Selected Phenolic Acids Inhibit the Initial Growth of Ambrosia artemisiifolia L.
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Collection
2.2. Seed Bioassay
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scavo, A.; Abbate, C.; Mauromicale, G. Plant allelochemicals: Agronomic, nutritional and ecological relevance in the soil system. Plant Soil 2019, 442, 23–48. [Google Scholar] [CrossRef]
- Li, Z.H.; Wang, Q.; Ruan, X.; Pan, C.D.; Jiang, D.A. Phenolics and plant allelopathy. Molecules 2010, 15, 8933–8952. [Google Scholar] [CrossRef] [PubMed]
- Einhellig, F.A. Mode of Allelochemical Action of Phenolic Compounds. In Allelopathy: Chemistry and Mode of Action of Allelochemicals; Galindo, J.C.G., Molinillo, J.M.G., Cutler, H.G., Eds.; CRC Press LLC: Boca Raton, FL, USA, 2004; pp. 217–238. [Google Scholar]
- Brown, P.D.; Morra, M.J. Glucosinolate-Containing Plant Tissues as Bioherbicides. J. Agric. Food Chem. 1995, 43, 3070–3074. [Google Scholar] [CrossRef]
- Li, H.H.; Inoue, M.; Nishimura, H.; Mizutani, J.; Tsuzuki, E. Interactions of trans-cinnamic acid, its related phenolic allelochemicals, and abscisic acid in seedling growth and seed germination of lettuce. J. Chem. Ecol. 1993, 19, 1775–1787. [Google Scholar] [CrossRef]
- Gomaa, N.H.; Hassan, M.O.; Fahmy, G.M.; González, L.; Hammouda, O.; Atteya, A.M. Allelopathic effects of Sonchus oleraceus L. on the germination and seedling growth of crop and weed species. Acta Bot. Bras. 2014, 28, 408–416. [Google Scholar] [CrossRef]
- Stupnicka-Rodzynkiewicz, E.; Dabkowska, T.; Stoklosa, A.; Hura, T.; Dubert, F.; Lepiarczyk, A. The effect of selected phenolic compounds on the initial growth of four weed species. J. Plant Dis. Prot. 2006, 20, 479–486. [Google Scholar]
- Almaghrabi, O.A. Control of wild oat (Avena fatua) using some phenolic compounds I—Germination and some growth parameters. Saudi J. Biol. Sci. 2012, 19, 17–24. [Google Scholar] [CrossRef][Green Version]
- Heidarzade, A.; Pirdashti, H.; Esmaeili, M.A.; Asghari, J. Inhibitory activity of allelochemicals on barnyardgrass (Echinochloa crus-galli L.) seed and seedling parameters. World Appl. Sci. J. 2012, 17, 1535–1540. [Google Scholar]
- Haramoto, E.R.; Gallandt, E.R. Brassica cover cropping: I. Effects on weed and crop establishment. Weed Sci. 2005, 53, 695–701. [Google Scholar] [CrossRef]
- Brijačak, E.; Košćak, L.; Šoštarčić, V.; Kljak, K.; Šćepanović, M. Sensitivity of yellow foxtail (Setaria glauca L.) and barnyardgrass (Echinochloa crus-galli L.) to aqueous extracts or dry biomass of cover crops. J. Sci. Food Agric. 2020, 100, 5510–5517. [Google Scholar] [CrossRef]
- Šćepanović, M.; Sarić-Krsmanović, M.; Šoštarčić, V.; Brijačak, E.; Lakić, J.; Špirović Trifunović, B.; Gajić Umiljendić, J.; Radivojević, L. Inhibitory effects of brassicaceae cover crop on Ambrosia artemisiifolia germination and early growth. Plants 2021, 10, 794. [Google Scholar] [CrossRef] [PubMed]
- European Comission. European Green Deal; European Comission: Maastricht, The Netherlands, 2019. [Google Scholar]
- Scavo, A.; Mauromicale, G. Integrated Weed Management in Herbaceous Field Crops. Agronomy 2020, 10, 466. [Google Scholar] [CrossRef]
- Essl, F.; Biró, K.; Brandes, D.; Broennimann, O.; Bullock, J.M.; Chapman, D.S.; Chauvel, B.; Dullinger, S.; Fumanal, B.; Guisan, A.; et al. Biological Flora of the British Isles: Ambrosia artemisiifolia. J. Ecol. 2015, 103, 1069–1098. [Google Scholar] [CrossRef]
- Šarić, T.; Ostojić, Z.; Stefanović, L.; Milanova, S.D.; Kazinczi, G.; Tyšer, L. The changes of the composition of weed flora in Southeastern and Central Europe as affected by cropping practices. Herbologia 2011, 12, 5–27. [Google Scholar]
- Lommen, S.T.E.; Hallmann, C.A.; Jongejans, E.; Chauvel, B.; Leitsch-Vitalos, M.; Aleksanyan, A.; Tóth, P.; Preda, C.; Šćepanović, M.; Onen, H.; et al. Explaining variability in the production of seed and allergenic pollen by invasive Ambrosia artemisiifolia across Europe. Biol. Invasions 2018, 20, 1475–1491. [Google Scholar] [CrossRef]
- Milakovic, I.; Fiedler, K.; Karrer, G. Management of roadside populations of invasive Ambrosia artemisiifolia by mowing. Weed Res. 2014, 54, 256–264. [Google Scholar] [CrossRef]
- Galzina, N.; Barić, K.; Šćepanović, M.; Goršić, M.; Ostojić, Z. Distribution of Invasive Weed Ambrosia artemisiifolia L. in Croatia. Agric. Conspec. Sci. 2010, 75, 75–81. [Google Scholar]
- Heap, I. The International Herbicide-Resistant Weed Database. Available online: www.weedscience.org. (accessed on 14 January 2022).
- Šćepanović, M.; Šoštarčić, V.; Lakić, J.; Pintar, A.; Barić, K. Prvo izvješće o procjeni rezistentnosti ambrozije (Ambrosia artemisiifolia L.) na ALS herbicide foramsulfuron, prosulfuron, tifensulfuron i imazamoks u Republici Hrvatskoj. In Proceedings of the 11th Weed Science Congress and Symposium on Herbicides and Growth Regulators, Palić, Serbia, 20–23 September 2021. [Google Scholar]
- Einhellig, F.A.; Rasmussen, J.A. Synergistic inhibitory effects of vanillic and p-hydrozbenzoic cids on radish and grain sorghum. J. Chem. Ecol. 1978, 4, 425–436. [Google Scholar] [CrossRef]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-Response Analysis Using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Alqarawi, A.A.; Abd Allah, E.F. Bioherbicides: Current knowledge on weed control mechanism. Ecotoxicol. Environ. Saf. 2018, 158, 131–138. [Google Scholar] [CrossRef]
- Reigosa, M.J.; Souto, X.C.; González, L. Effect of phenolic compounds on the germination of six weeds species. Plant Growth Regul. 1999, 28, 83–88. [Google Scholar] [CrossRef]
- Hassan, M.; Daffalla, H.; Yagoub, S. Allelopathic effects of some Botanical extracts on germination and seedling growth of Sorghum bicolor L. Int. J. Agron. Plant Prod. 2012, 3, 132–138. [Google Scholar]
- Guenzi, W.D.; McCalla, T.M. Phenolic Acids in Oats, Wheat, Sorghum, and Corn Residues and Their Phytotoxicity 1. Agron. J. 1966, 58, 303–304. [Google Scholar] [CrossRef]
- Pardo-Muras, M.; Puig, C.G.; Souto, X.C.; Pedrol, N. Water-soluble phenolic acids and flavonoids involved in the bioherbicidal potential of Ulex europaeus and Cytisus scoparius. S. Afr. J. Bot. 2020, 133, 201–211. [Google Scholar] [CrossRef]
- Hegab, M.M.; Khodary, S.E.A.; Hammouda, O.; Ghareib, H.R. Autotoxicity of chard and its allelopathic potentiality on germination and some metabolic activities associated with growth of wheat seedlings. Afr. J. Biotechnol. 2008, 7, 884–892. [Google Scholar]
- Nishida, N.; Tamotsu, S.; Nagata, N.; Saito, C.; Sakai, A. Allelopathic effects of volatile monoterpenoids produced by Salvia leucophylla: Inhibition of cell proliferation and DNA synthesis in the root apical meristem of Brassica campestris seedlings. J. Chem. Ecol. 2005, 31, 1187–1203. [Google Scholar] [CrossRef]
- Krogmeier, M.J.; Bremner, J.M. Effects of Aliphatic Acids on Seed Germination and Seedling Growth in Soil. Commun. Soil Sci. Plant Anal. 1990, 21, 547–555. [Google Scholar] [CrossRef]
- Whitehead, D.C. Identification of p-Hydroxybenxoic, Vanillic, p-Coumaric and Ferulic Acids in Soils. Nature 1964, 202, 417–418. [Google Scholar] [CrossRef]
- Blum, G. Effects of microbial utilization of phenolic acids and their phenolic acid breakdown products on alelopathic interaction. J. Chem. Ecol. 1998, 24, 685–708. [Google Scholar] [CrossRef]
- Anugroho, F.; Kitou, M.; Nagumo, F.; Kinjo, K.; Tokashiki, Y. Effect of the sowing date on the growth of hairy vetch (vicia villosa) as a cover crop influenced the weed biomass and soil chemical properties in a subtropical region. Weed Biol. Manag. 2009, 9, 129–136. [Google Scholar] [CrossRef]
- Clossais-Besnard, N.; Larher, F. Physiological role of glucosinolates in brassica napus. Concentration and distribution pattern of glucosinolates among plant organs during a complete life cycle. J. Sci. Food Agric. 1991, 56, 25–38. [Google Scholar] [CrossRef]
- Sturm, D.J.; Kunz, C.; Peteinatos, G.; Gerhards, R. Do cover crop sowing date and fertilization affect field weed suppression? Plant Soil Environ. 2017, 63, 82–88. [Google Scholar]
- Hussain, M.I.; Reigosa, M.J. Secondary metabolites, ferulic acid and p-hydroxybenzoic acid induced toxic effects on photosynthetic process in Rumex Acetosa L. Biomolecules 2021, 11, 233. [Google Scholar] [CrossRef] [PubMed]
- Liebman, M.; Davis, A. Integration of soil, crop and weed management in low-external-input farming systems. Weed Res. 2000, 40, 27–47. [Google Scholar] [CrossRef]
- Šoštarcic, V.; Masin, R.; Turcinov, M.; Carin, N.; Scepanovic, M. Morfološka i funkcionalna intrapopulacijska varijabilnost sjemena korovne vrste Ambrosia artemisiifolia L. J. Cent. Eur. Agric. 2020, 21, 366–378. [Google Scholar]
- Zhou, X.G.; Wu, F.Z.; Xiang, W.S. Syringic acid inhibited cucumber seedling growht and changed rhizosphere microbial communities. Plant Soil Environ. 2014, 4, 158–164. [Google Scholar]
- Mushtaq, M.N.; Cheema, Z.A.; Khaliq, A.; Naveed, M.R. A 75% reduction in herbicide use through integration with sorghum+sunflower extracts for weed management in wheat. J. Sci. Food Agric. 2010, 90, 1897–1904. [Google Scholar] [CrossRef]
- Sarić-Krsmanovic, M.; Radivojević, L.J.; Šantrić, L.R.; Đorđević, T.M.; Gajić Umiljendić, J. Effects of mixtures of allelopathic plant water extracts and a herbicide on weed suppression. J. Environ. Sci. Health—B Pestic. Food Contam. Agric. Wastes 2020, 56, 16–22. [Google Scholar] [CrossRef]
- Rama Devi, S.; Prasad, M.N.V. Ferulic acid mediated changes in oxidative enzymes of maize seedlings: Implications in growth. Biol. Plant. 1996, 38, 387–395. [Google Scholar]
- Patterson, D.T. Effects of Allelopathic Chemicals on Growth and Physiological Responses of Soybean (Glycine max). Weed Sci. 1981, 29, 53–59. [Google Scholar] [CrossRef]
- Cordeau, S.; Triolet, M.; Wayman, S.; Steinberg, C.; Guillemin, J.P. Bioherbicides: Dead in the water? A review of the existing products for integrated weed management. Crop Prot. 2016, 87, 44–49. [Google Scholar] [CrossRef]

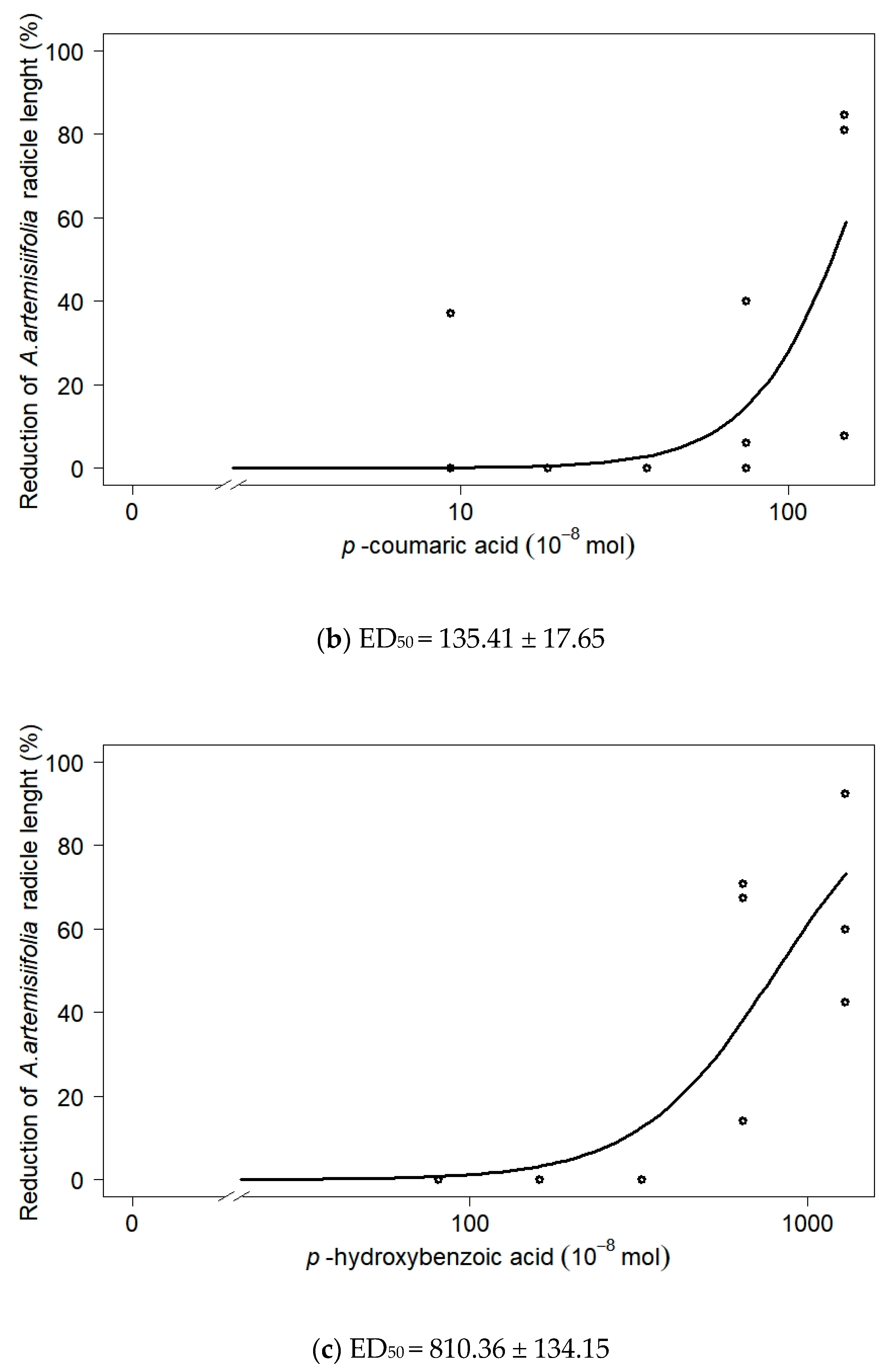
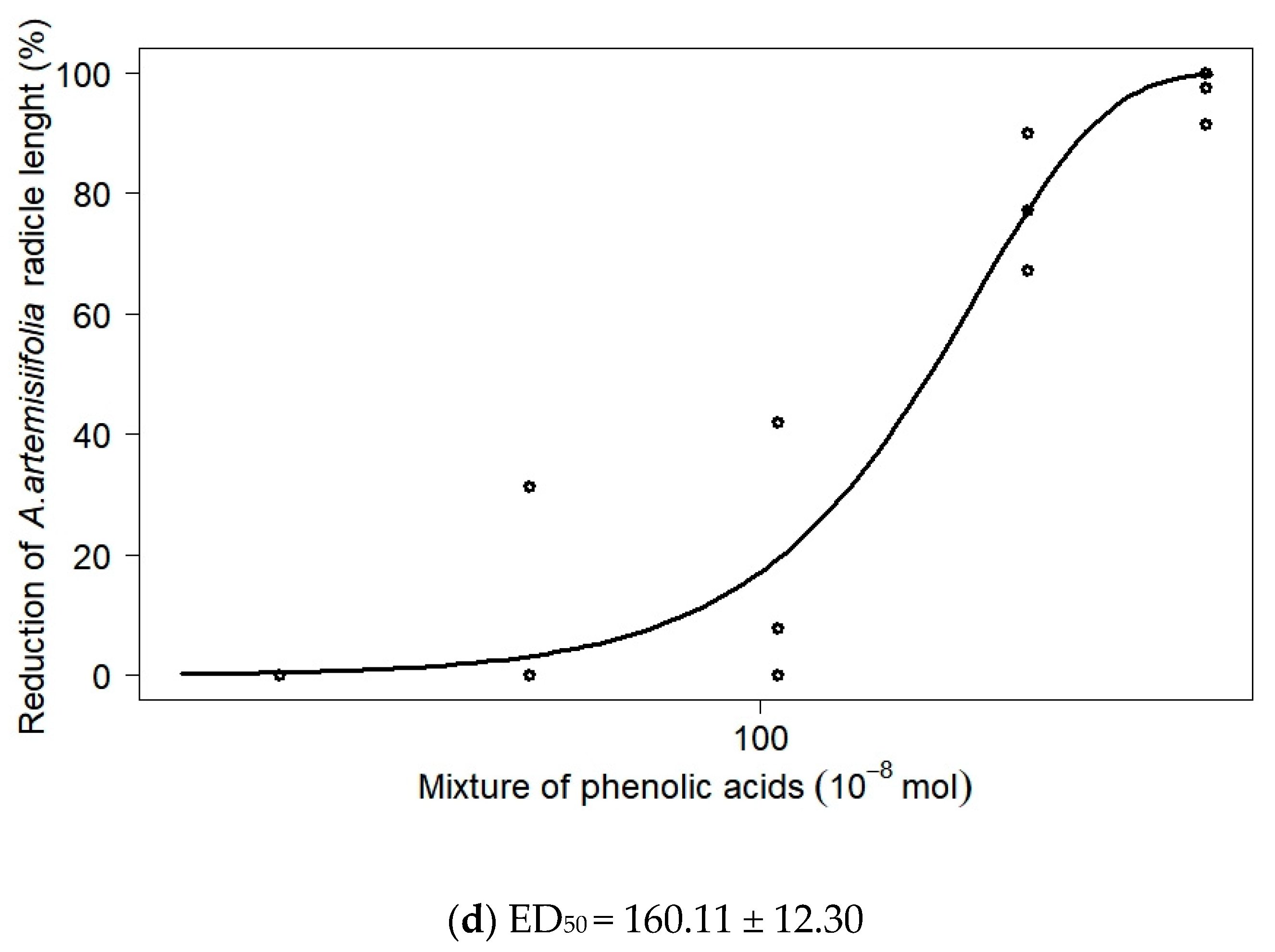
| Phenolic Acid | Dose * | ×10−8 mol | Inhibition Rate (%) | ||
|---|---|---|---|---|---|
| Shoot | Radicle | Biomass | |||
| Gallic acid | D1 | 19.3 | 0.3 ± 0.3 Aa | 4.6 ± 4.6 Ba | 0.9 ± 0.9 Ba |
| D2 | 38.5 | 0.0 ± 0.0 Ca | 0.0 ± 0.0 Ca | 1.8 ± 1.8 Ba | |
| D3 | 77.0 | 0.0 ± 0.0 Da | 0.2 ± 0.2 Ca | 1.2 ± 0.7 Ba | |
| D4 | 154.0 | 0.0 ± 0.0 Da | 0.0 ± 0.0 Ca | 6.5 ± 3.4 Ca | |
| D5 | 308.0 | 1.4 ± 1.4 Fa | 14.2 ± 7.1 Da | 11.1 ± 3.8 DEa | |
| Caffeic acid | D1 | 28.4 | 0.3 ± 0.3 Aa | 0.0 ± 0.0 Ba | 14.2 ± 2.5 ABab |
| D2 | 56.9 | 0.1 ± 0.1 BCa | 0.0 ± 0.0 Ca | 1.8 ± 1.3 Bc | |
| D3 | 113.8 | 0.0 ± 0.0 BCa | 0.2 ± 0.2 Ca | 10.2 ± 5.4 Bab | |
| D4 | 227.6 | 0.0 ± 0.0 Da | 0.0 ± 0.0 Ca | 19.8 ± 5.1 Ca | |
| D5 | 455.2 | 1.4 ± 1.4 DEa | 8.2 ± 8.2 Da | 25.3 ± 11.8 DEa | |
| Ferulic acid ** | D1 | 71.07 | 3.4 ± 3. Ac | 0.0 ± 0.0 Bc | 10.8 ± 9.1 ABc |
| D2 | 142.14 | 28.6 ± 12.9 Ab | 27.4 ± 13.9 Ab | 37.6 ± 2.6 Ab | |
| D3 | 284.27 | 37.89 ± 8.3 Ab | 29.6 ± 16.5 Ab | 36.3 ± 5.3 Ab | |
| D4 | 568.54 | 73.47 ± 9.2 Aa | 76.1 ± 9.0 Aa | 60.7 ± 8.8 Aa | |
| Vanillic acid | D1 | 23.6 | 8.4 ± 8.4 Ab | 4.2 ± 1.0 Ab | 6.71 ± 3.36 AB |
| D2 | 47.2 | 0.0 ± 0.0 Cb | 6.6 ± 6.6 ABb | 9.56 ± 6.37 Bb | |
| D3 | 94.3 | 0.2 ± 0.2 Db | 2.8 ± 2.8 Cb | 2.33 ± 2.33 Cb | |
| D4 | 188.6 | 0.0 ± 0.0 Db | 13.5 ± 7.2 Cb | 2.65 ± 2.65 Db | |
| D5 | 377.3 | 86.0 ± 0.9 ABa | 98.8 ± 0.3 Aa | 71.9 ± 2.6 Aba | |
| Syringic acid | D1 | 6.9 | 16.5 ± 6.1 Aa | 0.0 ± 0.0 Ba | 20.7 ± 6.0 Aa |
| D2 | 13.8 | 12.7 ± 4.4 Aba | 0.0 ± 0.0 Ca | 18.9 ± 9.7 Ba | |
| D3 | 27.6 | 1.9 ± 1.4 Da | 0.0 ± 0.0 Ca | 10.3 ± 6.9 Ba | |
| D4 | 55.1 | 6.3 ± 6.3 Da | 0.0 ± 0.0 Ca | 9.0 ± 7.2 Ca | |
| D5 | 110.2 | 7.6 ± 7.6 DEa | 1.6 ± 1.6 Da | 20.2 ± 7.87 DEa | |
| p-hydroxybenzoic acid | D1 | 80.5 | 0.6 ± 0.6 Ab | 0.0 ± 0.0 Bb | 7.7 ± 4.4 ABb |
| D2 | 160.9 | 6.6 ± 6.6 Cb | 0.0 ± 0.0 Cb | 11.7 ± 5.9 Bb | |
| D3 | 321.9 | 9.1 ± 3.5 BCb | 0.0 ± 0.0 Cb | 16.8 ± 6.7 Bb | |
| D4 | 643.8 | 46.5 ± 10.9 Ba | 50.7 ± 18.3 Ba | 41.9 ± 4.7 Ba | |
| D5 | 1287.6 | 56.9 ± 15.3 Ca | 64.9 ± 14.6 BCa | 47.7 ± 8.5 Ca | |
| Protocatechuic acid | D1 | 32.6 | 3.7 ± (1.9) Aab | 0.0 ± 0.0 Ba | 7.7 ± 1.4 ABa |
| D2 | 65.2 | 1.7 ± (1.7) Cc | 0.0 ± 0.0 Ca | 10.9 ± 6.2 Bc | |
| D3 | 130.4 | 13.1 ± 7.2 BCab | 8.9 ± 5.2 ABa | 17.5 ± 2.1 Bab | |
| D4 | 260.8 | 4.6 ± 1.8 Dab | 3.3 ± 1.6 Ca | 8.3 ± 5.3 Cc | |
| D5 | 521.7 | 19.7 ± 3.0 Da | 20.9 ± 13.0 Da | 29.0 ± 7.0 Da | |
| Chlorogenic acid | D1 | 14.1 | 5.4 ± 3.5 Aa | 0.0 ± 0.0 Ba | 13.0 ± 3.0 ABab |
| D2 | 28.2 | 6.9 ± 6.9 Ba | 0.3 ± 0.3 Ca | 13.2 ± 10.3 Bab | |
| D3 | 56.5 | 9.3 ± 9.30 BCa | 0.0 ± 0.0 Ca | 18.2 ± 10.0 Bab | |
| D4 | 113.0 | 7.5 ± 7.1 Da | 0.6 ± 0.6 Ca | 5.5 ± 5.3 Cc | |
| D5 | 225.9 | 20.2 ± 4.7 Da | 5.9 ± 5.9 Da | 23.2 ± 6.6 DEa | |
| p-coumaric acid | D1 | 9.3 | 0.0 ± 0.0 Ab | 12.4 ± 12.4 Bb | 1.5 ± 1.4 Bb |
| D2 | 18.5 | 0.2 ± 0.2 Cb | 0.0 ± 0.0 Cb | 5.1 ± 2.6 Bb | |
| D3 | 37.0 | 4.4 ± 4.4 BCb | 0.0 ± 0.0 Cb | 2.1 ± 2.1 Bb | |
| D4 | 74.1 | 8.1 ± 4.2 Da | 15.4 ± 12.5 Cb | 16.6 ± 8.3 Cb | |
| D5 | 148.2 | 59.9 ± 13.5 Ca | 57.8 ± 25.0 Da | 49.4 ± 5.8 Ca | |
| Mixture of all phenolic acids | D1 | 26.27 | 12.6 ± 3.7 Ac | 0.0 ± 0.0 Bb | 5.6 ± 5.6 ABc |
| D2 | 52.54 | 11.1 ± 11.1 Bc | 10.4 ± 10.4 ABb | 3.4 ± 3.4 Bc | |
| D3 | 105.09 | 19.8 ± 8.4 Bc | 16.6 ± 12.9 ABb | 8.3 ± 8.3 Bc | |
| D4 | 210.18 | 63.3 ± 2.7 ABb | 78.1 ± 6.61 Aa | 37.2 ± 3.9 Bb | |
| D5 | 343.85 | 97.2 ± 2.7 Aa | 96.3 ± 2.5 Aa | 88.5 ± 11.5 Aa | |
| Source of Variability | N-1 | Inhibition Rate (%) | ||
|---|---|---|---|---|
| Shoot | Radicle | Biomass | ||
| Phenolic acids | 9 | <0.001 | <0.001 | <0.001 |
| Dose level (Phenolic acids) | 40 | <0.001 | <0.001 | <0.001 |
| Residual | 100 | 114.32 | 181.85 | 112.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šćepanović, M.; Košćak, L.; Šoštarčić, V.; Pismarović, L.; Milanović-Litre, A.; Kljak, K. Selected Phenolic Acids Inhibit the Initial Growth of Ambrosia artemisiifolia L. Biology 2022, 11, 482. https://doi.org/10.3390/biology11040482
Šćepanović M, Košćak L, Šoštarčić V, Pismarović L, Milanović-Litre A, Kljak K. Selected Phenolic Acids Inhibit the Initial Growth of Ambrosia artemisiifolia L. Biology. 2022; 11(4):482. https://doi.org/10.3390/biology11040482
Chicago/Turabian StyleŠćepanović, Maja, Laura Košćak, Valentina Šoštarčić, Laura Pismarović, Ana Milanović-Litre, and Kristina Kljak. 2022. "Selected Phenolic Acids Inhibit the Initial Growth of Ambrosia artemisiifolia L." Biology 11, no. 4: 482. https://doi.org/10.3390/biology11040482
APA StyleŠćepanović, M., Košćak, L., Šoštarčić, V., Pismarović, L., Milanović-Litre, A., & Kljak, K. (2022). Selected Phenolic Acids Inhibit the Initial Growth of Ambrosia artemisiifolia L. Biology, 11(4), 482. https://doi.org/10.3390/biology11040482









