Snapshot of the Distribution and Biology of Alien Jellyfish Cassiopea andromeda (Forsskål, 1775) in a Mediterranean Touristic Harbour
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
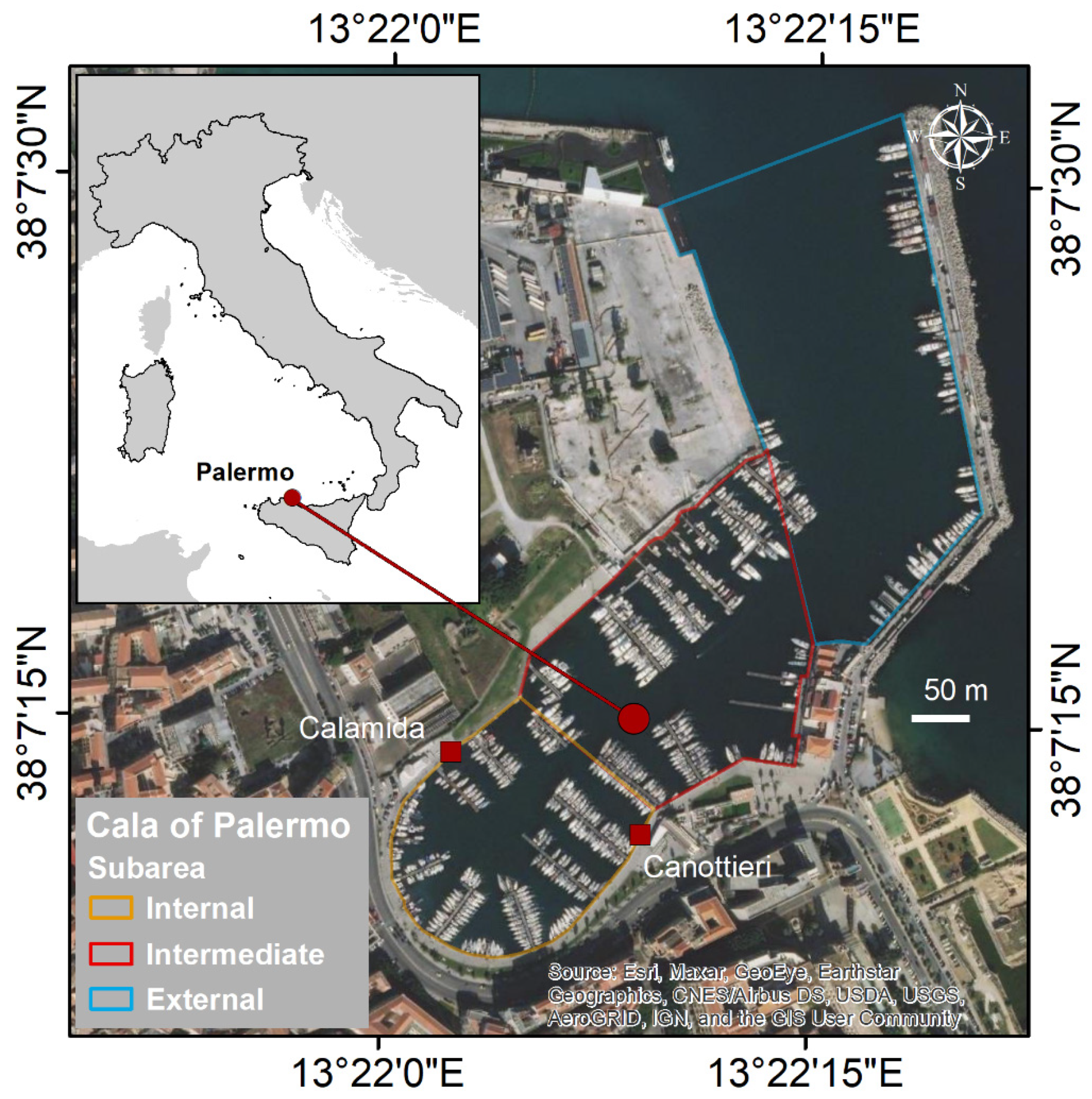
2.1. Study Area
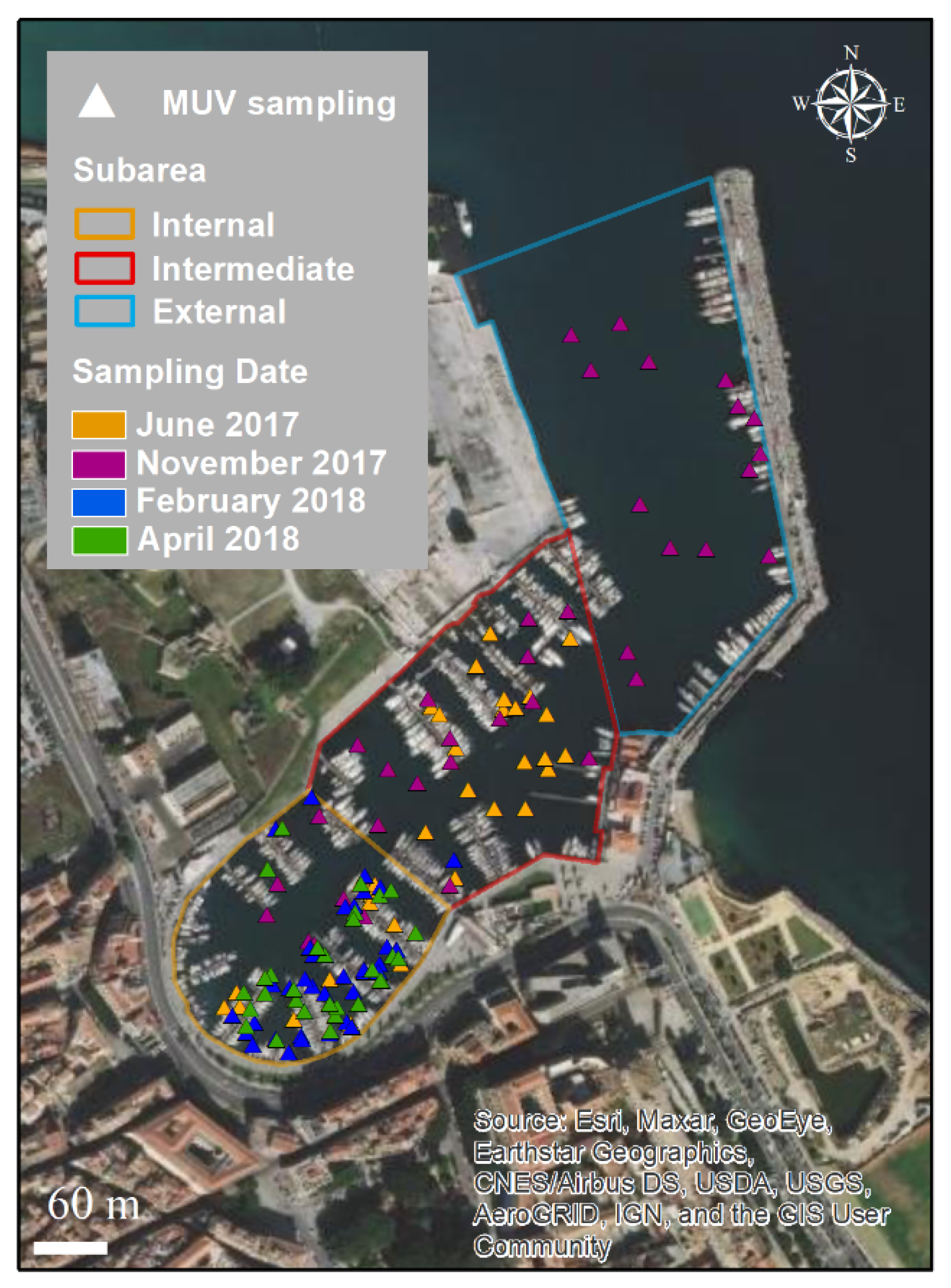
2.2. Visual Sampling Activities
2.3. Specimen Collection and Sample Preparation
2.4. Data Analysis
2.4.1. Visual Data Analysis
2.4.2. Spatial and Temporal Distribution Analysis
2.4.3. Stable Isotope
3. Results
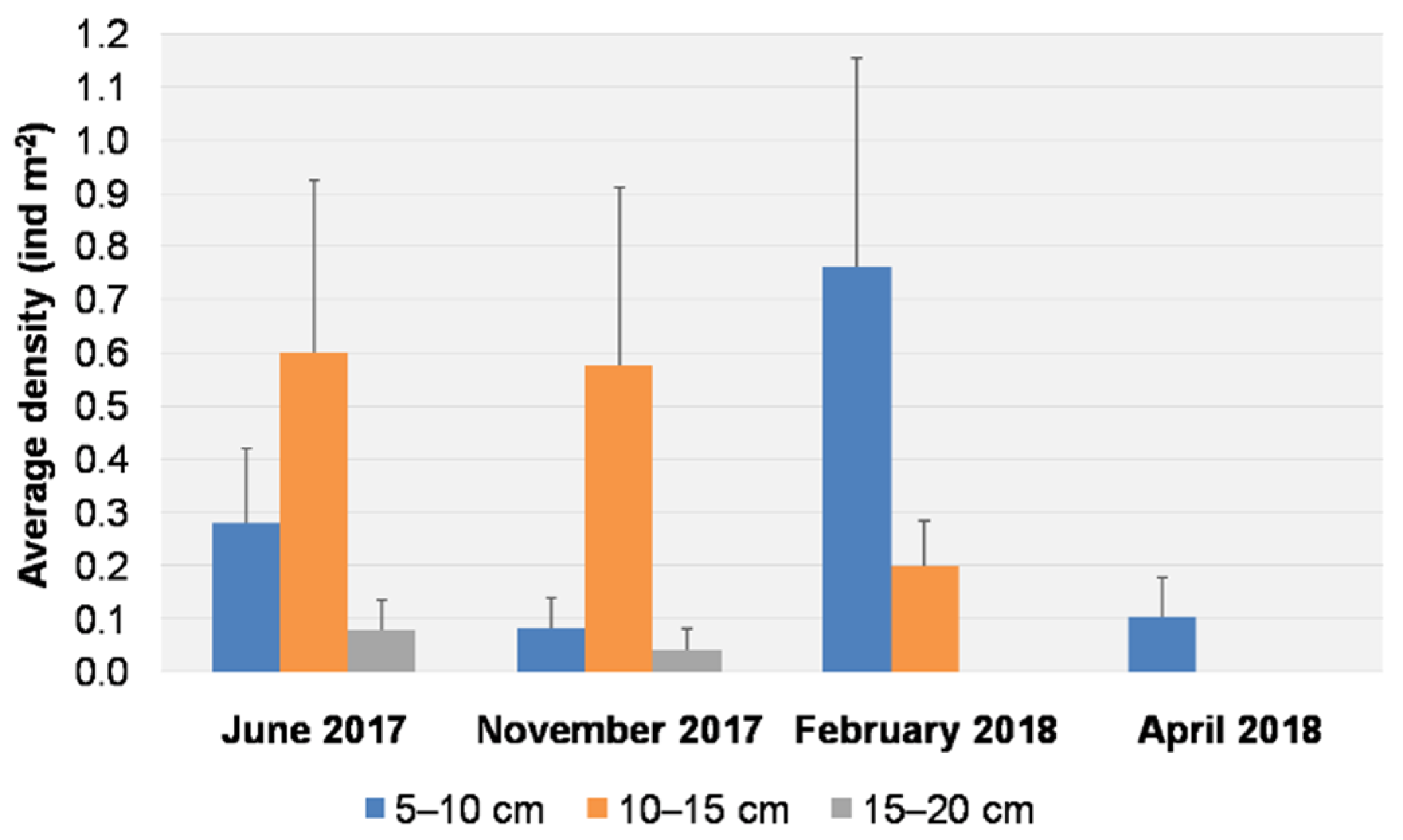
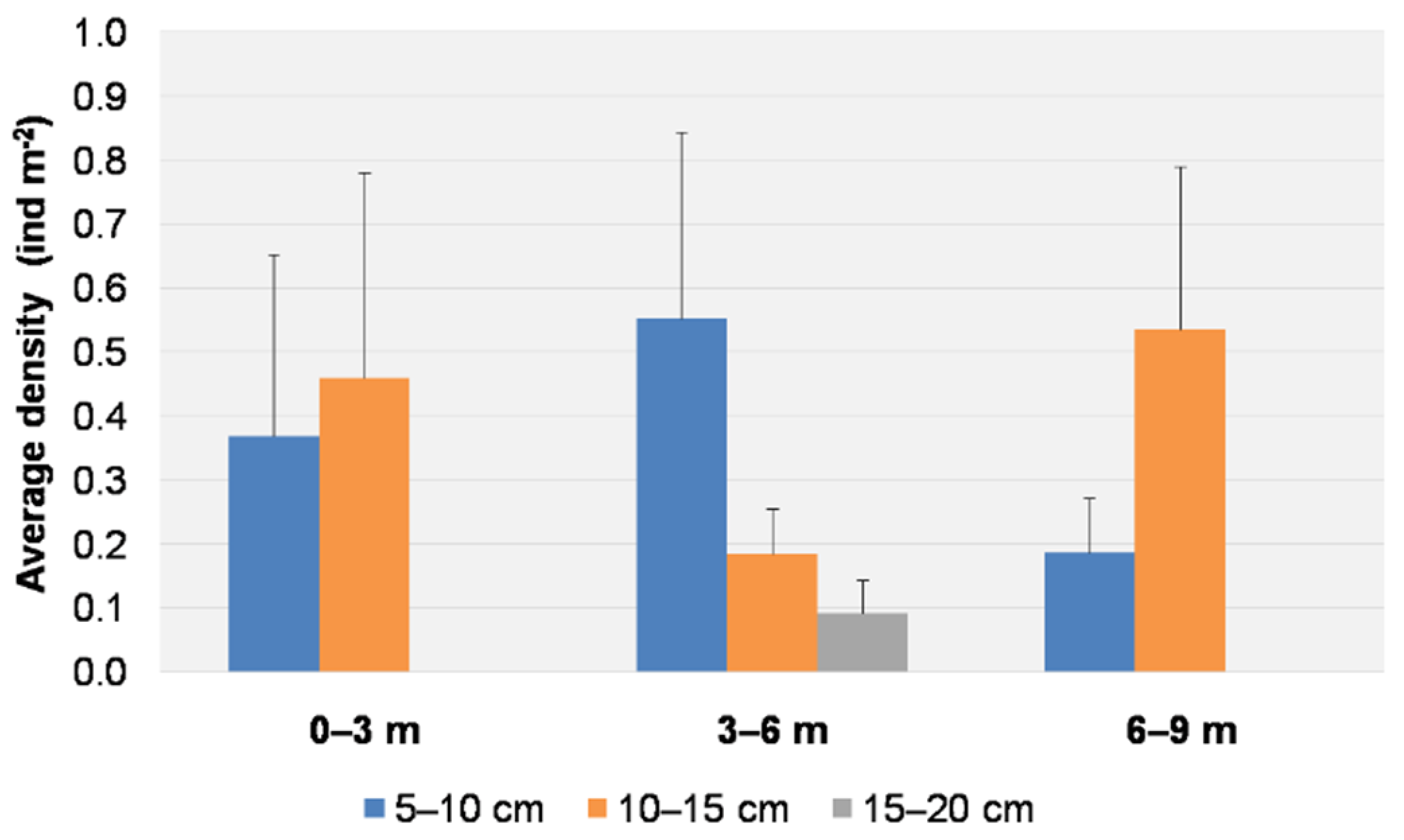
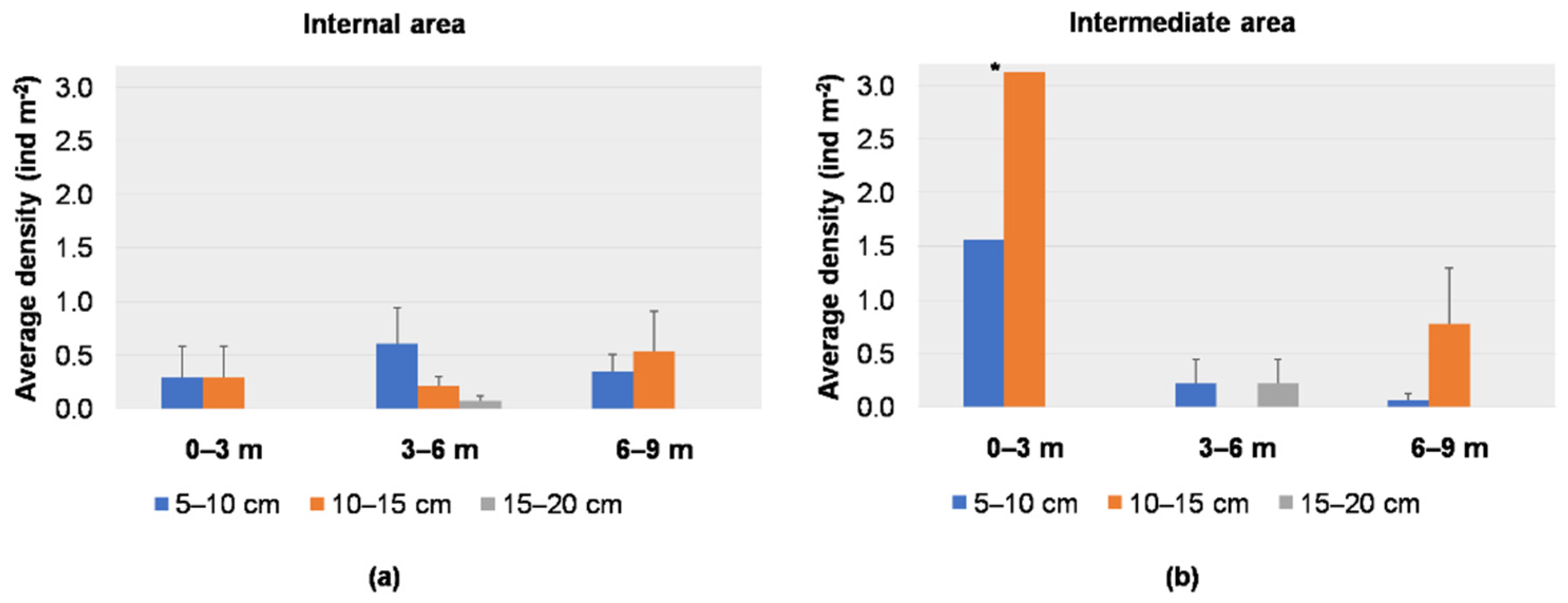
3.1. Visual Data Analysis
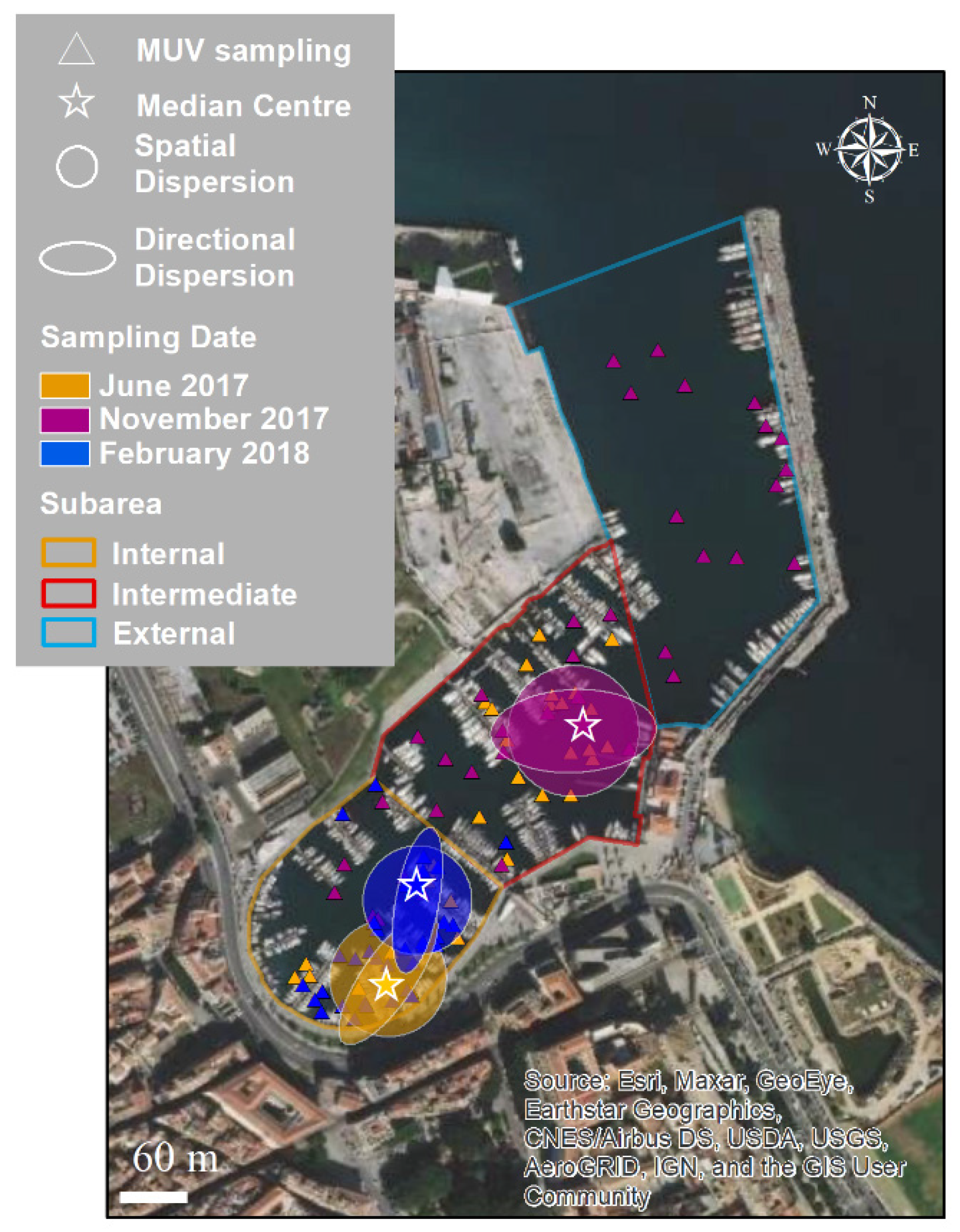
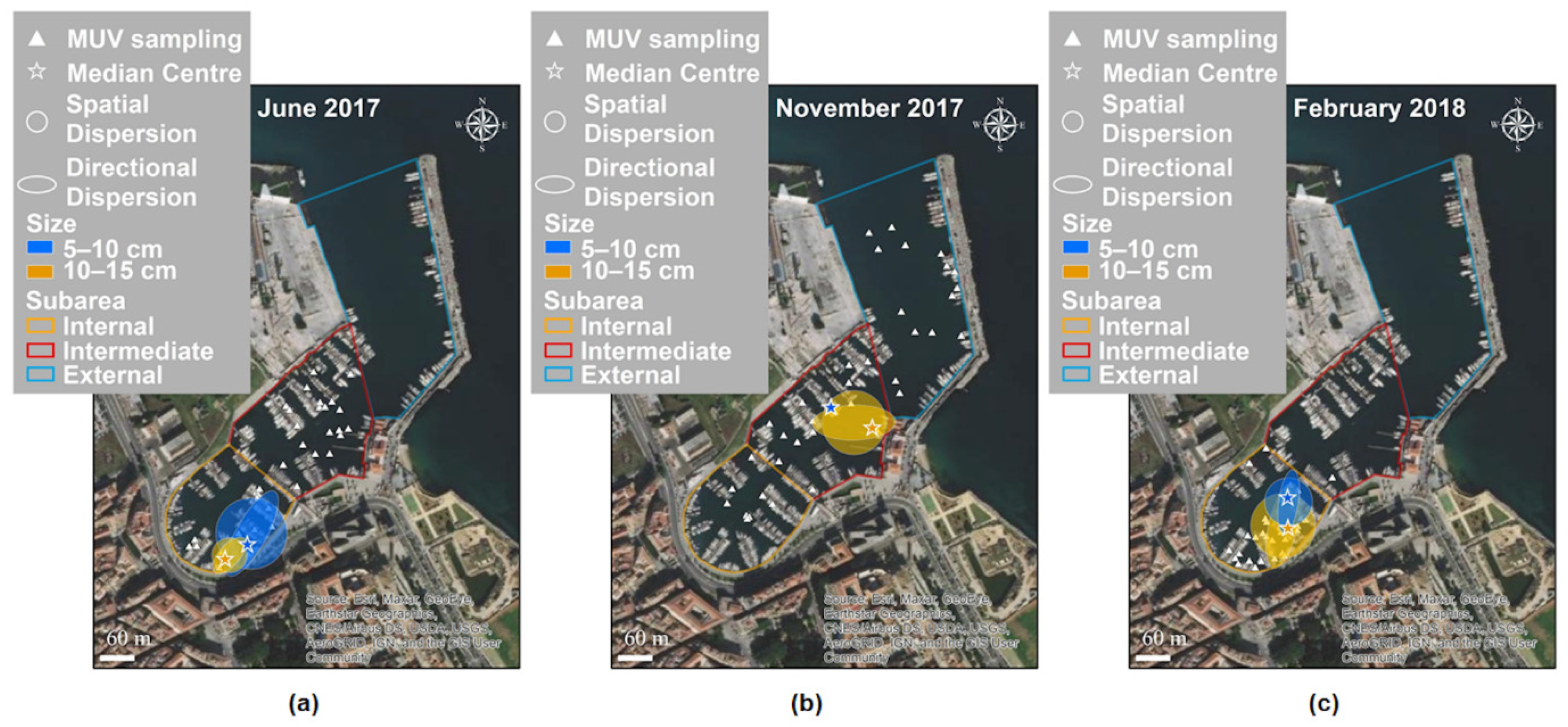
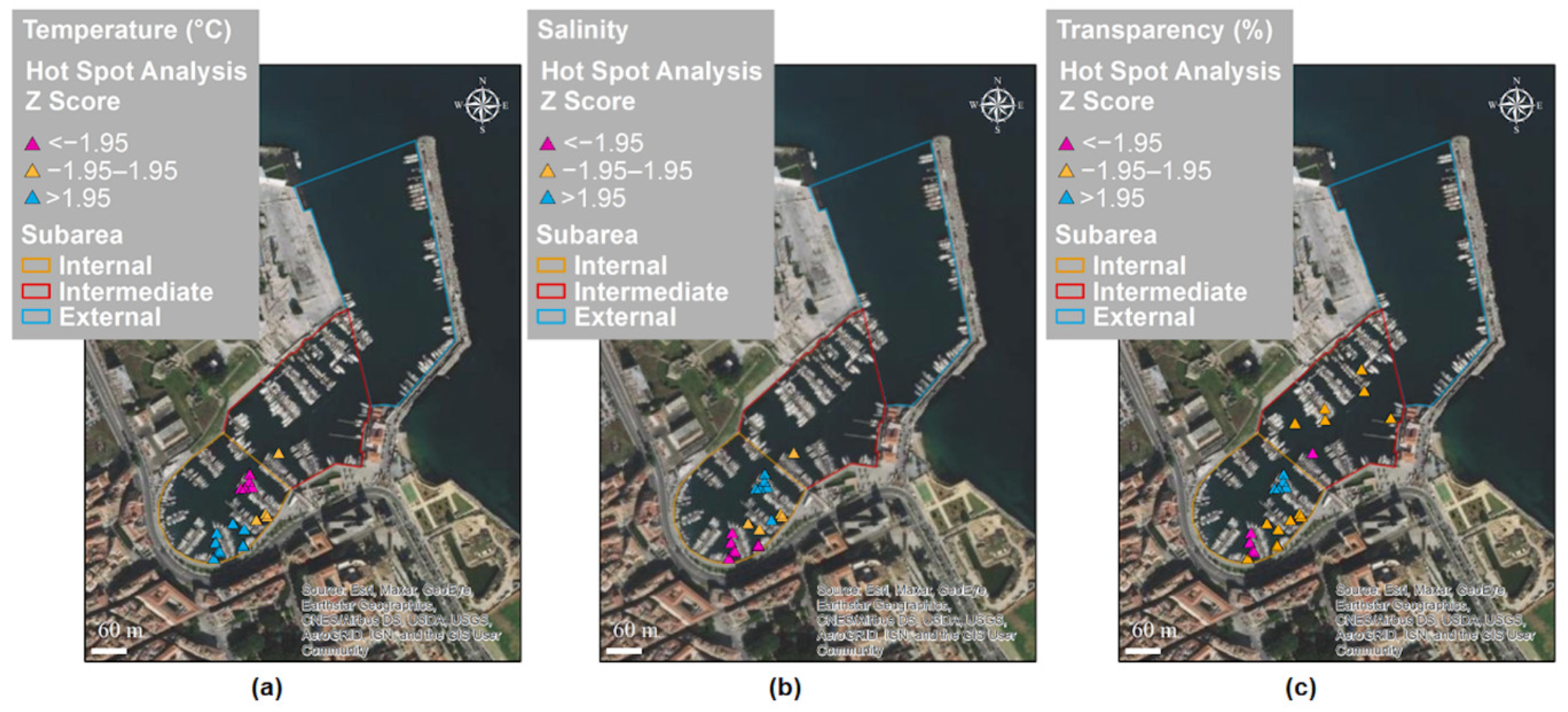
3.2. Spatial and Temporal Distributions
3.3. Stable Isotope Analysis
4. Discussion
4.1. Distribution of Cassiopea Andromeda
4.2. Trophic Behavior of Cassiopea Andromeda
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katsanevakis, S.; Wallentinus, I.; Zenetos, A.; Leppäkoski, E.; Çinar, M.E.; Oztürk, B.; Grabowski, M.; Golani, D.; Cardoso, A.N. Impacts of Invasive Alien Marine Species on Ecosystem Services and Biodiversity: A Pan-European Review. Aquat. Invasions 2014, 9, 391–423. [Google Scholar] [CrossRef]
- Bianchi, C.N. Biodiversity issues for the forthcoming tropical Mediterranean Sea. Hydrobiologia 2007, 580, 7. [Google Scholar] [CrossRef]
- Parravicini, V.; Mangialajo, L.; Mousseau, L.; Peirano, A.; Morri, C.; Montefalcone, M.; Francour, P.; Kulbicki, M.; Bianchi, C.N. Climate change and warm-water species at the north-western boundary of the Mediterranean Sea. Mar. Ecol. 2015, 36, 897–909. [Google Scholar] [CrossRef]
- Lasram, B.F.; Tomasini, J.A.; Guilhaumon, F.; Romdhane, M.S.; Do Chi, T.; Mouillot, D. Ecological correlates of dispersal success of Lessepsian fishes. Mar. Ecol. Prog. Ser. 2008, 363, 273–286. [Google Scholar] [CrossRef]
- Maggio, T.; Allegra, A.; Bosch-Belmar, M.; Cillari, T.; Cuttitta, A.; Falautano, M.; Milisenda, G.; Nicosia, A.; Perzia, P.; Sinopoli, M.; et al. Molecular identity of the non-indigenous species Cassiopea sp. from Palermo Harbour (central Mediterranean Sea). J. Mar. Biolog. Assoc. UK 2019, 99, 1765–1773. [Google Scholar] [CrossRef]
- Maas, O. Die Scyphomedusen der Siboga-Expedition; Siboga-Expeditie, Buchhandlung und Druckerei Vormals, EJ Brill: Leiden, The Netherlands, 1903; Volume 11, pp. 1–91. [Google Scholar] [CrossRef]
- Stampar, S.N.; Gamero-Mora, E.; Maronna, M.M.; Fritscher, J.M.; Oliveira, B.S.P.; Sampaio, C.L.S.; Morandini, A.C. The puzzling occurrence of the upside-down jellyfish Cassiopea (Cnidaria: Scyphozoa) along the Brazilian coast: A result of several invasion events? Zoologia 2020, 37, 1–10. [Google Scholar] [CrossRef]
- Stoner, E.W.; Layman, C.A.; Yeager, L.A.; Hassett, H.M. Effects of anthropogenic disturbance on the abundance and size of epibenthic jellyfish Cassiopea spp. Mar. Pollut. Bull. 2011, 62, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Heins, A.; Glatzel, T.; Holst, S. Revised descriptions of the nematocysts and the asexual reproduction modes of the scyphozoan jellyfish Cassiopea andromeda (Forsskål, 1775). Zoomorphology 2015, 134, 351–366. [Google Scholar] [CrossRef]
- Morandini, A.C.; Stampar, S.N.; Maronna, M.M.; Da Silveira, F.L. All non-indigenous species were introduced recently? The case study of Cassiopea (Cnidaria: Scyphozoa) in Brazilian waters. J. Mar. Biol. Assoc. UK 2017, 97, 321–328. [Google Scholar] [CrossRef]
- Ohdera, A.H.; Abrams, M.J.; Ames, C.L.; Baker, D.M.; Suescún-Bolívar, L.P.; Collins, A.G.; Freeman, C.J.; Gamero-Mora, E.; Goulet, T.L.; Hofmann, D.K.; et al. Upside-down but headed in the right direction: Review of the highly versatile Cassiopea xamachana system. Front. Ecol. Evol. 2018, 6, 1–15. [Google Scholar] [CrossRef]
- Kremer, P. Ingestion and elemental budgets for Linuche unguiculata, a scyphomedusa with zooxanthellae. J. Mar. Biol. Assoc. UK 2005, 85, 613–625. [Google Scholar] [CrossRef]
- Welsh, D.T.; Dunn, R.J.; Meziane, T. Oxygen and nutrient dynamics of the upside down jellyfish (Cassiopea sp.) and its influence on benthic nutrient exchanges and primary production. Hydrobiologia 2009, 635, 351–362. [Google Scholar] [CrossRef]
- Kremer, P.; Costello, J.; Kremer, J.; Canino, M. Significance of photosynthetic endosymbionts to the carbon budget of the scyphomedus Linuche unguiculata. Limnol. Oceanogr. 1990, 35, 609–624. [Google Scholar] [CrossRef]
- Kikinger, R. Cotylorhiza tuberculata (Cnidaria: Scyphozoa)—Life history of a stationary population. Mar. Ecol. 1992, 13, 333–362. [Google Scholar] [CrossRef]
- McCloskey, L.R.; Muscatine, L.; Wilkerson, F.P. Daily photosynthesis, respiration, and carbon budgets in a tropical marine jellyfish (Mastigias sp.). Mar. Biol. 1994, 119, 13–22. [Google Scholar] [CrossRef]
- Verde, E.A.; McCloskey, L.R. Production, respiration, and photophysiology of the mangrove jellyfish Cassiopea xamachana symbiotic with zooxanthellae: Effect of jellyfish size and season. Mar. Ecol. Prog. Ser. 1998, 168, 147–162. [Google Scholar] [CrossRef]
- Sugiura, Y. On the life-history of Rhizostome medusae V. On the relation between zooxanthellae and the strobilation of Cephea cephea. Bull. Mar. Biol. Stn. Asamushi 1969, 13, 227–233. [Google Scholar]
- Bolton, T.F.; Graham, W.M. Morphological variation among populations of an invasive jellyfish. Mar. Ecol. Prog. Ser. 2004, 278, 125–139. [Google Scholar] [CrossRef]
- Djeghri, N.; Stibor, H.; Lebeau, O.; Pondaven, P. δ13C, δ15N, and C:N ratios as nutrition indicators of zooxanthellate jellyfishes: Insights from an experimental approach. J. Exp. Mar. Biol. Ecol. 2020, 522, 151257. [Google Scholar] [CrossRef]
- Todd, B.D.; Thornhill, D.J.; Fitt, W.K. Patterns of inorganic phosphate uptake in Cassiopea xamachana: A bioindicator species. Mar. Pollut. Bull. 2006, 52, 515–521. [Google Scholar] [CrossRef]
- Muscatine, L.; Porter, J.W.; Kaplan, I.R. Resource partitioning by reef corals as determined from stable isotope composition I. δ13C of zooxanthellae and animal tissue versus depth. Mar. Biol. 1989, 100, 185–193. [Google Scholar] [CrossRef]
- Muscatine, L.; Kaplan, I.R. Resource partitioning by reef corals as determined from stable isotope composition II. δ15N of zooxanthellae and animal tissue versus depth. Pac. Sci. 1994, 48, 304–312. [Google Scholar]
- Ferrier-Pagès, C.; Peirano, A.; Abbate, M.; Cocito, S.; Negri, A.; Rottier, C.; Riera, P.; Metalpa, R.; Reynaud, S. Summer autotrophy and winter heterotrophy in the temperate symbiotic coral Cladocora caespitosa. Limnol. Oceanogr. 2011, 56, 1429–1438. [Google Scholar] [CrossRef]
- Yamamuro, M.; Kayanne, H.; Minagawa, M. Carbon and nitrogen stable isotopes of primary producers in coral reef ecosystems. Limnol. Oceanogr. 1995, 40, 617–621. [Google Scholar] [CrossRef]
- Sammarco, P.W.; Risk, M.J.; Schwarcz, H.P.; Heikoop, J.M. Cross-continental shelf trend in coral δ15N on the Great Barrier Reef: Further consideration of the reef nutrient paradox. Mar. Ecol. Prog. Ser. 1999, 180, 131–138. [Google Scholar] [CrossRef]
- Heikoop, J.M.; Dun, J.J.; Risk, M.J.; Tomascik, T.; Schwarz, H.P.; Sandeman, I.M.; Sammarco, P.W. Nitrogen-15 signals of anthropogenic nutrient loading in reef corals. Mar. Pollut. Bull. 2000, 40, 628–636. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Muscatine, L.; Goiran, C.; Siggaard, D.; Marion, G. Nutrient-induced perturbations to δ13C and δ15N in symbiotic dinoflagellates and their coral hosts. Mar. Ecol. Prog. Ser. 2004, 280, 105–114. [Google Scholar] [CrossRef]
- Marion, G.S.; Hoegh-Guldberg, O.; McCulloch, M.T.; Jupiter, S.D. Coral isotopic records (δ15N) of unprecedented land-use stress in Great Barrier Reef coastal communities. EOS Trans. Am. Geophys. Union Ocean Sci. Meet. Suppl. 2006, 87. [Google Scholar]
- Heikoop, J.M.; Dun, J.J.; Risk, M.J.; McConnaughey, T.A.; Sandman, I.M. Separation of kinetic and metabolic effect in carbon-13 records preserved in reef coral skeletons. Geochim. Cosmochim. Acta 2000, 64, 975–987. [Google Scholar] [CrossRef]
- Heikoop, J.M.; Dunn, J.J.; Risk, M.J.; Sandeman, I.M.; Schwartz, H.P.; Waltho, N. Relationship between light and the δ15N of coral tissue: Examples from Jamaica and Zanzibar. Limnol. Oceanogr. 1998, 43, 909–920. [Google Scholar] [CrossRef][Green Version]
- Rodrigues, L.; Grottoli, A. Calcification rate, and the stable carbon, oxygen and nitrogen isotopes in the skeleton, host tissue and zooxanthellae of bleached and recovering Hawaiian corals. Geochim. Cosmochim. Acta 2006, 70, 2781–2789. [Google Scholar] [CrossRef]
- Lampert, K.P. Cassiopea and its zooxanthellae. In The Cnidaria, Past, Present and Future; Springer: Cham, Switzerland, 2016; pp. 415–423. [Google Scholar]
- Schäfer, W. Eine Qualle aus dem Indischen Ozean in der Agais. Nat. Volk 1955, 85, 241–245. [Google Scholar]
- Schembri, P.J.; Deidun, A.; Vella, P.J. First record of Cassiopea andromeda (Scyphozoa: Rhizostomeae: Cassiopeidae) from the central Mediterranean Sea. Mar. Biodivers. Rec. 2010, 3, E6. [Google Scholar] [CrossRef][Green Version]
- Cillari, T.; Andaloro, F.; Castriota, L. First documented record of Cassiopea cfr andromeda (Cnidaria: Scyphozoa) in Italian waters. Cah. Biol. Mar. 2018, 59, 193–195. [Google Scholar]
- Thé, J.; de Sousa Barroso, H.; Mammone, M.; Viana, M.; Melo, C.S.B.; Mies, M.; Banha, T.N.S.; Morandini, A.C.; Rossi, S.; de Oliveira Soares, M. Aquaculture facilities promote populational stability throughout seasons and increase medusae size for the invasive jellyfish Cassiopea andromeda. Mar. Environ. Res. 2020, 162, 105161. [Google Scholar] [CrossRef]
- Mammone, M.; Ferrier-Pagés, C.; Lavorano, S.; Rizzo, L.; Piraino, S.; Rossi, S. High photosynthetic plasticity may reinforce invasiveness of upside-down zooxanthellate jellyfish in Mediterranean coastal waters. PLoS ONE 2021, 16, e0248814. [Google Scholar] [CrossRef]
- Autorità di Sistema Portuale del Mare di Sicilia Occidentale- Porti/Porto di Palermo/Diporto. Available online: https://www.adsppalermo.it/palermo-diporto (accessed on 30 November 2021).
- Cillari, T.; Allegra, A.; Bosch-Belmar, M.; Castriota, L.; Falautano, M.; Milisenda, G.; Maggio, T.; Perzia, P.; Sinopoli, M. Megabenthos Underwater Video (MUV): A new device to evaluate species distribution in hard-to-reach marine areas. In Proceedings of the International Workshop on Metrology for the Sea, Learning to Measure Sea Health Parameters (MetroSea), Virtual Conference, Milazzo, Italy, 4–6 October 2021; pp. 199–203. [Google Scholar] [CrossRef]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Perzia, P.; Battaglia, P.; Consoli, P.; Andaloro, F.; Romeo, T. Swordfish monitoring by a GIS-based spatial and temporal distribution analysis on harpoon fishery data: A case of study in the central Mediterranean Sea. Fish. Res. 2016, 183, 424–434. [Google Scholar] [CrossRef]
- Lipej, L.; Mavric, B.; Paliska, D. New northernmost record of the blunthead pufferfish, Sphoeroides pachygaster (osteichthyes: Tetraodontidae) in the Mediterranean Sea/Nuova segnalazione a nord del pesce palla liscio, Sphoeroides pachygaster (Osteichthyes: Tetraodontidae), nel mare Mediterraneo. Ann. Ser. Hist. Nat. 2013, 23, 103. [Google Scholar]
- Falautano, M.; Perzia, P.; Castriota, L. First record of the Lessepsian fish Parexocoetus mento in Italian waters and GIS-based spatial and temporal distribution in Mediterranean Sea. J. Mar. Biolog. Assoc. 2020, 100, 1163–1169. [Google Scholar] [CrossRef]
- Mitchell, A. The ESRI Guide to GIS Analysis; ESRI Press: Redlands, CA, USA, 2005; Volume 2, ISBN 1-58948-116-X. [Google Scholar]
- Scott, L.M.; Janikas, M.V. Spatial statistics in ArcGis. In Handbook of Applied Spatial Analysis: Software Tools, Methods and Applications; Fischer, M.M., Getis, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 27–41. [Google Scholar]
- ESRI ArcGIS. ArcGIS Desktop Help: Release 10.3; Environmental Systems Research Institute: Redlands, CA, USA, 2011. [Google Scholar]
- Alomar, C.; Deudero, S.; Andaloro, F.; Castriota, L.; Consoli, P.; Falautano, M.; Sinopoli, M. Caulerpa cylindracea Sonder invasion modifies trophic niche in infralittoral rocky benthic community. Mar. Environ. Res. 2016, 120, 86–92. [Google Scholar] [CrossRef]
- Ferrario, J.; Caronni, S.; Occhipinti-Ambrogi, A.; Marchini, A. Role of commercial harbours and recreational marinas in the spread of non-indigenous fouling species. Biofouling 2017, 33, 651–660. [Google Scholar] [CrossRef]
- Galil, B.S.; Marchini, A.; Occhipinti-Ambrogi, A. East is east and West is west? Management of marine bioinvasions in the Mediterranean Sea. Estuar. Coast. Shelf Sci. 2018, 201, 7–16. [Google Scholar] [CrossRef]
- Viana, I.G.; Bode, A.; Bartholomew, M.; Valiela, I. Experimental assessment of the macroalgae Ascophyllum nodosum and Fucus vesiculosus for monitoring N sources at different time-scales using stable isotope composition. J. Exp. Mar. Biol. Ecol. 2015, 466, 24–33. [Google Scholar] [CrossRef]
- McClellend, J.; Valiela, I. Linking nitrogen in estuarian producers to land-derived sources. Limnol. Oceanogr. 1998, 43, 577–585. [Google Scholar] [CrossRef]
- Risk, M. The reef crisis and the reef science crisis: Nitrogen isotopic ratios as an objective indicator of stress. Mar. Pollut. Bull. 2009, 58, 787–788. [Google Scholar] [CrossRef] [PubMed]
- Risk, M.J.; Sherwood, O.A.; Nairn, R.; Gibbons, C. Tracking the record of sewage discharge off Jeddah, Saudi Arabia, since 1950, using stable isotope records from antipatharians. Mar. Ecol. Prog. Ser. 2009, 397, 219–226. [Google Scholar] [CrossRef]
- Risk, M.J.; Erdmann, M.V. Isotopic composition of nitrogen in stomatopod (Crustacea) tissues as an indicator of human sewage impacts on Indonesian coral reefs. Mar. Pollut. Bull. 2000, 40, 50–58. [Google Scholar] [CrossRef]
- Cifuentes, L.A.; Fogel, M.L.; Pennock, J.R.; Sharp, J.H. Biogeochemical factors that influence the stable nitrogen isotope ratio of dissolved ammonium in the Delaware estuary. Geochim. Cosmochim. Acta 1989, 53, 2713–2721. [Google Scholar] [CrossRef]
- Montoya, P.; Korrigan, S.G.; McCarthy, J.J. Rapid, storm-induced changes in the natural abundance of 15N in a planktonic ecosystem, Chesapeake Bay, USA. Geochim. Cosmochim. Acta 1991, 55, 3627–3638. [Google Scholar] [CrossRef]
- Fogel, M.L.; Cifuentes, L.A. Isotope fractionation during primary production. In Organic Geochemistry; Engel, M.H., Macko, S.A., Eds.; Springer: Boston, MA, USA, 1993; Volume 11, pp. 73–98. [Google Scholar]
- Pennock, J.R.; Velinsky, D.J.; Ludlam, J.M.; Sharp, J.H.; Fogel, M.L. Isotopic fractionation of ammonium and nitrate during uptake by Skeletonema costatum: Implications for δ15N dynamics under bloom conditions. Limnol. Oceanogr. 1996, 41, 451–459. [Google Scholar] [CrossRef]
- Waser, N.A.D.; Harrison, P.J.; Nielsen, B.; Calvert, S.E.; Turpin, D.H. Nitrogen isotope fractionation during the uptake and assimilation of nitrate, nitrite, ammonium, and urea by a marine diatom. Limnol. Oceanogr. 1998, 43, 215–224. [Google Scholar] [CrossRef]
- Granger, J.; Sigman, D.M.; Needoba, J.A.; Harrison, P.J. Coupled nitrogen and oxygen isotope fractionation of nitrate during assimilation by cultures of marine phytoplankton. Limnol. Oceanogr. 2004, 49, 1763–1773. [Google Scholar] [CrossRef]
- Ferrier-Pagès, C.; Leal, M.C. Stable isotopes as tracers of trophic interactions in marine mutualistic symbioses. Ecol. Evol. 2018, 9, 723–740. [Google Scholar] [CrossRef] [PubMed]
- Freeman, C.J.; Stoner, E.W.; Easson, C.G.; Matterson, K.O.; Baker, D.M. Symbiont carbon and nitrogen assimilation in the Cassiopea–Symbiodinium mutualism. Mar. Ecol. Prog. Ser. 2016, 544, 281–286. [Google Scholar] [CrossRef]
- Freeman, C.J.; Stoner, E.W.; Easson, C.G.; Matterson, K.O.; Baker, D.M. Variation in δ13C and δ15N values suggests a coupling of host and symbiont metabolism in the Symbiodinium-Cassiopea mutualism. Mar. Ecol. Prog. Ser. 2017, 571, 245–251. [Google Scholar] [CrossRef]
- Cravotta, C.A. Use of Stable Isotopes of Carbon, Nitrogen, and Sulfur to Identify Sources of Nitrogen in Surface Waters in the Lower Susquehanna River Basin, Pennsylvania; U.S. Department of the Interior, U.S. Geological Survey: Denver, CO, USA, 2002; p. 99.
- Elliott, E.M.; Kendall, C.; Burns, D.A.; Boyer, E.W.; Harlin, K.; Wankel, S.D.; Butler, T.J.; Carlton, R. Nitrate isotopes in precipitation to distinguish NOx sources, atmospheric processes, and source areas in the United States. In AGU Fall Meeting Abstracts; American Geophysical Union: San Francisco, CA, USA, 2006; Volume 2007, abstract id. H52B-01. [Google Scholar]
- Hastings, M.G.; Sigman, D.M.; Lipschultz, F. Isotopic evidence for source changes of nitrate in rain at Bermuda. J. Geophys. Res. 2003, 108, 4790. [Google Scholar] [CrossRef]
- Hastings, M.G. Studies of Reactive Nitrogen in the Atmosphere Using Global Modelling and Stable Isotope Measurements. Ph.D. Thesis, Princeton University, Princeton, NJ, USA, 2004; 217p. [Google Scholar]
- Arai, M.N. A Functional Biology of the Scyphozoa; Chapman & Hall: London, UK, 1997; p. 300. [Google Scholar]
- Balderston, W.L.; Claus, G. Study of the symbiotic relationship between Symbiodinium microadriaticum Freudenthal, a Zooxanthella and the upside down jellyfish, Cassiopea sp. Nova Hedwigia 1969, 17, 373–382. [Google Scholar]
- Post, D.M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- Swart, P.K.; Saied, A.; Lamb, K. Temporal and spatial variation in the δ15N and δ13C of coral tissue and zooxanthellae in Montastraea faveolata collected from the Florida reef tract. Limnol. Oceanogr. 2005, 50, 1049–1058. [Google Scholar] [CrossRef]
- Alamaru, A.; Loya, Y.; Brokovich, E.; Yam, R.; Shemesh, A. Carbon and nitrogen utilization in two species of Red Sea corals along a depth gradient: Insights from stable isotope analysis of total organic material and lipids. Geochim. Cosmochim. Acta 2009, 73, 5333–5342. [Google Scholar] [CrossRef]
- Reynaud, S.; Martinez, P.; Houlbrèque, F.; Billy, I.; Allemand, D.; Ferrier-Pagès, C. Effect of light and feeding on the nitrogen isotopic composition of a zooxanthellate coral: Role of nitrogen recycling. Mar. Ecol. Prog. Ser. 2009, 392, 103–110. [Google Scholar] [CrossRef]
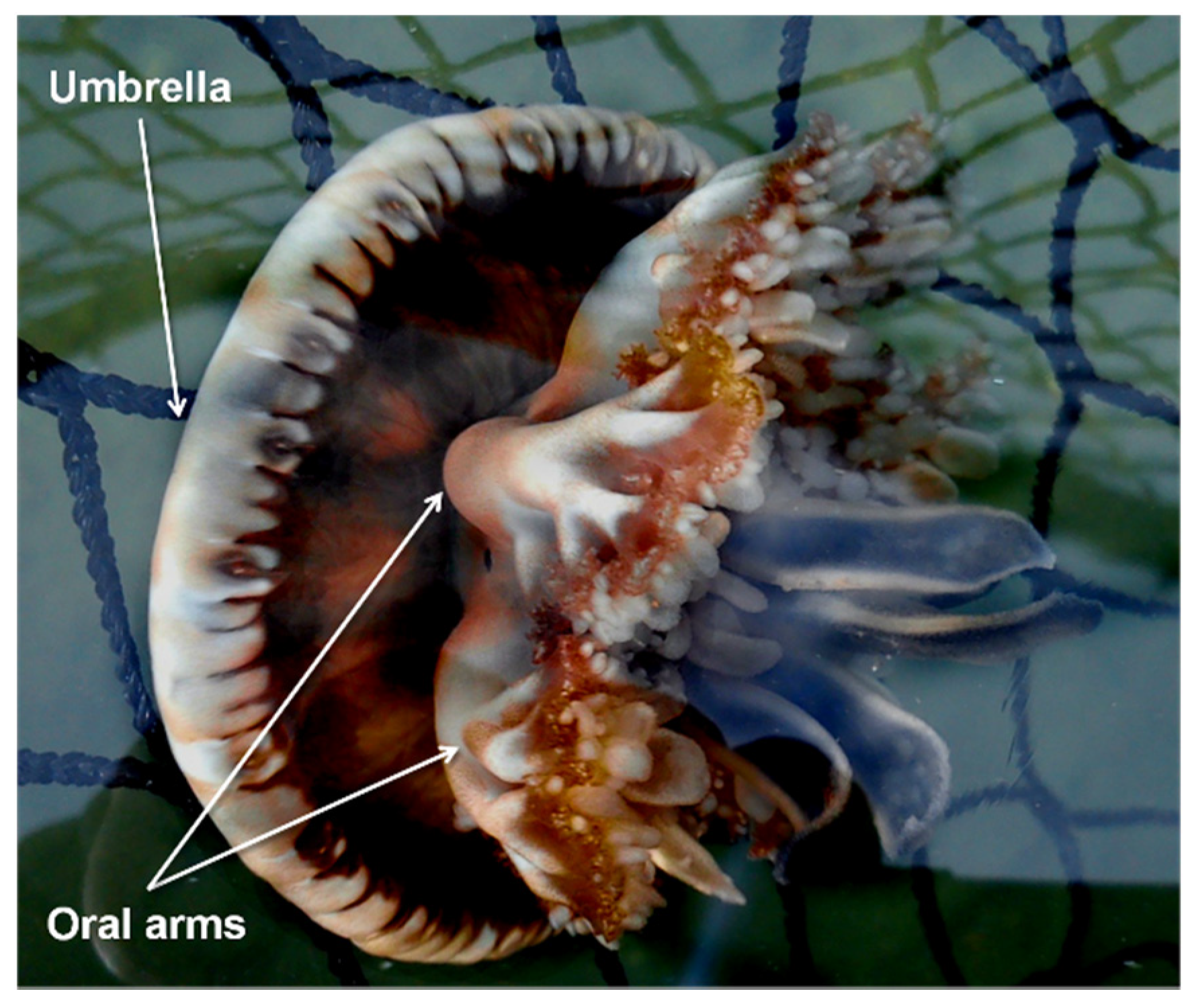

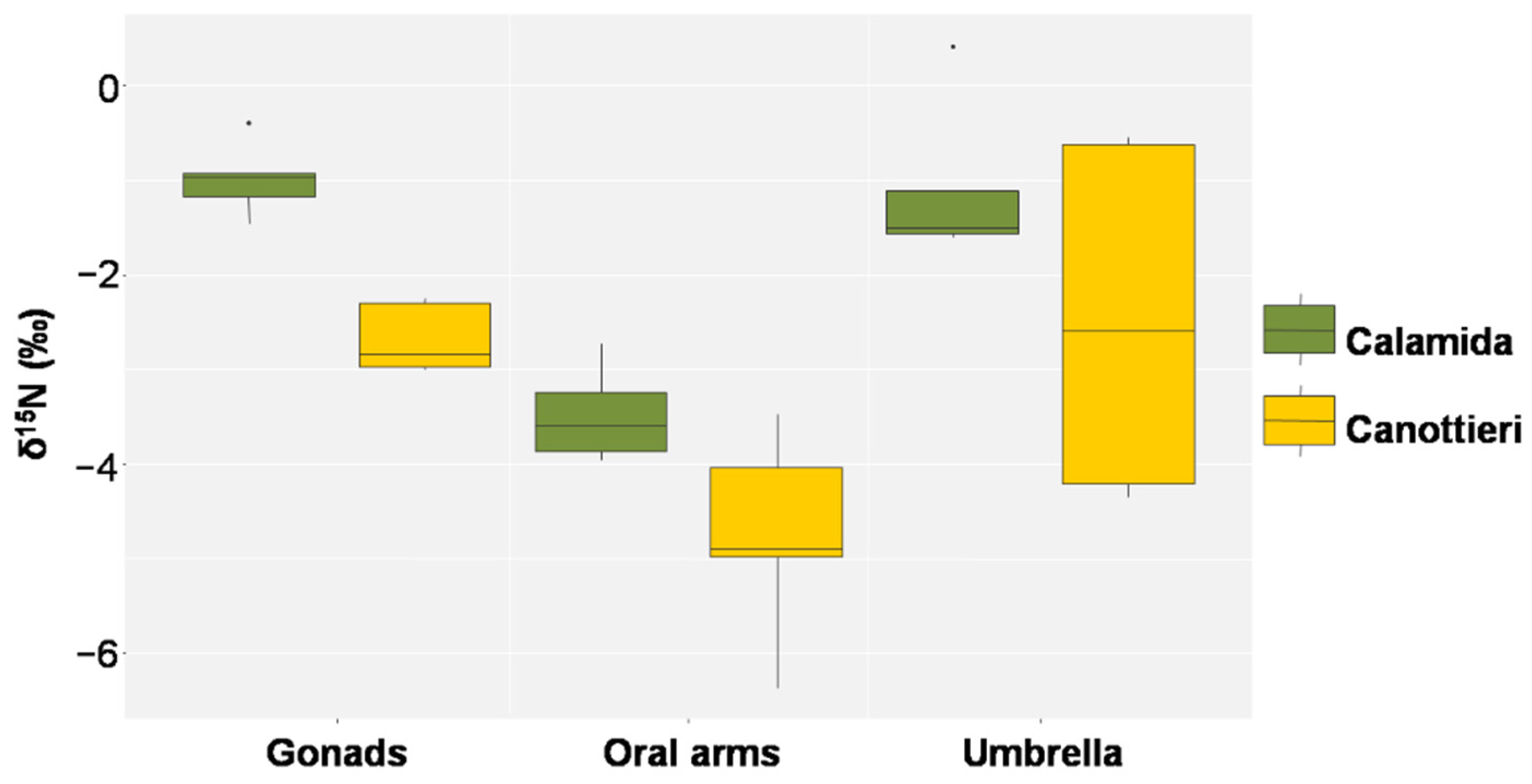

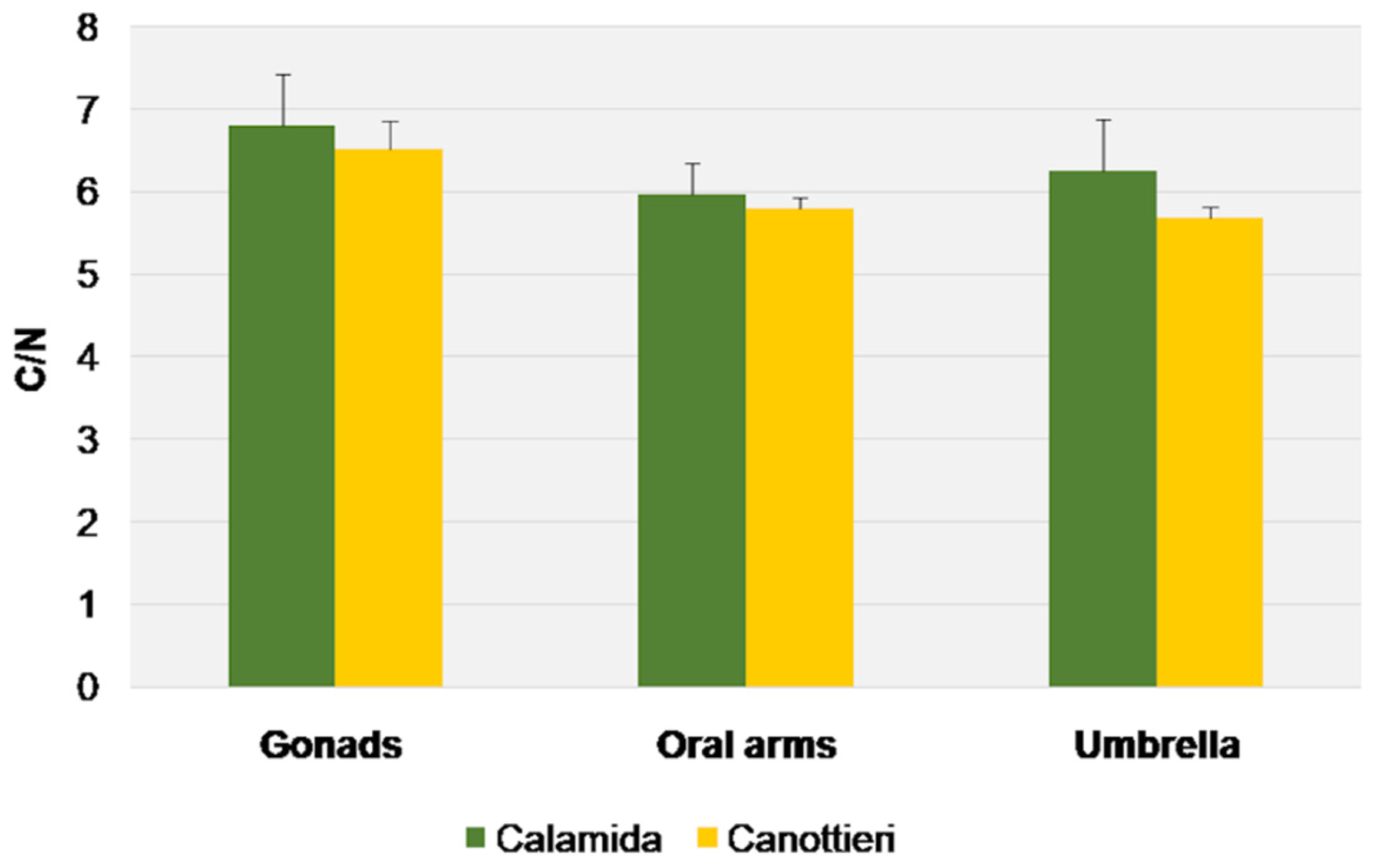
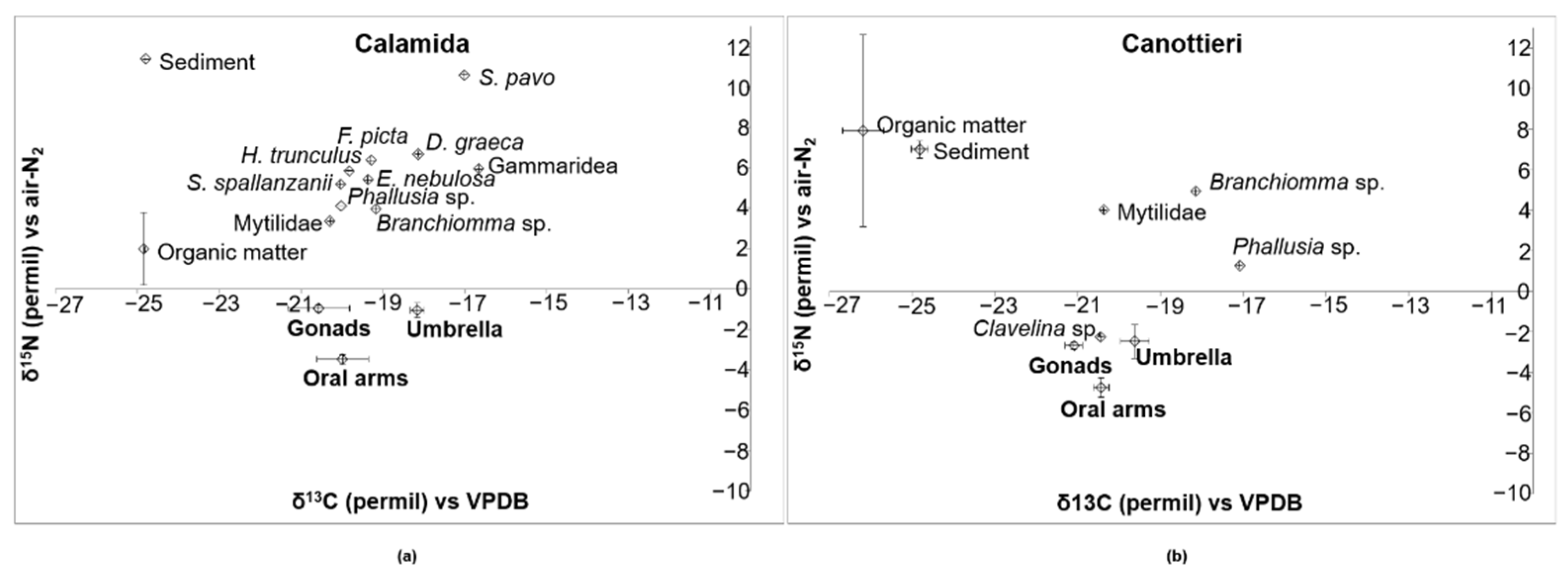
| Source | d.f. | MS | Pseudo-F | p-Value |
|---|---|---|---|---|
| SD | 3 | 0.51 | 0.764 | 0.57 |
| DR | 3 | 0.39 | 0.586 | 0.73 |
| SD × DR | 8 | 0.47 | 0.699 | 0.75 |
| SA | 2 | 0.88 | 1.342 | 0.24 |
| DR | 3 | 0.25 | 0.388 | 0.76 |
| SA | 2 | 0.12 | 0.193 | 0.78 |
| DR × SA | 3 | 0.08 | 0.133 | 0.93 |
| Moran’s Index | Expected Index | Variance | z-Score | p-Value | |
|---|---|---|---|---|---|
| Temperature (°C) | 0.89 | −0.02 | 0.006 | >2.58 | <0.01 |
| Salinity | 0.33 | −0.02 | 0.006 | >2.58 | <0.01 |
| Transparency (%) | 0.71 | −0.02 | 0.005 | >2.58 | <0.01 |
| Cold Spot | Hot Spot | z-Score | p-Value | |
|---|---|---|---|---|
| Temperature (°C) | 14.86 ± 1.96 | 21.52 ± 3.30 | >1.96 | <0.01 |
| Salinity | 35.07 ± 0.14 | 35.28 ± 0.16 | >1.96 | <0.01 |
| Transparency (%) | 93 ± 13 | 100 ± 0 | >1.96 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cillari, T.; Allegra, A.; Berto, D.; Bosch-Belmar, M.; Falautano, M.; Maggio, T.; Milisenda, G.; Perzia, P.; Rampazzo, F.; Sinopoli, M.; et al. Snapshot of the Distribution and Biology of Alien Jellyfish Cassiopea andromeda (Forsskål, 1775) in a Mediterranean Touristic Harbour. Biology 2022, 11, 319. https://doi.org/10.3390/biology11020319
Cillari T, Allegra A, Berto D, Bosch-Belmar M, Falautano M, Maggio T, Milisenda G, Perzia P, Rampazzo F, Sinopoli M, et al. Snapshot of the Distribution and Biology of Alien Jellyfish Cassiopea andromeda (Forsskål, 1775) in a Mediterranean Touristic Harbour. Biology. 2022; 11(2):319. https://doi.org/10.3390/biology11020319
Chicago/Turabian StyleCillari, Tiziana, Alessandro Allegra, Daniela Berto, Mar Bosch-Belmar, Manuela Falautano, Teresa Maggio, Giacomo Milisenda, Patrizia Perzia, Federico Rampazzo, Mauro Sinopoli, and et al. 2022. "Snapshot of the Distribution and Biology of Alien Jellyfish Cassiopea andromeda (Forsskål, 1775) in a Mediterranean Touristic Harbour" Biology 11, no. 2: 319. https://doi.org/10.3390/biology11020319
APA StyleCillari, T., Allegra, A., Berto, D., Bosch-Belmar, M., Falautano, M., Maggio, T., Milisenda, G., Perzia, P., Rampazzo, F., Sinopoli, M., & Castriota, L. (2022). Snapshot of the Distribution and Biology of Alien Jellyfish Cassiopea andromeda (Forsskål, 1775) in a Mediterranean Touristic Harbour. Biology, 11(2), 319. https://doi.org/10.3390/biology11020319







