.; Riley, R.L. Decreased E47 in Senescent B Cell Precursors Is Stage Specific and Regulated Posttranslationally by Protein Turnover. J. Immunol. 2004, 173, 818–827. [Google Scholar] [CrossRef] [Green Version]
- Clark, M.R.; Mandal, M.; Ochiai, K.; Singh, H. Orchestrating B cell lymphopoiesis through interplay of IL-7 receptor and pre-B cell receptor signalling. Nat. Rev. Immunol. 2014, 14, 69–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, K.; Hutter, C.; Sun, Q.; Bilic, I.; Cobaleda, C.; Malin, S.; Busslinger, M. Instructive Role of the Transcription Factor E2A in Early B Lymphopoiesis and Germinal Center B Cell Development. Immunity 2008, 28, 751–762. [Google Scholar] [CrossRef] [Green Version]
- Northrup, D.; Allman, D. Transcriptional regulation of early B cell development. Immunol. Res. 2008, 42, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Ratliff, M.; Alter, S.; McAvoy, K.; Frasca, D.; Wright, J.A.; Zinkel, S.S.; Khan, W.N.; Blomberg, B.B.; Riley, R.L. In aged mice, low surrogate light chain promotes pro- B -cell apoptotic resistance, compromises the P re BCR checkpoint, and favors generation of autoreactive, phosphorylcholine-specific B cells. Aging Cell 2015, 14, 382–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kogut, I.; Scholz, J.L.; Cancro, M.; Cambier, J. B cell maintenance and function in aging. Semin. Immunol. 2012, 24, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Szabo, P.; Zhao, K.; Kirman, I.; Le Maoult, J.; Dyall, R.; Cruikshank, W.; Weksler, E.M. Maturation of B cell precursors is impaired in thymic-deprived nude and old mice. J. Immunol. 1998, 161, 2248–2253. [Google Scholar] [PubMed]
- Ben-Yehuda, A.; Szabo, P.; Dyall, R.; Weksler, E.M. Bone marrow declines as a site of B-cell precursor differentiation with age: Relationship to thymus involution. Proc. Natl. Acad. Sci. USA 1994, 91, 11988–11992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeMaoult, J.; Szabo, P.; Weksler, M.E. Effect of age on humoral immunity, selection of the B-cell repertoire and B-cell development. Immunol. Rev. 1997, 160, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Kosan, C.; Saba, I.; Godmann, M.; Herold, S.; Herkert, B.; Eilers, M.; Möröy, T. Transcription Factor Miz-1 Is Required to Regulate Interleukin-7 Receptor Signaling at Early Commitment Stages of B Cell Differentiation. Immunity 2010, 33, 917–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toboso-Navasa, A.; Gunawan, A.; Morlino, G.; Nakagawa, R.; Taddei, A.; Damry, D.; Patel, Y.; Chakravarty, P.; Janz, M.; Kassiotis, G.; et al. Restriction of memory B cell differentiation at the germinal center B cell positive selection stage. J. Exp. Med. 2020, 217, e20191933. [Google Scholar] [CrossRef]
- Peukert, K.; Staller, P.; Schneider, A.; Carmichael, G.; Hänel, F.; Eilers, M. An alternative pathway for gene regulation by Myc. EMBO J. 1997, 16, 5672–5686. [Google Scholar] [CrossRef]
- Herold, S.; Wanzel, M.; Beuger, V.; Frohme, C.; Beul, D.; Hillukkala, T.; Syvaoja, J.; Saluz, H.-P.; Haenel, F.; Eilers, M. Negative Regulation of the Mammalian UV Response by Myc through Association with Miz-1. Mol. Cell 2002, 10, 509–521. [Google Scholar] [CrossRef]
- Stead, M.; Trinh, C.H.; Garnett, J.A.; Carr, S.B.; Baron, A.J.; Edwards, T.A.; Wright, S.C. A Beta-Sheet Interaction Interface Directs the Tetramerisation of the Miz-1 POZ Domain. J. Mol. Biol. 2007, 373, 820–826. [Google Scholar] [CrossRef] [Green Version]
- Stead, M.; Wright, S.C. Structures of heterodimeric POZ domains of Miz1/BCL6 and Miz1/NAC1. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2014, 70, 1591–1596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, J.P.; Cancro, M.P. B cells and aging: Balancing the homeostatic equation. Exp. Gerontol. 2007, 42, 396–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagasawa, T. Microenvironmental niches in the bone marrow required for B-cell development. Nat. Rev. Immunol. 2006, 6, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Mesin, L.; Ersching, J.; Victora, G.D. Germinal Center B Cell Dynamics. Immunity 2016, 45, 471–482. [Google Scholar] [CrossRef] [Green Version]
- Jung, C.; Hugot, J.-P.; Barreau, F. Peyer’s Patches: The Immune Sensors of the Intestine. Int. J. Inflamm. 2010, 2010, 823710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macpherson, A.J.; Hunziker, L.; McCoy, K.; Lamarre, A. IgA responses in the intestinal mucosa against pathogenic and non-pathogenic microorganisms. Microbes Infect. 2001, 3, 1021–1035. [Google Scholar] [CrossRef]
- Lycke, N.Y.; Bemark, M. The role of Peyer’s patches in synchronizing gut IgA responses. Front. Immunol. 2012, 3, 329. [Google Scholar] [CrossRef] [Green Version]
- Reboldi, A.; Cyster, J.G. Peyer’s patches: Organizing B-cell responses at the intestinal frontier. Immunol. Rev. 2016, 271, 230–245. [Google Scholar] [CrossRef]
- Pinti, M.; Appay, V.; Campisi, J.; Frasca, D.; Fülöp, T.; Sauce, D.; Larbi, A.; Weinberger, B.; Cossarizza, A. Aging of the immune system: Focus on inflammation and vaccination. Eur. J. Immunol. 2016, 46, 2286–2301. [Google Scholar] [CrossRef]
- Bulati, M.; Caruso, C.; Colonna-Romano, G. From lymphopoiesis to plasma cells differentiation, the age-related modifications of B cell compartment are influenced by “inflamm-ageing”. Ageing Res. Rev. 2017, 36, 125–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagen, M.; Derudder, E. Inflammation and the Alteration of B-Cell Physiology in Aging. Gerontology 2020, 66, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.L.; Kruger, M.G.; Elia, J. B cell precursors are decreased in senescent BALBc mice, but retain normal mitotic activity in vivo and in vitro. Clin. Immunol. Immunopathol. 1991, 59, 301–313. [Google Scholar] [CrossRef]
- Gao, B.; Lin, X.; Jing, H.; Fan, J.; Ji, C.; Jie, Q.; Zheng, C.; Wang, D.; Xu, X.; Hu, Y.; et al. Local delivery of tetramethylpyrazine eliminates the senescent phenotype of bone marrow mesenchymal stromal cells and creates an anti-inflammatory and angiogenic environment in aging mice. Aging Cell 2018, 17, e12741. [Google Scholar] [CrossRef] [PubMed]
- Rowh, M.A.W.; Demicco, A.; Horowitz, E.J.; Yin, B.; Yang-Iott, K.S.; Fusello, A.M.; Hobeika, E.; Reth, M.; Bassing, C.H. Tp53 deletion in B lineage cells predisposes mice to lymphomas with oncogenic translocations. Oncogene 2011, 30, 4757–4764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hobeika, E.; Thiemann, S.; Storch, B.; Jumaa, H.; Nielsen, P.J.; Pelanda, R.; Reth, M. Testing gene function early in the B cell lineage in mb1-cre mice. Proc. Natl. Acad. Sci. USA 2006, 103, 13789–13794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labrie, J.E.; Sah, A.P.; Allman, D.M.; Cancro, M.; Gerstein, R.M. Bone Marrow Microenvironmental Changes Underlie Reduced RAG-mediated Recombination and B Cell Generation in Aged Mice. J. Exp. Med. 2004, 200, 411–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weksler, M.E.; Goodhardt, M.; Szabo, P. The effect of age on B cell development and humoral immunity. Springer Semin. Immunopathol. 2002, 24, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Geiger, H.; Rudolph, K.L. Immunoaging induced by hematopoietic stem cell aging. Curr. Opin. Immunol. 2011, 23, 532–536. [Google Scholar] [CrossRef]
- Sherwood, E.M.; Xu, W.; Riley, R.L. B cell precursors in senescent mice exhibit decreased recruitment into proliferative compartments and altered expression of Bcl-2 family members. Mech. Ageing Dev. 2003, 124, 147–153. [Google Scholar] [CrossRef]
- Lu, L.; Chaudhury, P.; Osmond, D. Regulation of Cell Survival During B Lymphopoiesis: Apoptosis and Bcl-2/Bax Content of Precursor B cells in Bone Marrow of Mice with Altered Expression of IL-7 and Recombinase-Activating Gene-2. J. Immunol. 1999, 162, 1931–1940. [Google Scholar] [PubMed]
- Miao, L.; Song, Z.; Jin, L.; Zhu, Y.M.; Wen, L.; Wu, M. ARF antagonizes the ability of Miz-1 to inhibit p53-mediated transactivation. Oncogene 2010, 29, 711–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashkovan, M.; Vadnais, C.; Ross, J.; Gigoux, M.; Suh, W.-K.; Gu, W.; Kosan, C.; Möröy, T. Miz-1 regulates translation of Trp53 via ribosomal protein L22 in cells undergoing V(D)J recombination. Proc. Natl. Acad. Sci. USA 2014, 111, E5411–E5419. [Google Scholar] [CrossRef] [Green Version]
- Patel, J.H.; McMahon, S.B. BCL2 Is a Downstream Effector of MIZ-1 Essential for Blocking c-MYC-induced Apoptosis. J. Biol. Chem. 2007, 282, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Ji, M.; Klarmann, K.D.; Keller, J.R. Repression of Id2 expression by Gfi-1 is required for B-cell and myeloid development. Blood 2010, 116, 1060–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thal, M.A.; Carvalho, T.L.; He, T.; Kim, H.-G.; Gao, H.; Hagman, J.; Klug, C.A. Ebf1-mediated down-regulation of Id2 and Id3 is essential for specification of the B cell lineage. Proc. Natl. Acad. Sci. USA 2009, 106, 552–557. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Basu, S.; Qiu, Y.; Tang, F.; Dong, F. A role of Miz-1 in Gfi-1-mediated transcriptional repression of CDKN1A. Oncogene 2010, 29, 2843–2852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basu, S.; Liu, Q.; Qiu, Y.; Dong, F. Gfi-1 represses CDKN2B encoding p15 INK4B through interaction with Miz-1. Proc. Natl. Acad. Sci. USA 2009, 106, 1433–1438. [Google Scholar] [CrossRef] [Green Version]
- Lescale, C.; Dias, S.; Maës, J.; Cumano, A.; Szabo, P.; Charron, D.; Weksler, M.E.; Dosquet, C.; Vieira, P.; Goodhardt, M. Reduced EBF expression underlies loss of B-cell potential of hematopoietic progenitors with age. Aging Cell 2010, 9, 410–419. [Google Scholar] [CrossRef]
- Avivi, I.; Zisman-Rozen, S.; Naor, S.; Dai, I.; Benhamou, D.; Shahaf, G.; Tabibian-Keissar, H.; Rosenthal, N.; Rakovsky, A.; Hanna, A.; et al. Depletion of B cells rejuvenates the peripheral B-cell compartment but is insufficient to restore immune competence in aging. Aging Cell 2019, 18, e12959. [Google Scholar] [CrossRef] [PubMed]
- Frasca, D.; Diaz, A.; Romero, M.; Vazquez, T.; Blomberg, B.B. Obesity induces pro-inflammatory B cells and impairs B cell function in old mice. Mech. Ageing Dev. 2017, 162, 91–99. [Google Scholar] [CrossRef] [Green Version]
- Frasca, D.; Blomberg, B.B. Aging impairs murine B cell differentiation and function in primary and secondary lymphoid tissues. Aging Dis. 2011, 2, 361–373. [Google Scholar] [PubMed]
- Hao, Y.; O’Neill, P.; Naradikian, M.S.; Scholz, J.L.; Cancro, M.P. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood 2011, 118, 1294–1304. [Google Scholar] [CrossRef] [Green Version]
- Knox, J.J.; Myles, A.; Cancro, M.P. T-bet + memory B cells: Generation, function, and fate. Immunol. Rev. 2019, 288, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Rubtsova, K.; Rubtsov, A.V.; van Dyk, L.F.; Kappler, J.W.; Marrack, P. T-box transcription factor T-bet, a key player in a unique type of B-cell activation essential for effective viral clearance. Proc. Natl. Acad. Sci. USA 2013, 110, E3216–E3224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinn, W.J.; Scholz, J.L.; Cancro, M.P. Dwindling competition with constant demand: Can homeostatic adjustments explain age-associated changes in peripheral B cell selection? Semin. Immunol. 2005, 17, 362–369. [Google Scholar] [CrossRef]
- Muggen, A.F.; de Jong, M.; Wolvers-Tettero, I.L.M.; Kallemeijn, M.J.; Teodosio, C.; Darzentas, N.; Stadhouders, R.; Ijspeert, H.; Van Der Burg, M.; Van Ijcken, W.F.; et al. The presence of CLL-associated stereotypic B cell receptors in the normal BCR repertoire from healthy individuals increases with age. Immun. Ageing 2019, 16, 22. [Google Scholar] [CrossRef] [PubMed]
- Palomero, T.; Lim, W.K.; Odom, D.T.; Sulis, M.L.; Real, P.J.; Margolin, A.; Barnes, K.C.; O’Neil, J.; Neuberg, D.; Weng, A.P.; et al. NOTCH1 directly regulates c-MYC and activates a feed-forward-loop transcriptional network promoting leukemic cell growth. Proc. Natl. Acad. Sci. USA 2006, 103, 18261–18266. [Google Scholar] [CrossRef] [Green Version]
- Belver, L.; Yang, A.Y.; Albero, R.; Herranz, D.; Brundu, F.G.; Quinn, S.A.; Pérez-Durán, P.; Álvarez, S.; Gianni, F.; Rashkovan, M.; et al. GATA3-Controlled Nucleosome Eviction Drives MYC Enhancer Activity in T-cell Development and Leukemia. Cancer Discov. 2019, 9, 1774–1791. [Google Scholar] [CrossRef] [Green Version]
- Kyriakis, J.M.; Banerjee, P.; Nikolakaki, E.; Dai, T.; Rubie, E.A.; Ahmad, M.F.; Avruch, J.; Woodgett, J. The stress-activated protein kinase subfamily of c-Jun kinases. Nature 1994, 369, 156–160. [Google Scholar] [CrossRef]
- Hibi, M.; Lin, A.; Smeal, T.; Minden, A.; Karin, M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993, 7, 2135–2148. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zhao, Y.; Eilers, M.; Lin, A. Miz1 is a signal- and pathway-specific modulator or regulator (SMOR) that suppresses TNF-α-induced JNK1 activation. Proc. Natl. Acad. Sci. USA 2009, 106, 18279–18284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarubin, T.; Han, J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005, 15, 11–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, B.; Han, S.; Takahashi, Y.; Kelsoe, G. Immunosenescence and germinal center reaction. Immunol. Rev. 1997, 160, 63–77. [Google Scholar] [CrossRef]
- Phan, R.T.; Saito, M.; Basso, K.; Niu, H.; Dalla-Favera, R. BCL6 interacts with the transcription factor Miz-1 to suppress the cyclin-dependent kinase inhibitor p21 and cell cycle arrest in germinal center B cells. Nat. Immunol. 2005, 6, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Phan, R.T.; Dalla-Favera, R. The BCL6 proto-oncogene suppresses p53 expression in germinal-centre B cells. Nature 2004, 432, 635–639. [Google Scholar] [CrossRef]
- Linterman, A.M. How T follicular helper cells and the germinal centre response change with age. Immunol. Cell Biol. 2014, 92, 72–79. [Google Scholar] [CrossRef]
- Cortegano, I.; Rodríguez, M.; Martín, I.; Prado, M.C.; Ruíz, C.; Hortigüela, R.; Alía, M.; Vilar, M.; Mira, H.; Cano, E.; et al. Altered marginal zone and innate-like B cells in aged senescence-accelerated SAMP8 mice with defective IgG1 responses. Cell Death Dis. 2017, 8, e3000. [Google Scholar] [CrossRef]
- Hodge, D.L.; Berthet, C.; Coppola, V.; Kastenmüller, W.; Buschman, M.D.; Schaughency, P.M.; Shirota, H.; Scarzello, A.J.; Subleski, J.J.; Anver, M.R.; et al. IFN-gamma AU-rich element removal promotes chronic IFN-gamma expression and autoimmunity in mice. J. Autoimmun. 2014, 53, 33–45. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piskor, E.-M.; Ross, J.; Möröy, T.; Kosan, C. Myc-Interacting Zinc Finger Protein 1 (Miz-1) Is Essential to Maintain Homeostasis and Immunocompetence of the B Cell Lineage. Biology 2022, 11, 504. https://doi.org/10.3390/biology11040504
Piskor E-M, Ross J, Möröy T, Kosan C. Myc-Interacting Zinc Finger Protein 1 (Miz-1) Is Essential to Maintain Homeostasis and Immunocompetence of the B Cell Lineage. Biology. 2022; 11(4):504. https://doi.org/10.3390/biology11040504
Chicago/Turabian StylePiskor, Eva-Maria, Julie Ross, Tarik Möröy, and Christian Kosan. 2022. "Myc-Interacting Zinc Finger Protein 1 (Miz-1) Is Essential to Maintain Homeostasis and Immunocompetence of the B Cell Lineage" Biology 11, no. 4: 504. https://doi.org/10.3390/biology11040504
APA StylePiskor, E.-M., Ross, J., Möröy, T., & Kosan, C. (2022). Myc-Interacting Zinc Finger Protein 1 (Miz-1) Is Essential to Maintain Homeostasis and Immunocompetence of the B Cell Lineage. Biology, 11(4), 504. https://doi.org/10.3390/biology11040504