Expression of G-Protein-Coupled Estrogen Receptor (GPER) in Whole Testicular Tissue and Laser-Capture Microdissected Testicular Compartments of Men with Normal and Aberrant Spermatogenesis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristic of the Study Group
2.2. Histological Analysis
- 0 Leydig cells—0 points
- 1–5 Leydig cells—1 point
- 6–10 Leydig cells—2 points
- 11–30 Leydig cells—3 points
- >30 Leydig cells (nodules of Leydig cells)—4 points
2.3. Procedure of Tissue Sections Preparation for RNA Extraction
2.4. RNA Extraction and RT-PCR
2.5. Immunohistochemical Expression of GPER
2.6. Statistical Analysis
3. Results
3.1. Subjects and Histological Evaluation
3.2. Hormonal Profile
3.3. Expression of GPER
3.3.1. Expression of GPER mRNA in the Whole Testicular Tissue
3.3.2. Expression of GPER mRNA in Laser-Microdissected Testicular Compartments
3.3.3. Immunohistochemical Expression and Cellular Localization of GPER
3.4. Quantification of Sertoli and Leydig Cell Function Markers mRNA Transcripts in Laser-Microdissected Testicular Compartments
3.4.1. AMH, BMP4, SCF mRNA Transcripts in Laser-Microdissected Seminiferous Tubules
3.4.2. StAR, CYP17A1, HSD173B mRNA Transcripts in Laser-Microdissected Leydig Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Payne, A.H.; Perkins, L.M.; Georgiou, M.; Quinn, P.G. Intratesticular site of aromatase activity and possible function of testicular estradiol. Steroids 1987, 50, 435–448. [Google Scholar] [CrossRef][Green Version]
- Brodie, A.; Inkster, S.; Yue, W. Aromatase expression in the human male. Mol. Cell. Endocrinol. 2001, 178, 23–28. [Google Scholar] [CrossRef]
- Lambard, S.; Galeraud-Denis, I.; Saunders, P.T.; Carreau, S. Human immature germ cells and ejaculated spermatozoa contain aromatase and oestrogen receptors. J. Mol. Endocrinol. 2004, 32, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Lardone, M.C.; Castillo, P.; Valdevenito, R.; Ebensperger, M.; Ronco, A.M.; Pommer, R.; Piottante, A.; Castro, A. P450-aromatase activity and expression in human testicular tissues with severe spermatogenic failure. Int. J. Androl. 2010, 33, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Kula, K. Induction of precocious maturation of spermatogenesis in infant rats by human menopausal gonadotropin and inhibition by simultaneous administration of gonadotropins and testosterone. Endocrinology 1988, 122, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Kula, K.; Walczak-Jedrzejowska, R.; Slowikowska-Hilczer, J.; Oszukowska, E. Estradiol enhances the stimulatory effect of fsh on testicular maturation and contributes to precocious initiation of spermatogenesis. Mol. Cell. Endocrinol. 2001, 178, 89–97. [Google Scholar] [CrossRef]
- Ebling, F.J.; Brooks, A.N.; Cronin, A.S.; Ford, H.; Kerr, J.B. Estrogenic induction of spermatogenesis in the hypogonadal mouse. Endocrinology 2000, 141, 2861–2869. [Google Scholar] [CrossRef] [PubMed]
- Baines, H.; Nwagwu, M.O.; Hastie, G.R.; Wiles, R.A.; Mayhew, T.M.; Ebling, F.J. Effects of estradiol and fsh on maturation of the testis in the hypogonadal (hpg) mouse. Reprod. Biol. Endocrinol. 2008, 6, 4. [Google Scholar] [CrossRef]
- Pentikainen, V.; Erkkila, K.; Suomalainen, L.; Parvinen, M.; Dunkel, L. Estradiol acts as a germ cell survival factor in the human testis in vitro. J. Clin. Endocrinol. Metab. 2000, 85, 2057–2067. [Google Scholar] [CrossRef] [PubMed]
- Dostalova, P.; Zatecka, E.; Dvorakova-Hortova, K. Of oestrogens and sperm: A review of the roles of oestrogens and oestrogen receptors in male reproduction. Int. J. Mol. Sci. 2017, 18, 904. [Google Scholar] [CrossRef]
- Haverfield, J.T.; Ham, S.; Brown, K.A.; Simpson, E.R.; Meachem, S.J. Teasing out the role of aromatase in the healthy and diseased testis. Spermatogenesis 2011, 1, 240–249. [Google Scholar] [CrossRef][Green Version]
- Cooke, P.S.; Nanjappa, M.K.; Ko, C.; Prins, G.S.; Hess, R.A. Estrogens in male physiology. Physiol. Rev. 2017, 97, 995–1043. [Google Scholar] [CrossRef]
- Walczak-Jedrzejowska, R.; Marchlewska, K.; Oszukowska, E.; Filipiak, E.; Slowikowska-Hilczer, J.; Kula, K. Estradiol and testosterone inhibit rat seminiferous tubule development in a hormone-specific way. Reprod. Biol. 2013, 13, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Misiakiewicz-Has, K.; Pilutin, A.; Wiszniewska, B. Influence of hormonal imbalance on the integrity of seminiferous epithelium in the testes of adult rats chronically exposed to letrozole and rats exposed to soya isoflavones during the prenatal period, lactation, and up to sexual maturity. Reprod. Biol. 2021, 21, 100562. [Google Scholar] [CrossRef]
- Juul, A.; Almstrup, K.; Andersson, A.M.; Jensen, T.K.; Jorgensen, N.; Main, K.M.; Rajpert-De Meyts, E.; Toppari, J.; Skakkebaek, N.E. Possible fetal determinants of male infertility. Nat. Rev. Endocrinol. 2014, 10, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, R.M.; Skakkebaek, N.E. Testicular dysgenesis syndrome: Mechanistic insights and potential new downstream effects. Fertil. Steril. 2008, 89, e33–e38. [Google Scholar] [CrossRef] [PubMed]
- Amir, S.; Shah, S.T.A.; Mamoulakis, C.; Docea, A.O.; Kalantzi, O.I.; Zachariou, A.; Calina, D.; Carvalho, F.; Sofikitis, N.; Makrigiannakis, A.; et al. Endocrine disruptors acting on estrogen and androgen pathways cause reproductive disorders through multiple mechanisms: A review. Int. J. Environ. Res. Public Health 2021, 18, 1464. [Google Scholar] [CrossRef] [PubMed]
- Jungwirth, A.; Giwercman, A.; Tournaye, H.; Diemer, T.; Kopa, Z.; Dohle, G.; Krausz, C.; EAU Working Group on Male Infertility. European association of urology guidelines on male infertility: The 2012 update. Eur. Urol. 2012, 62, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Krausz, C. Male infertility: Pathogenesis and clinical diagnosis. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Cioppi, F.; Rosta, V.; Krausz, C. Genetics of azoospermia. Int. J. Mol. Sci. 2021, 22, 3264. [Google Scholar] [CrossRef] [PubMed]
- Adamczewska, D.; Slowikowska-Hilczer, J.; Marchlewska, K.; Walczak-Jedrzejowska, R. Features of gonadal dysgenesis and leydig cell impairment in testes with sertoli cell-only syndrome. Folia Histochem. Cytobiol. 2020, 58, 73–82. [Google Scholar] [CrossRef]
- Holm, M.; Rajpert-De Meyts, E.; Andersson, A.M.; Skakkebaek, N.E. Leydig cell micronodules are a common finding in testicular biopsies from men with impaired spermatogenesis and are associated with decreased testosterone/lh ratio. J. Pathol. 2003, 199, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Hauptman, D.; Peric, M.H.; Maric, T.; Bojanac, A.K.; Sincic, N.; Zimak, Z.; Kastelan, Z.; Jezek, D. Leydig cells in patients with non-obstructive azoospermia: Do they really proliferate? Life 2021, 11, 1266. [Google Scholar] [CrossRef] [PubMed]
- Lardone, M.C.; Argandona, F.; Florez, M.; Parada-Bustamante, A.; Ebensperger, M.; Palma, C.; Piottante, A.; Castro, A. Overexpression of cyp19a1 aromatase in leydig cells is associated with steroidogenic dysfunction in subjects with sertoli cell-only syndrome. Andrology 2017, 5, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Lan, K.C.; Chen, Y.T.; Chang, C.; Chang, Y.C.; Lin, H.J.; Huang, K.E.; Kang, H.Y. Up-regulation of sox9 in sertoli cells from testiculopathic patients accounts for increasing anti-mullerian hormone expression via impaired androgen receptor signaling. PLoS ONE 2013, 8, e76303. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Yang, S.; Zhang, Z.; Li, P.; Gong, Y.; Liu, L.; Zhu, Y.; Tian, R.; Liu, Y.; Wang, X.; et al. Sertoli cells from non-obstructive azoospermia and obstructive azoospermia patients show distinct morphology, raman spectrum and biochemical phenotype. Hum. Reprod. 2013, 28, 1863–1873. [Google Scholar] [CrossRef] [PubMed]
- Lardone, M.C.; Argandona, F.; Lorca, M.; Piottante, A.; Florez, M.; Palma, C.; Ebensperger, M.; Castro, A. Leydig cell dysfunction is associated with post-transcriptional deregulation of cyp17a1 in men with sertoli cell-only syndrome. Mol. Hum. Reprod. 2018, 24, 203–210. [Google Scholar] [CrossRef]
- Steger, K.; Rey, R.; Louis, F.; Kliesch, S.; Behre, H.M.; Nieschlag, E.; Hoepffner, W.; Bailey, D.; Marks, A.; Bergmann, M. Reversion of the differentiated phenotype and maturation block in sertoli cells in pathological human testis. Hum. Reprod. 1999, 14, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Kuyucu, Y.; Coskun, G.; Saker, D.; Karaoglan, O.; Urunsak, I.F.; Izol, V.; Aridogan, I.A.; Erdogan, S.; Ozgur, H.; Polat, S. Immunohistochemical examination of androgen receptor and estrogen receptor alpha expressions in obstructive and non-obstructive azoospermia. Syst. Biol. Reprod. Med. 2021, 67, 463–470. [Google Scholar] [CrossRef]
- Andersson, A.M.; Jorgensen, N.; Frydelund-Larsen, L.; Rajpert-De Meyts, E.; Skakkebaek, N.E. Impaired leydig cell function in infertile men: A study of 357 idiopathic infertile men and 318 proven fertile controls. J. Clin. Endocrinol. Metab. 2004, 89, 3161–3167. [Google Scholar] [CrossRef] [PubMed]
- Levalle, O.A.; Zylbersztein, C.; Aszpis, S.; Mariani, V.; Ponzio, R.; Aranda, C.; Guitelman, A.; Scaglia, H.E. Serum luteinizing hormone pulsatility and intratesticular testosterone and oestradiol concentrations in idiopathic infertile men with high and normal follicle stimulating hormone serum concentrations. Hum. Reprod. 1994, 9, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Yasar, P.; Ayaz, G.; User, S.D.; Gupur, G.; Muyan, M. Molecular mechanism of estrogen-estrogen receptor signaling. Reprod. Med. Biol. 2017, 16, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.R.; Hammes, S.R. Nuclear receptors outside the nucleus: Extranuclear signalling by steroid receptors. Nat. Rev. Mol. Cell Biol. 2016, 17, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Feng, H.L.; Sandlow, J.I.; Haines, C.J. Comparing expression of progesterone and estrogen receptors in testicular tissue from men with obstructive and nonobstructive azoospermia. J. Androl. 2009, 30, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Araujo, F.C.; Oliveira, C.A.; Reis, A.B.; Del Puerto, H.L.; Martins, A.S.; Reis, F.M. Expression of the proto-oncogene c-fos and the immunolocalization of c-fos, phosphorylated c-fos and estrogen receptor beta in the human testis. Histol. Histopathol. 2009, 24, 1515–1522. [Google Scholar]
- Cavaco, J.E.; Laurentino, S.S.; Barros, A.; Sousa, M.; Socorro, S. Estrogen receptors alpha and beta in human testis: Both isoforms are expressed. Syst. Biol. Reprod. Med. 2009, 55, 137–144. [Google Scholar] [CrossRef]
- Filipiak, E.; Suliborska, D.; Laszczynska, M.; Walczak-Jedrzejowska, R.; Oszukowska, E.; Marchlewska, K.; Kula, K.; Slowikowska-Hilczer, J. Estrogen receptor alpha localization in the testes of men with normal spermatogenesis. Folia Histochem. Cytobiol. 2013, 50, 340–345. [Google Scholar] [CrossRef]
- Pelletier, G.; El-Alfy, M. Immunocytochemical localization of estrogen receptors alpha and beta in the human reproductive organs. J. Clin. Endocrinol. Metab. 2000, 85, 4835–4840. [Google Scholar]
- Fietz, D.; Ratzenbock, C.; Hartmann, K.; Raabe, O.; Kliesch, S.; Weidner, W.; Klug, J.; Bergmann, M. Expression pattern of estrogen receptors alpha and beta and g-protein-coupled estrogen receptor 1 in the human testis. Histochem. Cell Biol. 2014, 142, 421–432. [Google Scholar] [CrossRef]
- Zimmerman, M.A.; Budish, R.A.; Kashyap, S.; Lindsey, S.H. GPER-novel membrane oestrogen receptor. Clin. Sci. 2016, 130, 1005–1016. [Google Scholar] [CrossRef]
- Sirianni, R.; Chimento, A.; Ruggiero, C.; De Luca, A.; Lappano, R.; Ando, S.; Maggiolini, M.; Pezzi, V. The novel estrogen receptor, g protein-coupled receptor 30, mediates the proliferative effects induced by 17beta-estradiol on mouse spermatogonial gc-1 cell line. Endocrinology 2008, 149, 5043–5051. [Google Scholar] [CrossRef] [PubMed]
- Chimento, A.; Sirianni, R.; Delalande, C.; Silandre, D.; Bois, C.; Ando, S.; Maggiolini, M.; Carreau, S.; Pezzi, V. 17 beta-estradiol activates rapid signaling pathways involved in rat pachytene spermatocytes apoptosis through gpr30 and er alpha. Mol. Cell Endocrinol. 2010, 320, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Chimento, A.; Sirianni, R.; Casaburi, I.; Ruggiero, C.; Maggiolini, M.; Ando, S.; Pezzi, V. 17beta-estradiol activates gper- and esr1-dependent pathways inducing apoptosis in gc-2 cells, a mouse spermatocyte-derived cell line. Mol. Cell Endocrinol. 2012, 355, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Lucas, T.F.; Royer, C.; Siu, E.R.; Lazari, M.F.; Porto, C.S. Expression and signaling of g protein-coupled estrogen receptor 1 (gper) in rat sertoli cells. Biol. Reprod. 2010, 83, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Vaucher, L.; Funaro, M.G.; Mehta, A.; Mielnik, A.; Bolyakov, A.; Prossnitz, E.R.; Schlegel, P.N.; Paduch, D.A. Activation of gper-1 estradiol receptor downregulates production of testosterone in isolated rat leydig cells and adult human testis. PLoS ONE 2014, 9, e92425. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Traverso, G.B.; Pearl, C.A. Immunolocalization of g protein-coupled estrogen receptor in the rat epididymis. Reprod. Biol. Endocrinol. 2015, 13, 48. [Google Scholar] [CrossRef]
- Chimento, A.; De Luca, A.; Nocito, M.C.; Avena, P.; La Padula, D.; Zavaglia, L.; Pezzi, V. Role of gper-mediated signaling in testicular functions and tumorigenesis. Cells 2020, 9, 2115. [Google Scholar] [CrossRef] [PubMed]
- Bouskine, A.; Nebout, M.; Mograbi, B.; Brucker-Davis, F.; Roger, C.; Fenichel, P. Estrogens promote human testicular germ cell cancer through a membrane-mediated activation of extracellular regulated kinase and protein kinase A. Endocrinology 2008, 149, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Rago, V.; Romeo, F.; Giordano, F.; Maggiolini, M.; Carpino, A. Identification of the estrogen receptor gper in neoplastic and non-neoplastic human testes. Reprod. Biol. Endocrinol. 2011, 9, 135. [Google Scholar] [CrossRef]
- Oliveira, P.F.; Alves, M.G.; Martins, A.D.; Correia, S.; Bernardino, R.L.; Silva, J.; Barros, A.; Sousa, M.; Cavaco, J.E.; Socorro, S. Expression pattern of g protein-coupled receptor 30 in human seminiferous tubular cells. Gen. Comp. Endocrinol. 2014, 201, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Bernardino, R.L.; Alves, M.G.; Silva, J.; Barros, A.; Ferraz, L.; Sousa, M.; Sa, R.; Oliveira, P.F. Expression of estrogen receptors alpha (er-alpha), beta (er-beta), and g protein-coupled receptor 30 (gpr30) in testicular tissue of men with klinefelter syndrome. Horm. Metab. Res. 2016, 48, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Sonne, S.B.; Dalgaard, M.D.; Nielsen, J.E.; Hoei-Hansen, C.E.; Rajpert-De Meyts, E.; Gjerdrum, L.M.; Leffers, H. Optimizing staining protocols for laser microdissection of specific cell types from the testis including carcinoma in situ. PLoS ONE 2009, 4, e5536. [Google Scholar] [CrossRef] [PubMed]
- Jezek, D.; Knuth, U.A.; Schulze, W. Successful testicular sperm extraction (tese) in spite of high serum follicle stimulating hormone and azoospermia: Correlation between testicular morphology, tese results, semen analysis and serum hormone values in 103 infertile men. Hum. Reprod. 1998, 13, 1230–1234. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, S.G. Testicular biopsy score count--a method for registration of spermatogenesis in human testes: Normal values and results in 335 hypogonadal males. Hormones 1970, 1, 2–25. [Google Scholar] [CrossRef]
- Guminska, A.; Oszukowska, E.; Kuzanski, W.; Sosnowski, M.; Wolski, J.K.; Walczak-Jedrzejowska, R.; Marchlewska, K.; Niedzielski, J.; Kula, K.; Slowikowska-Hilczer, J. Less advanced testicular dysgenesis is associated by a higher prevalence of germ cell neoplasia. Int. J. Androl. 2010, 33, e153–e162. [Google Scholar] [CrossRef]
- Holstein, A.F.; Roosen-Runge, E.C.; Schirren, C.S. The testis biopsy. In Ilustrated Pathology of Human Spermatogenesis; Holstein, A.F., Ed.; Grosse Verlag: Berlin, Germany, 1988; pp. 58–75. [Google Scholar]
- Guminska, A.; Slowikowska-Hilczer, J.; Kuzanski, W.; Sosnowski, M.; Oszukowska, E.; Marchlewska, K.; Walczak-Jedrzejowska, R.; Niedzielski, J.; Kula, K. Features of impaired seminiferous tubule differentiation are associated with germ cell neoplasia in adult men surgically treated in childhood because of cryptorchidism. Folia Histochem. Cytobiol. 2007, 45 (Suppl. 1), S163–S168. [Google Scholar]
- Svingen, T.; Jorgensen, A.; Rajpert-De Meyts, E. Validation of endogenous normalizing genes for expression analyses in adult human testis and germ cell neoplasms. Mol. Hum. Reprod. 2014, 20, 709–718. [Google Scholar] [CrossRef]
- Kotula-Balak, M.; Zielinska, R.; Glogowski, J.; Kowalski, R.K.; Sarosiek, B.; Bilinska, B. Aromatase expression in testes of xy, yy, and xx rainbow trout (oncorhynchus mykiss). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2008, 149, 188–196. [Google Scholar] [CrossRef]
- Lydka, M.; Kopera-Sobota, I.; Kotula-Balak, M.; Chojnacka, K.; Zak, D.; Bilinska, B. Morphological and functional alterations in adult boar epididymis: Effects of prenatal and postnatal administration of flutamide. Acta Vet. Scand. 2011, 53, 12. [Google Scholar] [CrossRef]
- Witkowski, M.; Pardyak, L.; Pawlicki, P.; Galuszka, A.; Profaska-Szymik, M.; Plachno, B.J.; Kantor, S.; Duliban, M.; Kotula-Balak, M. The g-protein-coupled membrane estrogen receptor is present in horse cryptorchid testes and mediates downstream pathways. Int. J. Mol. Sci. 2021, 22, 7131. [Google Scholar] [CrossRef]
- Smolen, A.J. Image analytic techniques for quantification of immunocytochemical staining in the nervous system. In Methods in Neurosciences; Conn, P.M., Ed.; Academic Press: New York, NY, USA, 1990. [Google Scholar]
- Filardo, E.J.; Graeber, C.T.; Quinn, J.A.; Resnick, M.B.; Giri, D.; DeLellis, R.A.; Steinhoff, M.M.; Sabo, E. Distribution of gpr30, a seven membrane-spanning estrogen receptor, in primary breast cancer and its association with clinicopathologic determinants of tumor progression. Clin. Cancer Res. 2006, 12, 6359–6366. [Google Scholar] [CrossRef] [PubMed]
- Andrade, D.L.; Viana, M.C.; Esteves, S.C. Differential diagnosis of azoospermia in men with infertility. J. Clin. Med. 2021, 10, 3144. [Google Scholar] [CrossRef] [PubMed]
- Lardone, M.C.; Piottante, A.; Valdevenito, R.; Ebensperger, M.; Castro, A. Histological and hormonal testicular function in oligo/azoospermic infertile men. Andrologia 2013, 45, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Luboshitzky, R.; Kaplan-Zverling, M.; Shen-Orr, Z.; Nave, R.; Herer, P. Seminal plasma androgen/oestrogen balance in infertile men. Int. J. Androl. 2002, 25, 345–351. [Google Scholar] [CrossRef]
- Chevalier, N.; Vega, A.; Bouskine, A.; Siddeek, B.; Michiels, J.F.; Chevallier, D.; Fenichel, P. Gpr30, the non-classical membrane g protein related estrogen receptor, is overexpressed in human seminoma and promotes seminoma cell proliferation. PLoS ONE 2012, 7, e34672. [Google Scholar] [CrossRef]
- Sandner, F.; Welter, H.; Schwarzer, J.U.; Kohn, F.M.; Urbanski, H.F.; Mayerhofer, A. Expression of the oestrogen receptor gper by testicular peritubular cells is linked to sexual maturation and male fertility. Andrology 2014, 2, 695–701. [Google Scholar] [CrossRef]
- Revankar, C.M.; Cimino, D.F.; Sklar, L.A.; Arterburn, J.B.; Prossnitz, E.R. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 2005, 307, 1625–1630. [Google Scholar] [CrossRef]
- Filardo, E.J.; Thomas, P. Minireview: G protein-coupled estrogen receptor-1, gper-1: Its mechanism of action and role in female reproductive cancer, renal and vascular physiology. Endocrinology 2012, 153, 2953–2962. [Google Scholar] [CrossRef]
- Ghanami Gashti, N.; Sadighi Gilani, M.A.; Abbasi, M. Sertoli cell-only syndrome: Etiology and clinical management. J. Assist. Reprod. Genet. 2021, 38, 559–572. [Google Scholar] [CrossRef]
- Zitzmann, M.; Aksglaede, L.; Corona, G.; Isidori, A.M.; Juul, A.; T’Sjoen, G.; Kliesch, S.; D’Hauwers, K.; Toppari, J.; Slowikowska-Hilczer, J.; et al. European academy of andrology guidelines on klinefelter syndrome endorsing organization: European society of endocrinology. Andrology 2021, 9, 145–167. [Google Scholar] [CrossRef]
- Giudice, M.G.; Vermeulen, M.; Wyns, C. Blood testis barrier and somatic cells impairment in a series of 35 adult klinefelter syndrome patients. Int. J. Mol. Sci. 2019, 20, 5717. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, B.Y.; Oh, A.R.; Park, S.Y.; Lee, H.S.; Seo, J.T. Clinical, hormonal, and genetic evaluation of idiopathic nonobstructive azoospermia and klinefelter syndrome patients. Cytogenet. Genome Res. 2017, 153, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Boscia, F.; Passaro, C.; Gigantino, V.; Perdona, S.; Franco, R.; Portella, G.; Chieffi, S.; Chieffi, P. High levels of gpr30 protein in human testicular carcinoma in situ and seminomas correlate with low levels of estrogen receptor-beta and indicate a switch in estrogen responsiveness. J. Cell Physiol. 2015, 230, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Lucas, T.F.; Pimenta, M.T.; Pisolato, R.; Lazari, M.F.; Porto, C.S. 17beta-estradiol signaling and regulation of sertoli cell function. Spermatogenesis 2011, 1, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Steger, K.; Rey, R.; Kliesch, S.; Louis, F.; Schleicher, G.; Bergmann, M. Immunohistochemical detection of immature sertoli cell markers in testicular tissue of infertile adult men: A preliminary study. Int. J. Androl. 1996, 19, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Hai, Y.; Hou, J.; Liu, Y.; Liu, Y.; Yang, H.; Li, Z.; He, Z. The roles and regulation of sertoli cells in fate determinations of spermatogonial stem cells and spermatogenesis. Semin. Cell Dev. Biol. 2014, 29, 66–75. [Google Scholar] [CrossRef]
- Mauduit, C.; Hamamah, S.; Benahmed, M. Stem cell factor/c-kit system in spermatogenesis. Hum. Reprod. Update 1999, 5, 535–545. [Google Scholar] [CrossRef]
- Feng, L.X.; Ravindranath, N.; Dym, M. Stem cell factor/c-kit up-regulates cyclin d3 and promotes cell cycle progression via the phosphoinositide 3-kinase/p70 s6 kinase pathway in spermatogonia. J. Biol. Chem. 2000, 275, 25572–25576. [Google Scholar] [CrossRef]
- Hasthorpe, S. Clonogenic culture of normal spermatogonia: In vitro regulation of postnatal germ cell proliferation. Biol. Reprod. 2003, 68, 1354–1360. [Google Scholar] [CrossRef]
- Correia, S.; Alves, M.R.; Cavaco, J.E.; Oliveira, P.F.; Socorro, S. Estrogenic regulation of testicular expression of stem cell factor and c-kit: Implications in germ cell survival and male fertility. Fertil. Steril. 2014, 102, 299–306. [Google Scholar] [CrossRef]
- Nagano, M.; Ryu, B.Y.; Brinster, C.J.; Avarbock, M.R.; Brinster, R.L. Maintenance of mouse male germ line stem cells in vitro. Biol. Reprod. 2003, 68, 2207–2214. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Grimaldi, P.; Rossi, P.; Geremia, R.; Dolci, S. Developmental expression of bmp4/alk3/smad5 signaling pathway in the mouse testis: A potential role of bmp4 in spermatogonia differentiation. J. Cell Sci. 2003, 116, 3363–3372. [Google Scholar] [CrossRef] [PubMed]
- Paduch, D.A.; Hilz, S.; Grimson, A.; Schlegel, P.N.; Jedlicka, A.E.; Wright, W.W. Aberrant gene expression by sertoli cells in infertile men with sertoli cell-only syndrome. PLoS ONE 2019, 14, e0216586. [Google Scholar] [CrossRef] [PubMed]
- Bialas, M.; Borczynska, A.; Rozwadowska, N.; Fiszer, D.; Kosicki, W.; Jedrzejczak, P.; Kurpisz, M. SCF and c-kit expression profiles in male individuals with normal and impaired spermatogenesis. Andrologia 2010, 42, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Lasala, C.; Schteingart, H.F.; Arouche, N.; Bedecarras, P.; Grinspon, R.P.; Picard, J.Y.; Josso, N.; di Clemente, N.; Rey, R.A. Sox9 and sf1 are involved in cyclic amp-mediated upregulation of anti-mullerian gene expression in the testicular prepubertal sertoli cell line SMAT1. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E539–E547. [Google Scholar] [CrossRef] [PubMed]
- Sansone, A.; Kliesch, S.; Isidori, A.M.; Schlatt, S. AMH and INSL3 in testicular and extragonadal pathophysiology: What do we know? Andrology 2019, 7, 131–138. [Google Scholar] [CrossRef]
- Lapoirie, M.; Dijoud, F.; Lejeune, H.; Plotton, I. Effect of androgens on sertoli cell maturation in human testis from birth to puberty. Basic Clin. Androl. 2021, 31, 31. [Google Scholar] [CrossRef]
- Edelsztein, N.Y.; Racine, C.; di Clemente, N.; Schteingart, H.F.; Rey, R.A. Androgens downregulate anti-mullerian hormone promoter activity in the sertoli cell through the androgen receptor and intact steroidogenic factor 1 sites. Biol. Reprod. 2018, 99, 1303–1312. [Google Scholar] [CrossRef]
- Edelsztein, N.Y.; Rey, R.A. Importance of the androgen receptor signaling in gene transactivation and transrepression for pubertal maturation of the testis. Cells 2019, 8, 861. [Google Scholar] [CrossRef]
- Maymon, B.B.; Yogev, L.; Paz, G.; Kleiman, S.E.; Schreiber, L.; Botchan, A.; Hauser, R.; Yavetz, H. Sertoli cell maturation in men with azoospermia of different etiologies. Fertil. Steril. 2002, 77, 904–909. [Google Scholar] [CrossRef]
- Van Saen, D.; Vloeberghs, V.; Gies, I.; De Schepper, J.; Tournaye, H.; Goossens, E. Characterization of the stem cell niche components within the seminiferous tubules in testicular biopsies of klinefelter patients. Fertil. Steril. 2020, 113, 1183–1195.e3. [Google Scholar] [CrossRef] [PubMed]
- Alfano, M.; Ventimiglia, E.; Locatelli, I.; Capogrosso, P.; Cazzaniga, W.; Pederzoli, F.; Frego, N.; Matloob, R.; Sacca, A.; Pagliardini, L.; et al. Anti-mullerian hormone-to-testosterone ratio is predictive of positive sperm retrieval in men with idiopathic non-obstructive azoospermia. Sci. Rep. 2017, 7, 17638. [Google Scholar] [CrossRef] [PubMed]
- Valeri, C.; Lovaisa, M.M.; Racine, C.; Edelsztein, N.Y.; Riggio, M.; Giulianelli, S.; Venara, M.; Bedecarras, P.; Ballerini, M.G.; di Clemente, N.; et al. Molecular mechanisms underlying AMH elevation in hyperoestrogenic states in males. Sci. Rep. 2020, 10, 15062. [Google Scholar] [CrossRef]
- Kotula-Balak, M.; Pawlicki, P.; Milon, A.; Tworzydlo, W.; Sekula, M.; Pacwa, A.; Gorowska-Wojtowicz, E.; Bilinska, B.; Pawlicka, B.; Wiater, J.; et al. The role of g-protein-coupled membrane estrogen receptor in mouse leydig cell function-in vivo and in vitro evaluation. Cell Tissue Res. 2018, 374, 389–412. [Google Scholar] [CrossRef] [PubMed]
- Gorowska-Wojtowicz, E.; Dutka, P.; Kudrycka, M.; Pawlicki, P.; Milon, A.; Plachno, B.J.; Tworzydlo, W.; Pardyak, L.; Kaminska, A.; Hejmej, A.; et al. Regulation of steroidogenic function of mouse leydig cells: G-coupled membrane estrogen receptor and peroxisome proliferator-activated receptor partnership. J. Physiol. Pharmacol. 2018, 69, 69. [Google Scholar]
- Duliban, M.; Gorowska-Wojtowicz, E.; Tworzydlo, W.; Rak, A.; Brzoskwinia, M.; Krakowska, I.; Wolski, J.K.; Kotula-Balak, M.; Plachno, B.J.; Bilinska, B. Interstitial leydig cell tumorigenesis-leptin and adiponectin signaling in relation to aromatase expression in the human testis. Int. J. Mol. Sci 2020, 21, 3649. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L.; Auchus, R.J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef] [PubMed]
- D’Aurora, M.; Ferlin, A.; Di Nicola, M.; Garolla, A.; De Toni, L.; Franchi, S.; Palka, G.; Foresta, C.; Stuppia, L.; Gatta, V. Deregulation of sertoli and leydig cells function in patients with klinefelter syndrome as evidenced by testis transcriptome analysis. BMC Genom. 2015, 16, 156. [Google Scholar] [CrossRef][Green Version]
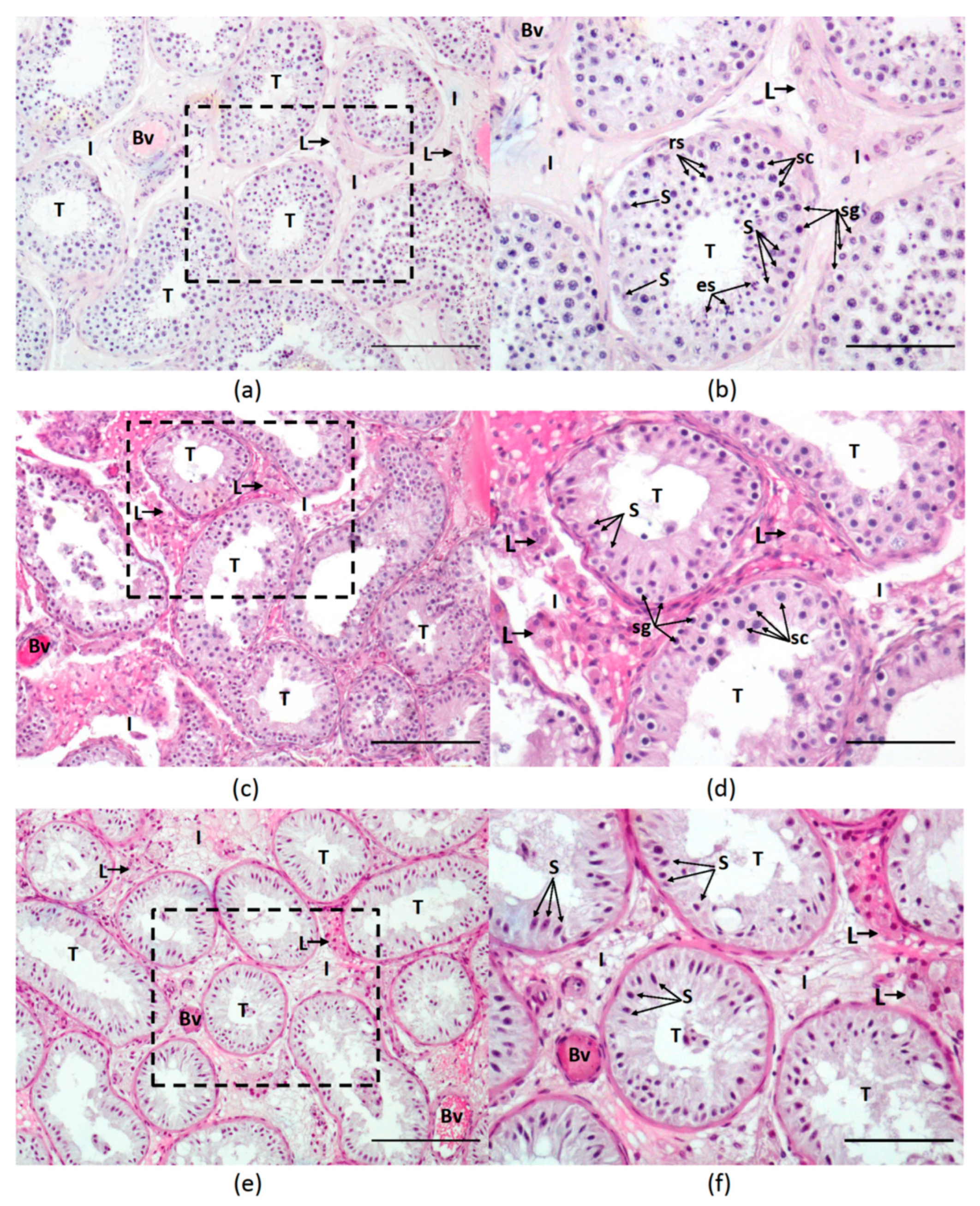
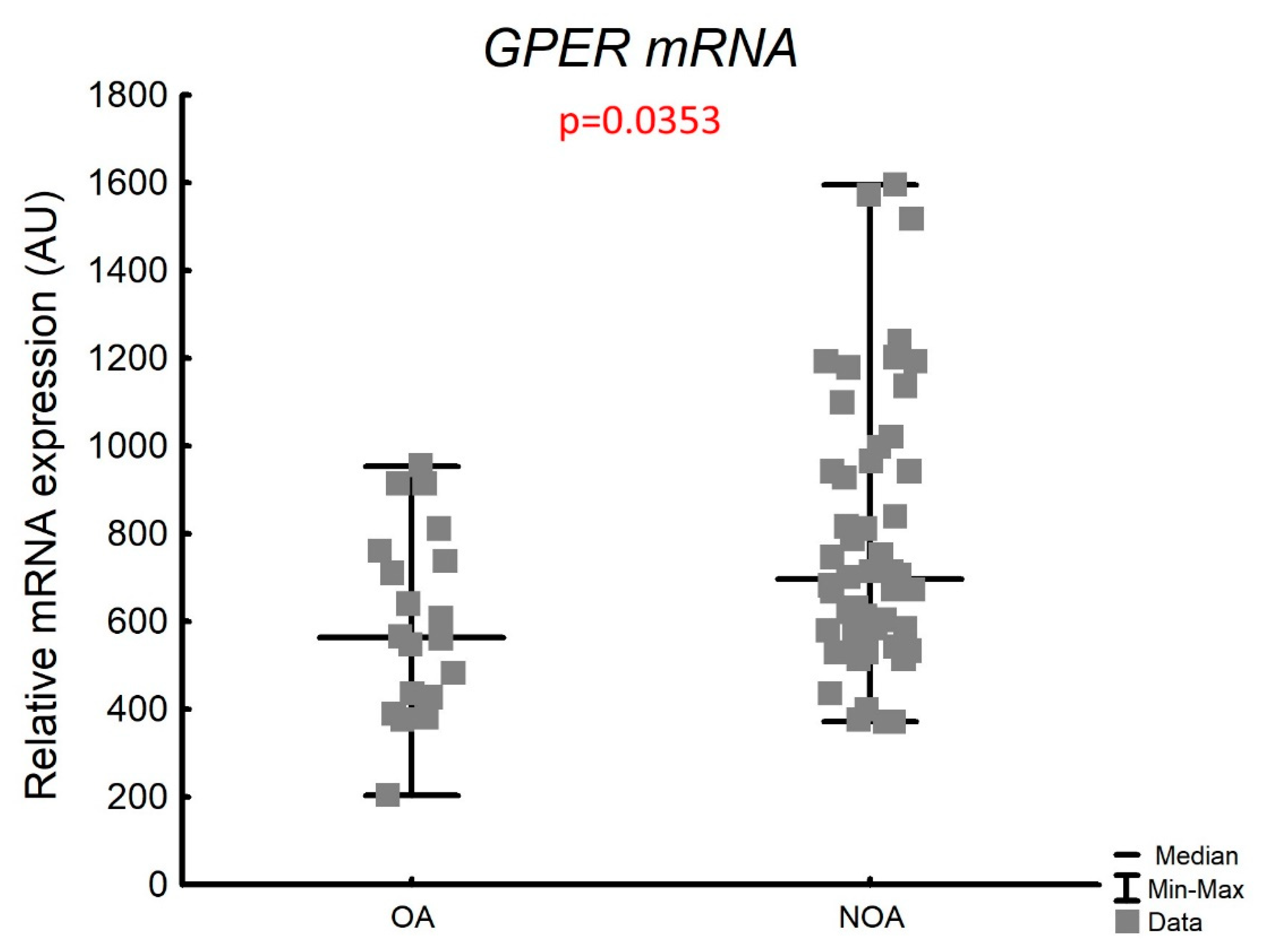
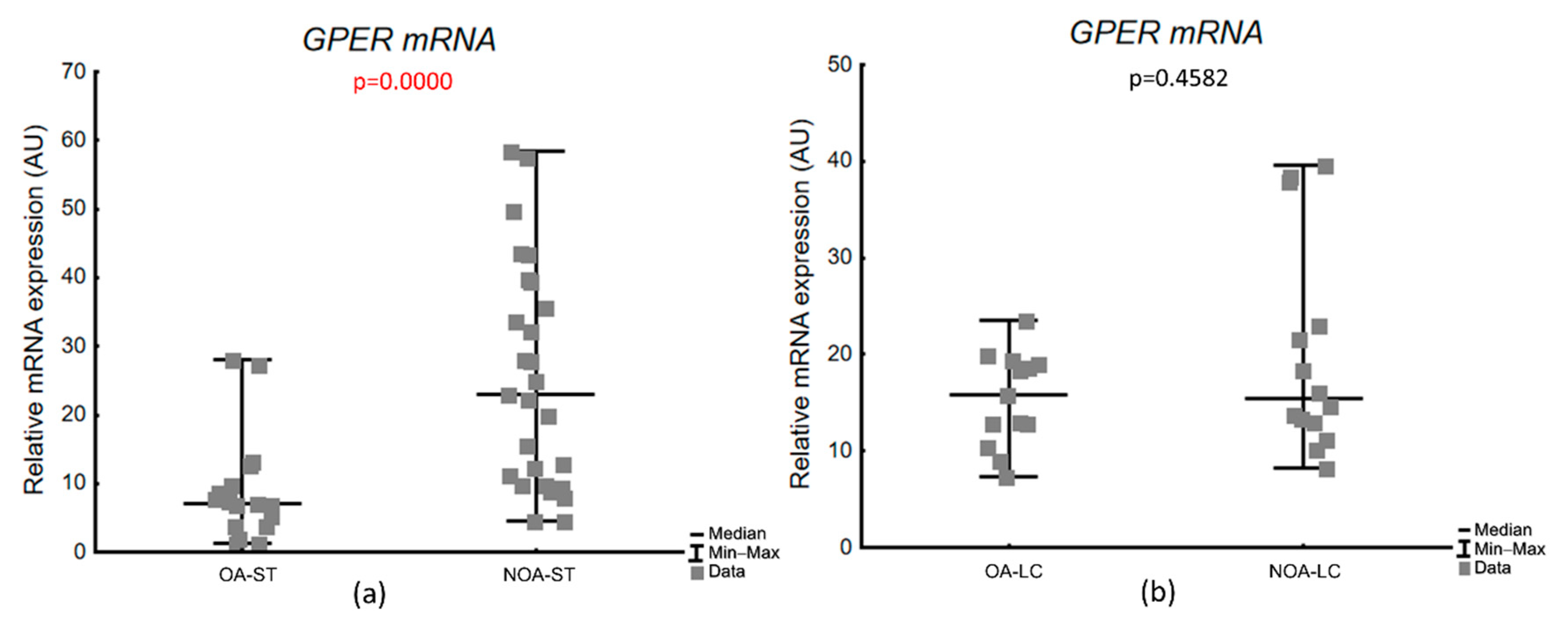
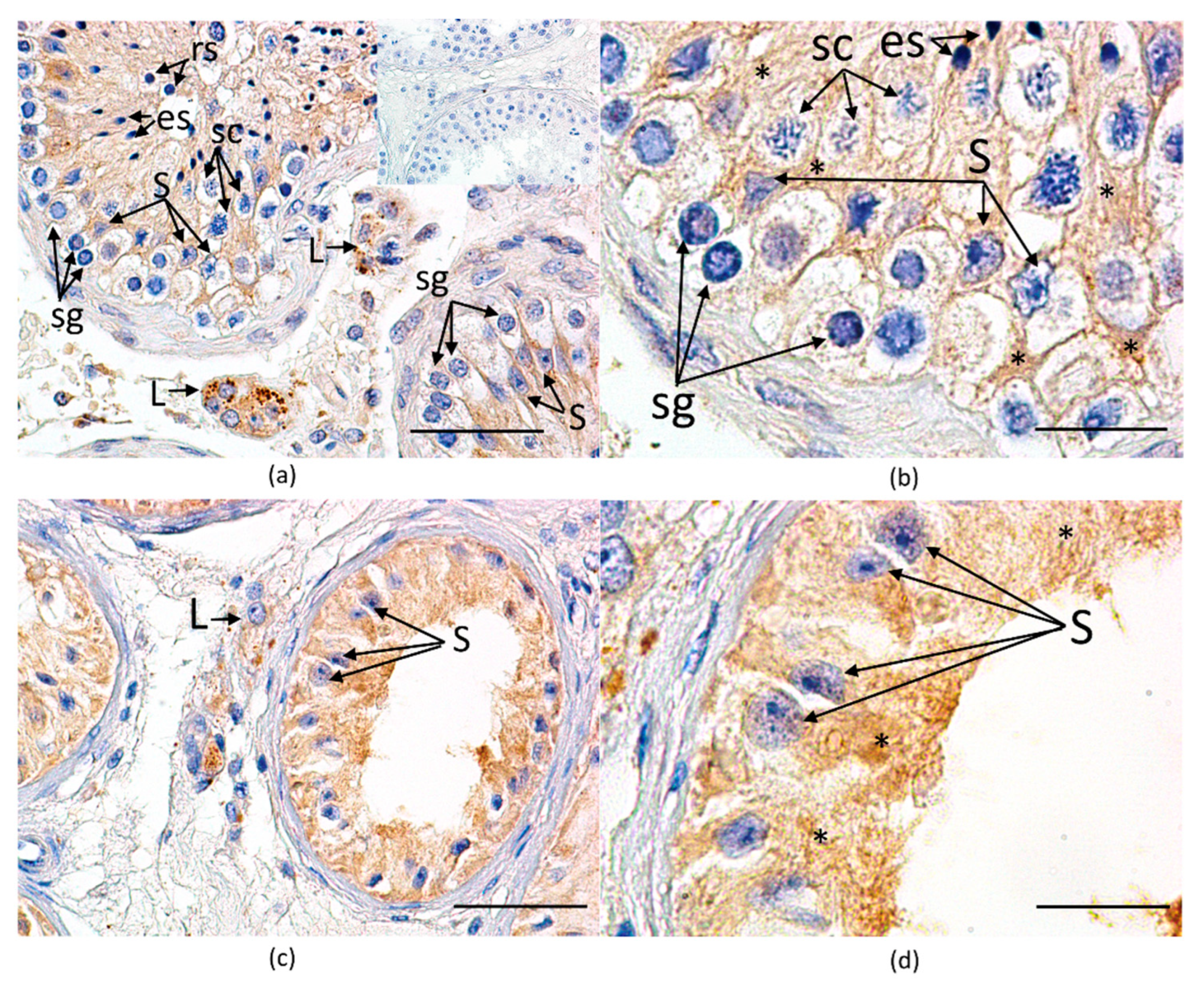
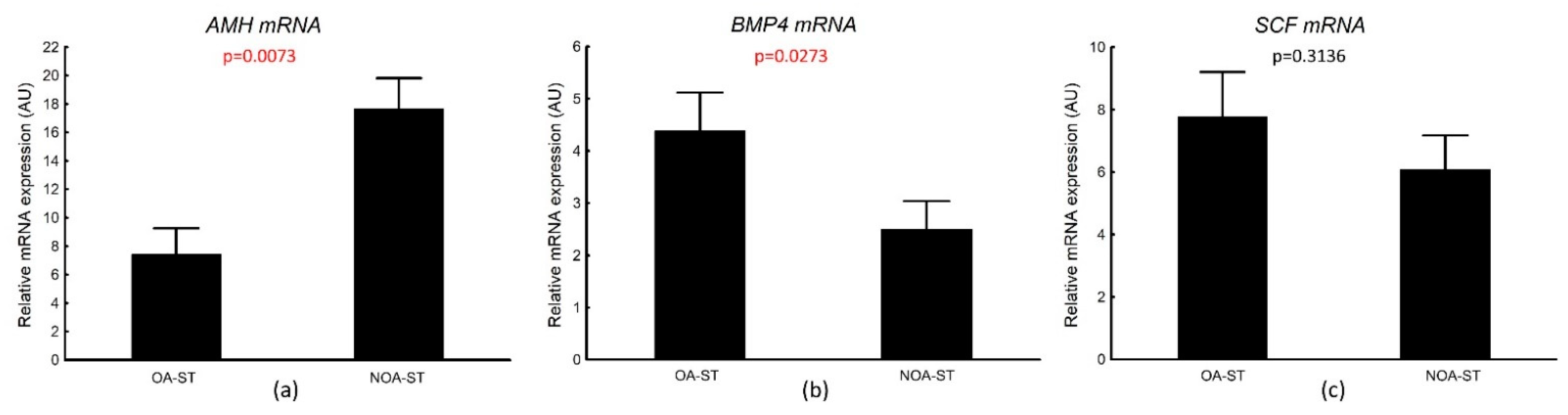
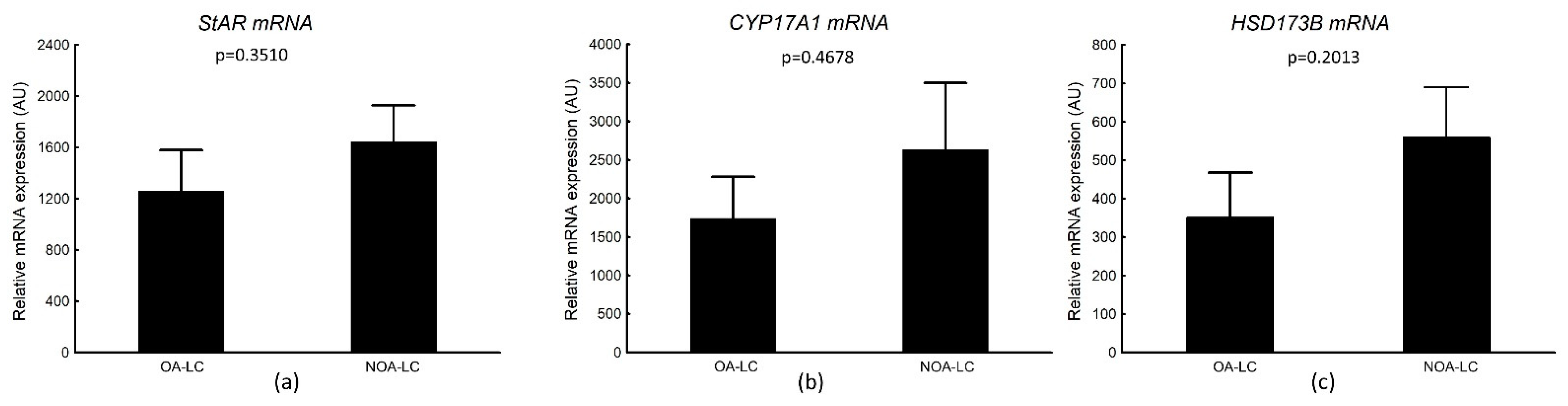
| Histological Group | Description | JS (Points) Median (Q1–Q3) |
|---|---|---|
| CS | All tubules with complete spermatogenesis or at least up to elongated spermatids | 8.5 (8.1–8.8) |
| ARR/MIX | Tubules with germ cells until spermatogonia or spermatocytes or immature spermatids/single tubules with few elongated spermatids and others presenting maturational arrest at different stages or SCOS | 4.5 (3.1–5.2) |
| SCOS | No tubules containing germ cells | 2.0 (1.6–2.0) |
| Target Gene | Assay Code | Whole Tissue | ST-LCM | LC-LCM |
|---|---|---|---|---|
| GPER | Hs00173506_m1 | + | + | + |
| RPS29 | Hs03004310_g1 | + | + | + |
| AMH | Hs01006984_g1 | − | + | − |
| BMP4 | Hs00370078_m1 | − | + | − |
| SCF | Hs00931798_m1 | − | + | − |
| StAR | Hs00986556_g1 | − | − | |
| CYP17A1 | Hs04981326_g1 | − | − | + |
| HSD173B | Hs00609319_m1 | − | − | + |
| OA n = 19 | NOA n = 53 | |
|---|---|---|
| Age (years) | 32.0 (27.0–36.0) | 31.0 (29.0–32.0) |
| Testicular volume (mL) * | 15.2 (11.5–16.3) | 10.1 (8.7–11.8) a |
| STD (µm) | 199.9 (188.5–208.0) | 159.6 (139.5–181.8) a |
| TM (µm) | 6.5 (6.0–7.5) | 8.0 (6.9–10.3) |
| LC-score (points) | 1.5 (1.3–1.8) | 2.2 (1.9–2.8) a |
| OA n = 19 | NOA n = 53 | |
|---|---|---|
| FSH (mIU/mL) | 3.5 (2.6–5.4) | 13.5 (10.0–19.9) a |
| LH (mIU/mL) | 4.5 (3.4–5.4) | 7.4 (5.8–9.8) a |
| Testosterone (T) (nmol/L) | 15.5 (10.9–20.1) | 15.9 (11.9–21.1) |
| Estradiol (E) (pmol/L) | 107.2(82.5–133.2) | 110.1 (82.2–151.9) |
| T/LH ratio | 4.0 (2.7–5.7) | 2.4 (1.5–3.1) a |
| E/T ratio | 7.2 (4.8–9.2) | 8.3 (6.9–10.2) |
| GPER mRNA | ||
|---|---|---|
| OA n = 19 rs (p) | NOA n = 53 rs (p) | |
| Age (years) | 0.091 (0.7111) | 0.106 (0.4486) |
| Testicular volume (mL) * | −0.142 (0.5620) | −0.283 (0.0586) |
| STD (µm) | 0.331 (0.1653) | −0.106 (0.4525) |
| TM (µm) | 0.058 (0.8105) | 0.157 (0.2635) |
| LC-score | 0.174 (0.4740) | 0.291 (0.0346) |
| FSH (mIU/mL) | 0.164 (0.5018) | 0.289 (0.0484) |
| LH (mIU/mL) | −0.236 (0.3305) | 0.055 (0.7001) |
| Testosterone (T) (nmol/L) | −0.254 (0.2928) | −0.175 (0.2089) |
| Estradiol (E) (pmol/L) | 0.137 (0.6545) | 0.183 (0.2915) |
| T/LH ratio | −0.061 (0.8054) | −0.110 (0.4391) |
| E/T ratio | 0.157 (0.5599) | 0.184 (0.2958) |
| GPER mRNA | ||
|---|---|---|
| OA-ST n = 16 rs (p) | NOA-ST n = 27 rs (p) | |
| Age (years) | 0.375 (0.1528) | −0.187 (0.3815) |
| Testicular volume (mL) * | −0.184 (0.4797) | −0.335 (0.1012) |
| STD (µm) | −0.208 (0.4238) | 0.182 (0.3839) |
| TM (µm) | 0.306 (0.2670) | 0.291 (0.1582) |
| LC-score | 0.294 (0.2513) | 0.174 (0.4043) |
| FSH (mIU/mL) | −0.158 (0.6627) | 0.627 (0.0008) |
| LH (mIU/mL) | 0.164 (0.6504) | 0.534 (0.0059) |
| Testosterone (T) (nmol/L) | 0.170 (0.6382) | 0.039 (0.8523) |
| Estradiol (E) (pmol/L) | 0.371 (0.4684) | 0.232 (0.3861) |
| T/LH ratio | −0.212 (0.5550) | −0.435 (0.0296) |
| E/T ratio | 0.285 (0.4927) | −0.044 (0.8711) |
| GPER mRNA | ||
|---|---|---|
| OA-LC n = 13 rs (p) | NOA-LC n = 14 rs (p) | |
| Age (years) | 0.259 (0.4406) | −0.103 (0.7375) |
| Testicular volume (mL) * | 0.082 (0.8084) | 0.262 (0.4094) |
| STD (µm) | −0.301 (0.3701) | 0.126 (0.6807) |
| TM (µm) | −0.329 (0.3528) | 0.192 (0.5291) |
| LC-score | 0.356 (0.2823) | 0.162 (0.6156) |
| FSH (mIU/mL) | 0.277 (0.4079) | −0.209 (0.4911) |
| LH (mIU/mL) | 0.323 (0.3318) | −0.197 (0.5171) |
| Testosterone (T) (nmol/L) | 0.059 (0.8626) | −0.461 (0.1123) |
| Estradiol (E) (pmol/L) | 0.203 (0.6286) | 0.103 (0.7769) |
| T/LH ratio | −0.123 (0.7186) | −0.192 (0.5291) |
| E/T ratio | 0.460 (0.2125) | 0.272 (0.4458) |
| GPER | OA n = 19 | NOA n = 53 |
|---|---|---|
| ROD Leydig cells | 10.4 (8.5–15.8) | 10.3 (6.9–14.9) |
| ROD Sertoli cells | 8.4 (5.9–9.8) | 9.1 (8.7–10.3) a |
| GPER IHC | OA-LC n = 13 | NOA-LC n = 14 |
| ROD Leydig cells | 9.5 (5.7–14.1) | 8.4 (7.0–14.9) |
| OA-ST n = 16 | NOA-ST n = 27 | |
| ROD Sertoli cells | 8.2 (5.8–10.3) | 10.9 (9.9–11.7) a |
| GPER mRNA | ||
|---|---|---|
| OA-ST n = 16 rs (p) | NOA-ST n = 27 rs (p) | |
| AMH | 0.167 (0.6914) | 0.562 (0.0052) |
| BMP4 | 0.2029 (0.6997) | 0.028 (0.8190) |
| SCF | −0.268 (0.4841) | 0.4148 (0.0867) |
| GPER mRNA | ||
|---|---|---|
| OA-LC n = 13 rs (p) | NOA-LC n = 14 rs (p) | |
| StAR-LC | 0.320 (0.3369) | 0.231 (0.5194) |
| CYP17A1-LC | 0.379 (0.2491) | 0.584 (0.0529) |
| HSD17B3-LC | 0.407 (0.2762) | 0.487 (0.1526) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walczak-Jędrzejowska, R.; Forma, E.; Oszukowska, E.; Bryś, M.; Marchlewska, K.; Kula, K.; Słowikowska-Hilczer, J. Expression of G-Protein-Coupled Estrogen Receptor (GPER) in Whole Testicular Tissue and Laser-Capture Microdissected Testicular Compartments of Men with Normal and Aberrant Spermatogenesis. Biology 2022, 11, 373. https://doi.org/10.3390/biology11030373
Walczak-Jędrzejowska R, Forma E, Oszukowska E, Bryś M, Marchlewska K, Kula K, Słowikowska-Hilczer J. Expression of G-Protein-Coupled Estrogen Receptor (GPER) in Whole Testicular Tissue and Laser-Capture Microdissected Testicular Compartments of Men with Normal and Aberrant Spermatogenesis. Biology. 2022; 11(3):373. https://doi.org/10.3390/biology11030373
Chicago/Turabian StyleWalczak-Jędrzejowska, Renata, Ewa Forma, Elżbieta Oszukowska, Magdalena Bryś, Katarzyna Marchlewska, Krzysztof Kula, and Jolanta Słowikowska-Hilczer. 2022. "Expression of G-Protein-Coupled Estrogen Receptor (GPER) in Whole Testicular Tissue and Laser-Capture Microdissected Testicular Compartments of Men with Normal and Aberrant Spermatogenesis" Biology 11, no. 3: 373. https://doi.org/10.3390/biology11030373
APA StyleWalczak-Jędrzejowska, R., Forma, E., Oszukowska, E., Bryś, M., Marchlewska, K., Kula, K., & Słowikowska-Hilczer, J. (2022). Expression of G-Protein-Coupled Estrogen Receptor (GPER) in Whole Testicular Tissue and Laser-Capture Microdissected Testicular Compartments of Men with Normal and Aberrant Spermatogenesis. Biology, 11(3), 373. https://doi.org/10.3390/biology11030373







