ATP Purinergic Receptor P2X1-Dependent Suicidal NETosis Induced by Cryptosporidium parvum under Physioxia Conditions
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statements
2.2. Cryptosporidium parvum Strain and Sporozoite Excystation
2.3. Bovine PMN Isolation
2.4. The Determination of Oxygen Consumption Rates (OCR) and Extracellular Acidification Rates (ECAR) in Bovine PMN That had Been Exposed to Cryptosporidium parvum
2.5. Inhibition of ATP Purinergic Receptor P2X1, MCT1, MCT2, and Glycolysis in Cryptosporidium parvum-Exposed Bovine PMN
2.6. Inhibition of Notch Signaling in Cryptosporidium parvum-Exposed Bovine PMN under Physioxia and Hyperoxia
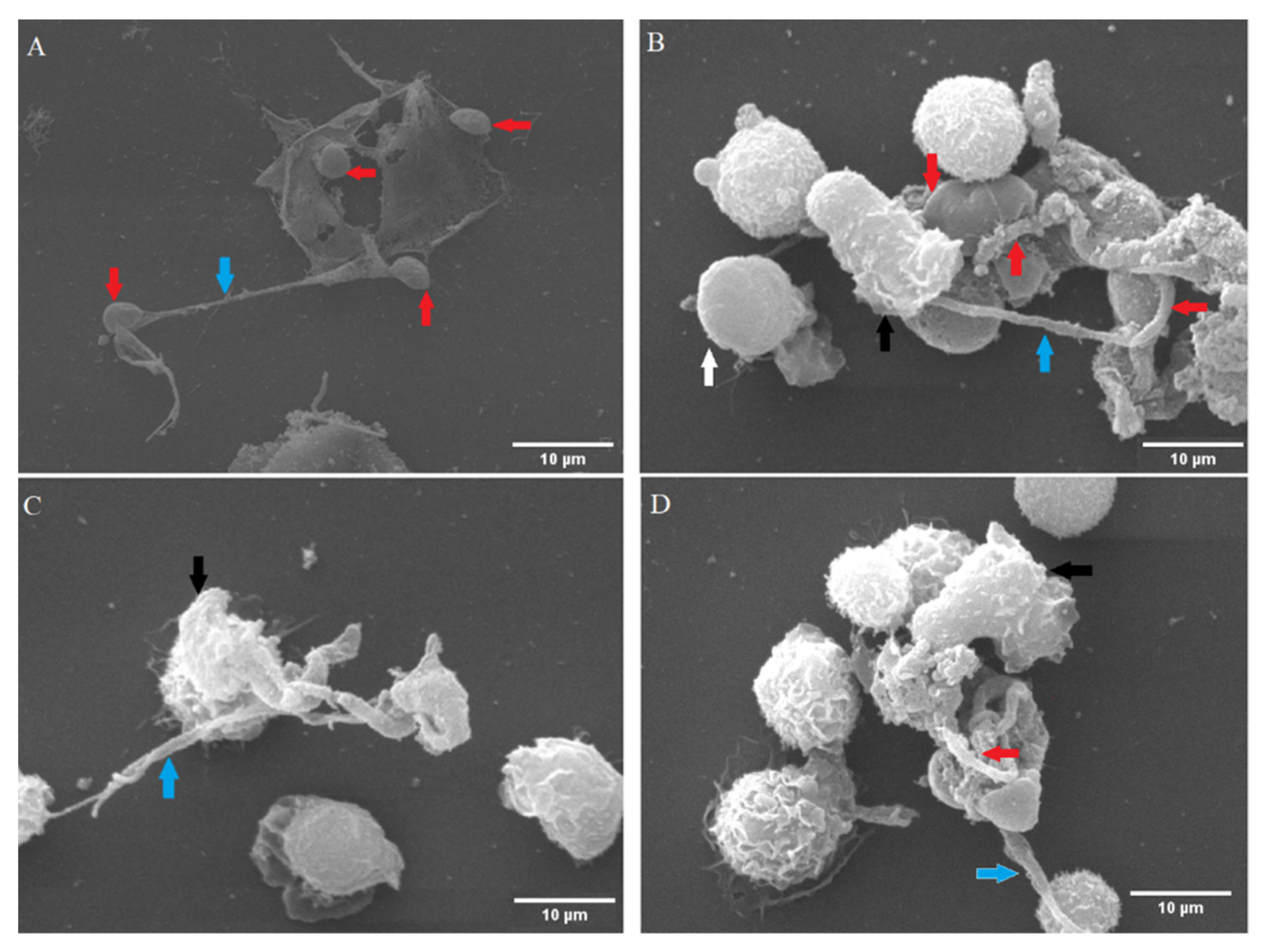
2.7. Examination of Cryptosporidium parvum-Induced NETs Using Scanning Electron Microscopy (SEM) Analysis
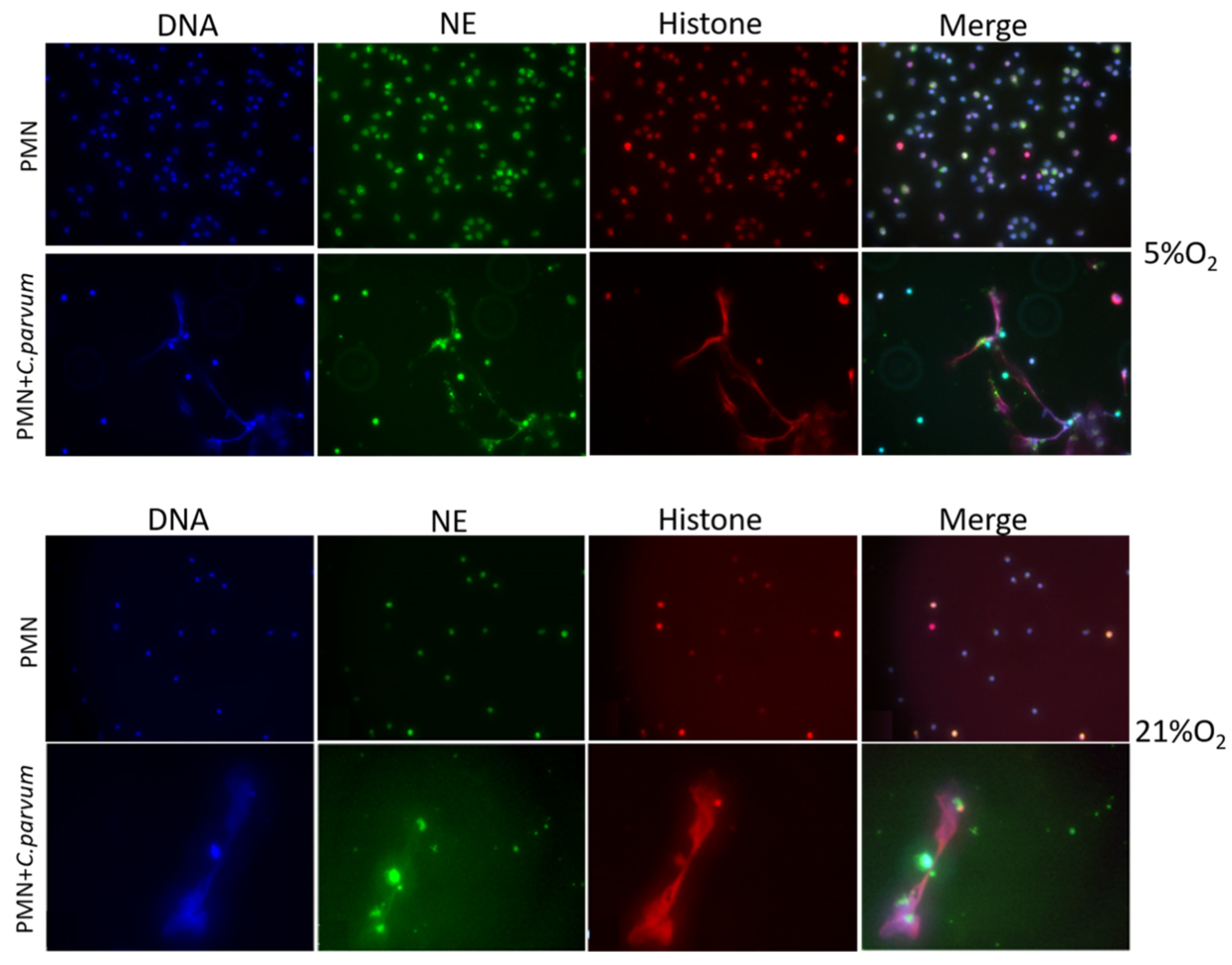
2.8. Cryptosporidium parvum-Induced Suicidal NETosis Visualization Using Immunofluorescence- and Confocal Microscopy Analyses
2.9. 3D Holotomographic Microscopy Investigation of Cryptosporidium parvum-Induced NETosis in Live Cells
2.10. Statistical Methods
3. Results
3.1. Cryptosporidium parvum-Oocysts and Sporozoites Induced Suicidal NETosis
3.2. Live Cell 3D-Holotomography Illustrated Cryptosporidium parvum-Mediated NETosis
3.3. Sporozoite Exposure had No Effects on Extracellular Acidification Rates (ECAR), Glycolysis, or Oxygen Consumption Rates (OCR) in Bovine PMN
3.4. Cryptosporidium parvum-Induced Bovine NETosis Is a MCT1- and MCT2-Independent Cell Death Process
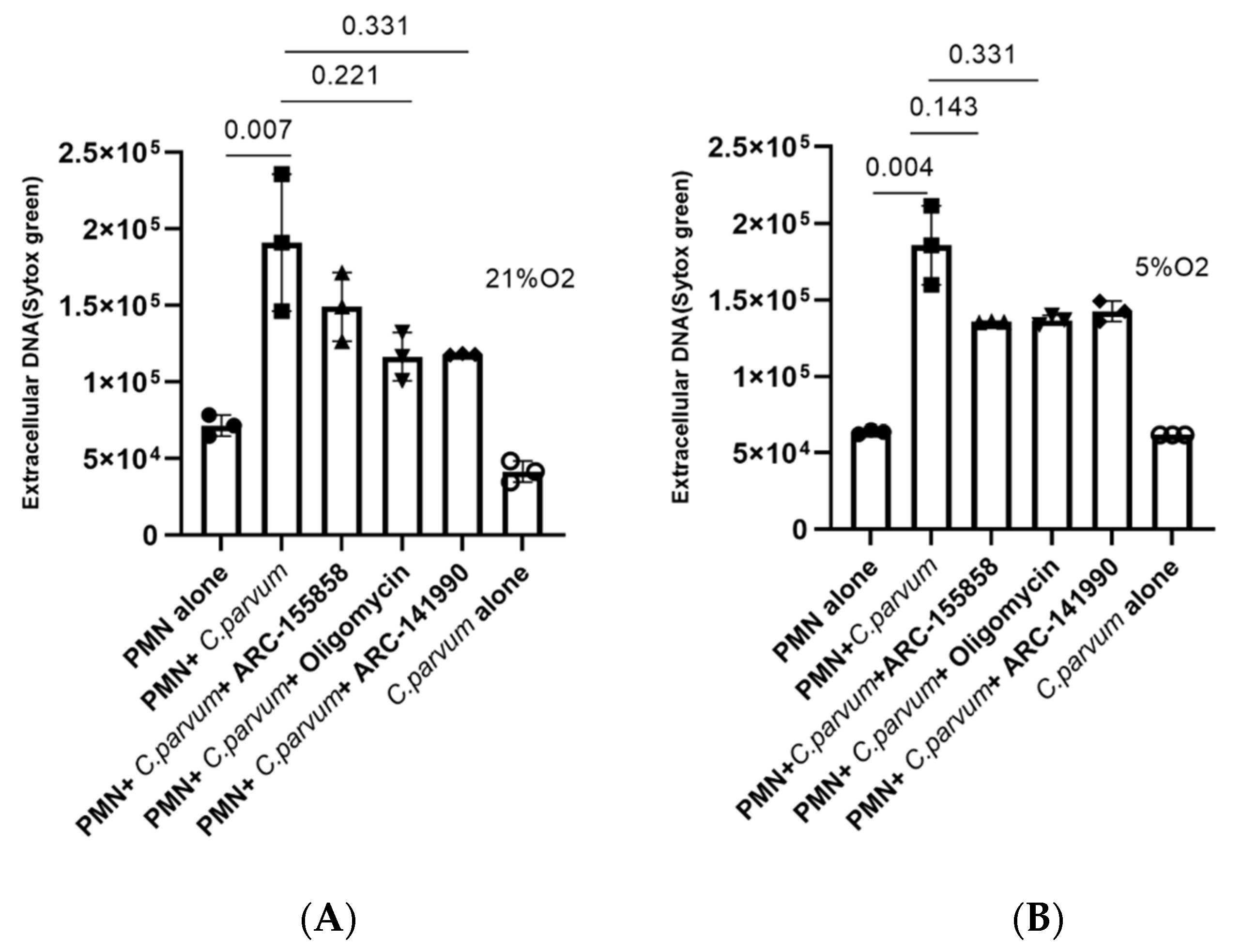
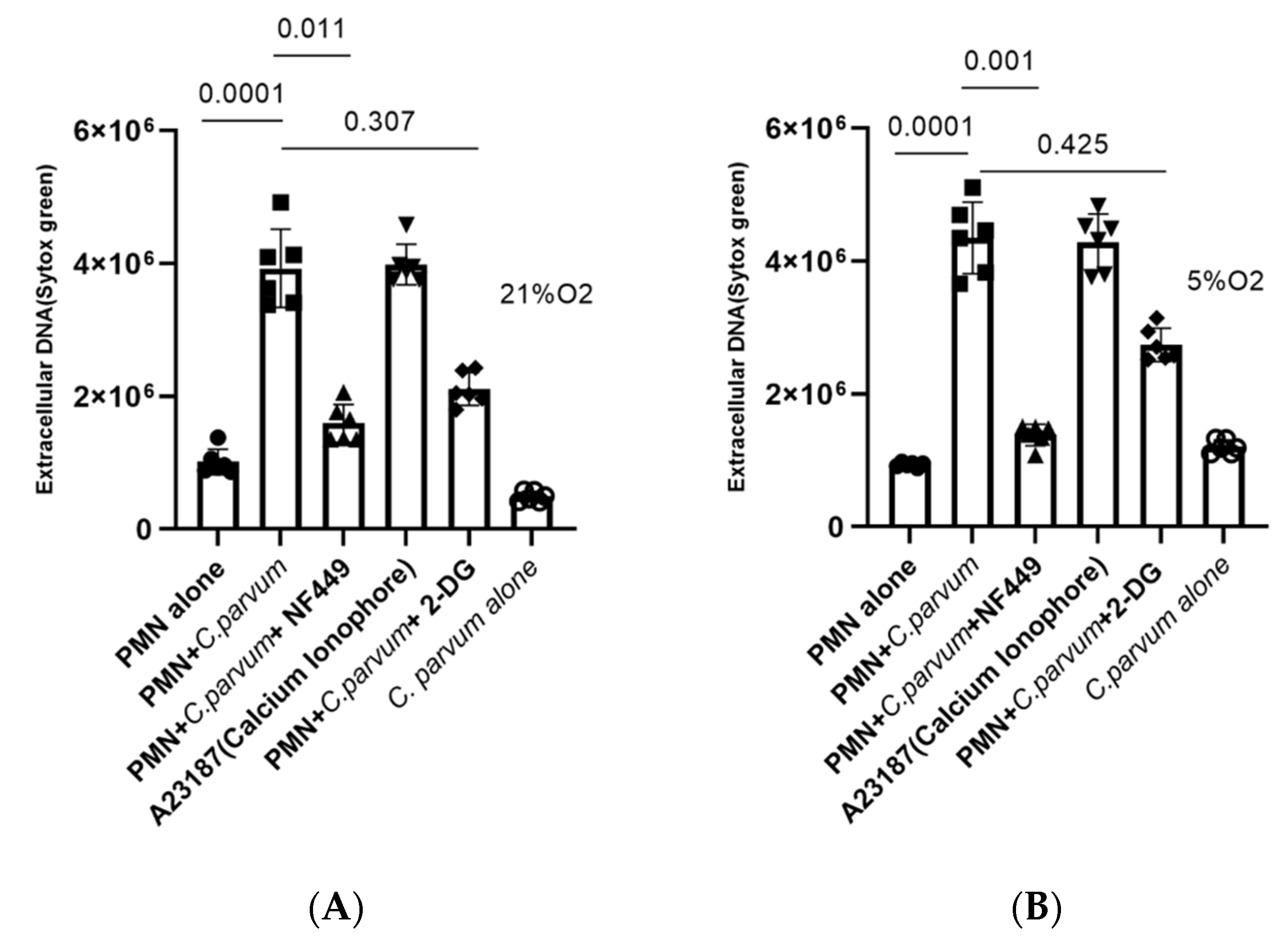
3.5. Cryptosporidium parvum-Induced NETosis Depends on P2X1-Mediated Purinergic Signaling but Not on Notch-Regulated Signal Transduction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Urquhart, G.M. (Ed.) Veterinary Parasitology; Longman: Harlow, UK, 1991; ISBN 978-0-582-40906-4. [Google Scholar]
- Muñoz-Caro, T.; Mena Huertas, S.; Conejeros, I.; Alarcón, P.; Hidalgo, M.A.; Burgos, R.A.; Hermosilla, C.; Taubert, A. Eimeria bovis-Triggered Neutrophil Extracellular Trap Formation Is CD11b-, ERK 1/2-, P38 MAP Kinase- and SOCE-Dependent. Vet. Res. 2015, 46, 23. [Google Scholar] [CrossRef] [PubMed]
- Ryan, U.; Fayer, R.; Xiao, L. Cryptosporidium Species in Humans and Animals: Current Understanding and Research Needs. Parasitology 2014, 141, 1667–1685. [Google Scholar] [CrossRef] [PubMed]
- Vélez, J.; Velasquez, Z.; Silva, L.M.R.; Gärtner, U.; Failing, K.; Daugschies, A.; Mazurek, S.; Hermosilla, C.; Taubert, A. Metabolic Signatures of Cryptosporidium parvum-Infected HCT-8 Cells and Impact of Selected Metabolic Inhibitors on C. Parvum Infection under Physioxia and Hyperoxia. Biology 2021, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Laurent, F.; Lacroix-Lamandé, S. Innate Immune Responses Play a Key Role in Controlling Infection of the Intestinal Epithelium by Cryptosporidium. Int. J. Parasitol. 2017, 47, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, R.; Silva, L.M.R.; López-Osório, S.; Zhou, E.; Gärtner, U.; Conejeros, I.; Taubert, A.; Hermosilla, C. Fasciola hepatica Induces Weak NETosis and Low Production of Intra- and Extracellular ROS in Exposed Bovine Polymorphonuclear Neutrophils. Dev. Comp. Immunol. 2021, 114, 103787. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Caro, T.; Machado Ribeiro da Silva, L.; Rentería-Solis, Z.; Taubert, A.; Hermosilla, C. Neutrophil Extracellular Traps in the Intestinal Mucosa of Eimeria-Infected Animals. Asian Pac. J. Trop. Biomed. 2016, 6, 301–307. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and Aetiology of Diarrhoeal Disease in Infants and Young Children in Developing Countries (the Global Enteric Multicenter Study, GEMS): A Prospective, Case-Control Study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef]
- McDonald, V.; Korbel, D.S.; Barakat, F.M.; Choudhry, N.; Petry, F. Innate Immune Responses against Cryptosporidium parvum Infection. Parasite Immunol. 2013, 35, 55–64. [Google Scholar] [CrossRef]
- Blackwelder, W.C.; Biswas, K.; Wu, Y.; Kotloff, K.L.; Farag, T.H.; Nasrin, D.; Graubard, B.I.; Sommerfelt, H.; Levine, M.M. Statistical Methods in the Global Enteric Multicenter Study (GEMS). Clin. Infect. Dis. 2012, 55, S246–S253. [Google Scholar] [CrossRef]
- Mauzy, M.J.; Enomoto, S.; Lancto, C.A.; Abrahamsen, M.S.; Rutherford, M.S. The Cryptosporidium parvum Transcriptome during In Vitro Development. PLoS ONE 2012, 7, e31715. [Google Scholar] [CrossRef]
- Vélez, J.; Lange, M.K.; Zieger, P.; Yoon, I.; Failing, K.; Bauer, C. Long-Term Use of Yeast Fermentation Products in Comparison to Halofuginone for the Control of Cryptosporidiosis in Neonatal Calves. Vet. Parasitol. 2019, 269, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Lendner, M.; Daugschies, A. Cryptosporidium Infections: Molecular Advances. Parasitology 2014, 141, 1511–1532. [Google Scholar] [CrossRef] [PubMed]
- Thomson, S.; Hamilton, C.A.; Hope, J.C.; Katzer, F.; Mabbott, N.A.; Morrison, L.J.; Innes, E.A. Bovine Cryptosporidiosis: Impact, Host-Parasite Interaction and Control Strategies. Vet. Res. 2017, 48, 42. [Google Scholar] [CrossRef] [PubMed]
- Shahiduzzaman, M.D.; Daugschies, A. Therapy and Prevention of Cryptosporidiosis in Animals. Vet. Parasitol. 2012, 188, 203–214. [Google Scholar] [CrossRef]
- Yildiz, K.; Gokpinar, S.; Gazyagci, A.N.; Babur, C.; Sursal, N.; Azkur, A.K. Role of NETs in the Difference in Host Susceptibility to Toxoplasma gondii between Sheep and Cattle. Vet. Immunol. Immunopathol. 2017, 189, 1–10. [Google Scholar] [CrossRef]
- Pantenburg, B.; Dann, S.M.; Wang, H.-C.; Robinson, P.; Castellanos-Gonzalez, A.; Lewis, D.E.; White, A.C. Intestinal Immune Response to Human Cryptosporidium Sp. Infection. Infect. Immun. 2008, 76, 23–29. [Google Scholar] [CrossRef]
- Codices, V.; Martins, C.; Novo, C.; De Sousa, B.; Lopes, Â.; Borrego, M.; Matos, O. Dynamics of Cytokines and Immunoglobulins Serum Profiles in Primary and Secondary Cryptosporidium parvum Infection: Usefulness of Luminex® XMAP Technology. Exp. Parasitol. 2013, 133, 106–113. [Google Scholar] [CrossRef]
- Lacroix-Lamandé, S.; Mancassola, R.; Naciri, M.; Laurent, F. Role of Gamma Interferon in Chemokine Expression in the Ileum of Mice and in a Murine Intestinal Epithelial Cell Line after Cryptosporidium parvum Infection. Infect. Immun. 2002, 70, 2090–2099. [Google Scholar] [CrossRef]
- Takeuchi, D.; Jones, V.C.; Kobayashi, M.; Suzuki, F. Cooperative Role of Macrophages and Neutrophils in Host Antiprotozoan Resistance in Mice Acutely Infected with Cryptosporidium parvum. Infect. Immun. 2008, 76, 3657–3663. [Google Scholar] [CrossRef]
- Zadrozny, L.M.; Stauffer, S.H.; Armstrong, M.U.; Jones, S.L.; Gookin, J.L. Neutrophils Do Not Mediate the Pathophysiological Sequelae of Cryptosporidium parvum Infection in Neonatal Piglets. Infect. Immun. 2006, 74, 5497–5505. [Google Scholar] [CrossRef]
- Tzipori, S. Cryptosporidiosis in Perspective. In Advances in Parasitology; Elsevier: Amsterdam, The Netherlands, 1988; Volume 27, pp. 63–129. ISBN 978-0-12-031727-1. [Google Scholar]
- Drinkall, E.; Wass, M.J.; Coffey, T.J.; Flynn, R.J. A Rapid IL-17 Response to Cryptosporidium parvum in the Bovine Intestine. Vet. Immunol. Immunopathol. 2017, 191, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Niine, T.; Dorbek-Kolin, E.; Lassen, B.; Orro, T. Cryptosporidium Outbreak in Calves on a Large Dairy Farm: Effect of Treatment and the Association with the Inflammatory Response and Short-Term Weight Gain. Res. Vet. Sci. 2018, 117, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel Cell Death Program Leads to Neutrophil Extracellular Traps. J. Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Conejeros, I.; Patterson, R.; Burgos, R.A.; Hermosilla, C.; Werling, D. Induction of Reactive Oxygen Species in Bovine Neutrophils Is CD11b, but Not Dectin-1-Dependent. Vet. Immunol. Immunopathol. 2011, 139, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Conejeros, I.; Velásquez, Z.D.; Grob, D.; Zhou, E.; Salecker, H.; Hermosilla, C.; Taubert, A. Histone H2A and Bovine Neutrophil Extracellular Traps Induce Damage of Besnoitia besnoiti-Infected Host Endothelial Cells but Fail to Affect Total Parasite Proliferation. Biology 2019, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; Brain, S.; Pearson, J.; Edgeworth, J.; Lewis, S.; Treacher, D. Neutrophils in Development of Multiple Organ Failure in Sepsis. Lancet 2006, 368, 157–169. [Google Scholar] [CrossRef]
- DuMont, A.L.; Yoong, P.; Day, C.J.; Alonzo, F.; McDonald, W.H.; Jennings, M.P.; Torres, V.J. Staphylococcus aureus LukAB Cytotoxin Kills Human Neutrophils by Targeting the CD11b Subunit of the Integrin Mac-1. Proc. Natl. Acad. Sci. USA 2013, 110, 10794–10799. [Google Scholar] [CrossRef]
- Kubelkova, K.; Macela, A. Innate Immune Recognition: An Issue More Complex Than Expected. Front. Cell. Infect. Microbiol. 2019, 9, 241. [Google Scholar] [CrossRef]
- Muñoz-Caro, T.; Gibson, A.J.; Conejeros, I.; Werling, D.; Taubert, A.; Hermosilla, C. The Role of TLR2 and TLR4 in Recognition and Uptake of the Apicomplexan Parasite Eimeria bovis and Their Effects on NET Formation. Pathogens 2021, 10, 118. [Google Scholar] [CrossRef]
- Pichyangkul, S.; Yongvanitchit, K.; Kum-arb, U.; Hemmi, H.; Akira, S.; Krieg, A.M.; Heppner, D.G.; Stewart, V.A.; Hasegawa, H.; Looareesuwan, S.; et al. Malaria Blood Stage Parasites Activate Human Plasmacytoid Dendritic Cells and Murine Dendritic Cells through a Toll-Like Receptor 9-Dependent Pathway. J. Immunol. 2004, 172, 4926–4933. [Google Scholar] [CrossRef] [PubMed]
- Lacy, P. Mechanisms of Degranulation in Neutrophils. Allergy Asthma Clin. Immunol. 2006, 2, 98. [Google Scholar] [CrossRef] [PubMed]
- Roma, E.H.; Macedo, J.P.; Goes, G.R.; Gonçalves, J.L.; De Castro, W.; Cisalpino, D.; Vieira, L.Q. Impact of Reactive Oxygen Species (ROS) on the Control of Parasite Loads and Inflammation in Leishmania amazonensis Infection. Parasit. Vectors 2016, 9, 193. [Google Scholar] [CrossRef] [PubMed]
- Bhaumik, P.; St-Pierre, G.; Milot, V.; St-Pierre, C.; Sato, S. Galectin-3 Facilitates Neutrophil Recruitment as an Innate Immune Response to a Parasitic Protozoa Cutaneous Infection. J. Immunol. 2013, 190, 630–640. [Google Scholar] [CrossRef]
- Villagra-Blanco, R.; Silva, L.M.R.; Muñoz-Caro, T.; Yang, Z.; Li, J.; Gärtner, U.; Taubert, A.; Zhang, X.; Hermosilla, C. Bovine Polymorphonuclear Neutrophils Cast Neutrophil Extracellular Traps against the Abortive Parasite Neospora caninum. Front. Immunol. 2017, 8, 606. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V. Neutrophil Extracellular Traps in the Second Decade. J. Innate Immun. 2018, 10, 414–421. [Google Scholar] [CrossRef]
- Zhao, G.H.; Fang, Y.Q.; Ryan, U.; Guo, Y.X.; Wu, F.; Du, S.Z.; Chen, D.K.; Lin, Q. Dynamics of Th17 Associating Cytokines in Cryptosporidium parvum-Infected Mice. Parasitol. Res. 2016, 115, 879–887. [Google Scholar] [CrossRef]
- Grob, D.; Conejeros, I.; Velásquez, Z.D.; Preußer, C.; Gärtner, U.; Alarcón, P.; Burgos, R.A.; Hermosilla, C.; Taubert, A. Trypanosoma brucei Brucei Induces Polymorphonuclear Neutrophil Activation and Neutrophil Extracellular Traps Release. Front. Immunol. 2020, 11, 559561. [Google Scholar] [CrossRef]
- Guimaraes-Costa, A.B.; Nascimento, M.T.C.; Froment, G.S.; Soares, R.P.P.; Morgado, F.N.; Conceicao-Silva, F.; Saraiva, E.M. Leishmania amazonensis Promastigotes Induce and Are Killed by Neutrophil Extracellular Traps. Proc. Natl. Acad. Sci. USA 2009, 106, 6748–6753. [Google Scholar] [CrossRef]
- Sousa-Rocha, D.; Thomaz-Tobias, M.; Diniz, L.F.A.; Souza, P.S.S.; Pinge-Filho, P.; Toledo, K.A. Trypanosoma cruzi and Its Soluble Antigens Induce NET Release by Stimulating Toll-Like Receptors. PLoS ONE 2015, 10, e0139569. [Google Scholar] [CrossRef]
- Rochael, N.C.; Guimarães-Costa, A.B.; Nascimento, M.T.C.; DeSouza-Vieira, T.S.; Oliveira, M.P.; Garcia e Souza, L.F.; Oliveira, M.F.; Saraiva, E.M. Classical ROS-Dependent and Early/Rapid ROS-Independent Release of Neutrophil Extracellular Traps Triggered by Leishmania Parasites. Sci. Rep. 2016, 5, 18302. [Google Scholar] [CrossRef] [PubMed]
- Zhou, E.; Conejeros, I.; Gärtner, U.; Mazurek, S.; Hermosilla, C.; Taubert, A. Metabolic Requirements of Besnoitia besnoiti Tachyzoite-Triggered NETosis. Parasitol. Res. 2020, 119, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Debierre-Grockiego, F.; Moiré, N.; Torres Arias, M.; Dimier-Poisson, I. Recent Advances in the Roles of Neutrophils in Toxoplasmosis. Trends Parasitol. 2020, 36, 956–958. [Google Scholar] [CrossRef]
- Chuah, C.; Jones, M.K.; Burke, M.L.; Owen, H.C.; Anthony, B.J.; McManus, D.P.; Ramm, G.A.; Gobert, G.N. Spatial and Temporal Transcriptomics of Schistosoma japonicum -Induced Hepatic Granuloma Formation Reveals Novel Roles for Neutrophils. J. Leukoc. Biol. 2013, 94, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Caro, T.; Lendner, M.; Daugschies, A.; Hermosilla, C.; Taubert, A. NADPH Oxidase, MPO, NE, ERK1/2, P38 MAPK and Ca2+ Influx Are Essential for Cryptosporidium parvum-Induced NET Formation. Dev. Comp. Immunol. 2015, 52, 245–254. [Google Scholar] [CrossRef]
- Zheng, L.; Kelly, C.J.; Colgan, S.P. Physiologic Hypoxia and Oxygen Homeostasis in the Healthy Intestine. A Review in the Theme: Cellular Responses to Hypoxia. Am. J. Physiol.-Cell Physiol. 2015, 309, C350–C360. [Google Scholar] [CrossRef]
- Morada, M.; Lee, S.; Gunther-Cummins, L.; Weiss, L.M.; Widmer, G.; Tzipori, S.; Yarlett, N. Continuous Culture of Cryptosporidium parvum Using Hollow Fiber Technology. Int. J. Parasitol. 2016, 46, 21–29. [Google Scholar] [CrossRef]
- Tammam, J.; Ware, C.; Efferson, C.; O’Neil, J.; Rao, S.; Qu, X.; Gorenstein, J.; Angagaw, M.; Kim, H.; Kenific, C.; et al. Down-Regulation of the Notch Pathway Mediated by a γ-Secretase Inhibitor Induces Anti-Tumour Effects in Mouse Models of T-Cell Leukaemia: Anti-Tumour Effects of GSI in Xenograft Model. Br. J. Pharmacol. 2009, 158, 1183–1195. [Google Scholar] [CrossRef]
- Wu, W.; Nie, L.; Zhang, L.; Li, Y. The Notch Pathway Promotes NF-ΚB Activation through Asb2 in T Cell Acute Lymphoblastic Leukemia Cells. Cell. Mol. Biol. Lett. 2018, 23, 37. [Google Scholar] [CrossRef]
- Amsen, D.; Blander, J.M.; Lee, G.R.; Tanigaki, K.; Honjo, T.; Flavell, R.A. Instruction of Distinct CD4 T Helper Cell Fates by Different Notch Ligands on Antigen-Presenting Cells. Cell 2004, 117, 515–526. [Google Scholar] [CrossRef]
- Fung, E.; Tang, S.-M.T.; Canner, J.P.; Morishige, K.; Arboleda-Velasquez, J.F.; Cardoso, A.A.; Carlesso, N.; Aster, J.C.; Aikawa, M. Delta-Like 4 Induces Notch Signaling in Macrophages: Implications for Inflammation. Circulation 2007, 115, 2948–2956. [Google Scholar] [CrossRef] [PubMed]
- Broglia, A.; Reckinger, S.; Cacció, S.M.; Nöckler, K. Distribution of Cryptosporidium parvum Subtypes in Calves in Germany. Vet. Parasitol. 2008, 154, 8–13. [Google Scholar] [CrossRef]
- Holzhausen, I.; Lendner, M.; Göhring, F.; Steinhöfel, I.; Daugschies, A. Distribution of Cryptosporidium parvum Gp60 Subtypes in Calf Herds of Saxony, Germany. Parasitol. Res. 2019, 118, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Plutzer, J.; Lassen, B.; Jokelainen, P.; Djurković-Djaković, O.; Kucsera, I.; Dorbek-Kolin, E.; Šoba, B.; Sréter, T.; Imre, K.; Omeragić, J.; et al. Review of Cryptosporidium and Giardia in the Eastern Part of Europe, 2016. Eurosurveillance 2018, 23, 4. [Google Scholar] [CrossRef]
- Najdrowski, M.; Joachim, A.; Daugschies, A. An Improved in Vitro Infection Model for Viability Testing of Cryptosporidium parvum Oocysts. Vet. Parasitol. 2007, 150, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Baker, V.S.; Imade, G.E.; Molta, N.B.; Tawde, P.; Pam, S.D.; Obadofin, M.O.; Sagay, S.A.; Egah, D.Z.; Iya, D.; Afolabi, B.B.; et al. Cytokine-Associated Neutrophil Extracellular Traps and Antinuclear Antibodies in Plasmodium falciparum Infected Children under Six Years of Age. Malar. J. 2008, 7, 41. [Google Scholar] [CrossRef]
- Shahiduzzaman, M.; Dyachenko, V.; Obwaller, A.; Unglaube, S.; Daugschies, A. Combination of Cell Culture and Quantitative PCR for Screening of Drugs against Cryptosporidium parvum. Vet. Parasitol. 2009, 162, 271–277. [Google Scholar] [CrossRef]
- Aronsen, L.; Orvoll, E.; Lysaa, R.; Ravna, A.W.; Sager, G. Modulation of High Affinity ATP-Dependent Cyclic Nucleotide Transporters by Specific and Non-Specific Cyclic Nucleotide Phosphodiesterase Inhibitors. Eur. J. Pharmacol. 2014, 745, 249–253. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Ma, D.; Lee, W.-N.P.; Xiao, J.; Zhao, Y.; Go, V.L.; Wang, Q.; Yen, Y.; Recker, R.; et al. Inhibition of Transketolase by Oxythiamine Altered Dynamics of Protein Signals in Pancreatic Cancer Cells. Exp. Hematol. Oncol. 2013, 2, 18. [Google Scholar] [CrossRef]
- Borggrefe, T.; Giaimo, B.D. (Eds.) Molecular Mechanisms of Notch Signaling; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2018; Volume 1066, ISBN 978-3-319-89511-6. [Google Scholar]
- Abi Abdallah, D.S.; Lin, C.; Ball, C.J.; King, M.R.; Duhamel, G.E.; Denkers, E.Y. Toxoplasma gondii Triggers Release of Human and Mouse Neutrophil Extracellular Traps. Infect. Immun. 2012, 80, 768–777. [Google Scholar] [CrossRef]
- Silva, L.M.R.; Muñoz Caro, T.; Gerstberger, R.; Vila-Viçosa, M.J.M.; Cortes, H.C.E.; Hermosilla, C.; Taubert, A. The Apicomplexan Parasite Eimeria arloingi Induces Caprine Neutrophil Extracellular Traps. Parasitol. Res. 2014, 113, 2797–2807. [Google Scholar] [CrossRef] [PubMed]
- DeSouza-Vieira, T.; Guimarães-Costa, A.; Rochael, N.C.; Lira, M.N.; Nascimento, M.T.; Lima-Gomez, P.d.S.; Mariante, R.M.; Persechini, P.M.; Saraiva, E.M. Neutrophil Extracellular Traps Release Induced by Leishmania: Role of PI3Kγ, ERK, PI3Kσ, PKC, and [Ca 2+]. J. Leukoc. Biol. 2016, 100, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Godínez, C.; Jorge-Rosas, J.F.; Néquiz, M.; Martínez-Calvillo, S.; Laclette, J.P.; Rosales, C.; Carrero, J.C. New Insights on NETosis Induced by Entamoeba histolytica: Dependence on ROS from Amoebas and Extracellular MPO Activity. Antioxidants 2021, 10, 974. [Google Scholar] [CrossRef]
- McCoy, C.J.; Reaves, B.J.; Giguère, S.; Coates, R.; Rada, B.; Wolstenholme, A.J. Human Leukocytes Kill Brugia Malayi Microfilariae Independently of DNA-Based Extracellular Trap Release. PLoS Negl. Trop. Dis. 2017, 11, e0005279. [Google Scholar] [CrossRef]
- Doolan, R.; Bouchery, T. Hookworm Infections: Reappraising the Evidence for a Role of Neutrophils in Light of NETosis. Parasite Immunol. 2022, e12911. [Google Scholar] [CrossRef]
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil Elastase and Myeloperoxidase Regulate the Formation of Neutrophil Extracellular Traps. J. Cell Biol. 2010, 191, 677–691. [Google Scholar] [CrossRef]
- Helander, H.F.; Fändriks, L. Surface Area of the Digestive Tract—Revisited. Scand. J. Gastroenterol. 2014, 49, 681–689. [Google Scholar] [CrossRef]
- Carreau, A.; Hafny-Rahbi, B.E.; Matejuk, A.; Grillon, C.; Kieda, C. Why Is the Partial Oxygen Pressure of Human Tissues a Crucial Parameter? Small Molecules and Hypoxia. J. Cell. Mol. Med. 2011, 15, 1239–1253. [Google Scholar] [CrossRef]
- Branitzki-Heinemann, K.; Möllerherm, H.; Völlger, L.; Husein, D.M.; De Buhr, N.; Blodkamp, S.; Reuner, F.; Brogden, G.; Naim, H.Y.; Von Köckritz-Blickwede, M. Formation of Neutrophil Extracellular Traps under Low Oxygen Level. Front. Immunol. 2016, 7, 518. [Google Scholar] [CrossRef]
- Semenza, G.L. Oxygen Sensing, Homeostasis, and Disease. N. Engl. J. Med. 2011, 365, 537–547. [Google Scholar] [CrossRef]
- Bartrons, R.; Caro, J. Hypoxia, Glucose Metabolism and the Warburg’s Effect. J. Bioenerg. Biomembr. 2007, 39, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Espinosa, O.; Rojas-Espinosa, O.; Moreno-Altamirano, M.M.B.; López-Villegas, E.O.; Sánchez-García, F.J. Metabolic Requirements for Neutrophil Extracellular Traps Formation. Immunology 2015, 145, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Halestrap, A.P.; Meredith, D. The SLC16 Gene Family? From Monocarboxylate Transporters (MCTs) to Aromatic Amino Acid Transporters and Beyond. Pflügers Arch. Eur. J. Physiol. 2004, 447, 619–628. [Google Scholar] [CrossRef]
- Dhup, S.; Kumar Dadhich, R.; Ettore Porporato, P.; Sonveaux, P. Multiple Biological Activities of Lactic Acid in Cancer: Influences on Tumor Growth, Angiogenesis and Metastasis. Curr. Pharm. Des. 2012, 18, 1319–1330. [Google Scholar] [CrossRef]
- Halestrap, A.P. The SLC16 Gene Family—Structure, Role and Regulation in Health and Disease. Mol. Aspects Med. 2013, 34, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, P.; Manosalva, C.; Conejeros, I.; Carretta, M.D.; Muñoz-Caro, T.; Silva, L.M.R.; Taubert, A.; Hermosilla, C.; Hidalgo, M.A.; Burgos, R.A. D (−) Lactic Acid-Induced Adhesion of Bovine Neutrophils onto Endothelial Cells Is Dependent on Neutrophils Extracellular Traps Formation and CD11b Expression. Front. Immunol. 2017, 8, 975. [Google Scholar] [CrossRef]
- Desgrandchamps, D.; Munzinger, J. Infectious gastroenteritis in the immunocompetent child. Significance of Cryptosporidium spp. and Aeromonas ssp. Schweiz. Med. Wochenschr. 1989, 119, 276–281. [Google Scholar]
- Wang, X.; Chen, D. Purinergic Regulation of Neutrophil Function. Front. Immunol. 2018, 9, 399. [Google Scholar] [CrossRef]
- Sofoluwe, A.; Bacchetta, M.; Badaoui, M.; Kwak, B.R.; Chanson, M. ATP Amplifies NADPH-Dependent and -Independent Neutrophil Extracellular Trap Formation. Sci. Rep. 2019, 9, 16556. [Google Scholar] [CrossRef]
- Kim, S.-W.; Davaanyam, D.; Seol, S.-I.; Lee, H.-K.; Lee, H.; Lee, J.-K. Adenosine Triphosphate Accumulated Following Cerebral Ischemia Induces Neutrophil Extracellular Trap Formation. Int. J. Mol. Sci. 2020, 21, 7668. [Google Scholar] [CrossRef]
- Shang, Y.; Smith, S.; Hu, X. Role of Notch Signaling in Regulating Innate Immunity and Inflammation in Health and Disease. Protein Cell 2016, 7, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Monsalve, E.; Ruiz-García, A.; Baladrón, V.; Ruiz-Hidalgo, M.J.; Sánchez-Solana, B.; Rivero, S.; García-Ramírez, J.J.; Rubio, A.; Laborda, J.; Díaz-Guerra, M.J.M. Notch1 Upregulates LPS-Induced Macrophage Activation by Increasing NF-ΚB Activity. Eur. J. Immunol. 2009, 39, 2556–2570. [Google Scholar] [CrossRef] [PubMed]
- Monsalve, E.; Pérez, M.A.; Rubio, A.; Ruiz-Hidalgo, M.J.; Baladrón, V.; García-Ramírez, J.J.; Gómez, J.C.; Laborda, J.; Díaz-Guerra, M.J.M. Notch-1 Up-Regulation and Signaling Following Macrophage Activation Modulates Gene Expression Patterns Known to Affect Antigen-Presenting Capacity and Cytotoxic Activity. J. Immunol. 2006, 176, 5362–5373. [Google Scholar] [CrossRef] [PubMed]
- Palaga, T.; Buranaruk, C.; Rengpipat, S.; Fauq, A.H.; Golde, T.E.; Kaufmann, S.H.E.; Osborne, B.A. Notch Signaling Is Activated by TLR Stimulation and Regulates Macrophage Functions. Eur. J. Immunol. 2008, 38, 174–183. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, C.; Liu, Z.; Liu, X.; Han, C.; Cao, X.; Li, N. Notch Signal Suppresses Toll-like Receptor-Triggered Inflammatory Responses in Macrophages by Inhibiting Extracellular Signal-Regulated Kinase 1/2-Mediated Nuclear Factor ΚB Activation. J. Biol. Chem. 2012, 287, 6208–6217. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasheminasab, S.S.; Conejeros, I.; D. Velásquez, Z.; Borggrefe, T.; Gärtner, U.; Kamena, F.; Taubert, A.; Hermosilla, C. ATP Purinergic Receptor P2X1-Dependent Suicidal NETosis Induced by Cryptosporidium parvum under Physioxia Conditions. Biology 2022, 11, 442. https://doi.org/10.3390/biology11030442
Hasheminasab SS, Conejeros I, D. Velásquez Z, Borggrefe T, Gärtner U, Kamena F, Taubert A, Hermosilla C. ATP Purinergic Receptor P2X1-Dependent Suicidal NETosis Induced by Cryptosporidium parvum under Physioxia Conditions. Biology. 2022; 11(3):442. https://doi.org/10.3390/biology11030442
Chicago/Turabian StyleHasheminasab, Seyed Sajjad, Iván Conejeros, Zahady D. Velásquez, Tilman Borggrefe, Ulrich Gärtner, Faustin Kamena, Anja Taubert, and Carlos Hermosilla. 2022. "ATP Purinergic Receptor P2X1-Dependent Suicidal NETosis Induced by Cryptosporidium parvum under Physioxia Conditions" Biology 11, no. 3: 442. https://doi.org/10.3390/biology11030442
APA StyleHasheminasab, S. S., Conejeros, I., D. Velásquez, Z., Borggrefe, T., Gärtner, U., Kamena, F., Taubert, A., & Hermosilla, C. (2022). ATP Purinergic Receptor P2X1-Dependent Suicidal NETosis Induced by Cryptosporidium parvum under Physioxia Conditions. Biology, 11(3), 442. https://doi.org/10.3390/biology11030442






