Mimicking CA3 Temporal Dynamics Controls Limbic Ictogenesis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Brain Slice Preparation and Maintenance
2.2. Microelectrode Array Recording
2.3. Electrical Stimulation
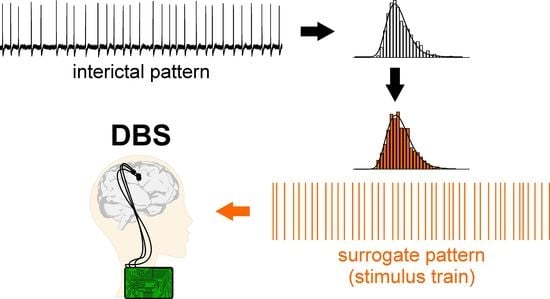
2.4. Surrogate Data Generation
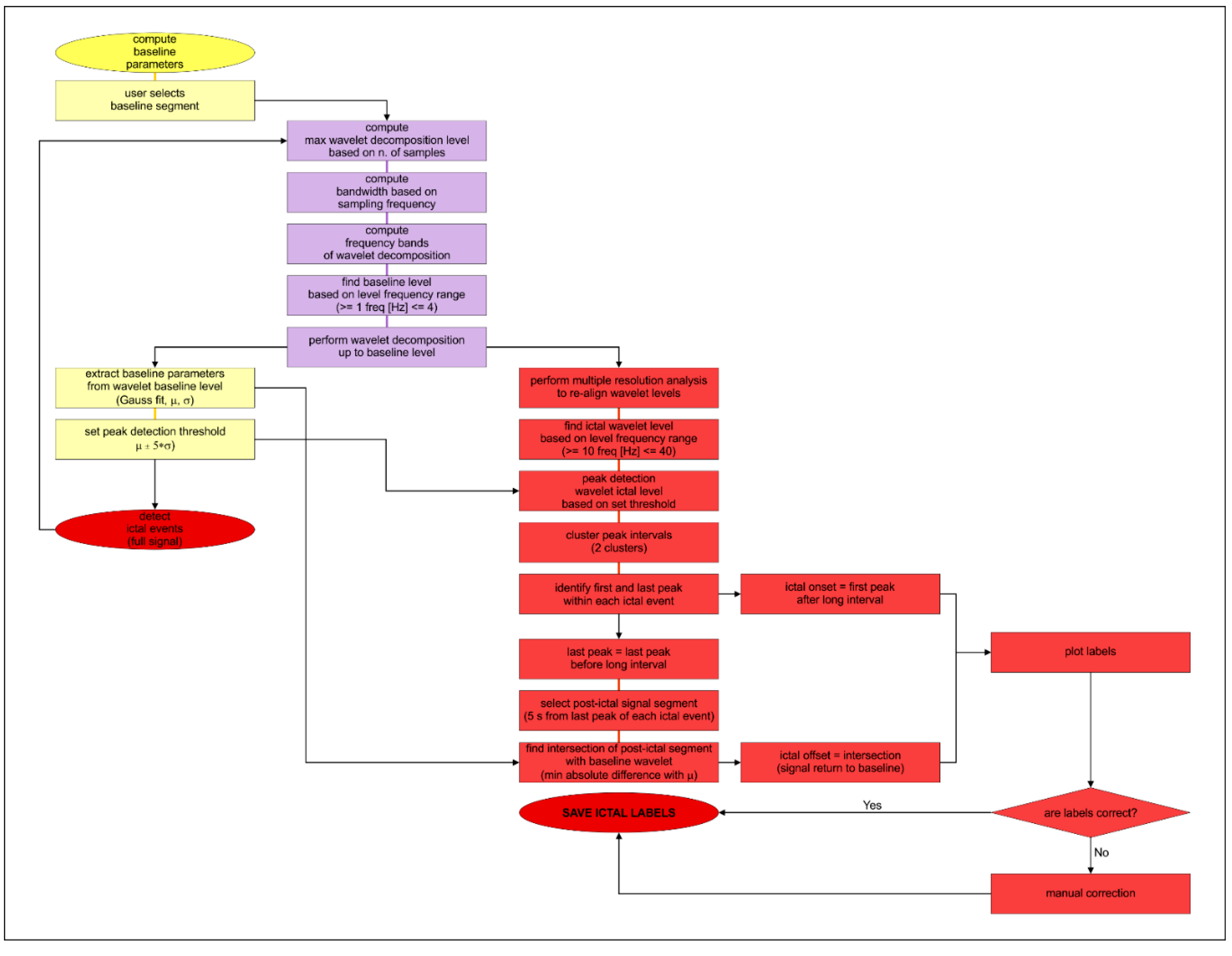
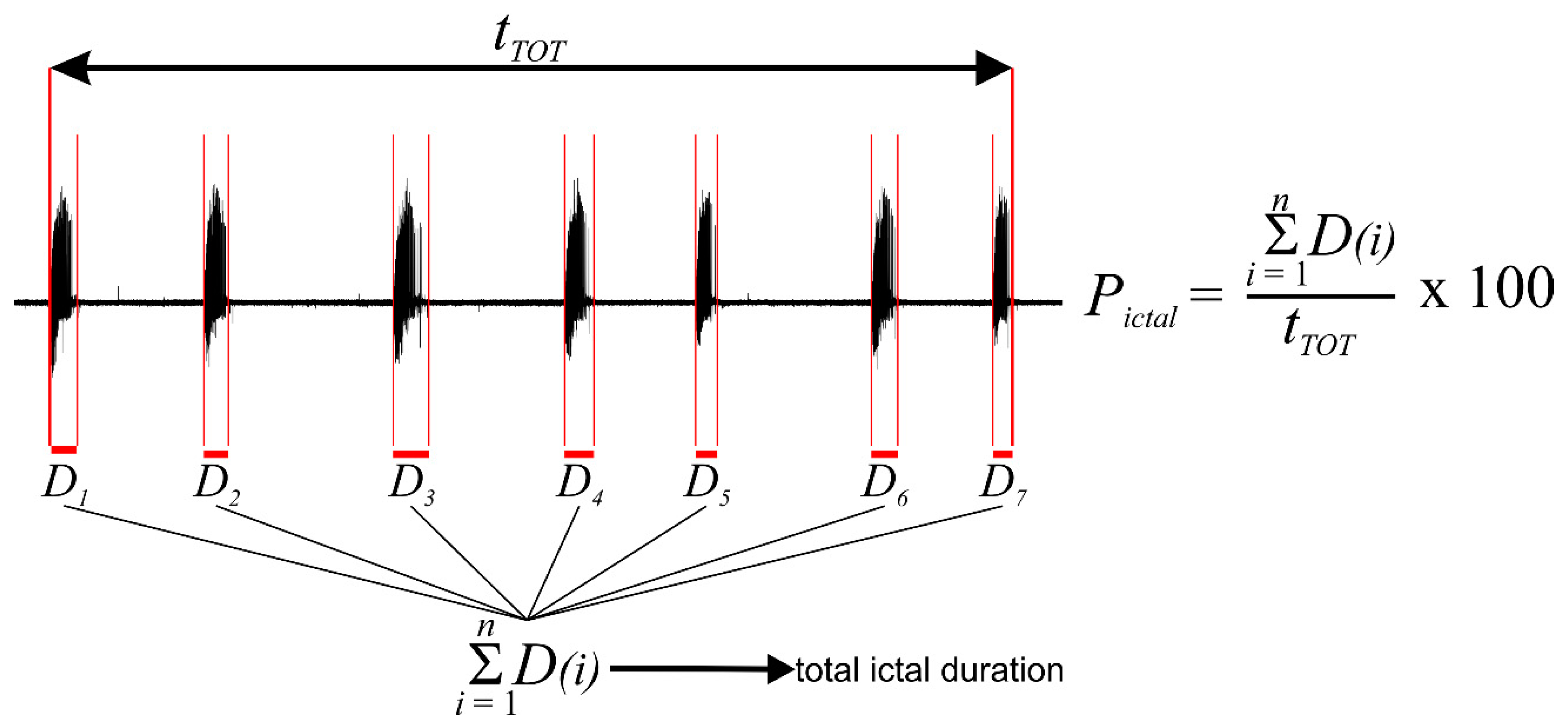
2.5. Data Analysis
2.6. Statistical Analysis
3. Results
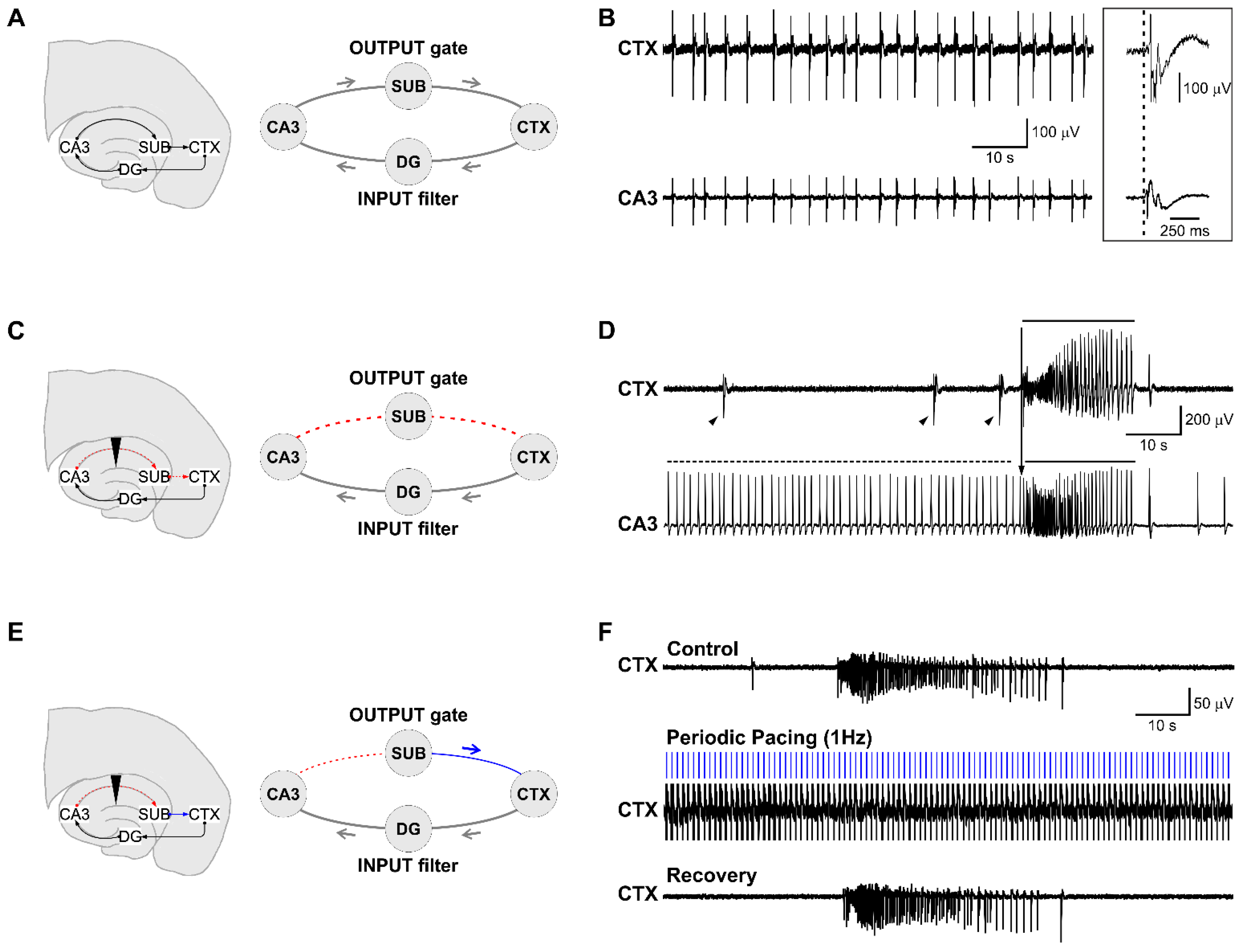
3.1. Epileptiform Activity Induced by 4-Aminopyridine in Hippocampus-Cortex Slices
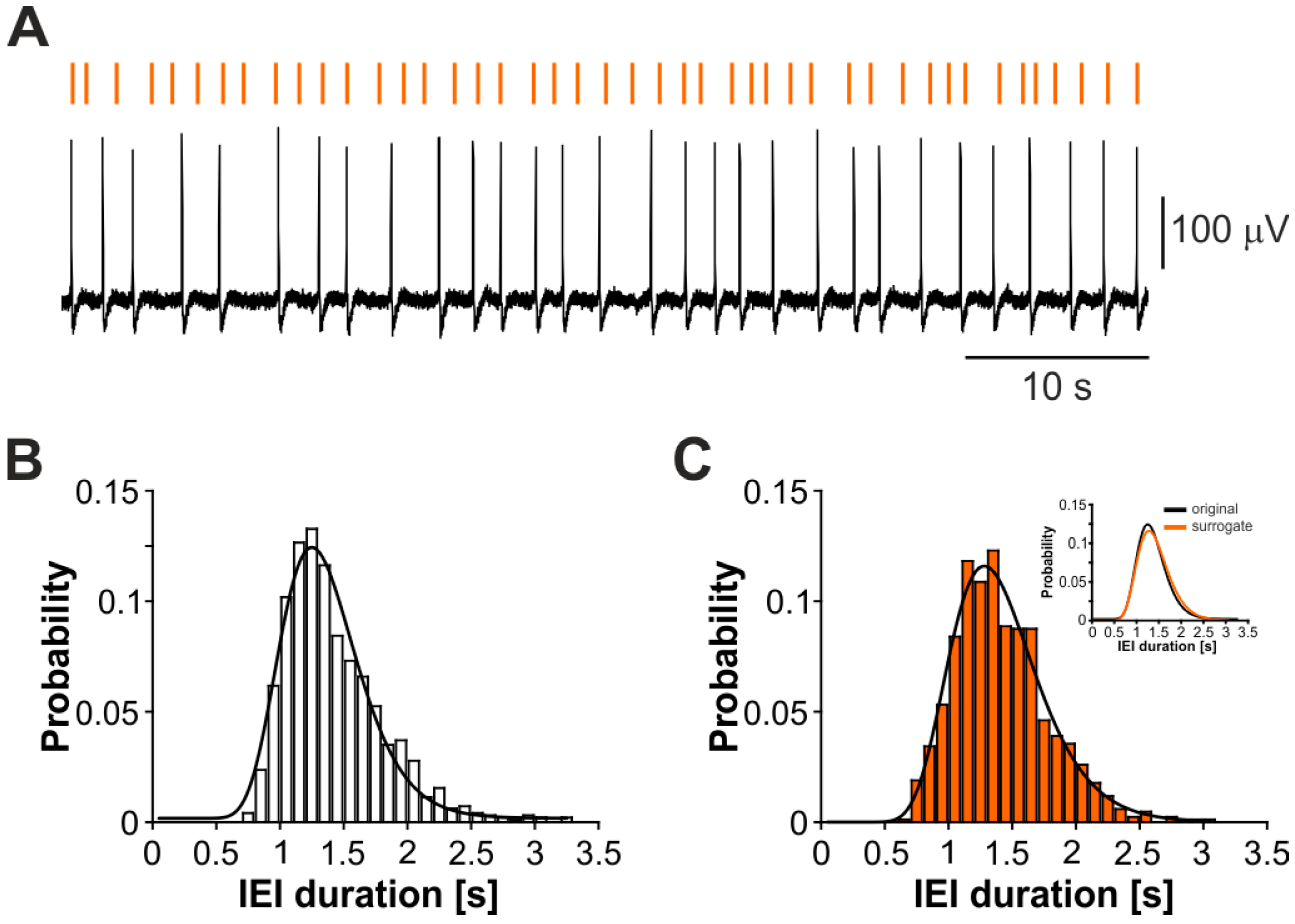
3.2. The CA3-Driven Interictal Activity Exhibits Lognormal Temporal Dynamics
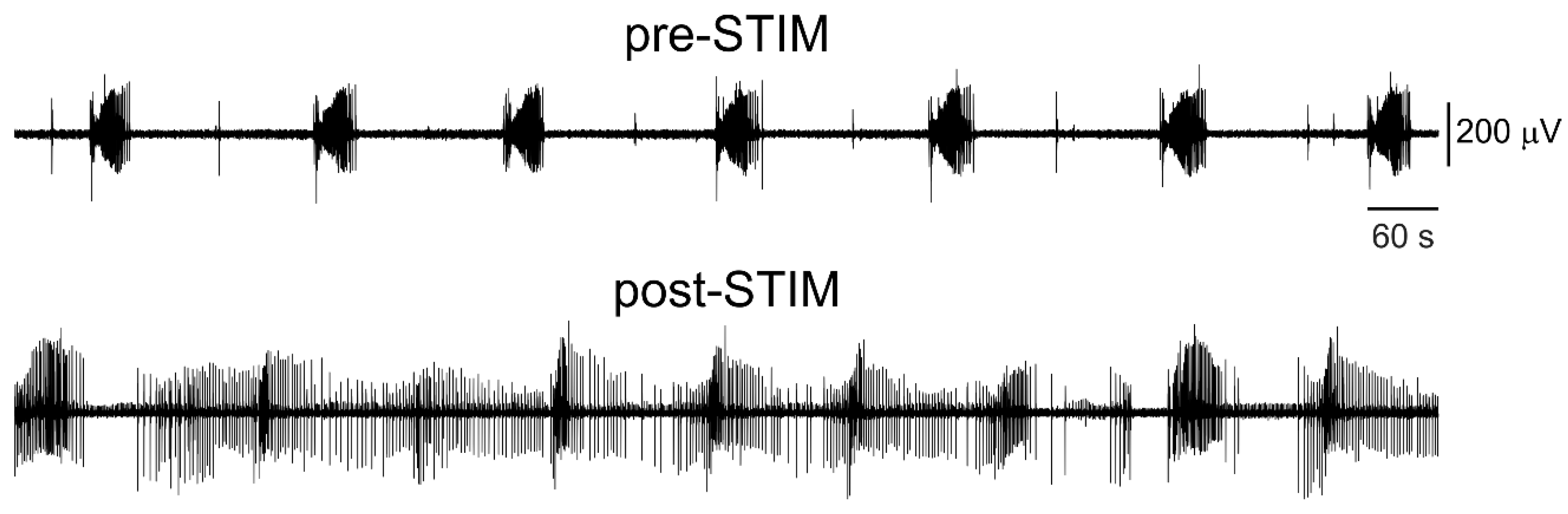
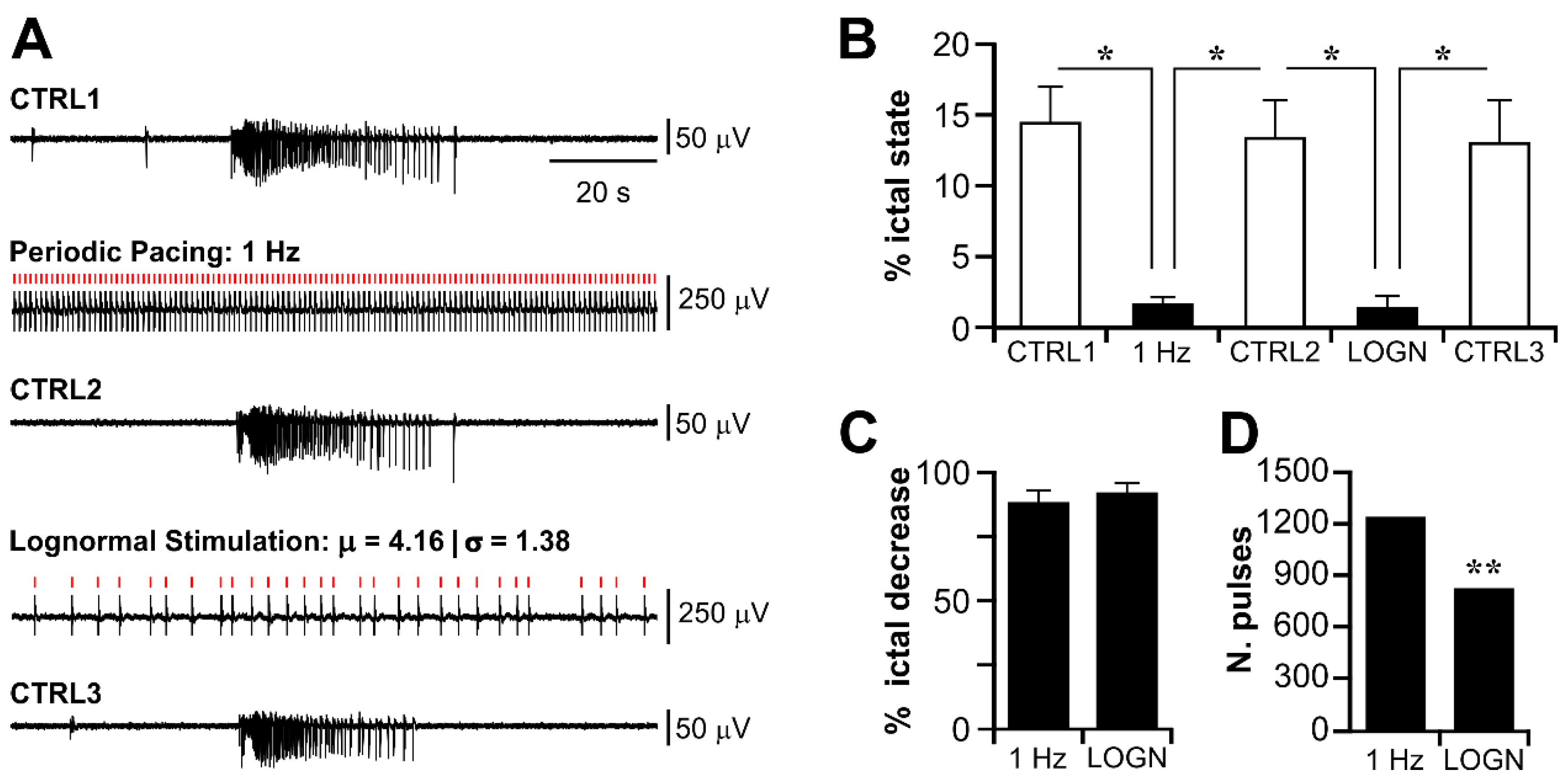
3.3. Electrical Stimulation Fashioned as a Surrogate CA3-Driven Interictal Pattern Controls Limbic Ictogenesis
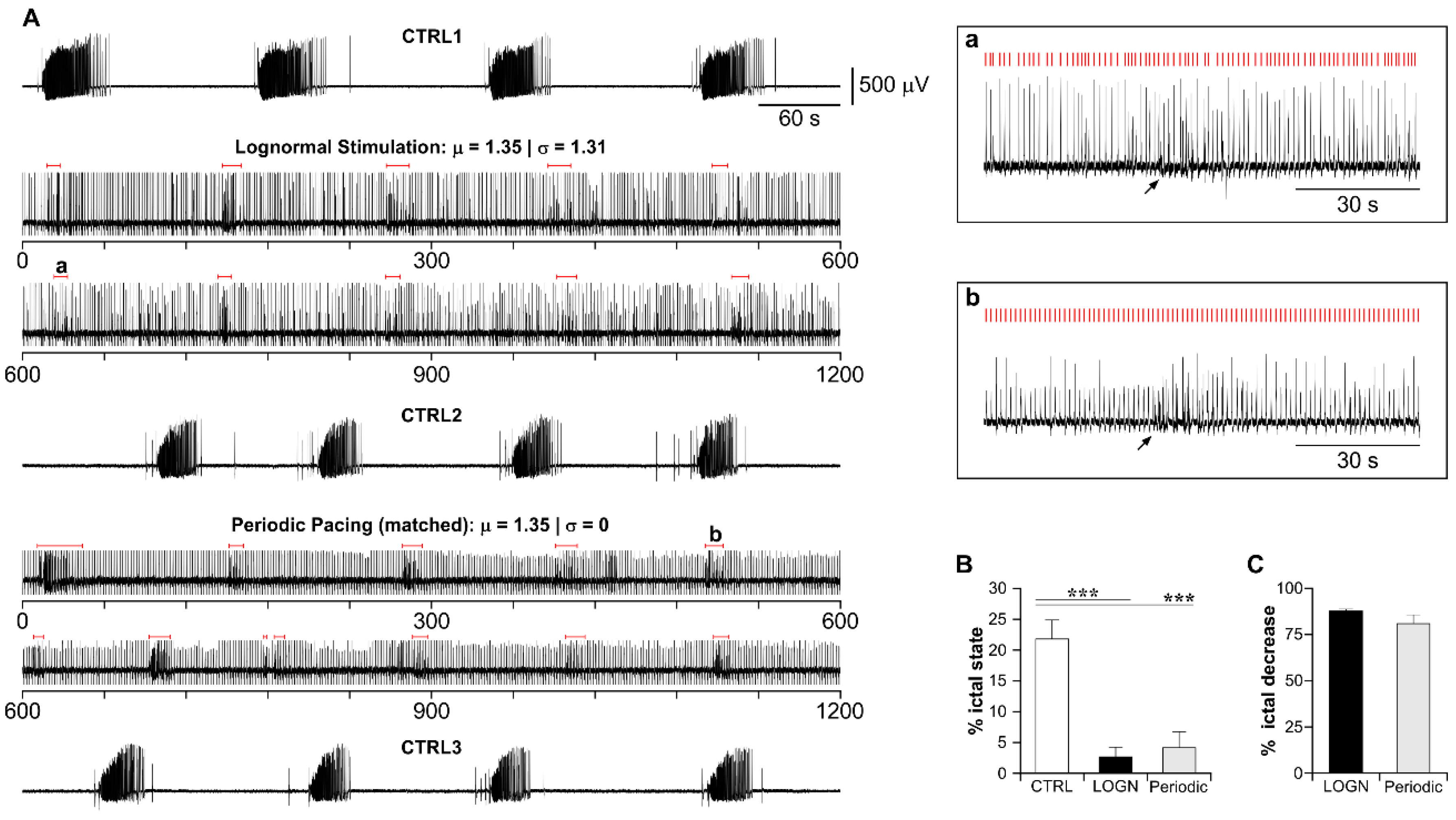
3.4. Lognormal Surrogate Pulse Trains Are More Efficient Than the Matched Periodic Stimulation
3.5. The Higher Efficiency of Lognormal Stimulation Reflects the Pulse Sparseness Determined by the Distribution Parameters
4. Discussion
4.1. The Temporal Dynamics of the CA3-Driven Interictal Activity Preserve the Lognormal Statistics of the Healthy Hippocampus
4.2. Surrogate Brain Patterns Are a Feasible, Effective and Efficient DBS Strategy
4.3. The Subiculum Is a More Suitable DBS Target Than the Hippocampus Proper in MTLE Patients
4.4. A Green Spikes-Based Grammar for DBS in Epilepsy
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Fisher, R.S.; Cross, J.H.; French, J.A.; Higurashi, N.; Hirsch, E.; Jansen, F.E.; Lagae, L.; Moshe, S.; Peltola, J.; Perez, E.R.; et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 522–530. [Google Scholar] [CrossRef]
- WHO. WHO—Epilepsy Facts Sheet. 2017. Available online: http://www.who.int/mediacentre/factsheets/fs999/en/ (accessed on 9 February 2022).
- Fiest, K.M.; Birbeck, G.L.; Jacoby, A.; Jette, N. Stigma in Epilepsy. Curr. Neurol. Neurosci. Rep. 2014, 14, 444. [Google Scholar] [CrossRef] [PubMed]
- Brodie, M.J.; Barry, S.J.E.; Bamagous, G.A.; Norrie, J.D.; Kwan, P. Patterns of treatment response in newly diagnosed epilepsy. Neurology 2012, 78, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Barbarosie, M.; Avoli, M. CA3-Driven Hippocampal-Entorhinal Loop Controls Rather than SustainsIn VitroLimbic Seizures. J. Neurosci. 1997, 17, 9308–9314. [Google Scholar] [CrossRef] [PubMed]
- D’Arcangelo, G.; Panuccio, G.; Tancredi, V.; Avoli, M. Repetitive low-frequency stimulation reduces epileptiform synchronization in limbic neuronal networks. Neurobiol. Dis. 2005, 19, 119–128. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wyckhuys, T.; Boon, P.; Raedt, R.; Van Nieuwenhuyse, B.; Vonck, K.; Wadman, W. Suppression of hippocampal epileptic seizures in the kainate rat by Poisson distributed stimulation. Epilepsia 2010, 51, 2297–2304. [Google Scholar] [CrossRef]
- Wyckhuys, T.; Raedt, R.; Vonck, K.; Wadman, W.; Boon, P. Comparison of hippocampal Deep Brain Stimulation with high (130 Hz) and low frequency (5 Hz) on afterdischarges in kindled rats. Epilepsy Res. 2010, 88, 239–246. [Google Scholar] [CrossRef]
- Rashid, S.; Pho, G.; Czigler, M.; Werz, M.A.; Durand, D.M. Low frequency stimulation of ventral hippocampal commissures reduces seizures in a rat model of chronic temporal lobe epilepsy. Epilepsia 2012, 53, 147–156. [Google Scholar] [CrossRef]
- Van Nieuwenhuyse, B.; Raedt, R.; Delbeke, J.; Wadman, W.; Boon, P.; Vonck, K. In Search of Optimal DBS Paradigms to Treat Epilepsy: Bilateral Versus Unilateral Hippocampal Stimulation in a Rat Model for Temporal Lobe Epilepsy. Brain Stimul. 2015, 8, 192–199. [Google Scholar] [CrossRef]
- Chen, N.; Gao, Y.; Yan, N.; Liu, C.; Zhang, J.-G.; Xing, W.-M.; Kong, D.-M.; Meng, F.-G. High-frequency stimulation of the hippocampus protects against seizure activity and hippocampal neuronal apoptosis induced by kainic acid administration in macaques. Neuroscience 2014, 256, 370–378. [Google Scholar] [CrossRef]
- Paschen, E.; Elgueta, C.; Heining, K.; Vieira, D.M.; Kleis, P.; Orcinha, C.; Häussler, U.; Bartos, M.; Egert, U.; Janz, P.; et al. Hippocampal low-frequency stimulation prevents seizure generation in a mouse model of mesial temporal lobe epilepsy. eLife 2020, 9, e54518. [Google Scholar] [CrossRef] [PubMed]
- Panuccio, G.; Guez, A.; Vincent, R.; Avoli, M.; Pineau, J. Adaptive control of epileptiform excitability in an in vitro model of limbic seizures. Exp. Neurol. 2013, 241, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Mangubat, E.Z.; Kellogg, R.G.; Harris, T.J., Jr.; Rossi, M.A. On-demand pulsatile intracerebral delivery of carisbamate with closed-loop direct neurostimulation therapy in an electrically induced self-sustained focal-onset epilepsy rat model. J. Neurosurg. 2015, 122, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Salam, M.T.; Perez Velazquez, J.L.; Genov, R. Seizure Suppression Efficacy of Closed-Loop Versus Open-Loop Deep Brain Stimulation in a Rodent Model of Epilepsy. IEEE Trans. Neural Syst. Rehabil. Eng. 2016, 24, 710–719. [Google Scholar] [CrossRef]
- Ii, B.B.; Netoff, T.I. Dynamic control of modeled tonic-clonic seizure states with closed-loop stimulation. Front. Neural Circuits 2013, 6, 126. [Google Scholar] [CrossRef]
- Pineau, J.; Guez, A.; Vincent, R.; Panuccio, G.; Avoli, M. Treating epilepsy via adaptive neurostimulation: A reinforcement learning approach. Int. J. Neural Syst. 2009, 19, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.A.; Green, A.L.; Stacey, R.J.; Aziz, T.Z. Refractory epilepsy and deep brain stimulation. J. Clin. Neurosci. 2012, 19, 27–33. [Google Scholar] [CrossRef]
- Klinger, N.V.; Mittal, S. Clinical efficacy of deep brain stimulation for the treatment of medically refractory epilepsy. Clin. Neurol. Neurosurg. 2016, 140, 11–25. [Google Scholar] [CrossRef]
- Ben-Menachem, E.; Krauss, G.L. Responsive neurostimulation—modulating the epileptic brain. Nat. Rev. Neurol. 2014, 10, 247–248. [Google Scholar] [CrossRef]
- Carrette, S.; Boon, P.; Sprengers, M.; Raedt, R.; Vonck, K. Responsive neurostimulation in epilepsy. Expert Rev. Neurother. 2015, 15, 1445–1454. [Google Scholar] [CrossRef]
- Morrell, M.J.; Halpern, C. Responsive Direct Brain Stimulation for Epilepsy. Neurosurg. Clin. N. Am. 2016, 27, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Serafini, R. Similarities and differences between the interictal epileptiform discharges of green-spikes and red-spikes zones of human neocortex. Clin. Neurophysiol. 2019, 130, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Avoli, M.; D’Antuono, M.; Louvel, J.; Köhling, R.; Biagini, G.; Pumain, R.; D’Arcangelo, G.; Tancredi, V. Network and pharmacological mechanisms leading to epileptiform synchronization in the limbic system in vitro. Prog. Neurobiol. 2002, 68, 167–207. [Google Scholar] [CrossRef]
- Avoli, M.; de Curtis, M.; Köhling, R. Does interictal synchronization influence ictogenesis? Neuropharmacology 2013, 69, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Buzsaki, G.; Mizuseki, K. The log-dynamic brain: How skewed distributions affect network operations. Nat. Rev. Neurosci. 2014, 15, 264–278. [Google Scholar] [CrossRef]
- Mizuseki, K.; Buzsáki, G. Preconfigured, Skewed Distribution of Firing Rates in the Hippocampus and Entorhinal Cortex. Cell Rep. 2013, 4, 1010–1021. [Google Scholar] [CrossRef]
- Boon, P.A.J.M. Hippocampal deep brain stimulation for epilepsy. Brain Stimul. 2017, 10, 431–432. [Google Scholar] [CrossRef]
- Jin, H.; Li, W.; Dong, C.; Wu, J.; Zhao, W.; Zhao, Z.; Ma, L.; Ma, F.; Chen, Y.; Liu, Q. Hippocampal deep brain stimulation in nonlesional refractory mesial temporal lobe epilepsy. Seizure 2016, 37, 1–7. [Google Scholar] [CrossRef]
- Vonck, K.; Boon, P.; Achten, E.; De Reuck, J.; Caemaert, J. Long-term amygdalohippocampal stimulation for refractory temporal lobe epilepsy. Ann. Neurol. 2002, 52, 556–565. [Google Scholar] [CrossRef]
- Velasco, M.; Velasco, F.; Velasco, A.L.; Boleaga, B.; Jimenez, F.; Brito, F.; Marquez, I. Subacute Electrical Stimulation of the Hippocampus Blocks Intractable Temporal Lobe Seizures and Paroxysmal EEG Activities. Epilepsia 2000, 41, 158–169. [Google Scholar] [CrossRef]
- Téllez-Zenteno, J.F.; McLachlan, R.S.; Parrent, A.; Kubu, C.S.; Wiebe, S. Hippocampal electrical stimulation in mesial temporal lobe epilepsy. Neurology 2006, 66, 1490–1494. [Google Scholar] [CrossRef] [PubMed]
- Boëx, C.; Seeck, M.; Vulliémoz, S.; Rossetti, A.O.; Staedler, C.; Spinelli, L.; Pegna, A.J.; Pralong, E.; Villemure, J.-G.; Foletti, G.; et al. Chronic deep brain stimulation in mesial temporal lobe epilepsy. Seizure 2011, 20, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Boon, P.; Vonck, K.; De Herdt, V.; Van Dycke, A.; Goethals, M.; Goossens, L.; Van Zandijcke, M.; De Smedt, T.; Dewaele, I.; Achten, R.; et al. Deep Brain Stimulation in Patients with Refractory Temporal Lobe Epilepsy. Epilepsia 2007, 48, 1551–1560. [Google Scholar] [CrossRef]
- McLachlan, R.S.; Pigott, S.; Tellez-Zenteno, J.F.; Wiebe, S.; Parrent, A. Bilateral hippocampal stimulation for intractable temporal lobe epilepsy: Impact on seizures and memory. Epilepsia 2010, 51, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Cukiert, A.; Cukiert, C.M.; Burattini, J.A.; Mariani, P.P.; Bezerra, D.F. Seizure outcome after hippocampal deep brain stimulation in patients with refractory temporal lobe epilepsy: A prospective, controlled, randomized, double-blind study. Epilepsia 2017, 58, 1728–1733. [Google Scholar] [CrossRef]
- Zhang, S.-H.; Sun, H.-L.; Fang, Q.; Zhong, K.; Wu, D.-C.; Wang, S.; Chen, Z. Low-frequency stimulation of the hippocampal CA3 subfield is anti-epileptogenic and anti-ictogenic in rat amygdaloid kindling model of epilepsy. Neurosci. Lett. 2009, 455, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Scott Swartzwelder, H.; Lewis, D.V.; Anderson, W.W.; Wilson, W.A. Seizure-like events in brain slices: Suppression by interictal activity. Brain Res. 1987, 410, 362–366. [Google Scholar] [CrossRef]
- Jensen, M.S.; Yaari, Y. The relationship between interictal and ictal paroxysms in an in vitro model of focal hippocampal epilepsy. Ann. Neurol. 1988, 24, 591–598. [Google Scholar] [CrossRef]
- Panuccio, G.; D’Antuono, M.; de Guzman, P.; De Lannoy, L.; Biagini, G.; Avoli, M. In vitro ictogenesis and parahippocampal networks in a rodent model of temporal lobe epilepsy. Neurobiol. Dis. 2010, 39, 372–380. [Google Scholar] [CrossRef]
- Panuccio, G.; Colombi, I.; Chiappalone, M. Recording and Modulation of Epileptiform Activity in Rodent Brain Slices Coupled to Microelectrode Arrays. J. Vis. Exp. 2018, e57548. [Google Scholar] [CrossRef]
- Rutecki, P.A.; Lebeda, F.J.; Johnston, D. 4-Aminopyridine produces epileptiform activity in hippocampus and enhances synaptic excitation and inhibition. J. Neurophysiol. 1987, 57, 1911–1924. [Google Scholar] [CrossRef] [PubMed]
- Sunderam, S.; Gluckman, B.; Reato, D.; Bikson, M. Toward rational design of electrical stimulation strategies for epilepsy control. Epilepsy Behav. 2010, 17, 6–22. [Google Scholar] [CrossRef]
- Su, Y.; Radman, T.; Vaynshteyn, J.; Parra, L.C.; Bikson, M. Effects of high-frequency stimulation on epileptiform activity in vitro: ON/OFF control paradigm. Epilepsia 2008, 49, 1586–1593. [Google Scholar] [CrossRef]
- Kozak, G.; Berényi, A. Sustained efficacy of closed loop electrical stimulation for long-term treatment of absence epilepsy in rats. Sci. Rep. 2017, 7, 6300. [Google Scholar] [CrossRef] [PubMed]
- Daubechies, I. Ten Lectures on Wavelets; Society for Industrial and Applied Mathematics: Philadelphia, PA, USA, 1992. [Google Scholar]
- Toprani, S.; Durand, D.M. Fiber tract stimulation can reduce epileptiform activity in an in-vitro bilateral hippocampal slice preparation. Exp. Neurol. 2013, 240, 28–43. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gal, A.; Marom, S. Entrainment of the Intrinsic Dynamics of Single Isolated Neurons by Natural-Like Input. J. Neurosci. 2013, 33, 7912–7918. [Google Scholar] [CrossRef]
- Scarsi, F.; Tessadori, J.; Pasquale, V.; Chiappalone, M. Impact of stimuli distribution on neural network responses. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 4761–4764. [Google Scholar] [CrossRef]
- Mitzenmacher, M. A Brief History of Generative Models for Power Law and Lognormal Distributions. Internet Math. 2004, 1, 226–251. [Google Scholar] [CrossRef]
- He, B.J. Scale-free brain activity: Past, present, and future. Trends Cogn. Sci. 2014, 18, 480–487. [Google Scholar] [CrossRef]
- Deniau, J.-M.; Degos, B.; Bosch-Bouju, C.; Maurice, N. Deep brain stimulation mechanisms: Beyond the concept of local functional inhibition. Eur. J. Neurosci. 2010, 32, 1080–1091. [Google Scholar] [CrossRef]
- Desai, S.A.; Rolston, J.D.; McCracken, C.E.; Potter, S.M.; Gross, R.E. Asynchronous Distributed Multielectrode Microstimulation Reduces Seizures in the Dorsal Tetanus Toxin Model of Temporal Lobe Epilepsy. Brain Stimul. 2016, 9, 86–100. [Google Scholar] [CrossRef]
- Tass, P.A.; Silchenko, A.N.; Hauptmann, C.; Barnikol, U.B.; Speckmann, E.-J. Long-lasting desynchronization in rat hippocampal slice induced by coordinated reset stimulation. Phys. Rev. E 2009, 80, 011902. [Google Scholar] [CrossRef] [PubMed]
- Sprengers, M.; Raedt, R.; Larsen, L.E.; Delbeke, J.; Wadman, W.J.; Boon, P.; Vonck, K. Deep brain stimulation reduces evoked potentials with a dual time course in freely moving rats: Potential neurophysiological basis for intermittent as an alternative to continuous stimulation. Epilepsia 2020, 61, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri, S.; Fathollahi, Y.; Semnanian, S.; Shojaei, A.; Asgari, A.; Amini, A.E.; Mirnajafi-Zadeh, J. Deep brain stimulation restores the glutamatergic and GABAergic synaptic transmission and plasticity to normal levels in kindled rats. PLoS ONE 2019, 14, e0224834. [Google Scholar] [CrossRef] [PubMed]
- Grosser, S.; Buck, N.; Braunewell, K.-H.; Gilling, K.E.; Wozny, C.; Fidzinski, P.; Behr, J. Loss of Long-Term Potentiation at Hippocampal Output Synapses in Experimental Temporal Lobe Epilepsy. Front. Mol. Neurosci. 2020, 13, 143. [Google Scholar] [CrossRef] [PubMed]
- Fisher, P.D.; Sperber, E.F.; Moshé, S.L. Hippocampal sclerosis revisited. Brain Dev. 1998, 20, 563–573. [Google Scholar] [CrossRef]
- Wozny, C.; Kivi, A.; Lehmann, T.-N.; Dehnicke, C.; Heinemann, U.; Behr, J. Comment on “On the Origin of Interictal Activity in Human Temporal Lobe Epilepsy in Vitro”. Science 2003, 301, 463. [Google Scholar] [CrossRef]
- Knopp, A.; Kivi, A.; Wozny, C.; Heinemann, U.; Behr, J. Cellular and network properties of the subiculum in the pilocarpine model of temporal lobe epilepsy. J. Comp. Neurol. 2005, 483, 476–488. [Google Scholar] [CrossRef]
- Benini, R.; Avoli, M. Rat subicular networks gate hippocampal output activity in anin vitromodel of limbic seizures. J. Physiol. 2005, 566, 885–900. [Google Scholar] [CrossRef]
- Cohen, I.; Navarro, V.; Clemenceau, S.; Baulac, M.; Miles, R. On the Origin of Interictal Activity in Human Temporal Lobe Epilepsy in Vitro. Science 2002, 298, 1418–1421. [Google Scholar] [CrossRef]
- de Guzman, P.; Inaba, Y.; Biagini, G.; Baldelli, E.; Mollinari, C.; Merlo, D.; Avoli, M. Subiculum network excitability is increased in a rodent model of temporal lobe epilepsy. Hippocampus 2006, 16, 843–860. [Google Scholar] [CrossRef]
- Stafstrom, C.E. The Role of the Subiculum in Epilepsy and Epileptogenesis. Epilepsy Curr. 2005, 5, 121–129. [Google Scholar] [CrossRef]
- Toyoda, I.; Bower, M.R.; Leyva, F.; Buckmaster, P.S. Early Activation of Ventral Hippocampus and Subiculum during Spontaneous Seizures in a Rat Model of Temporal Lobe Epilepsy. J. Neurosci. 2013, 33, 11100–11115. [Google Scholar] [CrossRef]
- Bartoli, A.; Tyrand, R.; Vargas, M.I.; Momjian, S.; Boëx, C. Low Frequency Microstimulation Is Locally Excitatory in Patients With Epilepsy. Front. Neural Circuits 2018, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Bondallaz, P.; Boëx, C.; Rossetti, A.O.; Foletti, G.; Spinelli, L.; Vulliemoz, S.; Seeck, M.; Pollo, C. Electrode location and clinical outcome in hippocampal electrical stimulation for mesial temporal lobe epilepsy. Seizure 2013, 22, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Velasco, A.L.; Saucedo-Alvarado, P.E.; Alejandre-Sánchez, M.; Guzmán-Jiménez, D.E.; González-Garcia, I.; Velasco, F. New Horizons in Temporal Lobe Seizure Control. J. Clin. Neurophysiol. 2021, 38, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Al-Otaibi, F.A.; Hamani, C.; Lozano, A.M. Neuromodulation in Epilepsy. Neurosurgery 2011, 69, 957–979. [Google Scholar] [CrossRef] [PubMed]

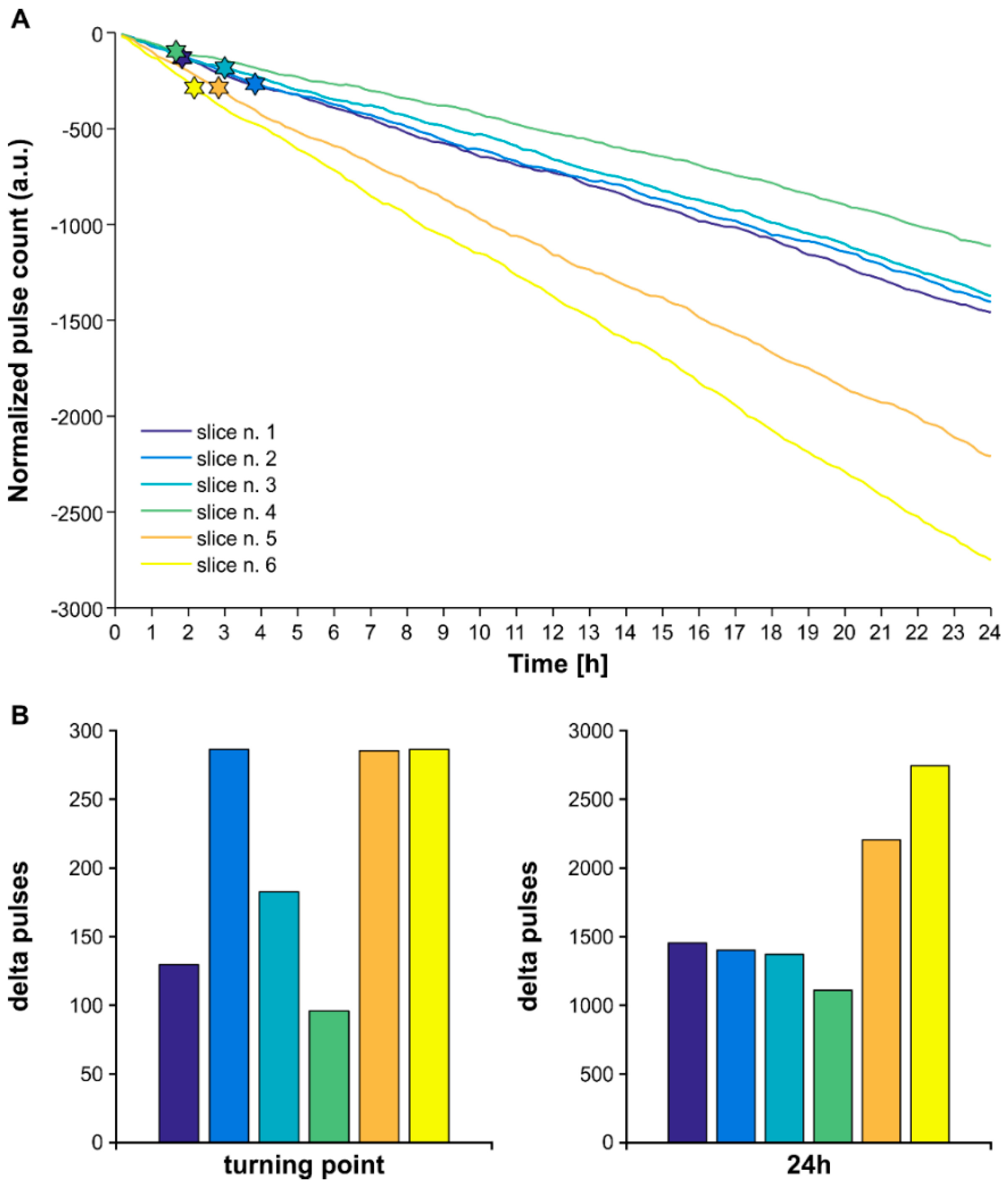
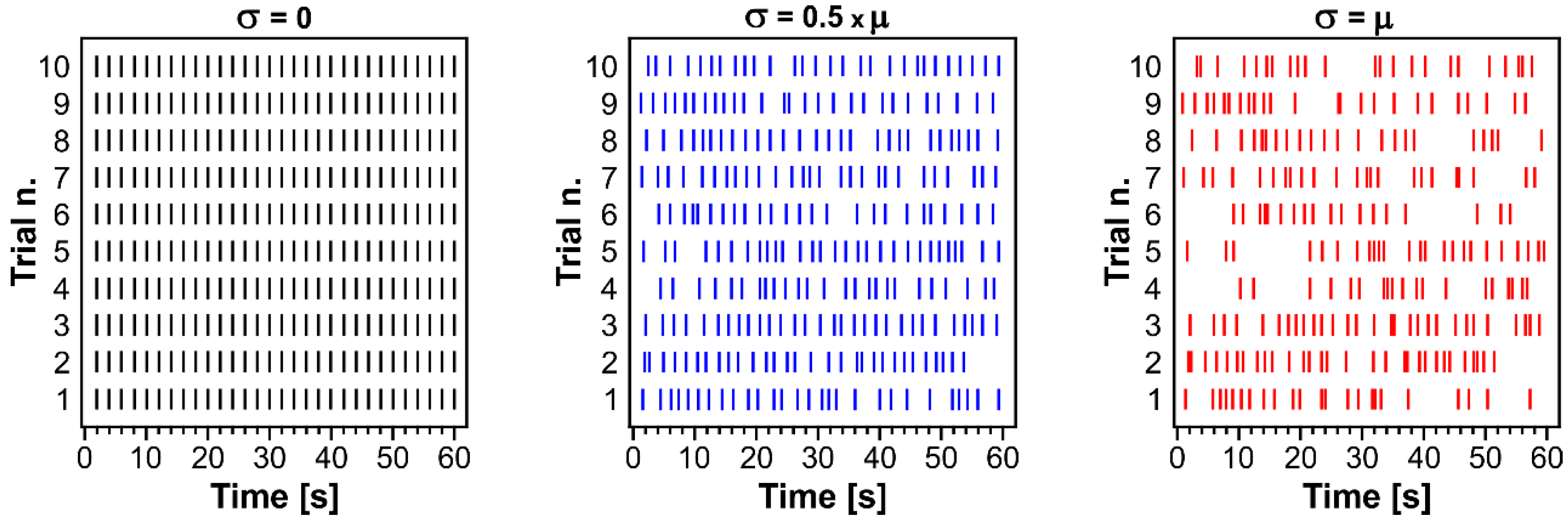
| Slice n. | μ | σ | Range [s] | n. Pulses LOGN | n. Pulses 1 Hz | Delta Pulses | Pulse Amplitude [μA] |
|---|---|---|---|---|---|---|---|
| 1 | 3.90 | 1.33 | 1.66–8.65 | 416 | 1800 | 1384 *** | ±250 |
| 2 | 1.86 | 1.36 | 0.59–5.71 | 618 | 1200 | 582 *** | ±300 |
| 3 | 2.76 | 1.56 | 1.01–12.27 | 414 | 1200 | 786 *** | ±200 |
| 4 | 3.64 | 1.48 | 0.57–12.67 | 290 | 1200 | 910 *** | ±250 |
| 5 | 1.12 | 1.27 | 0.26–2.11 | 1043 | 1200 | 157 *** | ±300 |
| Parameter | CTRL1 | CTRL2 | CTRL3 |
|---|---|---|---|
| Pictal | 23.05 ± 3.08 | 20.89 ± 2.6 | 21.46 ± 2.44 |
| Ictal amplitude [μV] | 858.47 ± 159.8 | 687.15 ± 110.48 | 701.7 ± 124.46 |
| Parameter | Pooled CTRL | STIM LOGN | STIM Matched Periodic |
|---|---|---|---|
| Pictal | 21.8 ± 1.54 | 2.68 ± 1.05 *** | 4.16 ± 1.5 *** |
| % ictal state reduction | - | 87.7 ± 0.4 | 80.91 ± 6.86 |
| Slice n. | μ | σ | Range [s] | n. Pulses LOGN | n. Pulses Matched Periodic | Delta PULSES | Pulse Amplitude [μA] |
|---|---|---|---|---|---|---|---|
| 1 | 1.09 | 1.22 | 0.5–2.28 | 1076 | 1095 | 19 | ±350 |
| 2 | 1.23 | 1.22 | 0.61–2.51 | 960 | 975 | 15 | ±250 |
| 3 | 0.65 | 1.15 | 0.39–0.97 | 1900 | 1949 | 49 | ±200 |
| 4 | 0.59 | 1.13 | 0.36–0.9 | 2012 | 2027 | 15 | ±250 |
| 5 | 1.35 | 1.31 | 0.31–3.22 | 862 | 888 | 26 | ±350 |
| 6 | 0.92 | 1.28 | 0.26–2.33 | 1272 | 1303 | 31 | ±350 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caron, D.; Canal-Alonso, Á.; Panuccio, G. Mimicking CA3 Temporal Dynamics Controls Limbic Ictogenesis. Biology 2022, 11, 371. https://doi.org/10.3390/biology11030371
Caron D, Canal-Alonso Á, Panuccio G. Mimicking CA3 Temporal Dynamics Controls Limbic Ictogenesis. Biology. 2022; 11(3):371. https://doi.org/10.3390/biology11030371
Chicago/Turabian StyleCaron, Davide, Ángel Canal-Alonso, and Gabriella Panuccio. 2022. "Mimicking CA3 Temporal Dynamics Controls Limbic Ictogenesis" Biology 11, no. 3: 371. https://doi.org/10.3390/biology11030371
APA StyleCaron, D., Canal-Alonso, Á., & Panuccio, G. (2022). Mimicking CA3 Temporal Dynamics Controls Limbic Ictogenesis. Biology, 11(3), 371. https://doi.org/10.3390/biology11030371