Differences in Corticosterone Release Rates of Larval Spring Salamanders (Gyrinophilus porphyriticus) in Response to Native Fish Presence
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
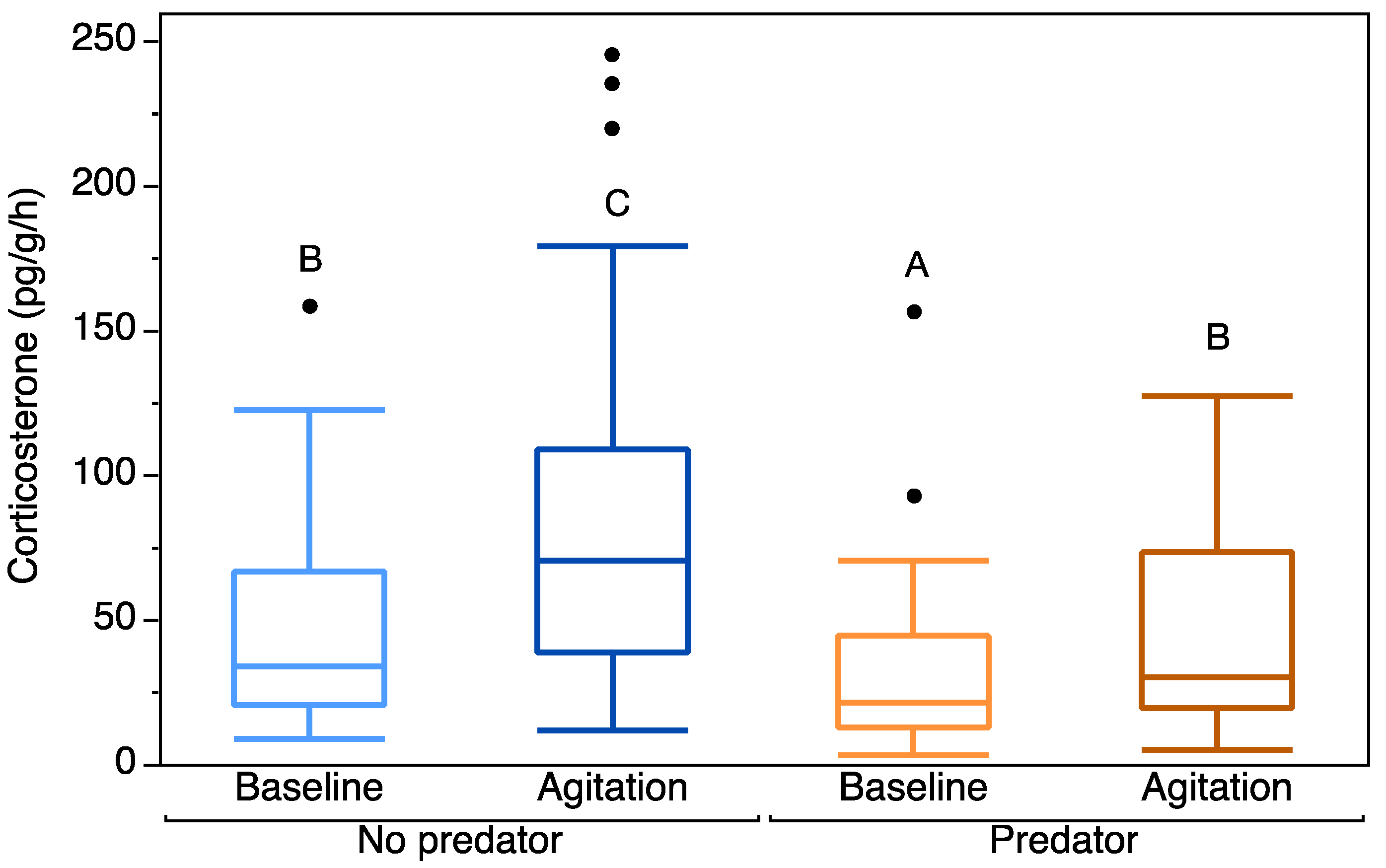
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stuart, S.N.; Chanson, J.S.; Cox, N.A.; Young, B.E.; Rodrigues, A.S.L.; Fischman, D.L.; Waller, R.W. Status and Trends of Amphibian Declines and Extinctions Worldwide. Science 2004, 306, 1783–1786. [Google Scholar] [CrossRef] [Green Version]
- Adams, M.J.; Miller, D.A.W.; Muths, E.; Corn, P.S.; Grant, E.H.C.; Bailey, L.L.; Fellers, G.M.; Fisher, R.N.; Sadinski, W.J.; Waddle, H.; et al. Trends in Amphibian Occupancy in the United States. PLoS ONE 2013, 8, e64347. [Google Scholar] [CrossRef]
- Falaschi, M.; Melotto, A.; Manenti, R.; Ficetola, G.F. Invasive Species and Amphibian Conservation. Herpetologica 2020, 76, 216–227. [Google Scholar] [CrossRef]
- Bucciarelli, G.M.; Blaustein, A.R.; Garcia, T.S.; Kats, L.B. Invasion Complexities: The Diverse Impacts of Nonnative Species on Amphibians. Copeia 2014, 2014, 611–632. [Google Scholar] [CrossRef]
- Remon, J.; Bower, D.S.; Gaston, T.F.; Clulow, J.; Mahony, M.J. Stable Isotope Analyses Reveal Predation on Amphibians by a Globally Invasive Fish (Gambusia holbrooki). Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 26, 724–735. [Google Scholar] [CrossRef]
- Preston, D.L.; Henderson, J.S.; Johnson, P.T. Community Ecology of Invasions: Direct and Indirect Effects of Multiple Invasive Species on Aquatic Communities. Ecology 2012, 93, 1254–1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Relyea, R.A. Trait-Mediated Indirect Effects in Larval Anurans: Reversing Competition with the Treat of Predation. Ecology 2000, 81, 2278–2289. [Google Scholar] [CrossRef]
- Denver, R.J. Stress Hormones Mediate Environment-Genotype Interactions during Amphibian Development. Gen. Comp. Endocrinol. 2009, 164, 20–31. [Google Scholar] [CrossRef]
- Knapp, R.A.; Matthews, K.R. Non-native Fish Introductions and the Decline of the Mountain Yellow-legged Frog from within Protected Areas. Conserv. Biol. 2000, 14, 428–438. [Google Scholar] [CrossRef] [Green Version]
- Segev, O.; Mangel, M.; Blaustein, L. Deleterious Effects by Mosquitofish (Gambusia affinis) on the Endangered Fire Salamander (Salamandra infraimmaculata). Anim. Conserv. 2009, 12, 29–37. [Google Scholar] [CrossRef]
- Hamer, A.J. Exotic Predatory Fish Reduce Amphibian Reproduction at Wetlands in an Urbanising Landscape. Hydrobiologia 2022, 849, 121–139. [Google Scholar]
- Smith, G.R.; Dibble, C.J. Effects of an Invasive Fish (Gambusia affinis) and Anthropogenic Nutrient Enrichment on American Toad (Anaxyrus americanus) Tadpoles. J. Herpetol. 2012, 46, 198–202. [Google Scholar] [CrossRef]
- Davis, D.R.; Gabor, C.R. Behavioral and Physiological Antipredator Responses of the San Marcos Salamander, Eurycea nana. Physiol. Behav. 2015, 139, 145–149. [Google Scholar] [CrossRef]
- Hau, M.; Casagrande, S.; Ouyang, J.Q.; Baugh, A.T. Glucocorticoid-Mediated Phenotypes in Vertebrates. In Advances in the Study of Behavior; Elsevier: Amsterdam, The Netherlands, 2016; Volume 48, pp. 41–115. ISBN 978-0-12-804787-3. [Google Scholar]
- Guindre-Parker, S. The Evolutionary Endocrinology of Circulating Glucocorticoids in Free-Living Vertebrates: Recent Advances and Future Directions across Scales of Study. Integr. Comp. Biol. 2018, 58, 814–825. [Google Scholar] [CrossRef] [Green Version]
- Székely, D.; Cogălniceanu, D.; Székely, P.; Armijos-Ojeda, D.; Espinosa-Mogrovejo, V.; Denoël, M. How to Recover from a Bad Start: Size at Metamorphosis Affects Growth and Survival in a Tropical Amphibian. BMC Ecol. 2020, 20, 24. [Google Scholar] [CrossRef] [Green Version]
- Gall, B.G.; Mathis, A. Innate Predator Recognition and the Problem of Introduced Trout. Ethology 2010, 116, 47–58. [Google Scholar] [CrossRef]
- Davis, D.R. Predator-Prey Interactions in the San Marcos Salamander (Eurycea nana): Predator Generalization and Stress Hormones in Response to Introduced Predators. Master’s Thesis, Texas State University-San Marcos, San Marcos, TX, USA, 2012. [Google Scholar]
- Davis, D.R.; Epp, K.J.; Gabor, C.R. Predator Generalization Decreases the Effect of Introduced Predators in the San Marcos Salamander, Eurycea nana. Ethology 2012, 118, 1191–1197. [Google Scholar] [CrossRef]
- Idler, D.R. Steroids in Non-Mammalian Vertebrates; Academic Press: New York, NY, USA, 1972. [Google Scholar]
- Romero, L.M. Physiological Stress in Ecology: Lessons from Biomedical Research. Trends Ecol. Evol. 2004, 19, 249–255. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How Do Glucocorticoids Influence Stress Responses? Integrating Permissive, Suppressive, Stimulatory, and Preparative Actions*. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [CrossRef] [Green Version]
- Jermacz, Ł.; Kletkiewicz, H.; Nowakowska, A.; Dzierżyńska-Białończyk, A.; Klimiuk, M.; Kobak, J. Continuity of Chronic Predation Risk Determines Changes in Prey Physiology. Sci. Rep. 2020, 10, 6972. [Google Scholar] [CrossRef] [Green Version]
- Bridges, C.M. Tadpoles Balance Foraging and Predator Avoidance: Effects of Predation, Pond Drying, and Hunger. J. Herpetol. 2002, 36, 627–634. [Google Scholar] [CrossRef]
- Rose, J.D.; Moore, F.L.; Orchinik, M. Rapid Neurophysiological Effects of Corticosterone on Medullary Neurons- Relationship to Stress-Induced Suppression of Courtship Clasping in an Amphibian. Neuroendocrinology 1993, 57, 815–824. [Google Scholar] [CrossRef]
- Cyr, N.E.; Romero, L.M. Identifying Hormonal Habituation in Field Studies of Stress. Gen. Comp. Endocrinol. 2009, 161, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Dahl, E.; Orizaola, G.; Winberg, S.; Laurila, A. Geographic Variation in Corticosterone Response to Chronic Predator Stress in Tadpoles: Geographical Variation in Corticosterone Response. J. Evol. Biol. 2012, 25, 1066–1076. [Google Scholar] [CrossRef] [PubMed]
- Glennemeier, K.A.; Denver, R.J. Small Changes in Whole-Body Corticosterone Content Affect Larval Rana pipiens Fitness Components. Gen. Comp. Endocrinol. 2002, 127, 16–25. [Google Scholar] [CrossRef]
- Wack, C.L.; DuRant, S.E.; Hopkins, W.A.; Lovern, M.B.; Feldhoff, R.C.; Woodley, S.K. Elevated Plasma Corticosterone Increases Metabolic Rate in a Terrestrial Salamander. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2012, 161, 153–158. [Google Scholar] [CrossRef]
- Altwegg, R.; Reyer, H.-U. Patterns of Natural Selection in Size at Metamorphosis in Water Frogs. Evolution 2003, 57, 872–882. [Google Scholar] [CrossRef]
- Scott, D.E.; Casey, E.D.; Donovan, M.F.; Lynch, T.K. Amphibian Lipid Levels at Metamorphosis Correlate to Post-Metamorphic Terrestrial Survival. Oecologia 2007, 153, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Crespi, E.J.; Denver, R.J. Programming Neuroendocrine Stress Axis Activity by Exposure to Glucocorticoids during Postembryonic Development of the Frog, Xenopus Laevis. Endocrinology 2008, 149, 5470–5481. [Google Scholar] [CrossRef]
- Warne, R.W.; Crespi, E.J. Larval Growth Rate and Sex Determine Resource Allocation and Stress Responsiveness across Life Stages in Juvenile Frogs. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2015, 323, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, R. Reality as the Leading Cause of Stress: Rethinking the Impact of Chronic Stress in Nature. Funct. Ecol. 2013, 27, 11–23. [Google Scholar] [CrossRef]
- Schoenle, L.A.; Zimmer, C.; Vitousek, M.N. Understanding Context Dependence in Glucocorticoid–Fitness Relationships: The Role of the Nature of the Challenge, the Intensity and Frequency of Stressors, and Life History. Integr. Comp. Biol. 2018, 58, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Middlemis Maher, J.; Werner, E.E.; Denver, R.J. Stress Hormones Mediate Predator-Induced Phenotypic Plasticity in Amphibian Tadpoles. Proc. R. Soc. B Biol. Sci. 2013, 280, 20123075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraker, M.E.; Hu, F.; Cuddapah, V.; McCollum, S.A.; Relyea, R.A.; Hempel, J.; Denver, R.J. Characterization of an Alarm Pheromone Secreted by Amphibian Tadpoles That Induces Behavioral Inhibition and Suppression of the Neuroendocrine Stress Axis. Horm. Behav. 2009, 55, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.M.; Longhi, J.N.; Chin, E.H.; Burness, G.; Kerr, L.R.; Murray, D.L. Acute Changes in Whole Body Corticosterone in Response to Perceived Predation Risk: A Mechanism for Anti-Predator Behavior in Anurans? Gen. Comp. Endocrinol. 2016, 229, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Greene, B.T.; Lowe, W.H.; Likens, G.E. Forest Succession and Prey Availability Influence the Strength and Scale of Terrestrial-aquatic Linkages in a Headwater Salamander System. Freshw. Biol. 2008, 53, 2234–2243. [Google Scholar] [CrossRef]
- Lowe, W.H.; Swartz, L.K.; Addis, B.R.; Likens, G.E. Hydrologic Variability Contributes to Reduced Survival through Metamorphosis in a Stream Salamander. Proc. Natl. Acad. Sci. USA 2019, 116, 19563. [Google Scholar] [CrossRef] [Green Version]
- Resetarits, W.J. Competitive Asymmetry and Coexistence in Size-Structured Populations of Brook Trout and Spring Salamanders. Oikos 1995, 73, 188. [Google Scholar] [CrossRef]
- Lowe, W.H.; Bolger, D.T. Local and Landscape-Scale Predictors of Salamander Abundance in New Hampshire Headwater Streams. Conserv. Biol. 2002, 16, 183–193. [Google Scholar] [CrossRef] [Green Version]
- Barr, G.E.; Babbitt, K.J. Trout Affect the Density, Activity and Feeding of a Larval Plethodontid Salamander. Freshw. Biol. 2007, 52, 1239–1248. [Google Scholar] [CrossRef]
- Currens, C.R.; Liss, W.J.; Hoffman, R.L. Impacts of a Gape Limited Brook Trout, Salvelinus fontinalis, on Larval Northwestern Salamander, Ambystoma gracile, Growth: A Field Enclosure Experiment. J. Herpetol. 2007, 41, 321–324. [Google Scholar] [CrossRef]
- Lowe, W.H.; Addis, B.R.; Smith, M.R.; Davenport, J.M. The Spatial Structure of Variation in Salamander Survival, Body Condition and Morphology in a Headwater Stream Network. Freshw. Biol. 2018, 63, 1287–1299. [Google Scholar] [CrossRef]
- Dickens, M.J.; Romero, L.M. A Consensus Endocrine Profile for Chronically Stressed Wild Animals Does Not Exist. Gen. Comp. Endocrinol. 2013, 191, 177–189. [Google Scholar] [CrossRef]
- Gabor, C.R.; Zabierek, K.C.; Kim, D.S.; da Barbiano, L.A.; Mondelli, M.J.; Bendik, N.F.; Davis, D.R. A Non-Invasive Water-Borne Assay of Stress Hormones in Aquatic Salamanders. Copeia 2016, 104, 172–181. [Google Scholar] [CrossRef]
- Forsburg, Z.R.; Goff, C.B.; Perkins, H.R.; Robicheaux, J.A.; Almond, G.F.; Gabor, C.R. Validation of Water-Borne Cortisol and Corticosterone in Tadpoles: Recovery Rate from an Acute Stressor, Repeatability, and Evaluating Rearing Methods. Gen. Comp. Endocrinol. 2019, 281, 145–152. [Google Scholar] [CrossRef]
- Gabor, C.R.; Bosch, J.; Fries, J.N.; Davis, D.R. A Non-Invasive Water-Borne Hormone Assay for Amphibians. Amphib.-Reptil. 2013, 34, 151–162. [Google Scholar] [CrossRef] [Green Version]
- Baugh, A.T.; Bastien, B.; Still, M.B.; Stowell, N. Validation of Water-Borne Steroid Hormones in a Tropical Frog (Physalaemus pustulosus). Gen. Comp. Endocrinol. 2018, 261, 67–80. [Google Scholar] [CrossRef]
- Narayan, E.J.; Forsburg, Z.R.; Davis, D.R.; Gabor, C.R. Non-Invasive Methods for Measuring and Monitoring Stress Physiology in Imperiled Amphibians. Front. Ecol. Evol. 2019, 7, 431. [Google Scholar] [CrossRef] [Green Version]
- McGuire, K.J.; Torgersen, C.E.; Likens, G.E.; Buso, D.C.; Lowe, W.H.; Bailey, S.W. Network Analysis Reveals Multiscale Controls on Streamwater Chemistry. Proc. Natl. Acad. Sci. USA 2014, 111, 7030–7035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kats, L.B.; Ferrer, R.P. Alien Predators and Amphibian Declines: Review of Two Decades of Science and the Transition to Conservation. Divers. Distrib. 2003, 9, 99–110. [Google Scholar] [CrossRef]
- Knapp, R.A. Effects of Nonnative Fish and Habitat Characteristics on Lentic Herpetofauna in Yosemite National Park, USA. Biol. Conserv. 2005, 121, 265–279. [Google Scholar] [CrossRef]
- Killen, S.S.; Marras, S.; Metcalfe, N.B.; McKenzie, D.J.; Domenici, P. Environmental Stressors Alter Relationships between Physiology and Behaviour. Trends Ecol. Evol. 2013, 28, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Warne, R.W.; Kardon, A.; Crespi, E.J. Physiological, Behavioral and Maternal Factors That Contribute to Size Variation in Larval Amphibian Populations. PLoS ONE 2013, 8, e76364. [Google Scholar] [CrossRef] [Green Version]
- Leary, C.J.; Garcia, A.M.; Knapp, R.; Hawkins, D.L. Relationships among Steroid Hormone Levels, Vocal Effort and Body Condition in an Explosive-Breeding Toad. Anim. Behav. 2008, 76, 175–185. [Google Scholar] [CrossRef]
- Crespi, E.J.; Warne, R.W. Environmental Conditions Experienced During the Tadpole Stage Alter Post-Metamorphic Glucocorticoid Response to Stress in an Amphibian. Integr. Comp. Biol. 2013, 53, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- Lowe, W.H.; Nislow, K.H.; Bolger, D.T. Stage-Specific and Interactive Effects of Sedimentation and Trout on Headwater Stream Salamander. Ecol. Appl. 2004, 14, 164–172. [Google Scholar] [CrossRef]
- Charbonnier, J.F.; Pearlmutter, J.; Vonesh, J.R.; Gabor, C.R.; Forsburg, Z.R.; Grayson, K.L. Cross-Life Stage Effects of Aquatic Larval Density and Terrestrial Moisture on Growth and Corticosterone in the Spotted Salamander. Diversity 2018, 10, 68. [Google Scholar] [CrossRef] [Green Version]
| N | Stream | Treatment | Mean SVL (mm) | SE | Mean Mass (mg) | SE |
|---|---|---|---|---|---|---|
| 20 | Bear | No predator | 47.80 | 2.03 | 2.37 | 0.30 |
| 18 | Zigzag | No predator | 54.72 | 2.81 | 4.03 | 0.56 |
| 20 | Bear | Predator | 47.70 | 2.71 | 2.78 | 0.41 |
| 15 | Zigzag | Predator | 50.67 | 2.43 | 2.75 | 0.32 |
| Fixed Effects | Estimate | SE | 2.5% CI | 97.5% CI | p-Value |
|---|---|---|---|---|---|
| (Intercept) | 4.090 | 0.128 | 3.84 | 4.35 | <0.0001 |
| Baseline CORT | −0.468 | 0.081 | −0.62 | −0.31 | <0.0001 |
| Predator Treatment | −0.605 | 0.176 | −0.96 | −0.24 | 0.0009 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bryant, A.R.; Gabor, C.R.; Swartz, L.K.; Wagner, R.; Cochrane, M.M.; Lowe, W.H. Differences in Corticosterone Release Rates of Larval Spring Salamanders (Gyrinophilus porphyriticus) in Response to Native Fish Presence. Biology 2022, 11, 484. https://doi.org/10.3390/biology11040484
Bryant AR, Gabor CR, Swartz LK, Wagner R, Cochrane MM, Lowe WH. Differences in Corticosterone Release Rates of Larval Spring Salamanders (Gyrinophilus porphyriticus) in Response to Native Fish Presence. Biology. 2022; 11(4):484. https://doi.org/10.3390/biology11040484
Chicago/Turabian StyleBryant, Amanda R., Caitlin R. Gabor, Leah K. Swartz, Ryan Wagner, Madaline M. Cochrane, and Winsor H. Lowe. 2022. "Differences in Corticosterone Release Rates of Larval Spring Salamanders (Gyrinophilus porphyriticus) in Response to Native Fish Presence" Biology 11, no. 4: 484. https://doi.org/10.3390/biology11040484
APA StyleBryant, A. R., Gabor, C. R., Swartz, L. K., Wagner, R., Cochrane, M. M., & Lowe, W. H. (2022). Differences in Corticosterone Release Rates of Larval Spring Salamanders (Gyrinophilus porphyriticus) in Response to Native Fish Presence. Biology, 11(4), 484. https://doi.org/10.3390/biology11040484







