Possible Ameliorative Effects of the Royal Jelly on Hepatotoxicity and Oxidative Stress Induced by Molybdenum Nanoparticles and/or Cadmium Chloride in Male Rats
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of MoO3-NPs and Their Characterization
2.2.1. MoO3-NPs Synthesis
2.2.2. MoO3-NPs Physicochemical Characterization Methods
2.3. Determination of Half Lethal Dose of MoO3-NPs
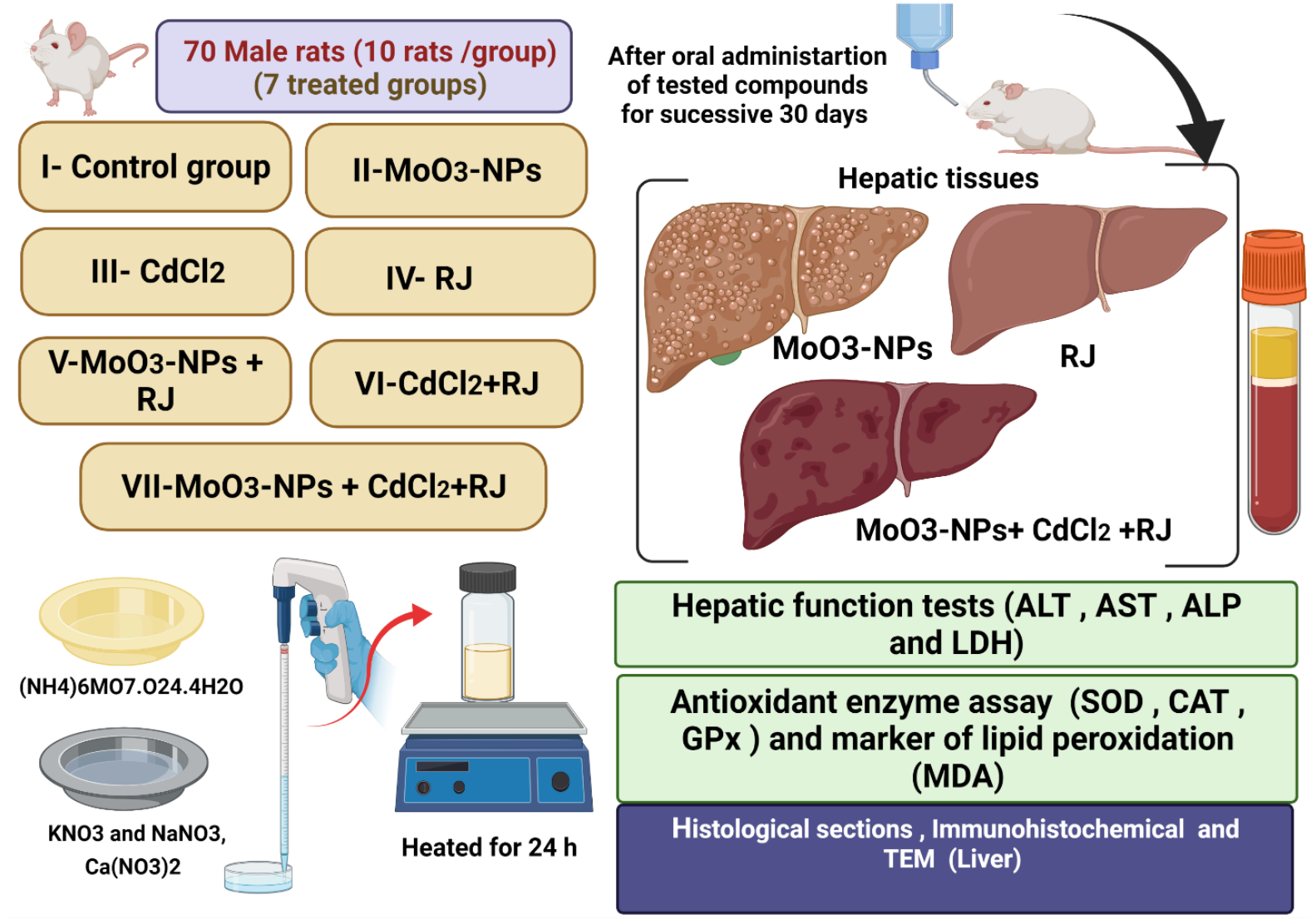
2.4. Animals Groups, Ethics, and Treatment
| Group I served as the control; the animals received 0.9% NaCl (physiological saline). |
| Group II was given 500 mg/kg of MoO3-NPs dispersed in saline. |
| Group III was administered with CdCl2 at a dose of 5 mg/kg dissolved in physiological saline [23]. |
| Group IV was given royal jelly (RJ; 85 mg/kg diluted in saline; this dose is equivalent to 250 mg crude RJ) [24]. |
| Group V received MoO3-NPs as the first dosage, followed by RJ 30 min after the MoO3-NPs were administered. |
| Group VI was given CdCl2 first, followed by RJ, as previously described. |
| Group VII was treated with a combination of MoO3-NPs and CdCl2, followed by RJ treatment at the same dosages (Figure 1). All animals were treated orally for 30 consecutive days. |
2.5. Sample Collection
2.6. Biochemical Investigation
2.6.1. Liver Function Assessment and Inflammation Markers
2.6.2. Assessment of Antioxidant and Oxidative Stress Indices
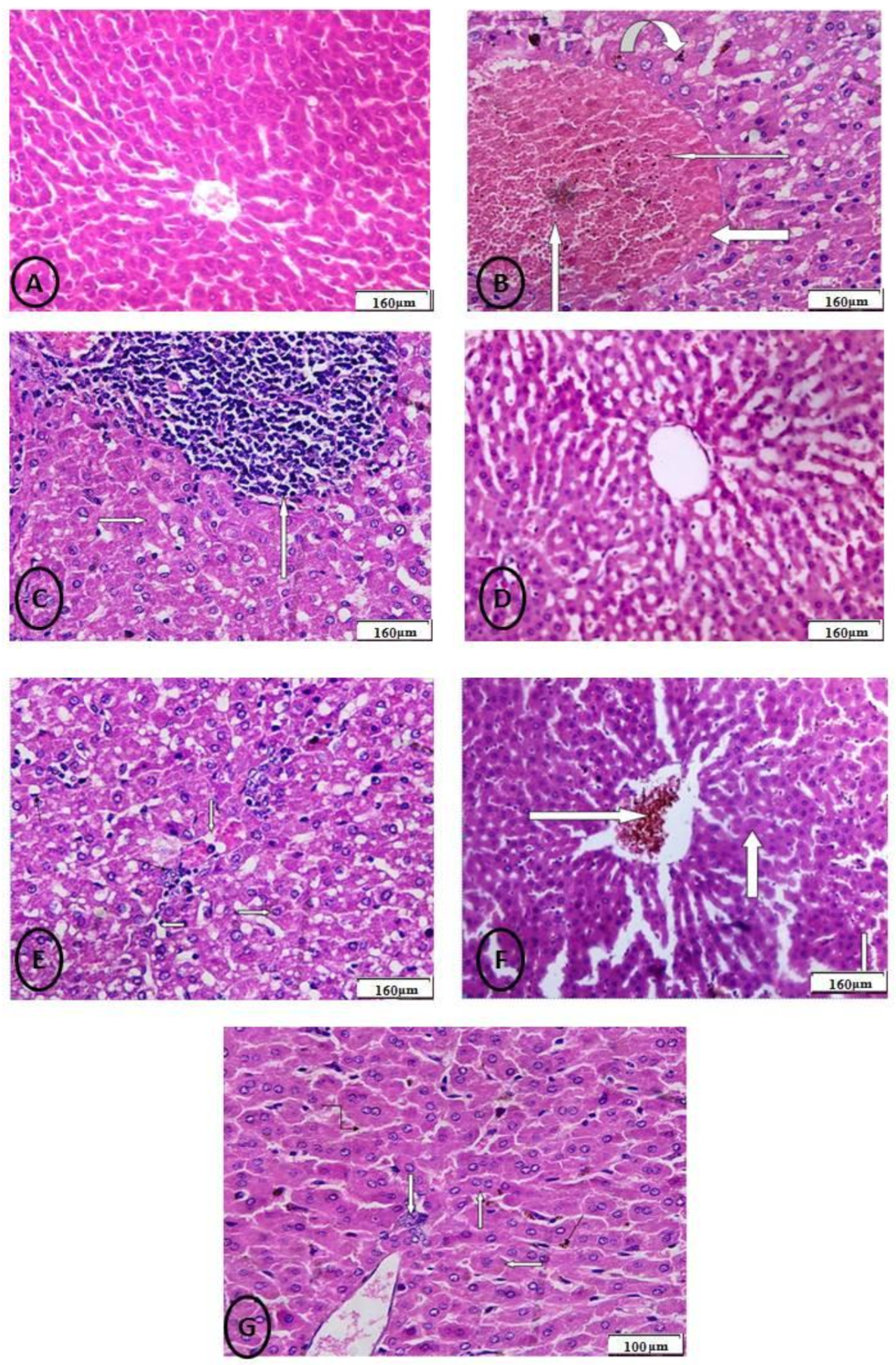
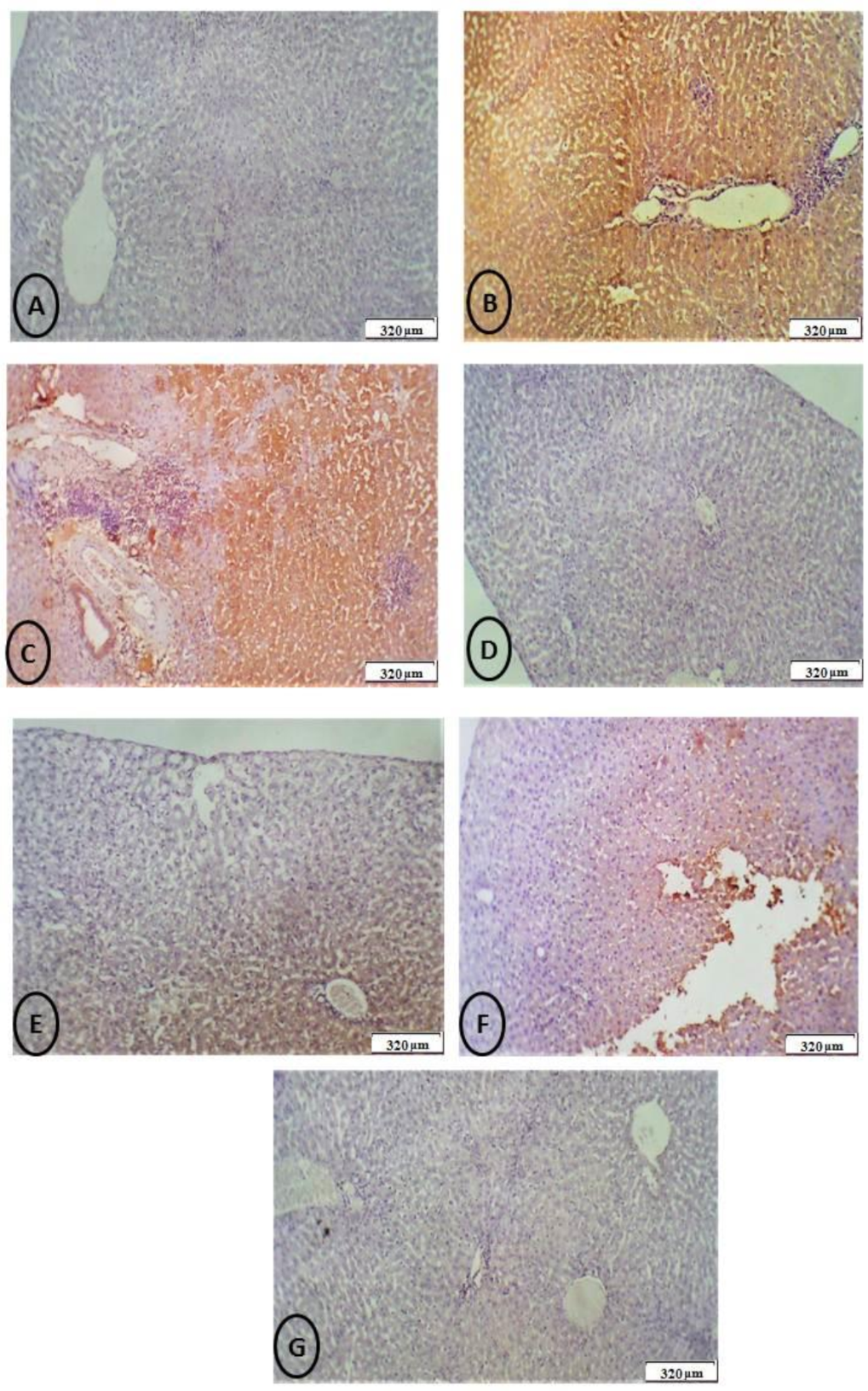
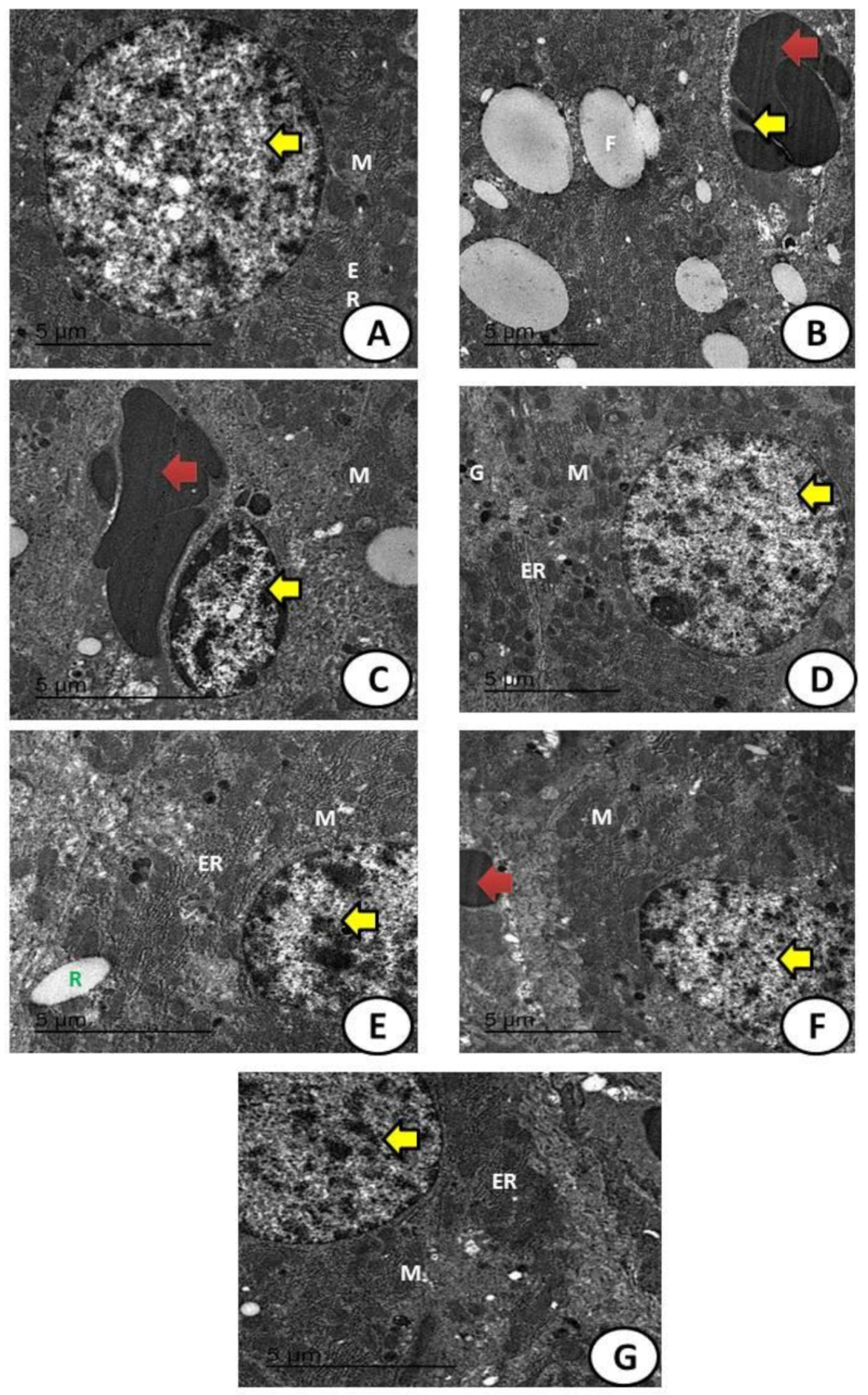
2.7. Histological, Apoptosis, and Immunohistochemically Assessment
2.8. Statistical and Data Analysis
3. Results
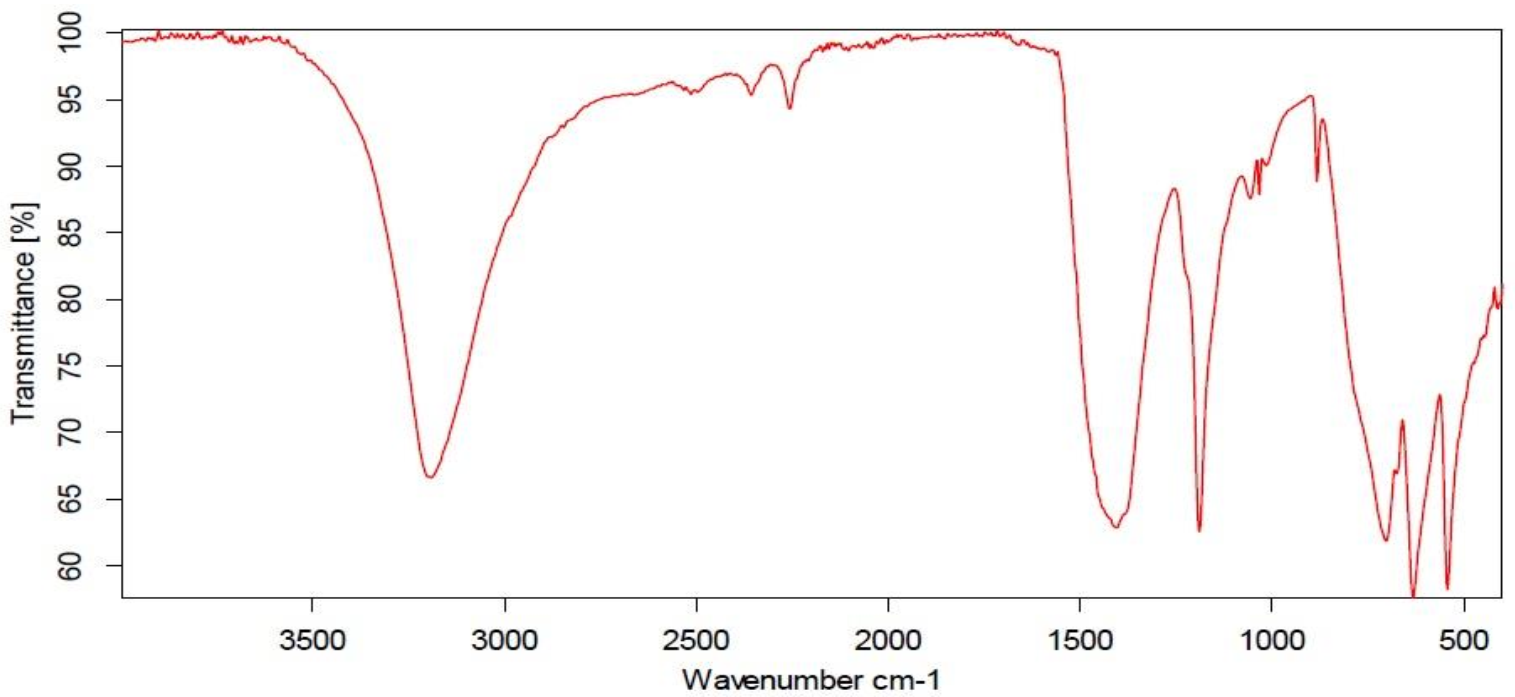
3.1. The Infrared Spectrum of Synthesized MoO3-NPs
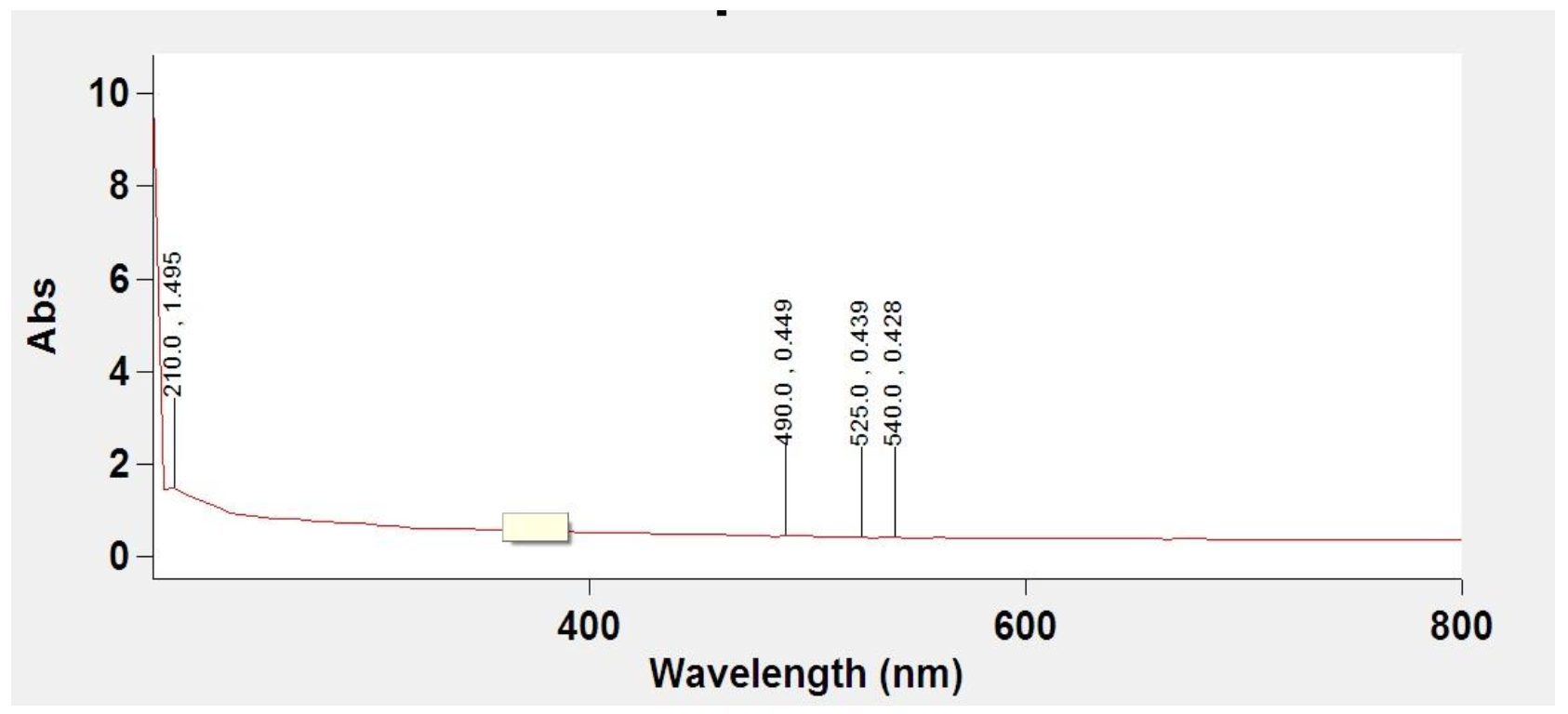
3.2. UV-Vis Spectrum of Synthesized MoO3-NPs
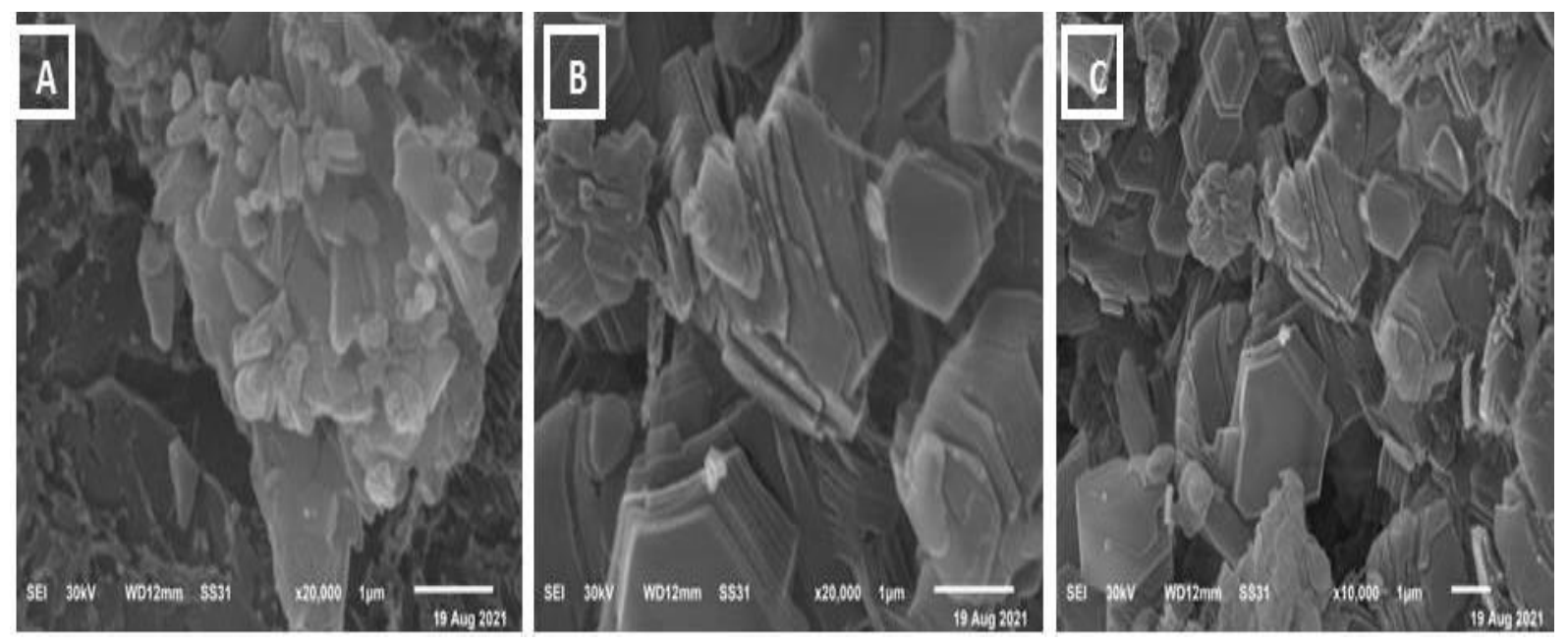
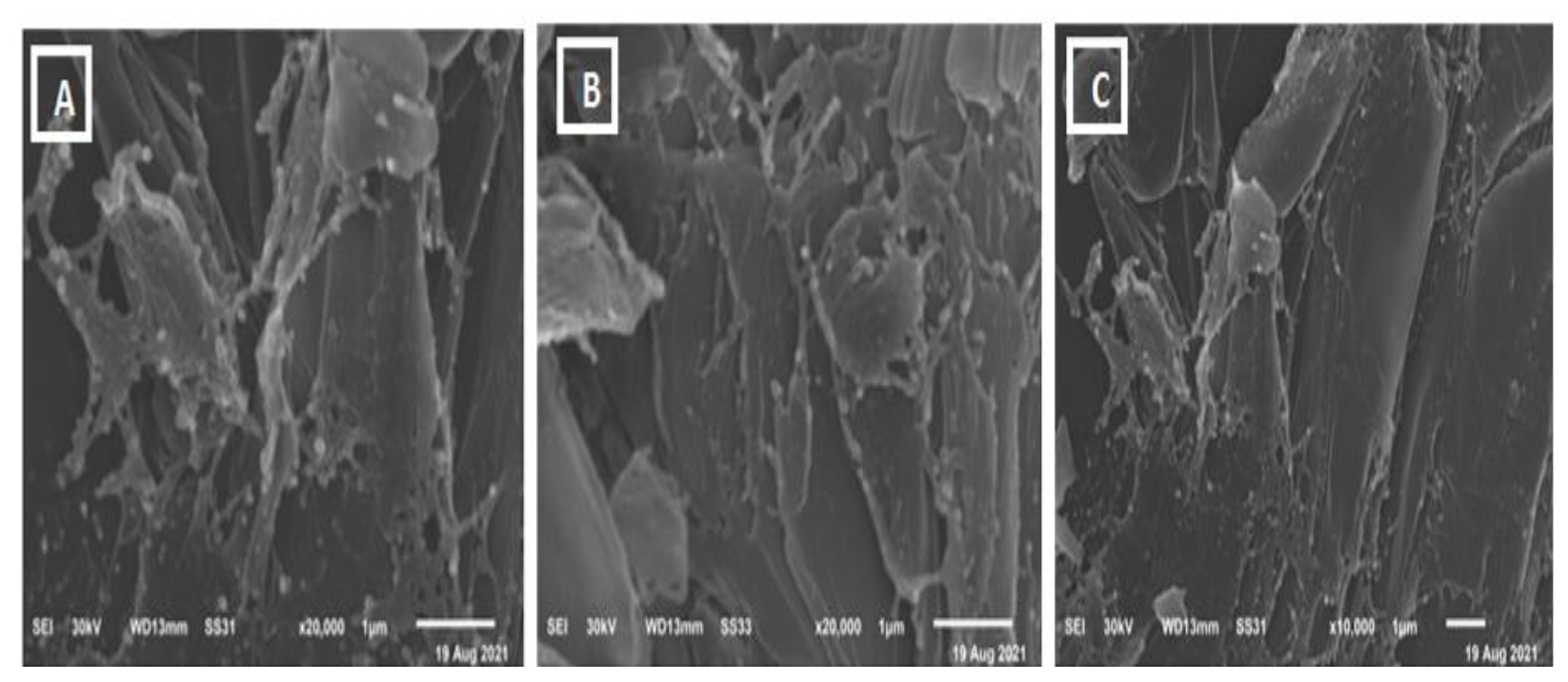
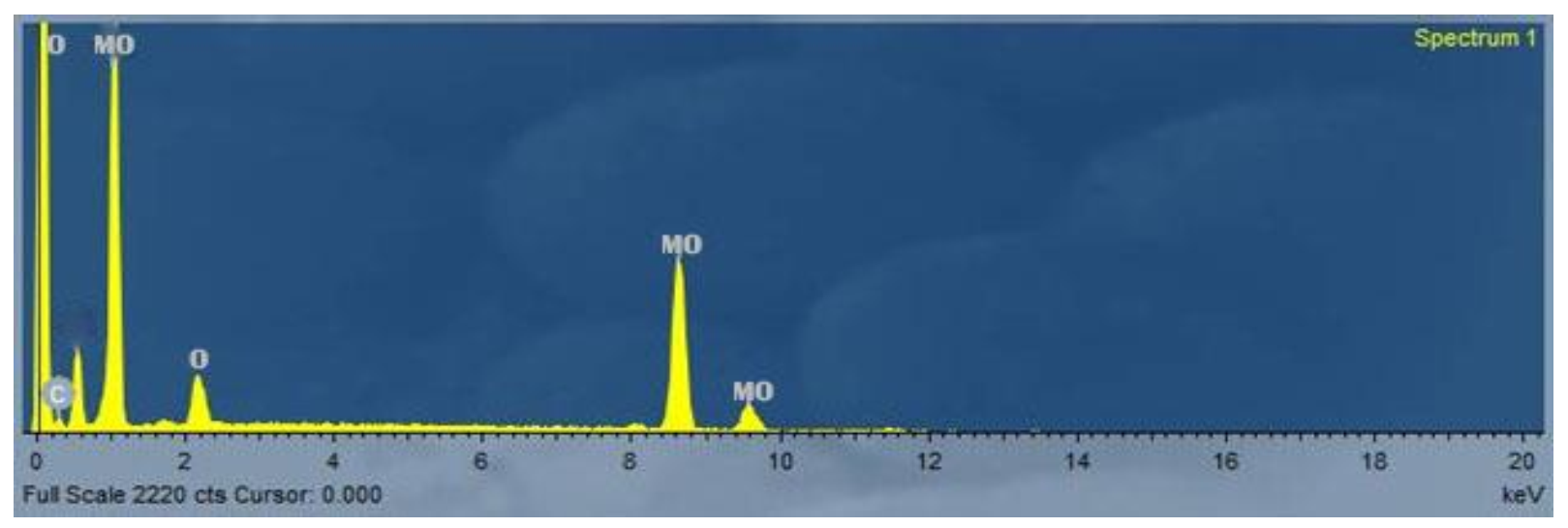
3.3. Scanning Electron Microscope (SEM)
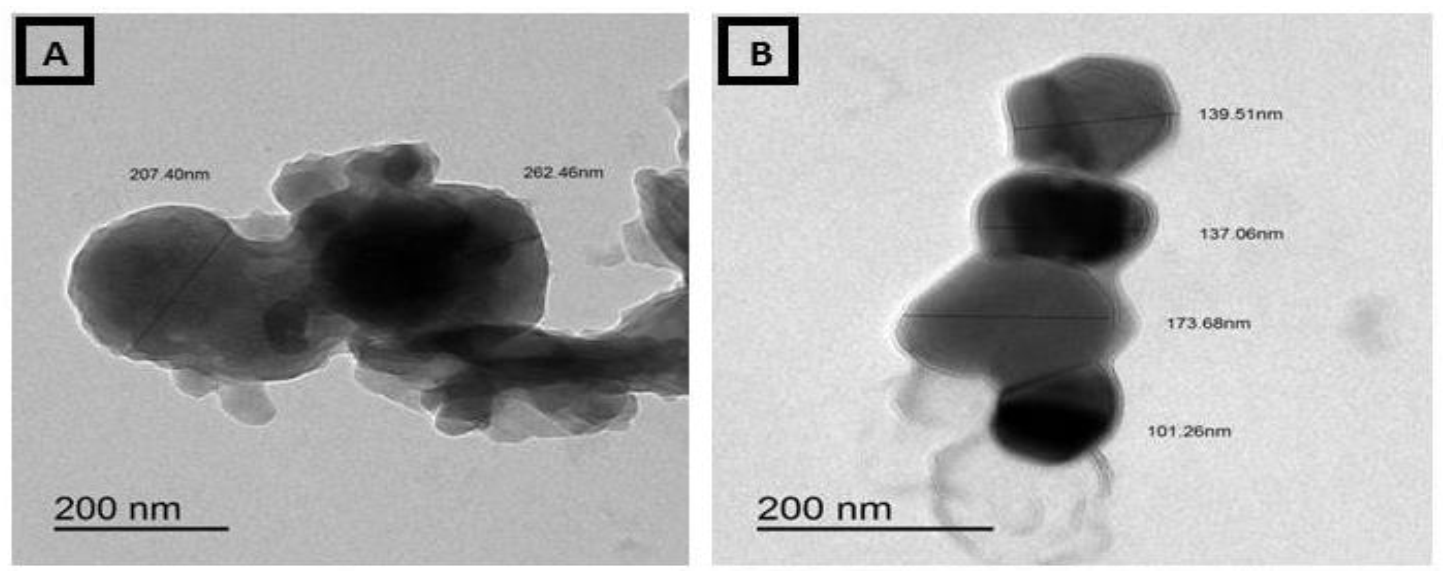
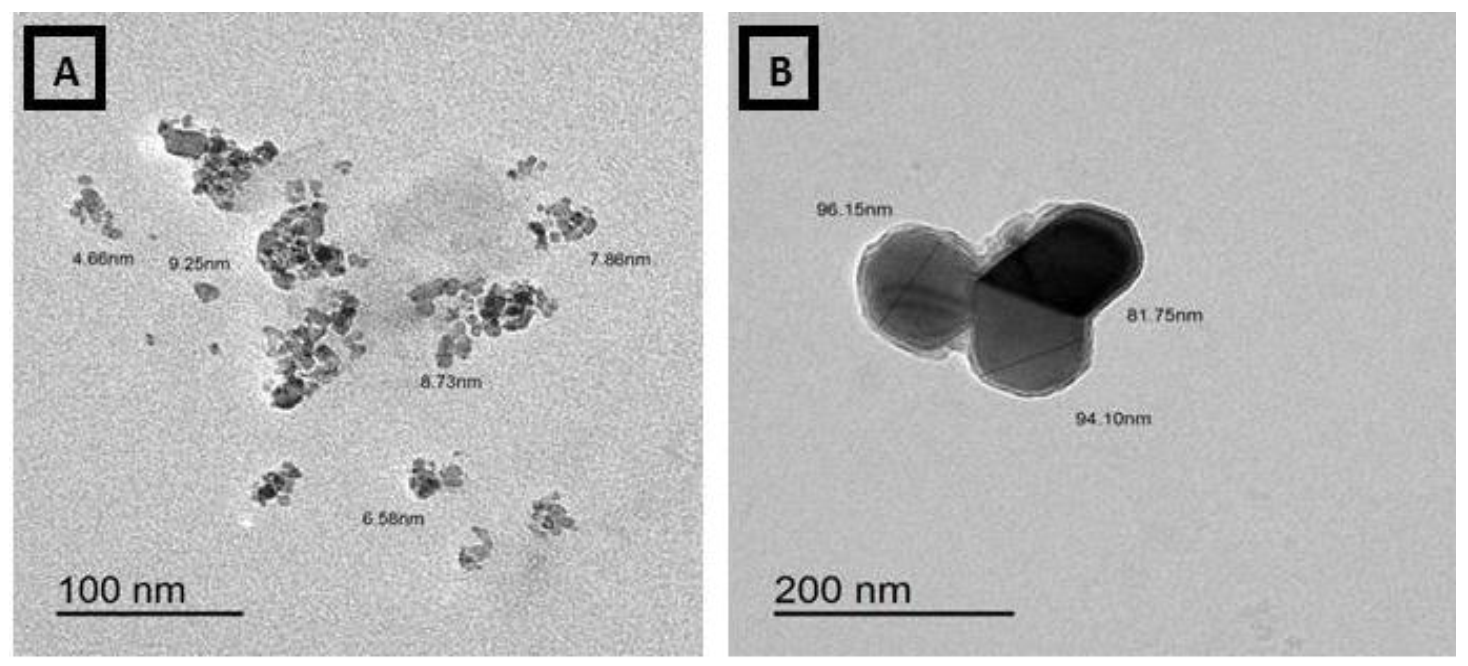
3.4. Transmission Electron Micrographs (TEM) of Ammonium Molybdate and MoO3-NPs
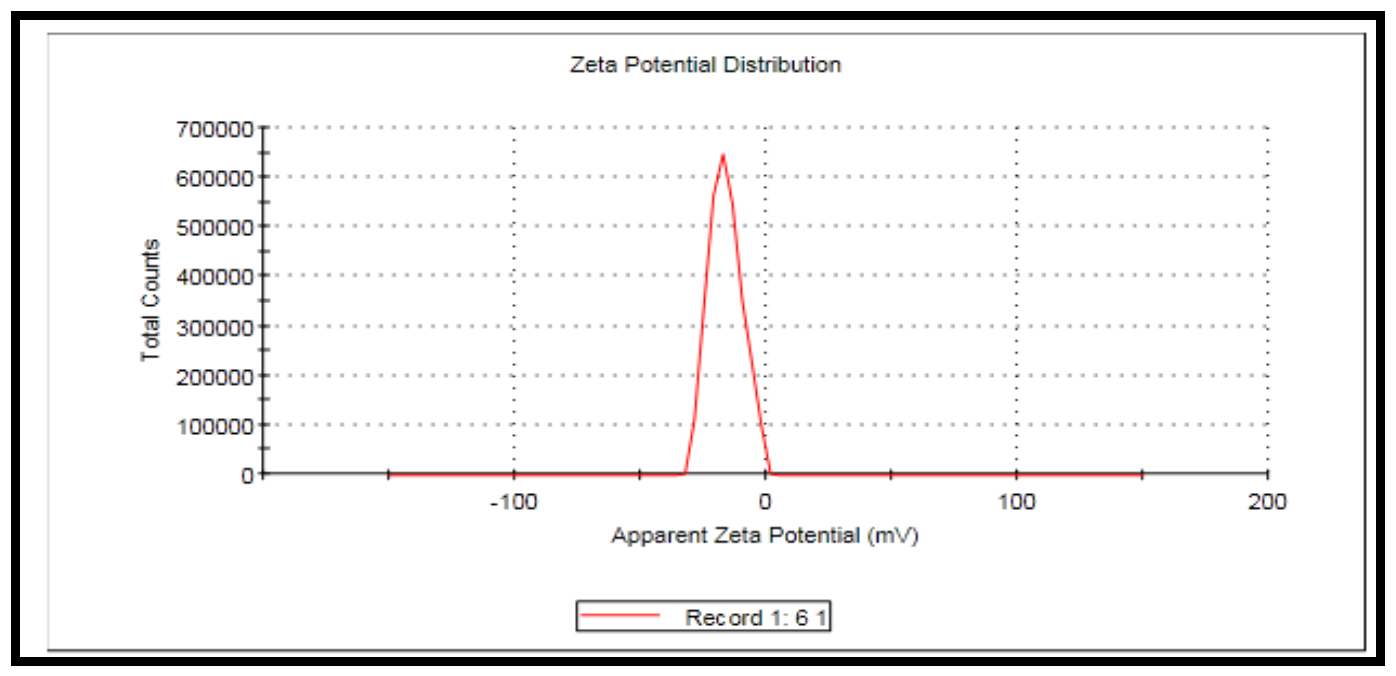
3.5. Zeta Deviation (6.49 mV)
3.6. Biochemical Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asadi, F.; Mohseni, M.; Noshahr, K.D.; Soleymani, F.H.; Jalilvand, A.; Heidari, A. Effect of molybdenum nanoparticles on blood cells, liver enzymes, and sexual hormones in male rats. Biol. Trace Elem. Res. 2017, 175, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Burguera, J.L.; Burguera, M. Molybdenum in human whole blood of adult residents of the Merida state (Venezuela). J. Trace Elem. Med. Biol. 2007, 21, 178–183. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmallah, M.I.Y.; Elkhadragy, M.F.; Al-Olayan, E.M.; Abdel Moneim, A.E. Protective effect of Fragaria ananassa crude extract on cadmium-induced lipid peroxidation, antioxidant enzymes suppression, and apoptosis in rat testes. Int. J. Mol. Sci. 2017, 18, 957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrivas, K.; Agrawal, K.; Harmukh, N. Trace level determination of molybdenum in environmental and biological samples using surfactant-mediated liquid-liquid extraction. J. Hazard Mater. 2009, 161, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.A.; Saquib, Q.; Ahamed, M.; Farshori, N.N.; Ahmad, J.; Wahab, R.; Khan, S.T.; Alhadlaq, H.A.; Musarrat, J.; Al-Khedhairy, A.A.; et al. Molybdenum nanoparticles-induced cytotoxicity, oxidative stress, G2/M arrest, and DNA damage in mouse skin fibroblast cells (L929). Colloid Surf. B 2015, 125, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Al-Salmi, F.A.; Hamza, R.Z.; El-Shenawy, N.S. The interaction of zinc oxide/green tea extract complex nanoparticles and its effect on monosodium glutamate toxicity in liver of rats. Curr. Pharm. Biotechnol. 2019, 20, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Hamza, R.Z.; Al-Salmi, F.A.; El-Shenawy, N.S. Antioxidant activities of green tea extract/zinc oxide nanoparticles against monosodium glutamate in different organs. Avicenna J. Med. Biochem. 2020, 8, 49–52. [Google Scholar] [CrossRef]
- Attia, A.; El-Hendawy, S.; Al-Suhaibani, N.; Tahir, M.U.; Mubushar, M.; Dos Santos Vianna, M.; Ullah, H.; Mansour, E.; Datta, A. Sensitivity of the DSSAT model in simulating maize yield and soil carbon dynamics in arid Mediterranean climate: Effect of soil, genotype and crop management. Field Crops Res. 2021, 260, 107981. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H.A. Antioxidative and cytoprotective response elicited by molybdenum nanoparticles in human cells. J. Colloid Interface Sci. 2015, 457, 370–377. [Google Scholar] [CrossRef]
- Refat, M.S.; Hamza, R.Z.; Adam, A.A.; Saad, H.A.; Gobouri, A.A.; Azab, E.; Al-SalmiF, A.; Altalhi, T.A.; Khojah, E.; Gaber, A.; et al. Antioxidant, antigenotoxic, and hepatic ameliorative effects of quercetin/zinc complex on cadmium-induced hepatotoxicity and alterations in hepatic tissue structure. Coatings 2021, 11, 501. [Google Scholar] [CrossRef]
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, propolis, and royal jelly: A comprehensive review of their biological actions and health. Oxidative Med. Cell. Longev. 2017, 2017, 1–21. [Google Scholar] [CrossRef]
- Spannhoff, A.; Kim, Y.K.; Raynal, N.J.; Gharibyan, V.; Su, M.B.; Zhou, Y.Y.; Li, J.; Castellano, S.; Sbardella, G.; Issa, J.P.; et al. Histone deacetylase inhibitor activity in royal jelly might facilitate caste switching in bees. EMBO Rep. 2011, 12, 238–243. [Google Scholar] [CrossRef] [Green Version]
- Yang, A.; Zhou, M.; Zhang, L.; Xie, G.; Chen, H.; Liu, Z.; Ma, W. Influence of royal jelly on the reproductive function of puberty male rats. Food Chem. Toxicol. 2012, 50, 1834–1840. [Google Scholar] [CrossRef]
- Nakajima, Y.; Tsuruma, K.; Shimazawa, M.; Mishima, S.; Hara, H. Comparison of bee products based on assays of antioxidant capacities. BMC Complement. Altern. Med. 2009, 9, 4. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Tian, Y.; Han, M.; Miao, X. Longevity extension of worker honey bees (Apis mellifera) by royal jelly: Optimal dose and active ingredient. Peer J. 2017, 5, e3118. [Google Scholar] [CrossRef] [Green Version]
- Filippo, F.; Giovanni, C.; Simone, M.; Antonio, F. Royal Jelly: An ancient remedy with remarkable antibacterial properties. Microbiol. Res. 2016, 192, 130–141. [Google Scholar]
- Habashy, N.H.; Abu-Serie, M.M. Major royal-jelly protein 2 and its isoform X1 are two novel safe inhibitors for hepatitis C and B viral entry and replication. Int. J. Biol. Macromol. 2019, 141, 1072–1087. [Google Scholar] [CrossRef]
- Ali, F.K.; Mohamed, A.; Hassan, A.A. Therapeutic effects of royal jelly on some biochemical parameters in nephrotoxicity induced by carbon tetrachloride (CCl4) In Albino Rats. Egypt. Acad. J. Biol. Sci. B Zool. 2021, 13, 279–286. [Google Scholar] [CrossRef]
- Poloyac, S.M. Drug and fatty acid cytochrome P450 metabolism in critical care. In Drug Metabolism in Diseases 2017; Academic Press: Boston, MA, USA, 2017; pp. 115–138. [Google Scholar]
- Tang, H.; Xu, M.; Luo, J.; Zhao, L.; Ye, G.; Shi, F.; Lv, C.; Chen, H.; Wang, Y.; Li, Y. Liver toxicity assessments in rats following sub-chronic oral exposure to copper nanoparticles. Environ. Sci. Eur. 2019, 31, 30. [Google Scholar] [CrossRef] [Green Version]
- Tice, R.R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Miyamae, Y.; Rojas, E.; Ryu, J.C.; Sasaki, Y.F. Single-cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagenes. 2000, 35, 206–221. [Google Scholar] [CrossRef]
- Al-Baqami, N.M.; Hamza, R.Z. Protective effect of resveratrol against hepatotoxicity of cadmium in male rats: Antioxidant and histopathological approaches. Coatings 2021, 11, 594. [Google Scholar] [CrossRef]
- Almeer, R.S.; Alarifi, S.; Alkahtani, S.; Ibrahim, S.R.; Abdel Moneim, D.A. The potential hepatoprotective effect of royal jelly against cadmium chloride-induced hepatotoxicity in mice is mediated by suppression of oxidative stress and upregulation of Nrf2 expression. Biomed. Pharmacother. 2018, 106, 1490–1498. [Google Scholar] [CrossRef] [PubMed]
- Carr, T.P.; Andresen, C.J.; Rudel, L.L. Enzymatic determination of triglyceride, free cholesterol, and total cholesterol in tissue lipid extracts. Clin. Biochem. 1993, 26, 39–42. [Google Scholar] [CrossRef]
- Warnick, G.; Benderson, J.; Albers, J.J. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin. Chem. 1993, 28, 1379–1388. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Norbert, W.T. Clinical Guide to Laboratory Tests, 3rd ed.; WB Saunders Company: Philadephi, PA, USA, 1995; Volume 35, p. 972. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Sun, Y.; Oberley, L.W.; Li, Y.A. Simple method for clinical assay of superoxide dismutase. Clin. Chem. 1988, 34, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Paglia, D.E.; Valentive, W.N. Studies on the avantitaue and gualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Kowalczyk, E.; Kopff, A.; Fijalkowski, P.; Kopff, M.; Niedworok, J.; Blaszczyk, J.; Kedziora, J.; Tyslerowicz, P. Effect of anthocyanins on selected biochemical parameters in rats exposed to cadmium. Acta Biochim. Pol. 2003, 50, 543–548. [Google Scholar] [CrossRef] [Green Version]
- Cotter, T.G.; Rinella, M. Nonalcoholic fatty liver disease. 2020: The State of the Disease. Gastroenterology 2020, 158, 1851–1864. [Google Scholar] [CrossRef]
- You, M.M.; Liu, Y.C.; Chen, Y.F.; Pan, Y.M.; Miao, Z.N.; Shi, Y.Z.; Si, J.J.; Chen, M.L.; Hu, F.L. Royal jelly attenuates nonalcoholic fatty liver disease by inhibiting oxidative stress and regulating the expression of circadian genes in ovariectomized rats. J. Food Biochem. 2020, 44, e13138. [Google Scholar] [CrossRef]
- Segun, L.; Figlaz, M.; Cavagnat, R.; Lassegues, J.C. Infrared and Raman spectra of MoO3 molybdenum trioxides and MoO3· xH2O molybdenum trioxide hydrates. Spectrochim. Acta Part A 1995, 51, 1323–1344. [Google Scholar] [CrossRef]
- Schoonman, J. Nanostructured materials in solid-state ionics. Solid State Ion. 2000, 135, 5–19. [Google Scholar] [CrossRef]
- Chithambararaj, A.; Bose, A.C. Investigation on structural, thermal, optical and sensing properties of meta-stable hexagonal MoO3 nanocrystals of one dimensional structure. Beilstein J. Nanotechnol. 2011, 2, 585–592. [Google Scholar] [CrossRef] [Green Version]
- Bagheri, K.G.S.; Chandrappa, S.; Hamid, S.B.A. Generation of Hematite Nanoparticles via Sol-Gel Method. Res. J. Chem. Sci. 2013, 3, 62–68. [Google Scholar]
- Natarajan, G.; Daniels, S.; Cameron, D.C.; Reilly, L.O.; Mitra, A.; McNally, P.J.; Lucas, O.F.; Rajendra Kumar, R.T.; Reid, I.; Bradley, A.L. Stoichiometry control of sputtered CuCl thin films: Influence on ultraviolet emission properties. J. Appl. Phys. 2006, 100, 033520. [Google Scholar] [CrossRef] [Green Version]
- Sobańska, Z.; Zapór, L.; Szparaga, M.; Stępnik, M. Biological effects of molybdenum compounds in nanosized forms under in vitro and in vivo conditions. Int. J. Occup. Med. Environ. Health 2020, 33, 1–19. [Google Scholar] [CrossRef]
- Mohamed, H.R.H.; El-Atawy, R.H.; Ghoneim, A.M.; El-Ghor, A.A. Induction of fetal abnormalities and genotoxicity by molybdenum nanoparticles in pregnant female mice and fetuses. Environ. Sci. Pollut. Res. Int. 2020, 27, 23950–23962. [Google Scholar] [CrossRef]
- Abu-El-Zahab, H.S.H.; Hamza, R.Z.; Montaser, M.M.; El-Mahdi, M.M.; Al-Harthi, W.A. Antioxidant, antiapoptotic, antigenotoxic, and hepatic ameliorative effects of L-carnitine and selenium on cadmium-induced hepatotoxicity and alterations in liver cell atructure in male mice. Ecotoxicol. Environ. Saf. 2019, 173, 419–428. [Google Scholar] [CrossRef]
- Collazo, N.; Carpena, M.; Nuñez-Estevez, B.; Otero, P.; Simal-Gandara, J.; Prieto, M.A. Health Promoting Properties of Bee Royal Jelly: Food of the Queens. Nutrients 2021, 13, 543. [Google Scholar] [CrossRef]
- Karadeniz, A.; Simsek, N.; Karakus, E.; Yildirim, S.; Kara, A.; Can, I.; Kisa, F.; Emre, H.; Turkeli, M. Royal jelly modulates oxidative stress and apoptosis in liver and kidneys of rats treated with cisplatin. Oxidative Med. Cell. Longev. 2011, 2011, 981793. [Google Scholar] [CrossRef] [Green Version]
- Kanbur, M.; Eraslan, G.; Beyaz, L.; Silici, S.; Liman, B.C.; Altinordulu, S.; Atasever, A. The effects of royal jelly on liver damage induced by paracetamol in mice. Exp. Toxicol. Pathol. 2009, 61, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Alharthi, W.A.; Hamza, R.Z.; Elmahdi, M.M.; Abuelzahab, H.S.H.; Saleh, H. Selenium and L-carnitine ameliorate reproductive toxicity induced by cadmium in male mice. Biol. Trace Elem. Res. 2020, 197, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Braydich-Stolle, L.; Hussain, S.; Schlager, J.J.; Hofmann, M.C. In Vitro cytotoxicity of nanoparticles in mammalian germline stem cells. Toxicol. Sci. 2005, 88, 412–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaskill, C.L.; Miller, L.M.; Mattoon, J.S.; Hoffmann, W.E.; Burton, S.A.; Gelens, H.; Cribb, A.E. Liver histopathology and liver and serum alanine aminotransferase and alkaline phosphatase activities in epileptic dogs receiving phenobarbital. Vet. Pathol. 2005, 42, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, G.D.; Kumar, P.R.; Bharavi, K.; Annapurna, P.; Rajendar, B.; Patel, P.T.; Rao, G.S. Protective effect of Tribulus terrestris Linn on liver and kidney in cadmium intoxicated rats. Indian J. Exp. Biol. 2012, 50, 141–146. [Google Scholar] [PubMed]
- Kyriakou, L.G.; Tzirogiannis, K.N.; Demonakou, M.D.; Kourentzi, K.T.; Mykoniatis, M.G.; Panoutsopoulos, G.I. Gadolinium chloride pretreatment ameliorates acute cadmium-induced hepatotoxicity. Toxicol. Ind. Health 2013, 29, 624–632. [Google Scholar] [CrossRef]
- Guo, H.; Ekusa, A.; Iwai, K.; Yonekura, M.; Takahata, Y.; Morimatsu, F. Royal jelly peptides inhibit lipid peroxidation in vitro and in vivo. J. Nutr. Sci. Vitaminol. 2008, 54, 191–195. [Google Scholar] [CrossRef] [Green Version]
- Kanbur, M.; Eraslan, G.; Silici, S.; Karabacak, M. Effects of sodium fluoride exposure on some biochemical parameters in mice: Evaluation of the ameliorative effect of royal jelly applications on these parameters. Food Chem. Toxicol. 2009, 47, 1184–1189. [Google Scholar] [CrossRef]
- Ahmad, S.; Campos, M.G.; Fratini, F.; Altaye, S.Z.; Li, J. New Insights into the Biological and Pharmaceutical Properties of Royal Jelly. Int. J. Mol. Sci. 2020, 21, 382. [Google Scholar] [CrossRef] [Green Version]
- Vazhacharickal, P.J. A review on health benefits and biological action of honey, propolis and royal jelly. J. Medicinal. Plants Studies 2021, 9, 1–13. [Google Scholar]
- Bogdanov, S. Royal Jelly, Bee Brood: Composition, Health, Medicine. J. Bee Prod. Sci. 2011, 3, 8–19. [Google Scholar]
- Yan, A.T.; Yan, R.T.; Tan, M.; Hackam, D.G.; Leblanc, K.L.; Kertland, H.; Tsang, J.L.; Jaffer, S.; Kates, M.L.; Leiter, L.A.; et al. Contemporary management of dyslipidemia in high-risk patients: Targets still not met. Am. J. Med. 2006, 119, 676–683. [Google Scholar] [CrossRef]
- Kunugi, H.; Mohammed Ali, A. Royal Jelly and Its Components Promote Healthy Aging and Longevity: From Animal Models to Humans. Int. J. Mol. Sci. 2019, 20, 4662. [Google Scholar] [CrossRef] [Green Version]
- Yoneshiro, T.; Kaede, R.; Nagaya, K.; Aoyama, J.; Saito, M.; Okamatsu-Ogura, Y.; Kimura, K.; Terao, A. Royal jelly ameliorates diet-induced obesity and glucose intolerance by promoting brown adipose tissue thermogenesis in mice. Obes. Res. Clin. Pract. 2018, 12, 127–137. [Google Scholar] [CrossRef]
- Kashima, Y.; Kanematsu, S.; Asai, S.; Kusada, M.; Watanabe, S.; Kawashima, T.; Nakamura, T.; Shimada, M.; Goto, T.; Nagaoka, S. Identification of a novel hypocholesterolemic protein, major royal jelly protein 1, derived from royal jelly. PLoS ONE 2014, 9, e105073. [Google Scholar] [CrossRef] [Green Version]
- Kamakura, M.; Moriyama, T.; Sakaki, T. Changes in hepatic gene expression associated with the hypocholesterolaemic activity of royal jelly. J. Pharm. Pharmacol. 2006, 58, 1683–1689. [Google Scholar] [CrossRef]
- You, M.M.; Chen, Y.F.; Pan, Y.M.; Liu, Y.C.; Tu, J.; Wang, K.; Hu, F.L. Royal jelly attenuates LPS-induced inflammation in BV-2 microglial cells through modulating NF-κB and p38/JNK signaling pathways. Mediat. Inflamm. 2018, 2018, 7834381. [Google Scholar] [CrossRef] [Green Version]
- Hadi, A.; Najafgholizadeh, A.; Aydenlu, E.S.; Shafiei, Z.; Pirivand, F.; Golpour, S.; Pourmasoumi, M. Royal jelly is an effective and relatively safe alternative approach to blood lipid modulation: A meta-analysis. J. Funct. Foods 2018, 41, 202–209. [Google Scholar] [CrossRef]
- Maleki, V.; Jafari-Vaughan, H.; Saleh-Ghadimi, S.; Adibian, M.; Kheirouri, S.; Alizadeh, M. Effects of Royal jelly on metabolic variables in diabetes mellitus: A systematic review. Complementary Ther. Med. 2019, 43, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Alghasham, A.; Tarek, A.S.; Abdel-Raheim, M.M. Effect of cadmium-polluted water on plasma levels of tumor necrosis factor-α, interleukin-6 and oxidative status biomarkers in rats: Protective effect of curcumin. Food Chem. Toxicol. 2013, 59, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Kohno, K.; Okamoto, I.; Sano, O.; Arai, N.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Royal Jelly Inhibits the Production of Proinflammatory Cytokines by Activated Macrophages. Biosci. Biotechnol. Biochem. 2004, 68, 138–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.H.; Shih, C.M.; Huang, C.J.; Lin, C.M.; Chou, C.M.; Tsai, M.L.; Liu, T.P.; Chiu, J.F.; Chen, C.T. Effects of cadmium on structure and enzymatic activity of Cu, Zn-SOD and oxidative status in neural cells. J. Cell. Biochem. 2006, 98, 577–589. [Google Scholar] [CrossRef]
- Donald, G. Molybdenum. Clin. Toxicol. 1999, 37, 231–237. [Google Scholar]
- El-Megharbel, S.M.; Al-Salmi, F.A.; Al-Harthi, S.; Alsolami, K.; Hamza, R.Z. Chitosan/Selenium Nanoparticles Attenuate Diclofenac Sodium-Induced Testicular Toxicity in Male Rats. Crystals 2021, 11, 1477. [Google Scholar] [CrossRef]
- Hamza, R.Z.; Al-Baqami, N.M.; Khojah, E.; Mansour, A.M.A.; Al-Motaani, S.E.; Al-Salmi, F.A.; El-Megharbel, S.M. Possible Antioxidant and Antidiabetic Effects of Combretum Molle Extract in a Diabetes Mellitus Experimental Model in Male Rats. Nat. Prod. Commun. 2021, 16, 1934578X211043964. [Google Scholar]
- Al-Salmi, F.A.; Hamza, R.Z. Efficacy of Vanadyl Sulfate and Selenium Tetrachloride as Anti-Diabetic Agents against Hyperglycemia and Oxidative Stress Induced by Diabetes Mellitus in Male Rats. Curr. Issues Mol. Biol. 2022, 44, 94–104. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.J.; Siddiqui, M.A.; Ahmad, J.; Musarrat, J.; Al-Khedhairy, A.A.; AlSalhi, M.S.; Alrokayan, S.A. Oxidative stress mediated apoptosis induced by nickel ferrite nanoparticles in cultured A549 cells. Toxicology 2011, 283, 101. [Google Scholar] [CrossRef]
- Hussain, S.M.; HessbJ, K.L.; GearhartcK, M.; GeissdJ, T.; Schlager, J. In Vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol. In Vitro 2005, 19, 975–983. [Google Scholar] [CrossRef]
- Mokaya, H.O.; Njeru, L.K.; Lattorff, H.M.G. African honeybee royal jelly: Phytochemical contents, free radical scavenging activity, and physicochemical properties. Food Biosci. 2020, 37, 100733. [Google Scholar] [CrossRef]
| Parameters | Control | MoO3-NPs | CdCl2 | RJ | MoO3-NPs + RJ | CdCl2 + RJ | MoO3-NPs + CdCl2+ RJ |
|---|---|---|---|---|---|---|---|
| ALT (U/L) | 12.12 ± 0.07 f | 156.88 ± 2.52 b | 184.30 ± 2.05 a | 13.25 ± 0.14 f | 29.28 ± 0.92 d | 35.39 ± 1.72 c | 23.64 ± 0.92 e |
| AST (U/L) | 12.45 ± 0.16 f | 282.22 ± 1.93 a | 273.40 ± 3.86 b | 12.45 ± 0.12 f | 34.17 ± 2.78 d | 37.12 ± 2.52 c | 27.30 ± 1.83 e |
| ALP (U/L) | 26.87 ± 0.44 f | 194.85 ± 2.32 a | 179.92 ± 3.07 b | 23.17 ± 1.40 g | 27.36 ± 0.58 ef | 37.23 ± 1.44 c | 30.11 ± 0.877 d |
| LDH (U/L) | 110.04 ± 4.29 d | 525.60 ± 18.66 b | 604.47 ± 36.14 a | 102.43 ± 1.51 d | 193.99 ± 7.96 c | 213.06 ± 13.12 c | 141.56 ± 14.311 d |
| Total proteins (g/dL) | 8.05 ± 0.15 a | 4.25 ± 0.28 c | 4.36 ± 0.41 c | 7.67 ± 0.20 a | 5.61 ± 0.34 b | 5.67 ± 0.26 b | 7.06 ± 0.57 a |
| Parameters (mg/dL) | Control | MoO3-NPs | CdCl2 | RJ | MoO3-NPs + RJ | CdCl2 + RJ | MoO3-NPs + CdCl2 + RJ |
|---|---|---|---|---|---|---|---|
| TG | 76.01 ± 1.66 c | 185.48 ± 5.72 a | 171.11 ± 12.66 a | 72.07 ± 2.04 c | 147.09 ± 5.57 b | 139.34 ± 4.47 b | 133.99 ± 10.23 b |
| TC | 134.19 ± 4.40 d | 270.91 ± 2.13 a | 266.76 ± 8.99 b | 132.96 ± 5.25 d | 173.26 ± 2.19 c | 196.24 ± 9.05 b | 146.86 ± 6.12 d |
| HDL-c | 39.30 ± 0.60 a | 26.30 ± 1.54 e | 23.18 ± 0.866 f | 36.88 ± 1.47 bc | 29.02 ± 1.17 de | 30.09 ± 1.02 d | 34.61 ± 0.799 c |
| LDL-c | 28.91 ± 1.02 d | 40.74 ± 1.28 a | 38.94 ± 1.84 b | 26.09 ± 0.96 d | 34.41 ± 0.82 c | 34.16 ± 1.20 c | 27.95 ± 1.01 d |
| vLDL-c | 15.41 ± 0.211 f | 35.79 ± 0.688 b | 38.34 ± 0.60 a | 14.33 ± 0.44 f | 28.93 ± 0.43 c | 26.81 ± 0.76 d | 22.14 ± 0.47 e |
| Groups | IL-6 (Pg/g) | TNF-α (Pg/g) | CRP (mg/L) |
|---|---|---|---|
| Control | 3.30 ± 0.13 d | 5.28 ± 0.10 e | 4.24 ± 0.11 e |
| MoO3-NPs | 27.40 ± 0.62 a | 32.15 ± 0.54 b | 26.40 ± 0.99 b |
| CdCl2 | 22.98 ± 0.59 b | 36.79 ± 0.69 a | 28.81 ± 0.49 a |
| RJ | 3.02 ± 0.03 d | 5.74 ± 0.28 e | 4.74 ± 0.27 e |
| MoO3-NPs + RJ | 8.06 ± 0.15 c | 19.92 ± 0.53 c | 17.30 ± 0.46 c |
| CdCl2 + RJ | 7.80 ± 0.28 c | 19.44 ± 1.18 c | 16.04 ± 0.499 c |
| MoO3-NPs + CdCl2 + RJ | 7.33 ± 0.53 c | 15.85 ± 0.399 d | 13.81 ± 0.91 d |
| Groups | MDA (nmoles of MDA/g) | CAT (nmol/g of Protein/min) | SOD (U/g of Protein) | GPx (nmol/g of Protein/min) |
|---|---|---|---|---|
| Control | 5.53 ± 0.22 e | 8.19 ± 0.27 a | 19.69 ± 0.62 b | 14.13 ± 0.69 a |
| MoO3-NPs | 33.12 ± 1.09 b | 3.09 ± 0.66 d | 7.57 ± 0.44 e | 6.52 ± 0.58 d |
| CdCl2 | 38.83 ± 0.65 a | 3.35 ± 0.44 d | 6.40 ± 0.39 e | 5.36 ± 0.98 d |
| RJ | 5.10 ± 0.065 e | 8.17 ± 0.21 a | 21.76 ± 0.65 a | 14.25 ± 1.69 a |
| MoO3-NPs +RJ | 18.24 ± 0.49 c | 5.02 ± 0.55 c | 11.41 ± 0.50 d | 8.28 ± 1.58 c |
| CdCl2 + RJ | 20.13 ± 0.68 c | 5.85 ± 0.48 c | 11.27 ± 0.89 d | 9.25 ± 1.65 c |
| MoO3-NPs + CdCl2 + RJ | 16.03 ± 0.96 d | 7.25 ± 0.85 bc | 16.70 ± 0.58 c | 12.65 ± 0.98 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamza, R.Z.; Al-Eisa, R.A.; El-Shenawy, N.S. Possible Ameliorative Effects of the Royal Jelly on Hepatotoxicity and Oxidative Stress Induced by Molybdenum Nanoparticles and/or Cadmium Chloride in Male Rats. Biology 2022, 11, 450. https://doi.org/10.3390/biology11030450
Hamza RZ, Al-Eisa RA, El-Shenawy NS. Possible Ameliorative Effects of the Royal Jelly on Hepatotoxicity and Oxidative Stress Induced by Molybdenum Nanoparticles and/or Cadmium Chloride in Male Rats. Biology. 2022; 11(3):450. https://doi.org/10.3390/biology11030450
Chicago/Turabian StyleHamza, Reham Z., Rasha A. Al-Eisa, and Nahla S. El-Shenawy. 2022. "Possible Ameliorative Effects of the Royal Jelly on Hepatotoxicity and Oxidative Stress Induced by Molybdenum Nanoparticles and/or Cadmium Chloride in Male Rats" Biology 11, no. 3: 450. https://doi.org/10.3390/biology11030450
APA StyleHamza, R. Z., Al-Eisa, R. A., & El-Shenawy, N. S. (2022). Possible Ameliorative Effects of the Royal Jelly on Hepatotoxicity and Oxidative Stress Induced by Molybdenum Nanoparticles and/or Cadmium Chloride in Male Rats. Biology, 11(3), 450. https://doi.org/10.3390/biology11030450







