Effects of Eccentric vs. Concentric Sports on Blood Muscular Damage Markers in Male Professional Players
Abstract
Simple Summary
Abstract
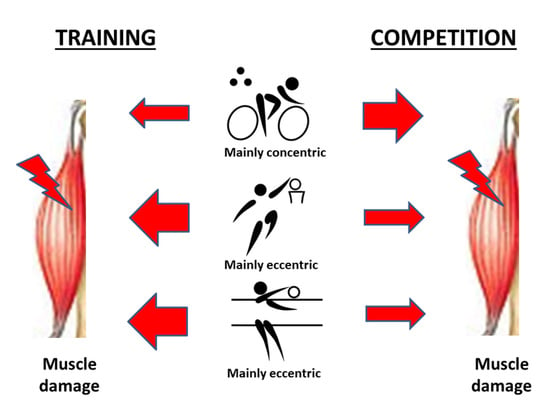
1. Introduction
2. Materials and Methods
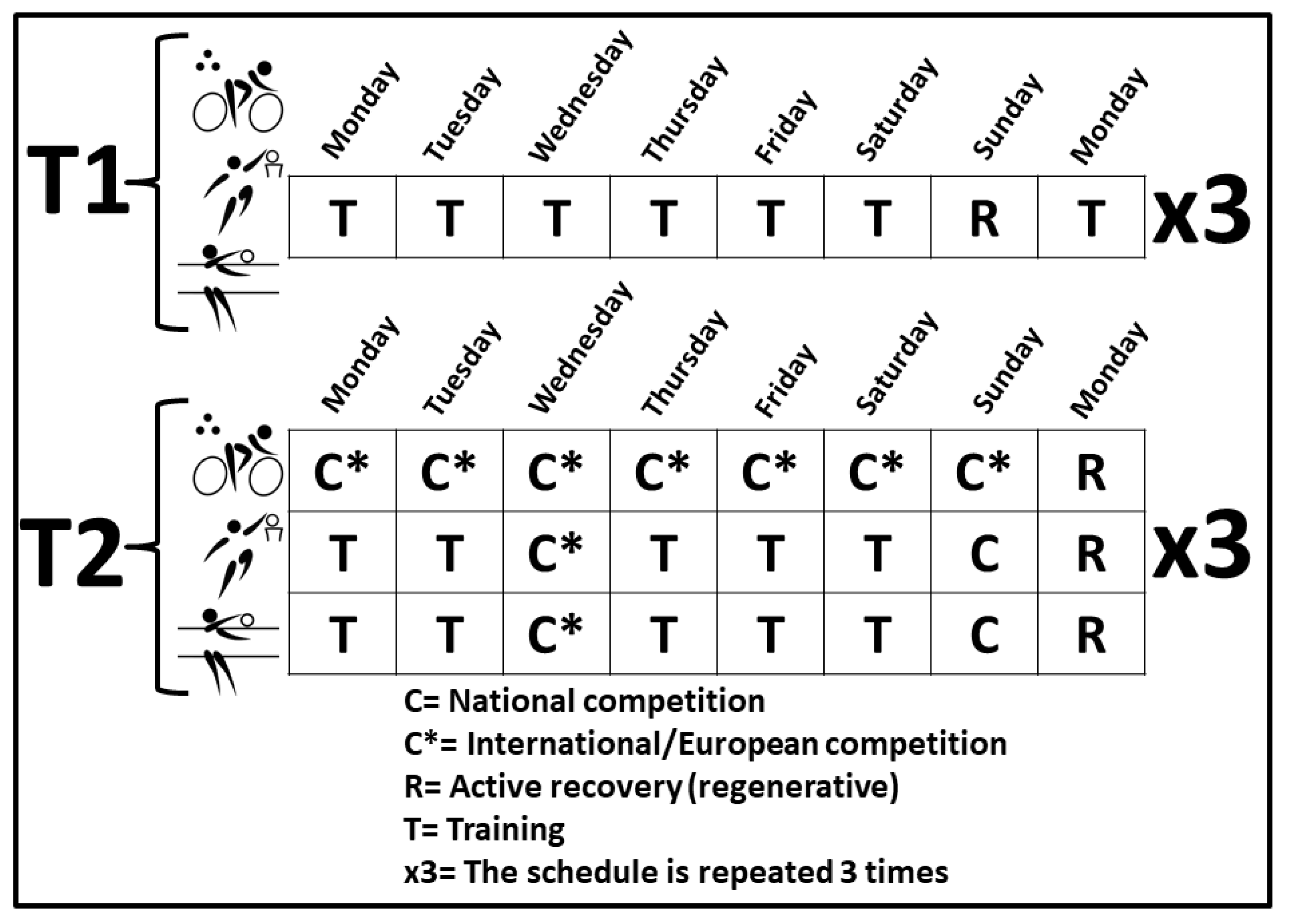
2.1. Participation in the Training and Competition Periods
2.2. Study Design
2.3. Determination of Blood Markers for Muscle Damage
2.4. Muscle Damage Assessment
2.5. Statistical Analysis
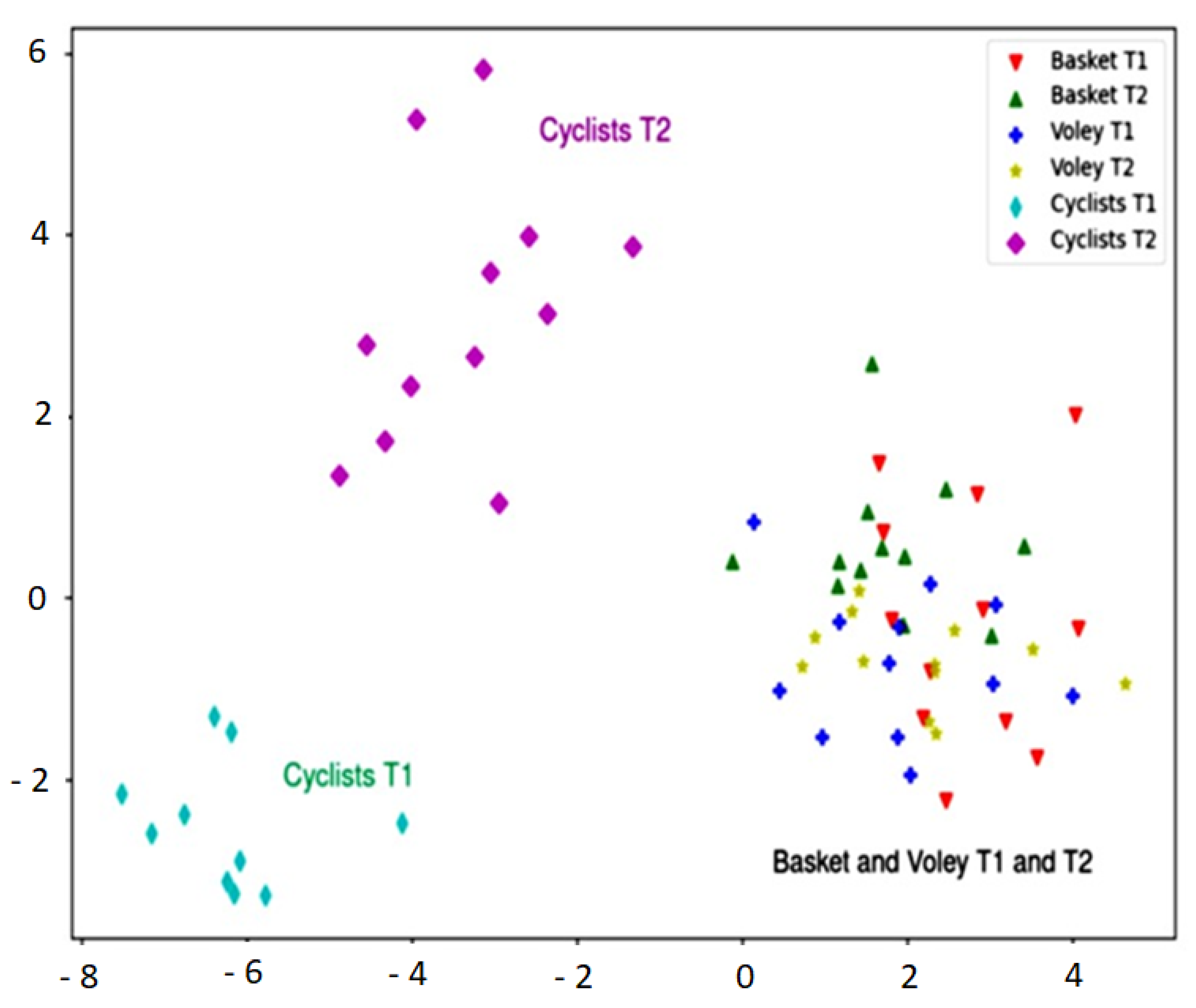
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Higbie, E.J.; Cureton, K.J.; Warren, G.L.; Prior, B.M. Effects of concentric and eccentric training on muscle strength, cross-sectional area, and neural activation. J. Appl. Physiol. 1996, 81, 2173–2181. [Google Scholar] [CrossRef] [PubMed]
- Franchi, M.V.; Reeves, N.D.; Narici, M.V. Skeletal muscle remodeling in response to eccentric vs concentric loading: Morphological, molecular and metabolic adaptations. Front. Psychol. 2017, 8, 447. [Google Scholar] [CrossRef] [PubMed]
- White, G.E.; Rhind, S.G.; Wells, G.D. The effect of various cold-water immersion protocols on exercise-induced inflammatory response and functional recovery from high-intensity sprint exercise. Eur. J. Appl. Physiol. 2014, 114, 2353–2367. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.J.; Twist, C.; Cobley, J.N.; Howatson, G.; Close, G.L. Exercise-induced muscle damage: What is it, what causes it and what are the nutritional solutions? Eur. J. Sport Sci. 2019, 19, 71–85. [Google Scholar] [CrossRef]
- Markus, I.; Constantini, K.; Hoffman, J.R.; Bartolomei, S.; Gepner, Y. Exercise-induced muscle damage: Mechanism, assessment and nutritional factors to accelerate recovery. Eur. J. Appl. Physiol. 2021, 121, 969–992. [Google Scholar] [CrossRef]
- Horská, A.; Fishbein, K.W.; Fleg, J.L.; Spencer, R.G. The relationship between creatine kinase kinetics and exercise intensity in human forearm is unchanged by age. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E333–E339. [Google Scholar] [CrossRef]
- Clarkson, P.M.; Hubal, M.J. Exercise-induced muscle damage in humans. Am. J. Phys. Med. Rehabil. 2002, 81, S52–S69. [Google Scholar] [CrossRef]
- Lee, J.; Clarkson, P.M. Plasma creatine kinase activity and glutathione after eccentric exercise. Med. Sci. Sports Exerc. 2003, 35, 930–936. [Google Scholar] [CrossRef]
- Brancaccio, P.; Maffulli, N.; Buonauro, R.; Limongelli, F.M. Serum enzyme monitoring in sports medicine. Clin. Sports Med. 2008, 27, 1–18. [Google Scholar] [CrossRef]
- Khan, F.Y. Rhabdomyolysis: A review of the literature. Neth. J. Med. 2009, 67, 272–283. [Google Scholar]
- Banfi, G.; Colombini, A.; Lombardi, G.; Lubkowska, A. Metabolic markers in sports medicine. Adv. Clin. Chem. 2012, 56, 1–54. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, G.; Mikkelsen, U.R.; Raastad, T.; Peake, J.M. Leucocytes, cytokines and satellite cells: What role do they play in muscle damage and regeneration following eccentric exercise? Exerc. Immunol. Rev. 2012, 18, 42–97. [Google Scholar] [PubMed]
- Hackney, A.C.; Walz, E.A. Hormonal adaptation and the stress of exercise training: The role of glucocorticoids. Trends Sport Sci. 2013, 20, 165–171. [Google Scholar]
- Córdova, A.; Martin, J.F.; Reyes, E.; Alvarez-Mon, M. Protection against muscle damage in competitive sports players: The effect of the immunomodulator AM3. J. Sports Sci. 2004, 22, 827–833. [Google Scholar] [CrossRef]
- Córdova, A.; Mielgo-Ayuso, J.; Fernandez-Lazaro, C.I.; Caballero-García, A.; Roche, E.; Fernandez-Lazaro, D. Effect of iron supplementation on the modulation of iron metabolism, muscle damage biomarkers and cortisol in professional cyclists. Nutrients 2019, 11, 500. [Google Scholar] [CrossRef] [PubMed]
- Córdova, A.; Mielgo-Ayuso, J.; Roche, E.; Caballero-Garcia, A.; Fernandez-Lazaro, D. Impact of magnesium supplementation in muscle damage of professional cyclists competing in a stage race. Nutrients 2019, 11, 1927. [Google Scholar] [CrossRef]
- Córdova-Martinez, A.; Seco-Calvo, J.; Tur-Mari, J.A.; Abecia-Inchaurregui, L.C.; Echevarría-Orella, E.; Pons-Biescas, A. Testosterone and cortisol changes in professional basketball players through a season competition. J. Strength Cond. Res. 2010, 24, 1102–1108. [Google Scholar] [CrossRef]
- Brancaccio, P.; Maffulli, N.; Limongelli, F.M. Creatine kinase monitoring in sport medicine. Br. Med. Bull. 2007, 81–82, 209–230. [Google Scholar] [CrossRef]
- Bessa, A.L.; Oliveira, V.N.; Agostini, G.G.; Oliveira, R.J.S.; Oliveira, A.C.S.; White, G.E.; Wells, G.D.; Teixeira, D.N.S.; Espindola, F.S. Exercise intensity and recovery: Biomarkers of injury, inflammation and oxidative stress. J. Strength Cond. Res. 2013, 30, 311–319. [Google Scholar] [CrossRef]
- Fry, C.S.; Drummond, M.J.; Glynn, E.L.; Dickinson, J.M.; Gundermann, D.M.; Timmerman, K.L.; Walker, D.K.; Volpi, E.; Rasmussen, B.B. Skeletal muscle autophagy and protein breakdown following resistance exercise are similar in younger and older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 599–607. [Google Scholar] [CrossRef]
- Smith, L.L. Acute inflammation: The underlying mechanism in delayed onset muscle soreness? Med. Sci. Sports Exerc. 1991, 23, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Fatouros, I.G.; Jamurtas, A.Z. Insights into the molecular etiology of exercise-induced inflammation: Opportunities for optimizing performance. J. Inflamm. Res. 2016, 9, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Hellebrandt, F.A.; Houtz, S.J.; Hockman, D.E.; Partridge, M.J. Physiological effects of simultaneous static and dynamic exercise. Am. J. Phys. Med. 1956, 35, 106–117. [Google Scholar] [PubMed]
- Talag, T.S. Residual muscular soreness as influenced by concentric, eccentric, and static contractions. Res. Q. 1973, 44, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, P.M.; Tremblay, I. Exercise-induced muscle damage, repair, and adaptation in humans. J. Appl. Physiol. 1988, 65, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cleak, M.J.; Eston, R.G. Muscle soreness, swelling, stiffness and strength loss after intense eccentric exercise. Br. J. Sports Med. 1992, 26, 267–272. [Google Scholar] [CrossRef]
- Clarkson, P.M.; Newham, D.J. Associations between muscle soreness, damage, and fatigue. Adv. Exp. Med. Biol. 1995, 384, 457–469. [Google Scholar] [CrossRef]
- Eston, R.G.; Finney, S.; Baker, S.; Baltzopoulos, V. Muscle tenderness and peak torque changes after downhill running following a prior bout of isokinetic eccentric exercise. J. Sports Sci. 1996, 14, 291–299. [Google Scholar] [CrossRef]
- Fridén, J.; Lieber, R.L. Eccentric exercise-induced injuries to contractile and cytoskeletal muscle fibre components. Acta Physiol. Scand. 2001, 171, 321–326. [Google Scholar] [CrossRef]
- Proske, U.; Allen, T.J. Damage to skeletal muscle from eccentric exercise. Exerc. Sport Sci. Rev. 2005, 33, 98–104. [Google Scholar] [CrossRef]
- Nicol, C.; Avela, J.; Komi, P.V. The stretch-shortening cycle: A model to study naturally occurring neuromuscular fatigue. Sports Med. 2006, 36, 977–999. [Google Scholar] [CrossRef] [PubMed]
- Pyne, D.B. Exercise-induced muscle damage and inflammation: A review. Aust. J. Sci. Med. Sport 1994, 26, 49–58. [Google Scholar] [PubMed]
- Baird, M.F.; Graham, S.M.; Baker, J.S.; Bickerstaff, G.F. Creatine-kinase- and exercise-related muscle damage implications for muscle performance and recovery. J. Nutr. Metab. 2012, 2012, 960363. [Google Scholar] [CrossRef] [PubMed]
- Hough, J.; Corney, R.; Kouris, A.; Gleeson, M. Salivary cortisol and testosterone responses to highintensity cycling before and after an 11-day intensified training period. J. Sports Sci. 2013, 31, 1614–1623. [Google Scholar] [CrossRef]
- Overgaard, K.; Fredsted, A.; Hyldal, A.; Ingemann-Hansen, T.; Gissel, H.; Clausen, T. Effects of running distance and training on Ca2+ content and damage in human muscle. Med. Sci. Sports Exerc. 2004, 36, 821–829. [Google Scholar] [CrossRef]
- Gibson, A.S.C.; Lambert, M.I.; Noakes, T.D. Age-related decrements in cycling and running performance. S. Afr. J. Sports Med. 2004, 2, 8–11. [Google Scholar] [CrossRef][Green Version]
- Córdova, A.; Monserrat, J.; Villa, G.; Reyes, E.; Soto, M.A. Effects of AM3 (Inmunoferon) on increased serum concentrations of interleukin-6 and tumour necrosis factor receptors I and II in cyclists. J. Sports Sci. 2006, 24, 565–573. [Google Scholar] [CrossRef]
- Córdova, A.; Sureda, A.; Pons, A.; Alvarez-Mon, M. Modulation of TNF-α, TNF-α receptors and IL-6 after treatment with AM3 in professional cyclists. J. Sports Med. Phys. Fit. 2015, 55, 345–351. [Google Scholar]
- Córdova-Martínez, A.; Martorell-Pons, M.; Sureda-Gomila, A.; Tur-Marí, J.A.; Pons-Biescas, A. Changes in circulating cytokines and markers of muscle damage in elite cyclists during a multistage competition. Clin. Physiol. Funct. Imaging 2015, 35, 351–358. [Google Scholar] [CrossRef]
- Spiteri, T.; Binetti, M.; Scanlan, A.T.; Dalbo, V.J.; Dolci, F.; Specos, C. Physical determinants of Division 1 Collegiate basketball, Women’s National Basketball League and Women’s National Basketball Association athletes: With reference to lower body sidedness. J. Strength Cond. Res. 2019, 33, 159–166. [Google Scholar] [CrossRef]
- Ebbeling, C.B.; Clarkson, P.M. Exercise-induced muscle damage and adaptation. Sports Med. 1989, 7, 207–234. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.; Eston, R. Maximal-intensity isometric and dynamic exercise performance after eccentric muscle actions. J. Sports Sci. 2002, 20, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, P.X.; Fusco, A.; Bell, J.W.; von Duvillard, S.P.; Cortis, C.; Wagner, H. Movement characteristics of volleyball spike jump performance in females. J. Sci. Med. Sport 2019, 22, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Spiteri, T.; Newton, R.U.; Binetti, M.; Hart, N.H.; Sheppard, J.M.; Nimphius, S. Mechanical determinants of faster change of direction and agility performance in female basketball athletes. J. Strength Cond. Res. 2015, 29, 2205–2214. [Google Scholar] [CrossRef] [PubMed]
- Lidor, R.; Ziv, G. Physical and physiological attributes of female volleyball players—A review. J. Strength Cond. Res. 2010, 24, 1963–1973. [Google Scholar] [CrossRef] [PubMed]
- Grgantov, Z.; Milić, M.; Katić, R. Identification of explosive power factors as predictors of player quality in young female volleyball players. Coll. Antropol. 2010, 37, 61–68. [Google Scholar]
- Burke, L.M. Nutritional practices of male and female endurance cyclists. Sports Med. 2001, 31, 521–532. [Google Scholar] [CrossRef]
- Vaquera, A.; Santos, S.; Villa-José, G.; Morante, J.C.; García-Tormo, V. Anthropometric characteristics of spanish professional basketball players. J. Hum. Kinet. 2015, 27, 99–106. [Google Scholar] [CrossRef]
- Ferro, A.; Garrido, G.; Villacieros, J.; Pérez, J.; Grams, L. Nutritional habits and performance in male elite wheelchair basketball players during a precompetitive period. Adapt. Phys. Act. Q. 2017, 34, 295–310. [Google Scholar] [CrossRef]
- Tsoufi, A.; Maraki, M.I.; Dimitrakopoulos, L.; Famisis, K.; Grammatikopoulou, M.G. The effect of professional dietary counseling: Elite basketball players eat healthier during competition days. J. Sports Med. Phys. Fit. 2017, 57, 1305–1310. [Google Scholar] [CrossRef]
- Kreider, R.B.; Wilborn, C.D.; Taylor, L.; Campbell, B.; Almada, A.L.; Collins, R.; Cooke, M.; Earnest, C.P.; Greenwood, M.; Kalman, D.S.; et al. ISSN exercise & sport nutrition review: Research & recommendations. J. Int. Soc. Sports Nutr. 2010, 7, 2–43. [Google Scholar]
- Dudley, G.A.; Tesch, P.A.; Miller, B.J.; Buchanan, P. Importance of eccentric actions in adaptations to resistance training. Aviat. Space Environ. Med. 1991, 62, 543–550. [Google Scholar] [PubMed]
- Mielgo-Ayuso, J.; Zourdos, M.C.; Urdampilleta, A.; Calleja-Gonzalez, J.; Seco, J.; Cordova, A. Relationship of long-term macronutrients intake on anabolic-catabolic hormones in female elite volleyball players. Nutr. Hosp. 2017, 34, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Norrbrand, L.; Fluckey, J.D.; Pozzo, M.; Tesch, P.A. Resistance training using eccentric overload induces early adaptations in skeletal muscle size. Eur. J. Appl. Physiol. 2008, 102, 271–281. [Google Scholar] [CrossRef]
- Colliander, E.B.; Tesch, P.A. Effects of eccentric and concentric muscle actions in resistance training. Acta Physiol. Scand. 1990, 140, 31–39. [Google Scholar] [CrossRef]
- Krentz, J.R.; Farthing, J.P. Neural and morphological changes in response to a 20-day intense eccentric training protocol. Eur. J. Appl. Physiol. 2010, 110, 333–340. [Google Scholar] [CrossRef]
- Souglis, A.; Bogdanis, G.; Giannopoulou, I.; Papadopoulos, C.; Apostolidis, N. Comparison of inflammatory responses and muscle damage indices following a soccer, basketball, volleyball and handball game at an elite competitive level. Res. Sports Med. 2015, 23, 59–72. [Google Scholar] [CrossRef]
- Vervoorn, C.; Quist, A.M.; Vermulst, L.J.; Erich, W.B.; de Vries, W.R.; Thijssen, J.H. The behaviour of the plasma free testosterone/cortisol ratio during a season of elite rowing training. Int. J. Sports Med. 1991, 12, 257–263. [Google Scholar] [CrossRef]
- Carrasco, G.A.; Van de Kar, L.D. Neuroendocrine pharmacology of stress. Eur. J. Pharmacol. 2003, 463, 235–272. [Google Scholar] [CrossRef]
- Engelmann, M.; Landgraf, R.; Wotjak, C.T. The hypothalamic-neurohypophysial system regulates the hypothalamic-pituitary-adrenal axis under stress: An old concept revisited. Front. Neuroendocrinol. 2004, 25, 132–149. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Ratamess, N.A. Hormonal responses and adaptations to resistance exercise and training. Sports Med. 2005, 35, 339–361. [Google Scholar] [CrossRef] [PubMed]
- Oltras, C.M.; Mora, F.; Vives, F. Beta-endorphin and ACTH in plasma: Effects of physical and psychological stress. Life Sci. 1987, 40, 1683–1686. [Google Scholar] [CrossRef]
| Sport | Cycling (n = 18) | Basket-Ball (n = 12) | Volley-Ball (n = 14) |
|---|---|---|---|
| Age (years) | 26.2 ± 1.8 | 25.3 ± 4.4 | 25.7 ± 2.1 |
| (23.7–31.4) | (18.0–32.3) | (21.0–29.7) | |
| Height (cm) | 176.2 ± 3.4 | 198.0 ± 9.9 | 189.0 ± 0.1 |
| (168.5–183.4) | (181.2–214.5) | (188.8–189.1) | |
| Weight (kg) | 66.1 ± 3.6 | 96.8 ± 13.0 | 87.2 ± 4.1 |
| (56.4–71.5) | (75.4–121.6) | (79.8–94.5) | |
| VO2 max (mL kg−1 min−1) | 83.6 ± 2.7 | 56.5 ± 7.7 | 65.3 ± 4.2 |
| (75.5–91.5) | (38.2–78.2) | (60.4–76.3) |
| Cyclists | Basketball Players | Volleyball Players | Two-Way Anova | ||||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T1 | T2 | T1 | T2 | ||
| TPP (g/dL) | 6.7 ± 0.4 | 6.2 ± 0.5 * | 7.3 ± 0.6 † | 7.3 ± 0.5 ‡ | 7.5 ± 0.6 † | 7.6 ± 0.6 ‡ | S, T×S |
| (5.9–7.1) | (5.4–7.1) | (6.7–7.9) | (6.7–7.7) | (7.0–7.9) | (7.1–8.1) | ||
| Urea (mg/dL) | 40.7 ± 5.1 | 45.8 ± 4.9 | 41.8 ± 5.2 | 43.8 ± 5.7 | 41.7± 5.2 | 44.8 ± 5.0 | |
| (31–48) | (31–63) | (28–59) | (27–60) | (30–55) | (27–63) | ||
| Creatinine (mg/dL) | 0.8 ± 0.1 | 1.0 ± 0.1 | 1.2 ± 0.1 † | 1.2 ± 0.1 ‡ | 1.2 ± 0.1 † | 1.3 ± 0.1 ‡ | S |
| (0.6–1.0) | (0.7–1.1) | (1.1–1.5) | (1.1–1.4) | (1.0–1.4) | (1.2–1.7) | ||
| LDH (U/L) | 122 ± 19 | 359 ± 29 * | 375 ± 31 † | 383 ± 33 ‡ | 349 ± 38 † | 330 ± 32 | T×S |
| (86–162) | (239–472) | (308–506) | (300–526) | (281–457) | (266–396) | ||
| Cycling | Basketball | Volleyball | Two-Way Anova | ||||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T1 | T2 | T1 | T2 | ||
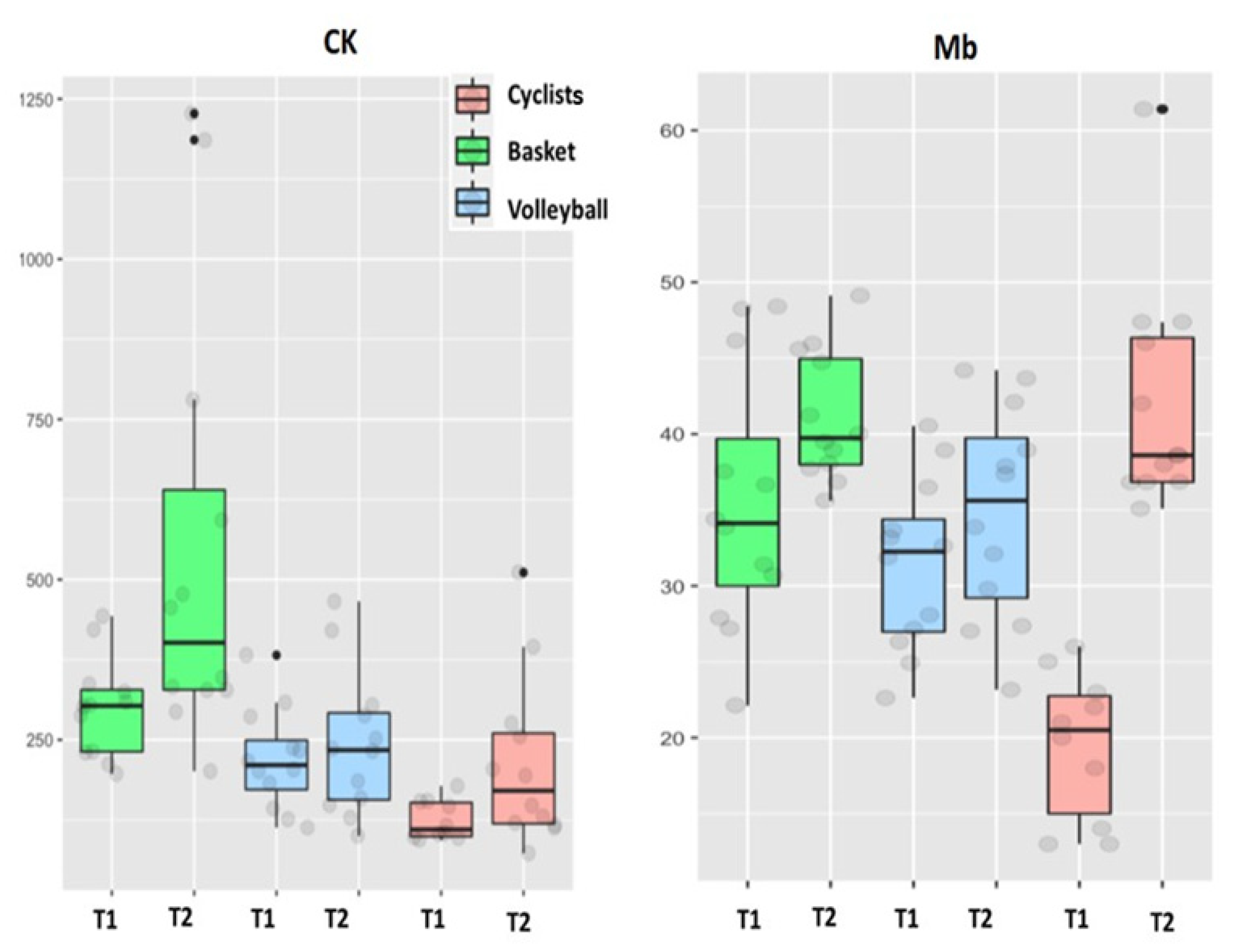
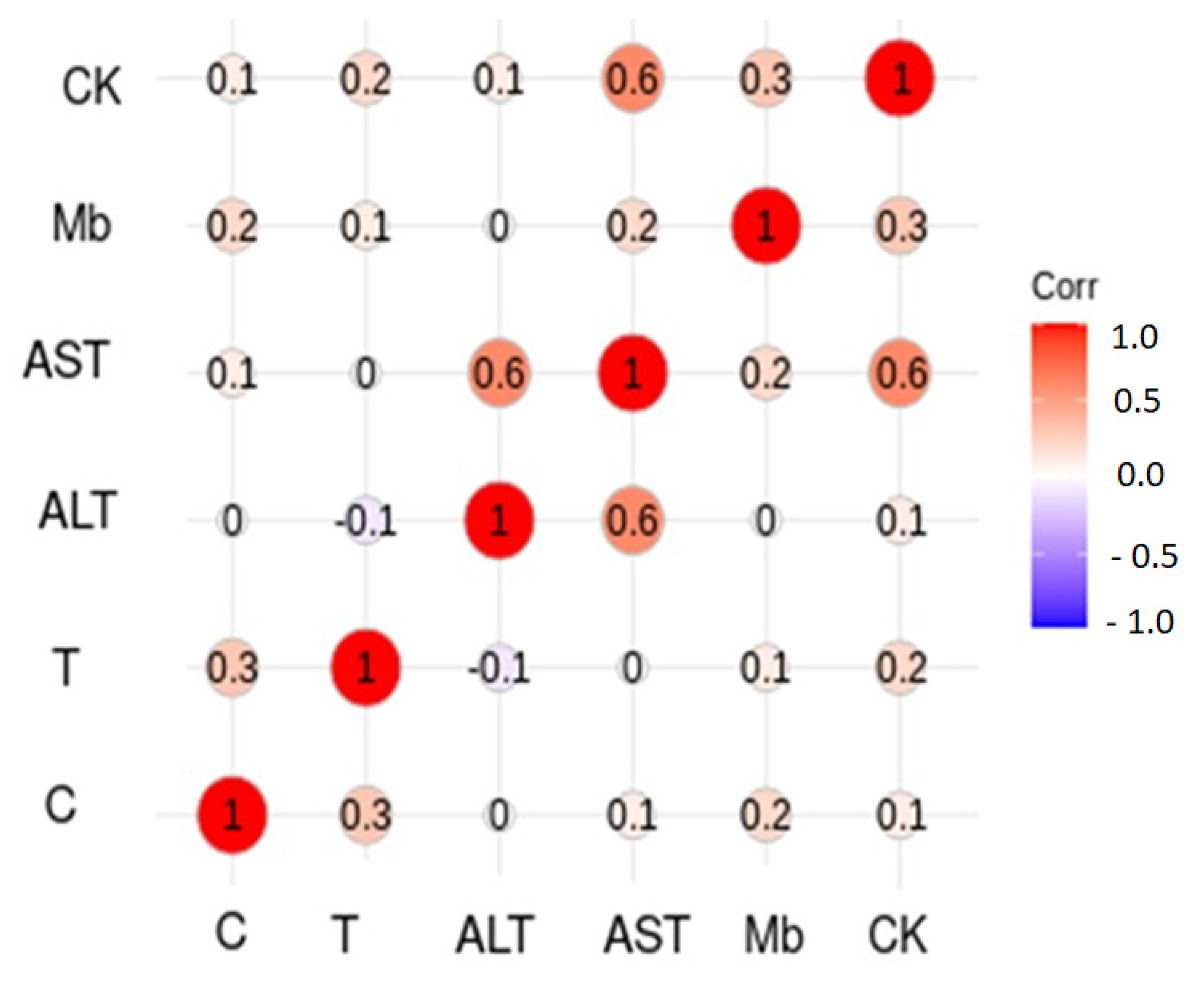
| CK (IU/L) | 125 + 22 (94–178) | 221 + 83 * (73–511) | 226 + 123 # (197–443) | 546 + 219 * (201–227) | 220 + 50 (113–382) | 243 + 72 (100–466) | T×S |
| Mb (ng/mL) | 19.5 + 3.5 (13.0–26.0) | 42.1 + 4.8 * (35.1–61.4) | 35.4 + 5.4 † (22.1–48.4) | 41.1 + 2.7 (35.6–49.1) | 31.4 + 3.6 † (22.6–40.5) | 34.75 + 4.5 (23.2–44.2) | T |
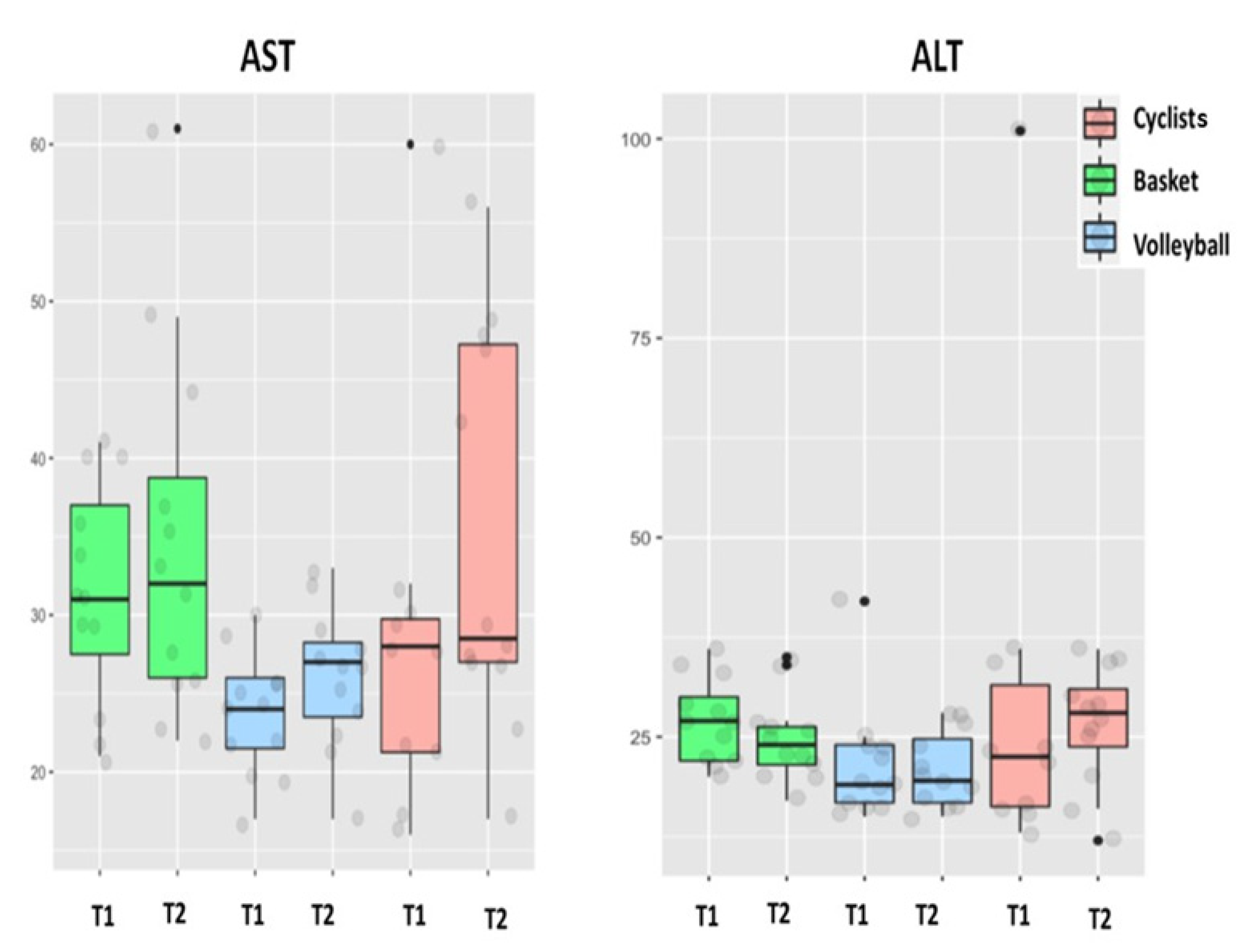
| AST (IU/L) | 28 + 9 (16–60) | 35 + 8 * (17–56) | 31 + 5 (21–41) | 35 + 7 (22–61) | 24 + 3 † (17–30) | 26 + 3 ‡ (17–33) | T×S |
| ALT (IU/L) | 30 + 19 (13–101) | 27 + 5 (12–36) | 27 + 3 (20–36) | 25 + 3 (17–35) | 22 + 6 (15–42) | 21 + 3 (15–28) | |
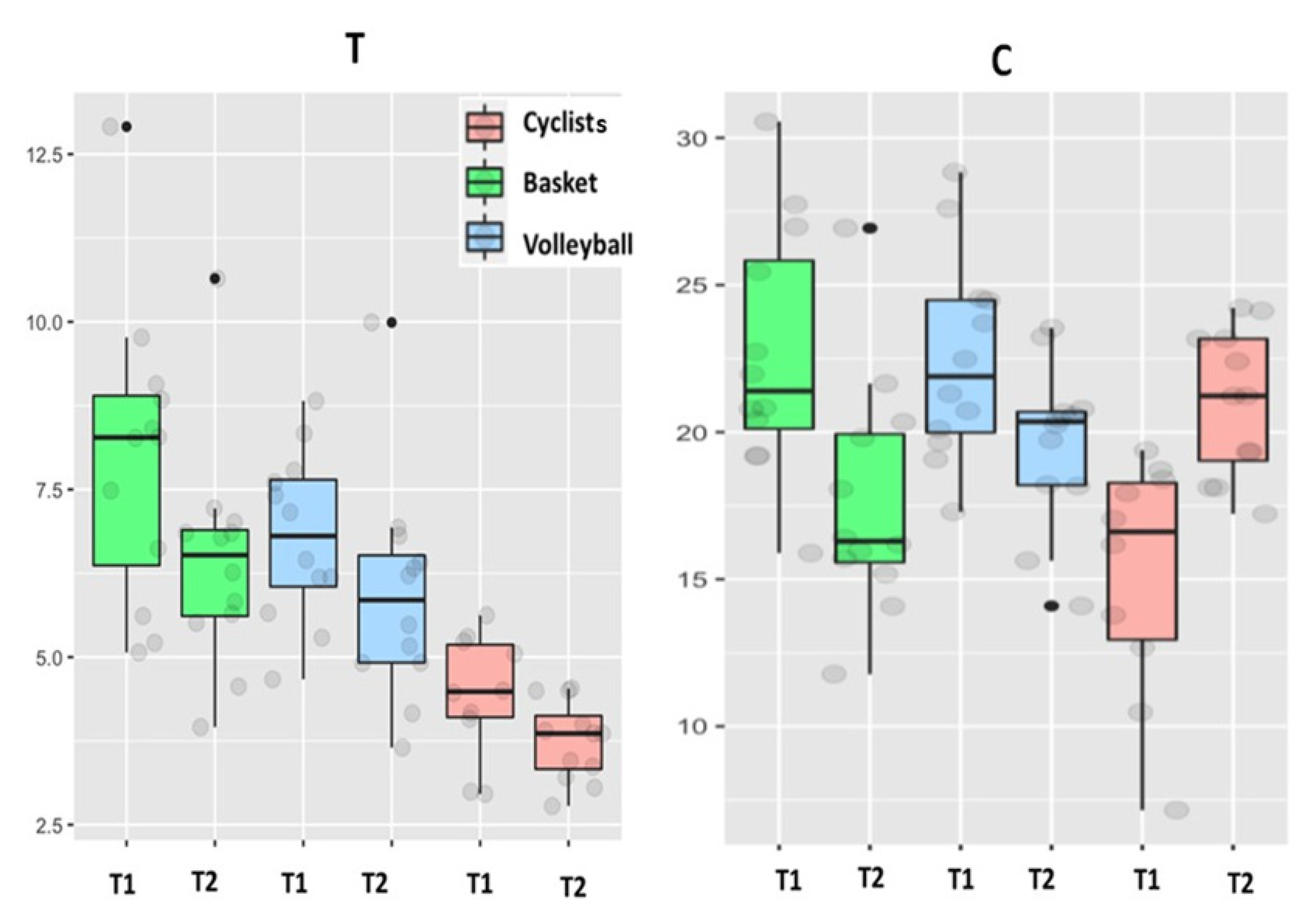
| Testosterone (ng/mL) | 4.4 + 0.7 (3.0–5.7) | 3.7 + 0.4 * (2.8–4.5) | 7.9 + 1.4 † (5.1–12.9) | 6.4 + 1.1 (4.0–10.6) | 6.8 + 0.8 † (4.7–8.8) | 5.9 + 1.0 * (3.7–10.0) | T, S |
| Cortisol (µg/dL) | 15.2 + 2.9 (7.2–19.4) | 21 + 1.6 * (17.2–24.2) | 22.7 + 2.7 † (15.9–30.6) | 17.7 + 2.6 * (11.8–26.9) | 22.5 + 2.2 † (17.3–28.8) | 19.7 + 1.8 (14.1–23.5) | T×S |
| Ciclyng | Basketball | Volleyball | |
|---|---|---|---|
| Δ (%) | Δ (%) | Δ (%) | |
| CK | 100 | 81.6 | 11 |
| Mb | 20 | 15.8 | 12.9 |
| AST | 23.6 | 12.9 | 83 |
| ALT | −12 | −8.1 | −0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Córdova-Martínez, A.; Caballero-García, A.; Bello, H.J.; Perez-Valdecantos, D.; Roche, E. Effects of Eccentric vs. Concentric Sports on Blood Muscular Damage Markers in Male Professional Players. Biology 2022, 11, 343. https://doi.org/10.3390/biology11030343
Córdova-Martínez A, Caballero-García A, Bello HJ, Perez-Valdecantos D, Roche E. Effects of Eccentric vs. Concentric Sports on Blood Muscular Damage Markers in Male Professional Players. Biology. 2022; 11(3):343. https://doi.org/10.3390/biology11030343
Chicago/Turabian StyleCórdova-Martínez, Alfredo, Alberto Caballero-García, Hugo J. Bello, Daniel Perez-Valdecantos, and Enrique Roche. 2022. "Effects of Eccentric vs. Concentric Sports on Blood Muscular Damage Markers in Male Professional Players" Biology 11, no. 3: 343. https://doi.org/10.3390/biology11030343
APA StyleCórdova-Martínez, A., Caballero-García, A., Bello, H. J., Perez-Valdecantos, D., & Roche, E. (2022). Effects of Eccentric vs. Concentric Sports on Blood Muscular Damage Markers in Male Professional Players. Biology, 11(3), 343. https://doi.org/10.3390/biology11030343