Impact of Red Complex Bacteria and TNF-α Levels on the Diabetic and Renal Status of Chronic Kidney Disease Patients in the Presence and Absence of Periodontitis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
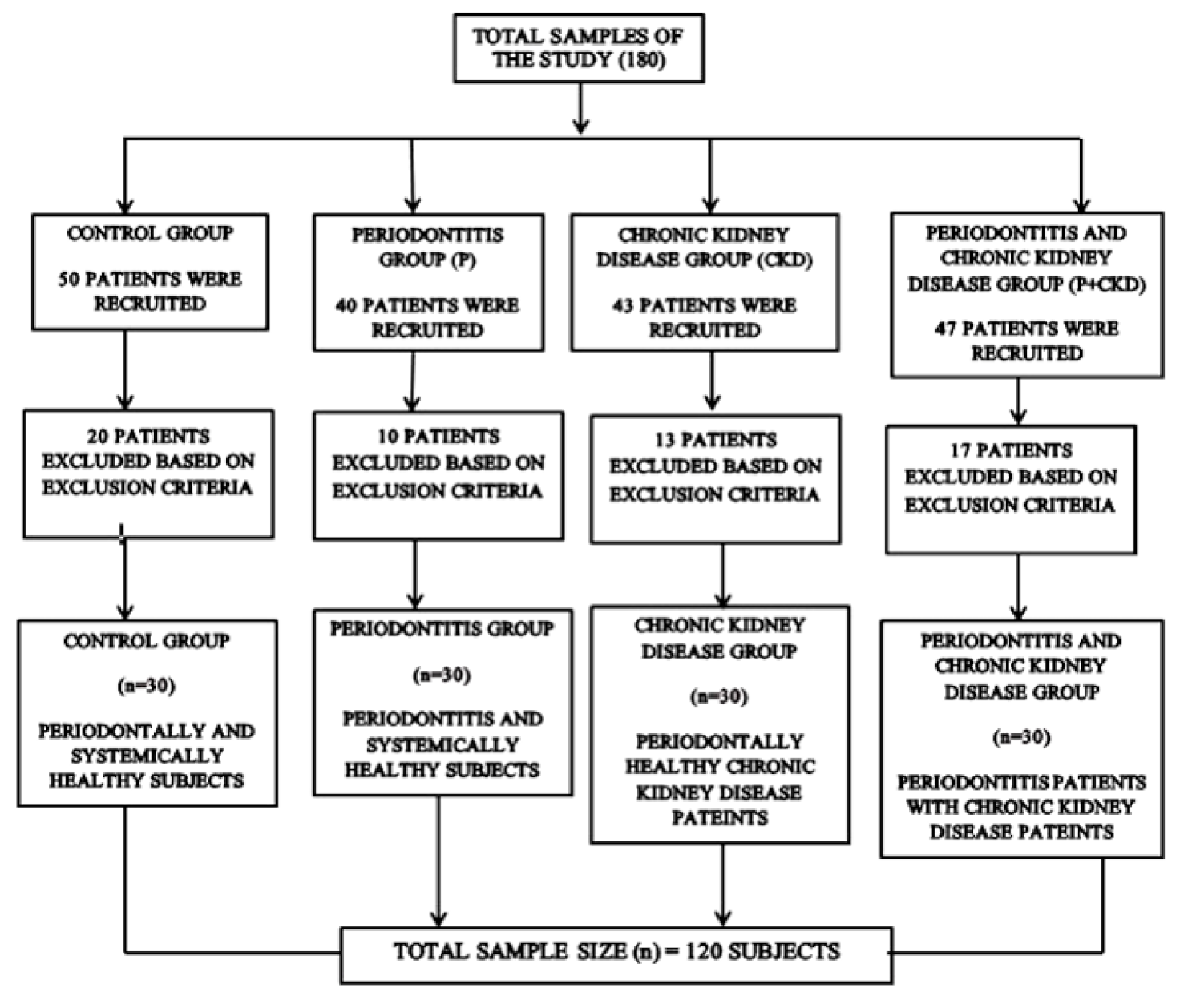
2.1. Study Design
2.2. Sample Collection
2.2.1. Blood Collection
2.2.2. Subgingival Plaque Sample Collection
2.3. Assessed Study Parameters
2.3.1. Demographic Variables
2.3.2. Periodontal Parameters
2.3.3. Diabetic Assessment
2.3.4. Renal Parameters
2.3.5. Tumour Necrosis Factor-α
2.4. Molecular Analysis
RT-PCR Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomas, R.; Kanso, A.; Sedor, J.R. Chronic Kidney Disease and Its Complications. Prim. Care Clin. Off. Pract. 2008, 35, 329–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D.R. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malekmakan, L.; Khajehdehi, P.; Pakfetrat, M.; Malekmakan, A.; Mahdaviazad, H.; Roozbeh, J. Prevalence of Chronic Kidney Disease and Its Related Risk Factors in Elderly of Southern Iran: A Population-Based Study. ISRN Nephrol. 2013, 2013, 427230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, P.S. From focal sepsis to periodontal medicine: A century of exploring the role of the oral microbiome in systemic disease. J. Physiol. 2017, 595, 465–476. [Google Scholar] [CrossRef]
- Manakil, J. (Ed.) Periodontal Diseases—A Clinician’s Guide; Books on Demand: Norderstedt, Germany, 2012; ISBN 978-953-307-818-2. [Google Scholar]
- Kim, J.; Amar, S. Periodontal disease and systemic conditions: A bidirectional relationship. Odontology 2006, 94, 10–21. [Google Scholar] [CrossRef] [Green Version]
- Hajishengallis, G.; Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 2021, 21, 426–440. [Google Scholar] [CrossRef]
- Nazir, M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. (Qassim) 2017, 11, 72–80. [Google Scholar]
- Grubbs, V.; Vittinghoff, E.; Taylor, G.; Kritz-Silverstein, D.; Powe, N.; Bibbins-Domingo, K.; Ishani, A.; Cummings, S.R. The association of periodontal disease with kidney function decline: A longitudinal retrospective analysis of the MrOS dental study. Nephrol. Dial. Transplant. 2016, 31, 466–472. [Google Scholar] [CrossRef] [Green Version]
- Lertpimonchai, A.; Rattanasiri, S.; Tamsailom, S.; Champaiboon, C.; Ingsathit, A.; Kitiyakara, C.; Limpianunchai, A.; Attia, J.; Sritara, P.; Thakkinstian, A. Periodontitis as the risk factor of chronic kidney disease: Mediation analysis. J. Clin. Periodontol. 2019, 46, 631–639. [Google Scholar] [CrossRef] [Green Version]
- Silva, N.; Abusleme, L.; Bravo, D.; Dutzan, N.; Garcia-Sesnich, J.; Vernal, R.; Hernández, M.; Gamonal, J. Host response mechanisms in periodontal diseases. J. Appl. Oral Sci. 2015, 23, 329–355. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Kabi, D. Severe periodontitis associated with chronic kidney disease. J. Indian Soc. Periodontol. 2013, 17, 128. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, Y.-L.; Liu, X.-Y.; Meng, X.; Zhao, R.-Q.; Ou, L.-L.; Li, B.-Z.; Xing, T. Periodontitis Exacerbates and Promotes the Progression of Chronic Kidney Disease Through Oral Flora, Cytokines, and Oxidative Stress. Front. Microbiol. 2021, 12, 656372. [Google Scholar] [CrossRef] [PubMed]
- Deschamps-Lenhardt, S.; Martin-Cabezas, R.; Hannedouche, T.; Huck, O. Association between periodontitis and chronic kidney disease: Systematic review and meta-analysis. Oral Dis. 2019, 25, 385–402. [Google Scholar] [CrossRef] [PubMed]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Periodontol. 2018, 89, S1–S8. [Google Scholar] [CrossRef]
- Levin, A.; Stevens, P.E.; Bilous, R.W.; Coresh, J.; De Francisco, A.L.; De Jong, P.E.; Griffith, K.E.; Hemmelgarn, B.R.; Iseki, K.; Lamb, E.J.; et al. Chapter 1: Definition and classification of CKD. Kidney Int. Suppl. 2013, 3, 19–62. [Google Scholar] [CrossRef] [Green Version]
- Löe, H. The Gingival Index, the Plaque Index and the Retention Index Systems. J. Periodontol. 1967, 38, 610–616. [Google Scholar] [CrossRef]
- Cekici, A.; Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontology 2000 2014, 64, 57–80. [Google Scholar] [CrossRef] [Green Version]
- Falcao, A.; Bullón, P. A review of the influence of periodontal treatment in systemic diseases. Periodontology 2000 2019, 79, 117–128. [Google Scholar] [CrossRef]
- Ismail, G.; Dumitriu, H.T.; Dumitriu, A.S.; Ismail, F.B. Periodontal Disease: A Covert Source of Inflammation in Chronic Kidney Disease Patients. Int. J. Nephrol. 2013, 2013, 515796. [Google Scholar] [CrossRef]
- Hickey, N.A.; Shalamanova, L.; Whitehead, K.A.; Dempsey-Hibbert, N.; van der Gast, C.; Taylor, R.L. Exploring the putative interactions between chronic kidney disease and chronic periodontitis. Crit. Rev. Microbiol. 2020, 46, 61–77. [Google Scholar] [CrossRef]
- Mahendra, J.; Parthiban, P.S.; Mahendra, L.; Balakrishnan, A.; Shanmugam, S.; Junaid, M.; Romanos, G.E. Evidence Linking the Role of Placental Expressions of Peroxisome Proliferator-Activated Receptor-γ and Nuclear Factor-Kappa B in the Pathogenesis of Preeclampsia Associated With Periodontitis. J. Periodontol. 2016, 87, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Ilango, P.; Mahendra, J.; Mahendra, L.; Cherian, S.M.; Kathaperumal, K.; Suresh, V.; Mahalingam, A. Evidence linking the role of periodontal viruses in coronary artery disease with and without periodontitis. J. Periodontol. 2021, 92, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, A.C.; Athavale, A.; Chen, J.; Hampole, H.; Garside, D.; Marucha, P.; Lash, J.P. Periodontal disease, chronic kidney disease and mortality: Results from the third national health and nutrition examination survey. BMC Nephrol. 2015, 16, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borawski, J.; Wilczynska-Borawska, M.; Stokowska, W.; Mysliwiec, M. The periodontal status of pre-dialysis chronic kidney disease and maintenance dialysis patients. Nephrol. Dial. Transplant. 2006, 22, 457–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Souza, C.M.; Braosi, A.P.R.; Luczyszyn, S.M.; Olandoski, M.; Kotanko, P.; Craig, R.G.; Trevilatto, P.C.; Pecoits-Filho, R. Association Among Oral Health Parameters, Periodontitis, and Its Treatment and Mortality in Patients Undergoing Hemodialysis. J. Periodontol. 2014, 85, e169–e178. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.; Sun, L.; Klein, B.E.K.; Lee, K.E.; Muntner, P.; Nieto Javier, F.; Tsai, M.Y.; Cruickshanks, K.J.; Schubert, C.R.; Brazy, P.C.; et al. Markers of inflammation predict the long-term risk of developing chronic kidney disease: A population-based cohort study. Kidney Int. 2011, 80, 1231–1238. [Google Scholar] [CrossRef] [Green Version]
- Kapellas, K.; Singh, A.; Bertotti, M.; Nascimento, G.G.; Jamieson, L.M. Periodontal and chronic kidney disease association: A systematic review and meta-analysis. Nephrology 2019, 24, 202–212. [Google Scholar] [CrossRef]
- Chang, J.-F.; Yeh, J.-C.; Chiu, Y.-L.; Liou, J.-C.; Hsiung, J.-R.; Tung, T.-H. Periodontal Pocket Depth, Hyperglycemia, and Progression of Chronic Kidney Disease: A Population-Based Longitudinal Study. Am. J. Med. 2017, 130, 61–69.e1. [Google Scholar] [CrossRef] [Green Version]
- George, C.; Matsha, T.E.; Korf, M.; Zemlin, A.E.; Erasmus, R.T.; Kengne, A.P. The agreement between fasting glucose and markers of chronic glycaemic exposure in individuals with and without chronic kidney disease: A cross-sectional study. BMC Nephrol. 2020, 21, 32. [Google Scholar] [CrossRef] [Green Version]
- Naghsh, N.; Sabet, N.K.; Vahidi, F.; Mogharehabed, A.; Yaghini, J. Relationship Between Periodontal Disease and Serum Factors in Patients Undergoing Hemodialysis. Open Dent. J. 2017, 11, 701–709. [Google Scholar] [CrossRef]
- Balu, P.; Muthu, J.; Velayutham, A.; Ravindran, S. Association between Serum Creatinine and Periodontal Disease Severity—A Comparative Clinicobiochemical Study. J. Sci. Dent. 2020, 10, 3–6. [Google Scholar] [CrossRef]
- Iwasaki, M.; Taylor, G.W.; Nesse, W.; Vissink, A.; Yoshihara, A.; Miyazaki, H. Periodontal Disease and Decreased Kidney Function in Japanese Elderly. Am. J. Kidney Dis. 2012, 59, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.A.; Taylor, G.W.; Shelton, B.J.; Jamerson, K.A.; Rahman, M.; Ojo, A.O.; Sehgal, A.R. Periodontal Disease and Other Nontraditional Risk Factors for CKD. Am. J. Kidney Dis. 2008, 51, 45–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.-F.; Lin, C.-L.; Lin, M.-C.; Lin, S.-Y.; Sung, F.-C.; Kao, C.-H. Surgical Treatment for Patients With Periodontal Disease Reduces Risk of End-Stage Renal Disease: A Nationwide Population-Based Retrospective Cohort Study. J. Periodontol. 2014, 85, 50–56. [Google Scholar] [CrossRef]
- Chambrone, L.; Foz, A.M.; Guglielmetti, M.R.; Pannuti, C.M.; Artese, H.P.C.; Feres, M.; Romito, G.A. Periodontitis and chronic kidney disease: A systematic review of the association of diseases and the effect of periodontal treatment on estimated glomerular filtration rate. J. Clin. Periodontol. 2013, 40, 443–456. [Google Scholar] [CrossRef]
- Pan, W.; Wang, Q.; Chen, Q. The cytokine network involved in the host immune response to periodontitis. Int. J. Oral Sci. 2019, 11, 30. [Google Scholar] [CrossRef] [Green Version]
- Ficek, R.; Kokot, F.; Chudek, J.; Adamczak, M.; Ficek, J.; Więcek, A. Plasma Concentrations of Tumor Necrosis Factor Alpha May Predict the Outcome of Patients with Acute Renal Failure. Kidney Blood Press. Res. 2006, 29, 203–209. [Google Scholar] [CrossRef]
- Kir, H.M.; Eraldemir, C.; Dervisoglu, E.; Caglayan, C.; Kalender, B. Effects of chronic kidney disease and type of dialysis on serum levels of adiponectin, TNF-alpha and high sensitive C-reactive protein. Clin. Lab. 2012, 58, 495–500. [Google Scholar]
- Lee, B.T.; Ahmed, F.A.; Hamm, L.L.; Teran, F.J.; Chen, C.-S.; Liu, Y.; Shah, K.; Rifai, N.; Batuman, V.; Simon, E.E.; et al. Association of C-reactive protein, tumor necrosis factor-alpha, and interleukin-6 with chronic kidney disease. BMC Nephrol. 2015, 16, 77. [Google Scholar] [CrossRef] [Green Version]
- Fisher, M.A.; Taylor, G.W.; Papapanou, P.N.; Rahman, M.; Debanne, S.M. Clinical and Serologic Markers of Periodontal Infection and Chronic Kidney Disease. J. Periodontol. 2008, 79, 1670–1678. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Ketteler, M.; Johnson, R.J.; Lindholm, B.; Pecoits-Filho, R.; Riella, M.; Heimbürger, O.; Cederholm, T.; Girndt, M. IL-10, IL-6, and TNF-α: Central factors in the altered cytokine network of uremia—The good, the bad, and the ugly. Kidney Int. 2005, 67, 1216–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Artese, H.P.C.; de Sousa, C.O.; de Barros Torres, M.C.M.; Silva-Boghossian, C.M.; Colombo, A.P.V. Effect of non-surgical periodontal treatment on the subgingival microbiota of patients with chronic kidney disease. Braz. Oral Res. 2012, 26, 366–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Kshirsagar, A.V.; Craig, R.G.; Moss, K.L.; Beck, J.D.; Offenbacher, S.; Kotanko, P.; Klemmer, P.J.; Yoshino, M.; Levin, N.W.; Yip, J.K.; et al. Periodontal disease adversely affects the survival of patients with end-stage renal disease. Kidney Int. 2009, 75, 746–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- França, L.F.C.; Vasconcelos, A.C.C.G.; da Silva, F.R.P.; Alves, E.H.P.; Carvalho, J.S.; Lenardo, D.D.; de Souza, L.K.M.; Barbosa, A.L.R.; Medeiros, J.-V.R.; de Oliveira, J.S.; et al. Periodontitis changes renal structures by oxidative stress and lipid peroxidation. J. Clin. Periodontol. 2017, 44, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, M.; Mochizuki, Y.; Miyata, Y.; Obata, Y.; Mitsunari, K.; Matsuo, T.; Ohba, K.; Mukae, H.; Yoshimura, A.; Nishino, T.; et al. Pathological Characteristics of Periodontal Disease in Patients with Chronic Kidney Disease and Kidney Transplantation. Int. J. Mol. Sci. 2019, 20, 3413. [Google Scholar] [CrossRef] [Green Version]
- Bastos, J.A.; Diniz, C.G.; Bastos, M.G.; Vilela, E.M.; Silva, V.L.; Chaoubah, A.; Souza-Costa, D.C.; Andrade, L.C.F. Identification of periodontal pathogens and severity of periodontitis in patients with and without chronic kidney disease. Arch. Oral Biol. 2011, 56, 804–811. [Google Scholar] [CrossRef] [Green Version]
- Schenkein, H.A.; Koertge, T.E.; Brooks, C.N.; Sabatini, R.; Purkall, D.E.; Tew, J.G. IL-17 in Sera from Patients with Aggressive Periodontitis. J. Dent. Res. 2010, 89, 943–947. [Google Scholar] [CrossRef] [Green Version]
- Dorn, B.R.; Harris, L.J.; Wujick, C.T.; Vertucci, F.J.; Progulske-Fox, A. Invasion of vascular cells in vitro by Porphyromonas endodontalis. Int. Endod. J. 2002, 35, 366–371. [Google Scholar] [CrossRef]
- Takahashi, Y.; Davey, M.; Yumoto, H.; Gibson, F.C., III; Genco, C.A. Fimbria-dependent activation of pro-inflammatory molecules in Porphyromonas gingivalis infected human aortic endothelial cells. Cell. Microbiol. 2006, 8, 738–757. [Google Scholar] [CrossRef]
- Takeuchi, H.; Furuta, N.; Morisaki, I.; Amano, A. Exit of intracellular Porphyromonas gingivalis from gingival epithelial cells is mediated by endocytic recycling pathway. Cell. Microbiol. 2011, 13, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Tomás, I.; Diz, P.; Tobías, A.; Scully, C.; Donos, N. Periodontal health status and bacteraemia from daily oral activities: Systematic review/meta-analysis. J. Clin. Periodontol. 2012, 39, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Reyes, L.; Herrera, D.; Kozarov, E.; Roldán, S.; Progulske-Fox, A. Periodontal bacterial invasion and infection: Contribution to atherosclerotic pathology. J. Clin. Periodontol. 2013, 40, S30–S50. [Google Scholar] [CrossRef] [PubMed]
- Shultis, W.A.; Weil, E.J.; Looker, H.C.; Curtis, J.M.; Shlossman, M.; Genco, R.J.; Knowler, W.C.; Nelson, R.G. Effect of Periodontitis on Overt Nephropathy and End-Stage Renal Disease in Type 2 Diabetes. Diabetes Care 2007, 30, 306–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Krauss, J.L.; Domon, H.; Hosur, K.B.; Liang, S.; Magotti, P.; Triantafilou, M.; Triantafilou, K.; Lambris, J.D.; Hajishengallis, G. Microbial Hijacking of Complement–Toll-Like Receptor Crosstalk. Sci. Signal. 2010, 3, ra11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrera, B.S.; Martins-Porto, R.; Campi, P.; Holzhausen, M.; Teixeira, S.A.; Mendes, G.D.; Costa, S.K.P.; Gyurko, R.; Van Dyke, T.E.; Spolidório, L.C.; et al. Local and cardiorenal effects of periodontitis in nitric oxide-deficient hypertensive rats. Arch. Oral Biol. 2011, 56, 41–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ksiazek, M.; Mizgalska, D.; Enghild, J.J.; Scavenius, C.; Thogersen, I.B.; Potempa, J. Miropin, a Novel Bacterial Serpin from the Periodontopathogen Tannerella forsythia, Inhibits a Broad Range of Proteases by Using Different Peptide Bonds within the Reactive Center Loop. J. Biol. Chem. 2015, 290, 658–670. [Google Scholar] [CrossRef] [Green Version]
- Chukkapalli, S.S.; Ambadapadi, S.; Varkoly, K.; Jiron, J.; Aguirre, J.I.; Bhattacharyya, I.; Morel, L.M.; Lucas, A.R.; Lakshmyya, K. Impaired innate immune signaling due to combined Toll-like receptor 2 and 4 deficiency affects both periodontitis and atherosclerosis in response to polybacterial infection. Pathog. Dis. 2018, 76, fty076. [Google Scholar] [CrossRef]
- Isola, G.; Polizzi, A.; Santonocito, S.; Dalessandri, D.; Migliorati, M.; Indelicato, F. New Frontiers on Adjuvants Drug Strategies and Treatments in Periodontitis. Sci. Pharm. 2021, 89, 46. [Google Scholar] [CrossRef]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Maiorani, C.; Milone, A.; Alovisi, M.; Scribante, A. Paraprobiotics in Non-Surgical Periodontal Therapy: Clinical and Microbiological Aspects in a 6-Month Follow-up Domiciliary Protocol for Oral Hygiene. Microorganisms 2022, 10, 337. [Google Scholar] [CrossRef]
| Variable | C | P | CKD | P + CKD | ANOVA | p Value |
|---|---|---|---|---|---|---|
| Age (mean + SD) | 37.63 + 10.26 | 54.03 + 9.10 | 59.27 + 10.90 | 61.47 + 10.99 | 32.56 | 0.004 * |
| Male (%) | 22.2 | 20.6 | 27 | 30.2 | 0.39 | 0.173 (NS) |
| BMI | 25.70 + 3.48 | 26.39 + 4.39 | 25.81 + 3.79 | 26.48 + 4.81 | 0.272 | 0.846 (NS) |
| Socioeconomic status | 18,084 ± 5020 | 19,059 ± 4085 | 19,076± 4097 | 19,069 ± 4084 | 0.546 | 0.3 (NS) |
| Plaque index | 0.79 + 0.29 | 1.79 + 0.22 | 0.88 + 0.29 | 2.27 + 0.23 | 225.748 | 0.001 * |
| Gingival index | 0.91 + 0.34 | 1.92 + 0.35 | 1.05 + 0.30 | 2.42 + 0.18 | 172.28 | 0.001 * |
| PPD | 1.33 + 0.17 | 2.77 + 0.27 | 1.29 + 0.09 | 3.18 + 0.24 | 675.859 | 0.002 * |
| Site percentage with PPD ≥ 5 mm | 0 + 0 | 12.03 + 9.29 | 0 + 0 | 21.87 + 7.49 | 94.276 | 0.003 * |
| CAL | 0 + 0 | 0.72 + 0.26 | 0 + 0 | 1.14 + 0.48 | 126.427 | 0.001 * |
| Site percentage with CAL ≥ 3 mm | 0 + 0 | 19.36 + 7.72 | 0 + 0 | 30.43 + 11.00 | 150.83 | 0.00 * |
| FBS | 89.43 ± 9.72 | 111.17 ± 48.08 | 103.73 ± 41.57 | 130.03 ± 52.07 | 5.008 | 0.003 * |
| HBA1C | 5.28 ± 0.22 | 5.97 ± 1.16 | 6.01 ± 1.22 | 7.62 ± 1.46 | 7.439 | 0.000 * |
| Serum creatinine | 0.80 + 0.14 | 0.72 + 0.11 | 1.20 + 0.43 | 1.08 + 0.19 | 25.20 | 0.00 * |
| eGFR | 107.67 + 14.81 | 101 + 7.10 | 64.77 + 18.94 | 68.43 + 12.45 | 74.154 | 0.005 * |
| TNF-α | 53.06 ± 25.51 | 72.75 ± 22.86 | 63.05 ± 29.90 | 69.28 ± 18.07 | 3.743 | 0.013 |
| P.g | 29.77 + 1.20 | 25.99 + 2.09 | 27 + 1.54 | 24.33 + 2.39 | 44.662 | 0.001 * |
| T.d | 28.58 + 1.28 | 26.1 + 1.67 | 27.10 + 1.72 | 25.94 + 1.01 | 21.047 | 0.003 * |
| T.f | 31.33 + 1.27 | 29.52 + 2.46 | 29.30 + 1.60 | 27.90 + 2.02 | 17.717 | 0.002 * |
| P.g CT Value | T.d CT Value | T.f CT Value | ||||
|---|---|---|---|---|---|---|
| Pearson Correlation | p Value | Pearson Correlation | p Value | Pearson Correlation | p Value | |
| PI score | −0.616 | 0.001 * | −0.455 | 0.004 * | −0.433 | 0.00 * |
| GI score | −0.571 | 0.003 * | −0.467 | 0.002 * | −0.432 | 0.002 * |
| TNF-A value (Pg/mL) | −0.244 | 0.007 * | −0.223 | 0.014 * | −0.200 | 0.03 * |
| Mean probing pocket depth (Mm) | −0.567 | 0.004 * | −0.473 | 0.001 * | −0.396 | 0.001 * |
| Mean clinical attachment loss | −0.504 | 0.002 * | −0.407 | 0.003 * | −0.361 | 0.003 * |
| E GFR value | 0.479 | 0.000 * | .271 | 0.003 * | .473 | 0.00 * |
| Fasting blood sugar value (Mg/dL) | −0.197 | 0.031 * | −0.182 | 0.046 * | −0.208 | 0.02 * |
| Glycated haemoglobin score (%) | −0.243 | 0.008 * | −0.193 | 0.034 * | −0.267 | 0.00 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahendra, J.; Palathingal, P.; Mahendra, L.; Alzahrani, K.J.; Banjer, H.J.; Alsharif, K.F.; Halawani, I.F.; Muralidharan, J.; Annamalai, P.T.; Verma, S.S.; et al. Impact of Red Complex Bacteria and TNF-α Levels on the Diabetic and Renal Status of Chronic Kidney Disease Patients in the Presence and Absence of Periodontitis. Biology 2022, 11, 451. https://doi.org/10.3390/biology11030451
Mahendra J, Palathingal P, Mahendra L, Alzahrani KJ, Banjer HJ, Alsharif KF, Halawani IF, Muralidharan J, Annamalai PT, Verma SS, et al. Impact of Red Complex Bacteria and TNF-α Levels on the Diabetic and Renal Status of Chronic Kidney Disease Patients in the Presence and Absence of Periodontitis. Biology. 2022; 11(3):451. https://doi.org/10.3390/biology11030451
Chicago/Turabian StyleMahendra, Jaideep, Plato Palathingal, Little Mahendra, Khalid J. Alzahrani, Hamsa Jameel Banjer, Khalaf F. Alsharif, Ibrahim Faisal Halawani, Janani Muralidharan, Pandapulaykal T. Annamalai, Shyam Sankar Verma, and et al. 2022. "Impact of Red Complex Bacteria and TNF-α Levels on the Diabetic and Renal Status of Chronic Kidney Disease Patients in the Presence and Absence of Periodontitis" Biology 11, no. 3: 451. https://doi.org/10.3390/biology11030451
APA StyleMahendra, J., Palathingal, P., Mahendra, L., Alzahrani, K. J., Banjer, H. J., Alsharif, K. F., Halawani, I. F., Muralidharan, J., Annamalai, P. T., Verma, S. S., Sharma, V., Varadarajan, S., Bhandi, S., & Patil, S. (2022). Impact of Red Complex Bacteria and TNF-α Levels on the Diabetic and Renal Status of Chronic Kidney Disease Patients in the Presence and Absence of Periodontitis. Biology, 11(3), 451. https://doi.org/10.3390/biology11030451







