Blue Laser Irradiation Decreases the ATP Level in Mouse Skin and Increases the Production of Superoxide Anion and Hypochlorous Acid in Mouse Fibroblasts
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Measurement of the ATP Levels in Mouse Skin
2.2. Cell Culture
2.3. Fluorescent Probes for Detecting ROS and RNS
2.4. Laser Irradiation
2.5. Fluorescence Analyses
2.6. MTT Assay
2.7. Statistical Analysis
3. Results
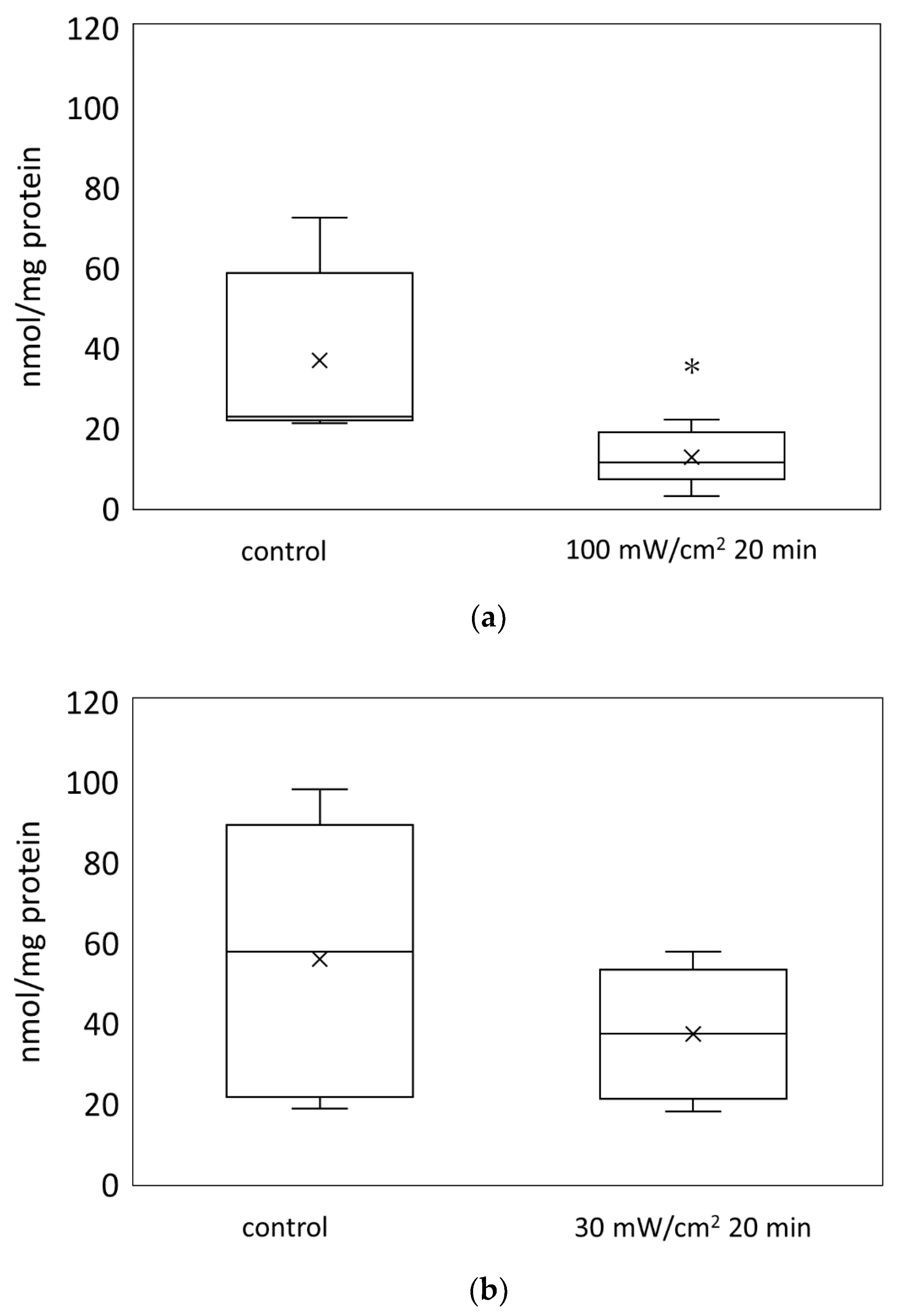
3.1. Blue Laser Irradiation Reduced the ATP Levels in the Mouse Skin
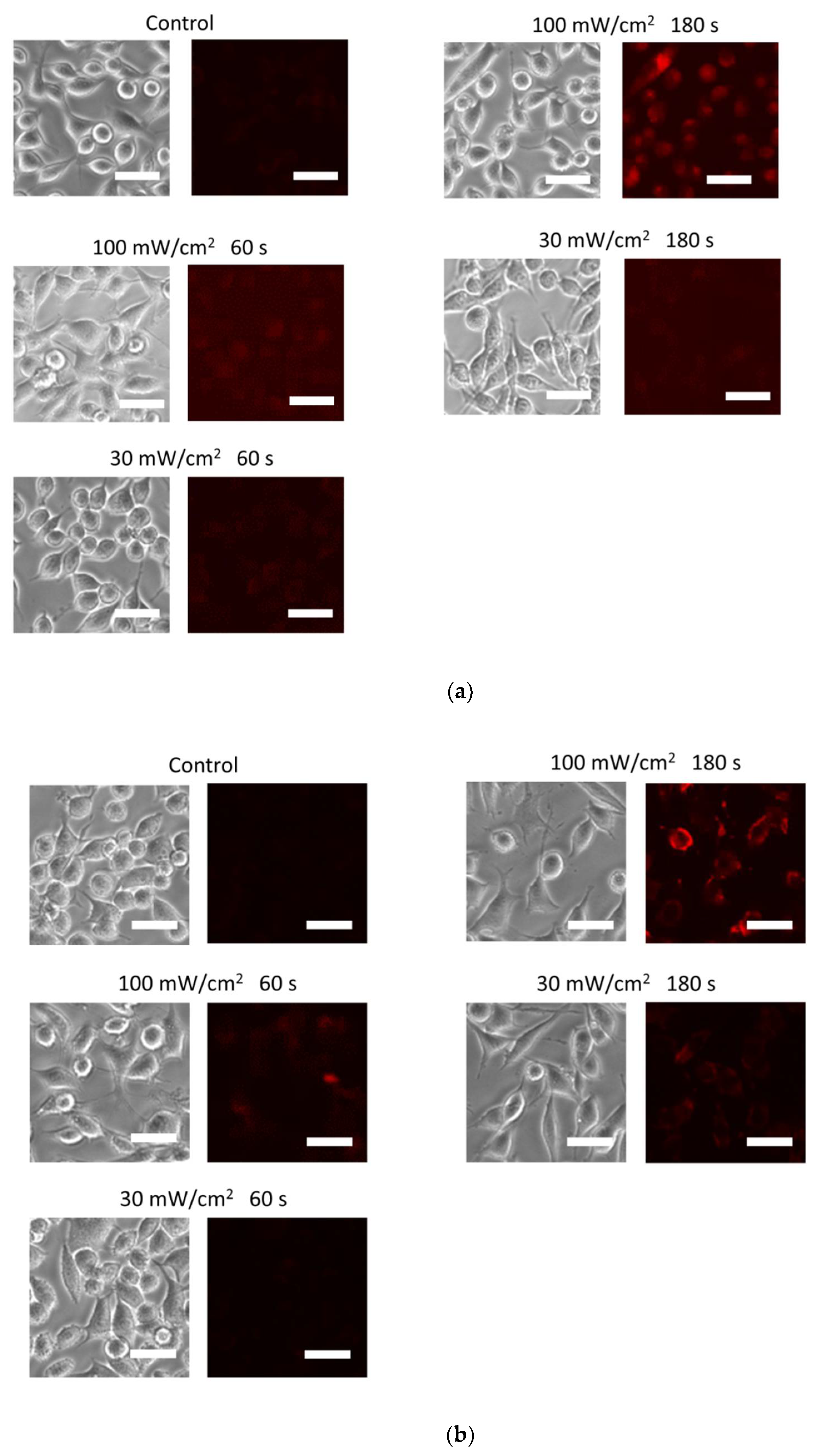
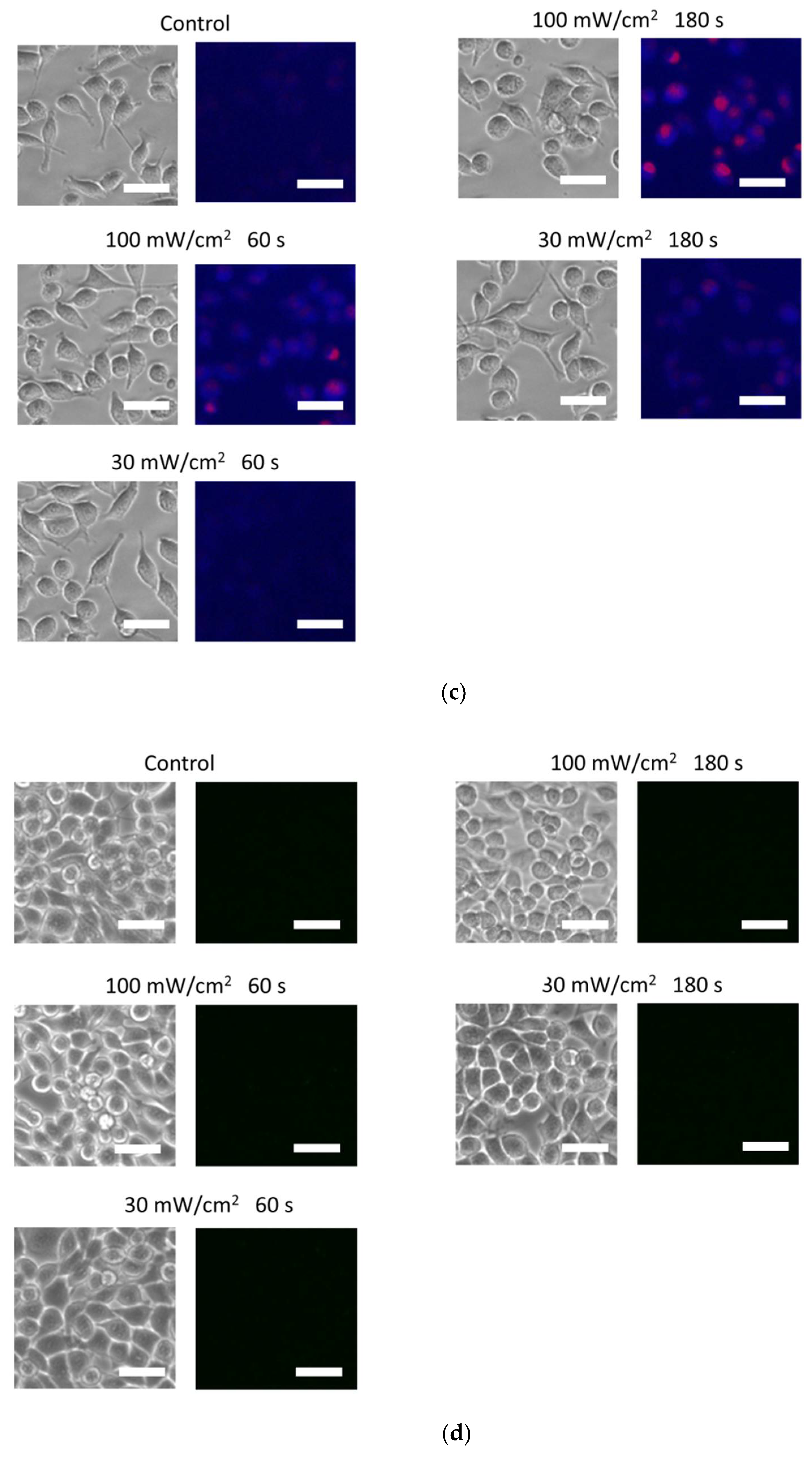
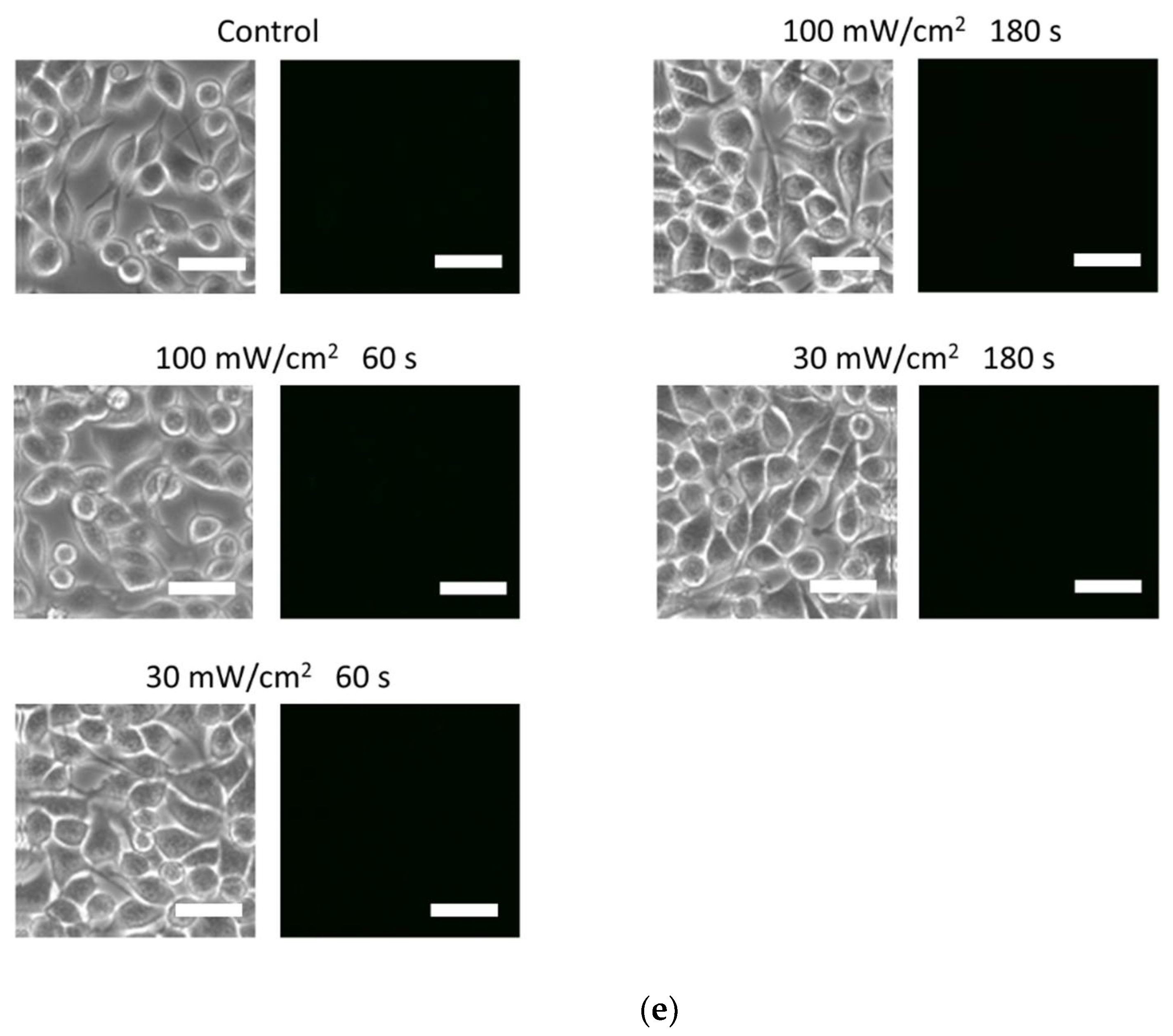
3.2. Blue Laser Irradiation Induces the Generation of O2−• and HClO, but Not the Generation of NO• and ONOO−
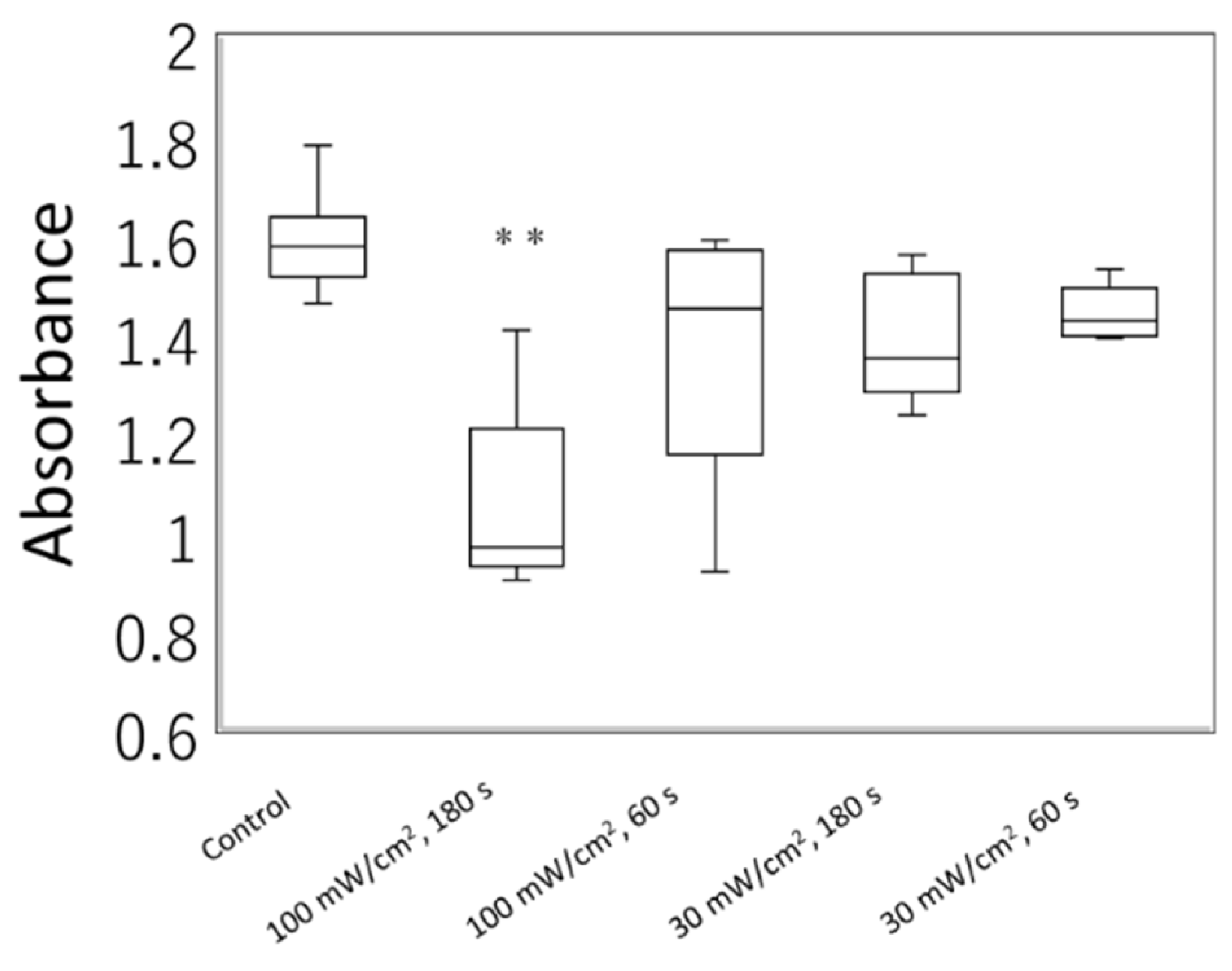
3.3. Irradiation of a Blue Laser Reduces Cell Viability of L929 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Avci, P.; Gupta, A.; Sadasivam, M.; Vecchio, D.; Pam, Z.; Pam, N.; Hamblin, M.R. Low-level laser (light) therapy (LLLT) in skin: Stimulating, healing, restoring. Semin. Cutan. Med. Surg. 2013, 32, 41–52. [Google Scholar]
- Scott, A.M.; Stehlik, P.; Clark, J.; Zhang, D.; Yang, Z.; Hoffmann, T.; Mar, C.D.; Glasziou, P. Blue-Light Therapy for Acne Vulgaris: A Systematic Review and Meta-Analysis. Ann. Fam. Med. 2019, 17, 545–553. [Google Scholar] [CrossRef] [Green Version]
- Pfaff, S.; Liebmann, J.; Born, M.; Merk, H.F.; von Felbert, V. Prospective Randomized Long-Term Study on the Efficacy and Safety of UV-Free Blue Light for Treating Mild Psoriasis Vulgaris. Dermatology 2015, 231, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, F.; Yoshida, A. Effects of blue-light irradiation during dental treatment. Jpn. Dent. Sci. Rev. 2018, 54, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; You, C.E.; Park, M.Y. Blue and red light combination LED phototherapy for acne vulgaris in patients with skin phototype IV. Lasers Surg. Med. 2007, 39, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Elman, M.; Lebzelter, J. Light therapy in the treatment of acne vulgaris. Dermatol. Surg. 2004, 30, 139–146. [Google Scholar] [CrossRef]
- Sadowska, M.; Narbutt, J.; Lesiak, A. Blue Light in Dermatology. Life 2021, 11, 670. [Google Scholar] [CrossRef]
- Yoshida, A.; Yoshino, F.; Makita, T.; Maehata, Y.; Higashi, K.; Miyamoto, C.; Wada-Takahashi, S.; Takahashi, S.S.; Takahashi, O.; Lee, M.C. Reactive oxygen species production in mitochondria of human gingival fibroblast induced by blue light irradiation. J. Photochem. Photobiol. B 2013, 129, 1–5. [Google Scholar] [CrossRef]
- Nakashima, Y.; Ohta, S.; Wolf, A.M. Blue light-induced oxidative stress in live skin. Free Radic. Biol. Med. 2017, 108, 300–310. [Google Scholar] [CrossRef]
- Hamblin, M.R. Mechanisms and Mitochondrial Redox Signaling in Photobiomodulation. Photochem. Photobiol. 2018, 94, 199–212. [Google Scholar] [CrossRef] [Green Version]
- Nakanishi-Ueda, T.; Majima, H.J.; Watanabe, K.; Ueda, T.; Indo, H.P.; Suenaga, S.; Hisamitsu, T.; Ozawa, T.; Yasuhara, H.; Koide, R. Blue LED light exposure develops intracellular reactive oxygen species, lipid peroxidation, and subsequent cellular injuries in cultured bovine retinal pigment epithelial cells. Free Radic. Res. 2013, 47, 774–780. [Google Scholar] [CrossRef]
- Arthaut, L.D.; Jourdan, N.; Mteyrek, A.; Procopio, M.; El-Esawi, M.; d’Harlingue, A.; Bouchet, P.E.; Witczak, J.; Ritz, T.; Klarsfeld, A.; et al. Blue-light induced accumulation of reactive oxygen species is a consequence of the Drosophila cryptochrome photocycle. PLoS ONE 2017, 12, e0171836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudryavtseva, A.V.; Krasnov, G.S.; Dmitriev, A.A.; Alekseev, B.Y.; Kardymon, O.L.; Sadritdinova, A.F.; Fedorova, M.S.; Pokrovsky, A.V.; Melnikova, N.V.; Kaprin, A.D.; et al. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget 2016, 7, 44879–44905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karu, T.I. Mitochondrial signaling in mammalian cells activated by red and near-IR radiation. Photochem. Photobiol. 2008, 84, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.; Franco, M.C.; Estevez, A.G. Reactive nitrogen species in cellular signaling. Exp. Biol. Med. (Maywood) 2015, 240, 711–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Colavitti, R.; Rovira, I.I.; Finkel, T. Redox-dependent transcriptional regulation. Circ. Res. 2005, 97, 967–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, X.; Xing, D. Molecular mechanisms of cell proliferation induced by low power laser irradiation. J. Biomed. Sci. 2009, 16, 4. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.Y.; Chen, C.H.; Wang, C.Z.; Ho, M.L.; Yeh, M.L.; Wang, Y.H. Low-power laser irradiation suppresses inflammatory response of human adipose-derived stem cells by modulating intracellular cyclic AMP level and NF-kappaB activity. PLoS ONE 2013, 8, e54067. [Google Scholar] [CrossRef]
- Jagdeo, J.; Austin, E.; Mamalis, A.; Wong, C.; Ho, D.; Siegel, D.M. Light-emitting diodes in dermatology: A systematic review of randomized controlled trials. Lasers Surg. Med. 2018, 50, 613–628. [Google Scholar] [CrossRef]
- Diogo, M.L.G.; Campos, T.M.; Fonseca, E.S.R.; Pavani, C.; Horliana, A.; Fernandes, K.P.S.; Bussadori, S.K.; Fantin, F.; Leite, D.P.V.; Yamamoto, A.T.A.; et al. Effect of Blue Light on Acne Vulgaris: A Systematic Review. Sensors 2021, 21, 6943. [Google Scholar] [CrossRef]
- Kushibiki, T.; Hirasawa, T.; Okawa, S.; Ishihara, M. Blue laser irradiation generates intracellular reactive oxygen species in various types of cells. Photomed. Laser Surg. 2013, 31, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Kushibiki, T.; Hirasawa, T.; Okawa, S.; Ishihara, M. Low Reactive Level Laser Therapy for Mesenchymal Stromal Cells Therapies. Stem Cells Int. 2015, 2015, 974864. [Google Scholar] [CrossRef]
- Park, C.; Cha, H.J.; Hong, S.H.; Kim, G.Y.; Kim, S.; Kim, H.S.; Kim, B.W.; Jeon, Y.J.; Choi, Y.H. Protective Effect of Phloroglucinol on Oxidative Stress-Induced DNA Damage and Apoptosis through Activation of the Nrf2/HO-1 Signaling Pathway in HaCaT Human Keratinocytes. Mar. Drugs 2019, 17, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, D.; Fu, C.; Xiao, J.; Zhang, Z.; Zou, J.; Ye, Z.; Zhang, X. SGK1 Attenuates Oxidative Stress-Induced Renal Tubular Epithelial Cell Injury by Regulating Mitochondrial Function. Oxid. Med. Cell. Longev. 2019, 2019, 2013594. [Google Scholar] [CrossRef]
- Cui, L.; Li, Z.; Chang, X.; Cong, G.; Hao, L. Quercetin attenuates vascular calcification by inhibiting oxidative stress and mitochondrial fission. Vasc. Pharmacol. 2017, 88, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Al Musawi, M.S.; Al-Gailani, B.T. ATP level in red blood cells improves by altering the low-level DPSS laser irradiation condition. Appl. Nanosc. 2021. [Google Scholar] [CrossRef]
- Buscone, S.; Mardaryev, A.N.; Raafs, B.; Bikker, J.W.; Sticht, C.; Gretz, N.; Farjo, N.; Uzunbajakava, N.E.; Botchkareva, N.V. A new path in defining light parameters for hair growth: Discovery and modulation of photoreceptors in human hair follicle. Lasers Surg. Med. 2017, 49, 705–718. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Branicky, R.; Noe, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Hawkins, C.L.; Pattison, D.I.; Davies, M.J. Hypochlorite-induced oxidation of amino acids, peptides and proteins. Amino Acids 2003, 25, 259–274. [Google Scholar] [CrossRef]
- Lindgard, A.; Hulten, L.M.; Svensson, L.; Soussi, B. Irradiation at 634 nm releases nitric oxide from human monocytes. Lasers Med. Sci. 2007, 22, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, M.; Migliario, M.; Tonello, S.; Rocchetti, V.; Reno, F. Photobiomodulation induces in vitro re-epithelialization via nitric oxide production. Lasers Med. Sci. 2018, 33, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Mio, Y.; Pratt, P.F.; Lohr, N.; Warltier, D.C.; Whelan, H.T.; Zhu, D.; Jacobs, E.R.; Medhora, M.; Bienengraeber, M. Near infrared light protects cardiomyocytes from hypoxia and reoxygenation injury by a nitric oxide dependent mechanism. J. Mol. Cell. Cardiol. 2009, 46, 4–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oplander, C.; Hidding, S.; Werners, F.B.; Born, M.; Pallua, N.; Suschek, C.V. Effects of blue light irradiation on human dermal fibroblasts. J. Photochem. Photobiol. B 2011, 103, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Karu, T.I.; Pyatibrat, L.V.; Afanasyeva, N.I. Cellular effects of low power laser therapy can be mediated by nitric oxide. Lasers Surg. Med. 2005, 36, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Dai, T.; Sharma, S.K.; Huang, Y.Y.; Carroll, J.D.; Hamblin, M.R. The nuts and bolts of low-level laser (light) therapy. Ann. Biomed. Eng. 2012, 40, 516–533. [Google Scholar] [CrossRef] [Green Version]
- Nauser, T.; Koppenol, W. The Rate Constant of the Reaction of Superoxide with Nitrogen Monoxide: Approaching the Diffusion Limit. J. Phys. Chem. A 2002, 106, 4084–4086. [Google Scholar] [CrossRef]
- Terakita, A. The opsins. Genome Biol. 2005, 6, 213. [Google Scholar] [CrossRef] [Green Version]
- Castellano-Pellicena, I.; Uzunbajakava, N.E.; Mignon, C.; Raafs, B.; Botchkarev, V.A.; Thornton, M.J. Does blue light restore human epidermal barrier function via activation of Opsin during cutaneous wound healing? Lasers Surg. Med. 2019, 51, 370–382. [Google Scholar] [CrossRef] [Green Version]
- Poletini, M.O.; Moraes, M.N.; Ramos, B.C.; Jeronimo, R.; Castrucci, A.M. TRP channels: A missing bond in the entrainment mechanism of peripheral clocks throughout evolution. Temperature (Austin) 2015, 2, 522–534. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Huang, Y.Y.; Wang, Y.; Lyu, P.; Hamblin, M.R. Red (660 nm) or near-infrared (810 nm) photobiomodulation stimulates, while blue (415 nm), green (540 nm) light inhibits proliferation in human adipose-derived stem cells. Sci. Rep. 2017, 7, 7781. [Google Scholar] [CrossRef]
- Swartz, T.E.; Corchnoy, S.B.; Christie, J.M.; Lewis, J.W.; Szundi, I.; Briggs, W.R.; Bogomolni, R.A. The photocycle of a flavin-binding domain of the blue light photoreceptor phototropin. J. Biol. Chem. 2001, 276, 36493–36500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.Y.; Chang, C.J.; Chen, L.Y. Blue light induced reactive oxygen species from flavin mononucleotide and flavin adenine dinucleotide on lethality of HeLa cells. J. Photochem. Photobiol. B 2017, 173, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Shu, M.; Kuo, S.; Wang, Y.; Jiang, Y.; Liu, Y.T.; Gallo, R.L.; Huang, C.M. Porphyrin metabolisms in human skin commensal Propionibacterium acnes bacteria: Potential application to monitor human radiation risk. Curr. Med. Chem. 2013, 20, 562–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsolekile, N.; Nelana, S.; Oluwafemi, O.S. Porphyrin as Diagnostic and Therapeutic Agent. Molecules 2019, 24, 2669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krammer, B.; Plaetzer, K. ALA and its clinical impact, from bench to bedside. Photochem. Photobiol. Sci. 2008, 7, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Amin, R.M.; Bhayana, B.; Hamblin, M.R.; Dai, T. Antimicrobial blue light inactivation of Pseudomonas aeruginosa by photo-excitation of endogenous porphyrins: In vitro and in vivo studies. Lasers Surg. Med. 2016, 48, 562–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imlay, J.A. Pathways of oxidative damage. Annu. Rev. Microbiol. 2003, 57, 395–418. [Google Scholar] [CrossRef]
- Buettner, G.R. Superoxide dismutase in redox biology: The roles of superoxide and hydrogen peroxide. Anticancer Agents Med. Chem. 2011, 11, 341–346. [Google Scholar] [CrossRef]
- Wiecek, M.; Szymura, J.; Maciejczyk, M.; Kantorowicz, M.; Szygula, Z. Anaerobic Exercise-Induced Activation of Antioxidant Enzymes in the Blood of Women and Men. Front. Physiol. 2018, 9, 1006. [Google Scholar] [CrossRef]
- Roehlecke, C.; Schumann, U.; Ader, M.; Brunssen, C.; Bramke, S.; Morawietz, H.; Funk, R.H. Stress reaction in outer segments of photoreceptors after blue light irradiation. PLoS ONE 2013, 8, e71570. [Google Scholar] [CrossRef] [PubMed]
- Zielonka, J.; Kalyanaraman, B. Hydroethidine- and MitoSOX-derived red fluorescence is not a reliable indicator of intracellular superoxide formation: Another inconvenient truth. Free Radic. Biol. Med. 2010, 48, 983–1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Rogers, S.C.; Kavdia, M. Analysis of kinetics of dihydroethidium fluorescence with superoxide using xanthine oxidase and hypoxanthine assay. Ann. Biomed. Eng. 2013, 41, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, A.; Shiotsu-Ogura, Y.; Wada-Takahashi, S.; Takahashi, S.S.; Toyama, T.; Yoshino, F. Blue light irradiation-induced oxidative stress in vivo via ROS generation in rat gingival tissue. J. Photochem. Photobiol. B 2015, 151, 48–53. [Google Scholar] [CrossRef]
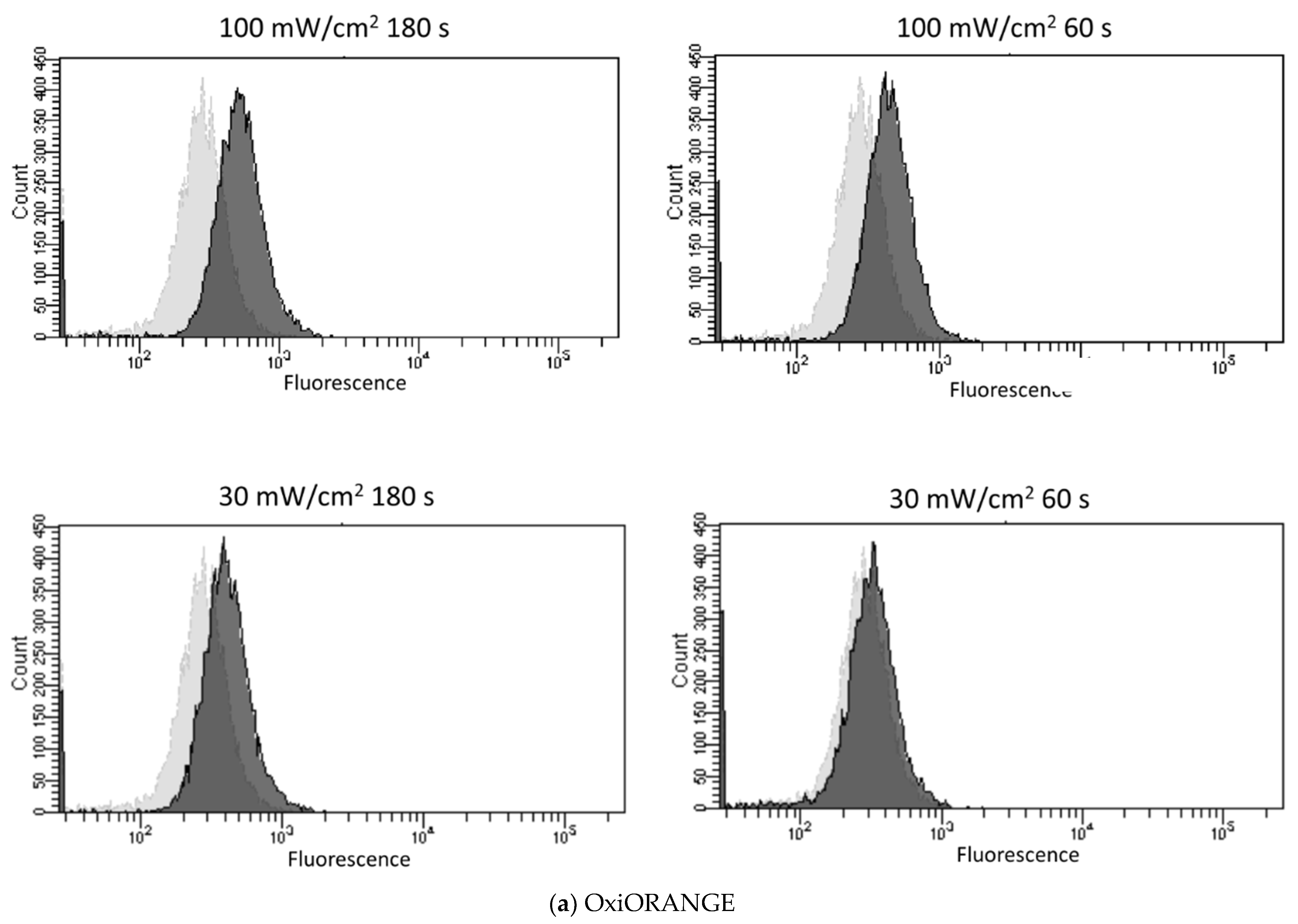
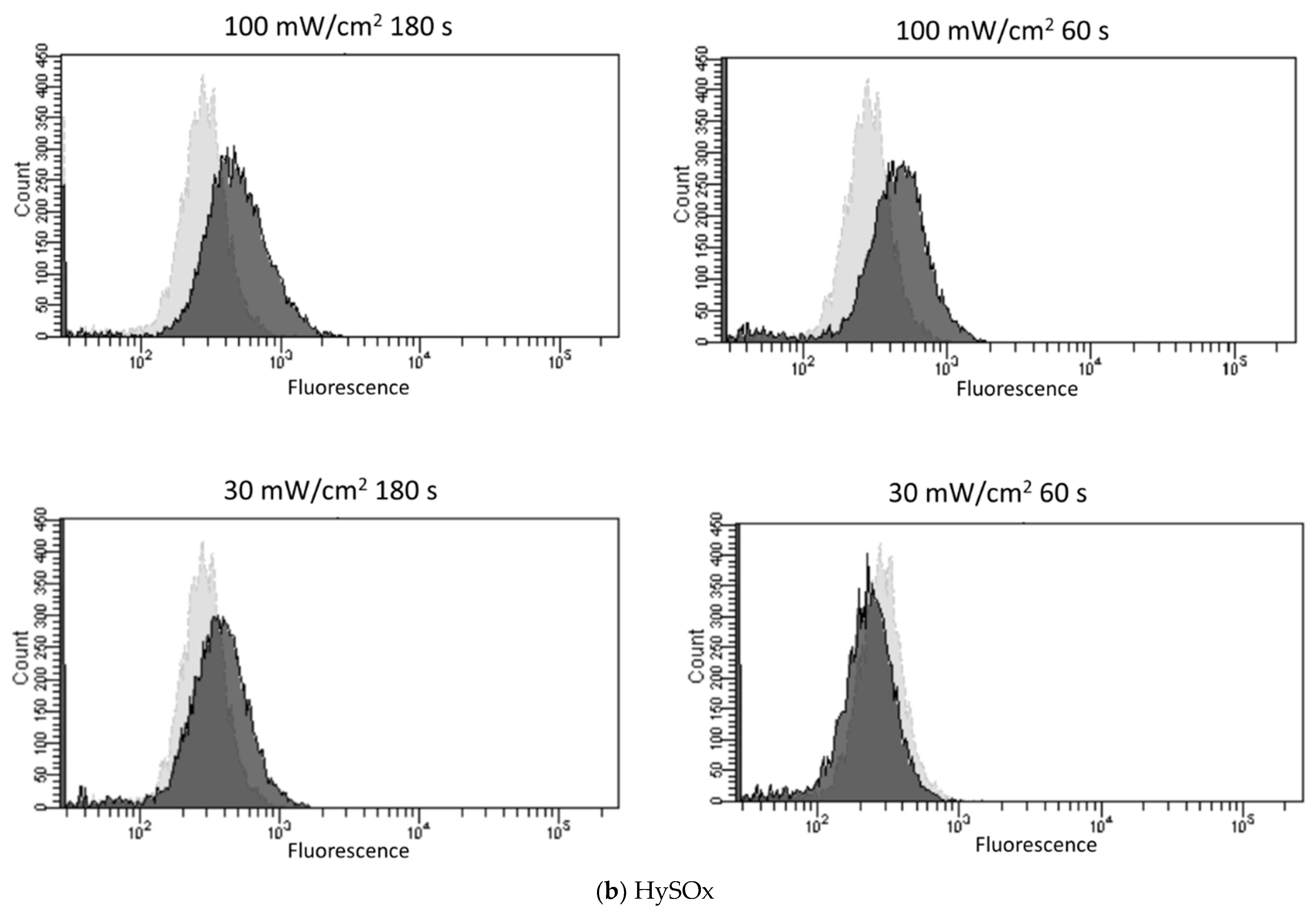
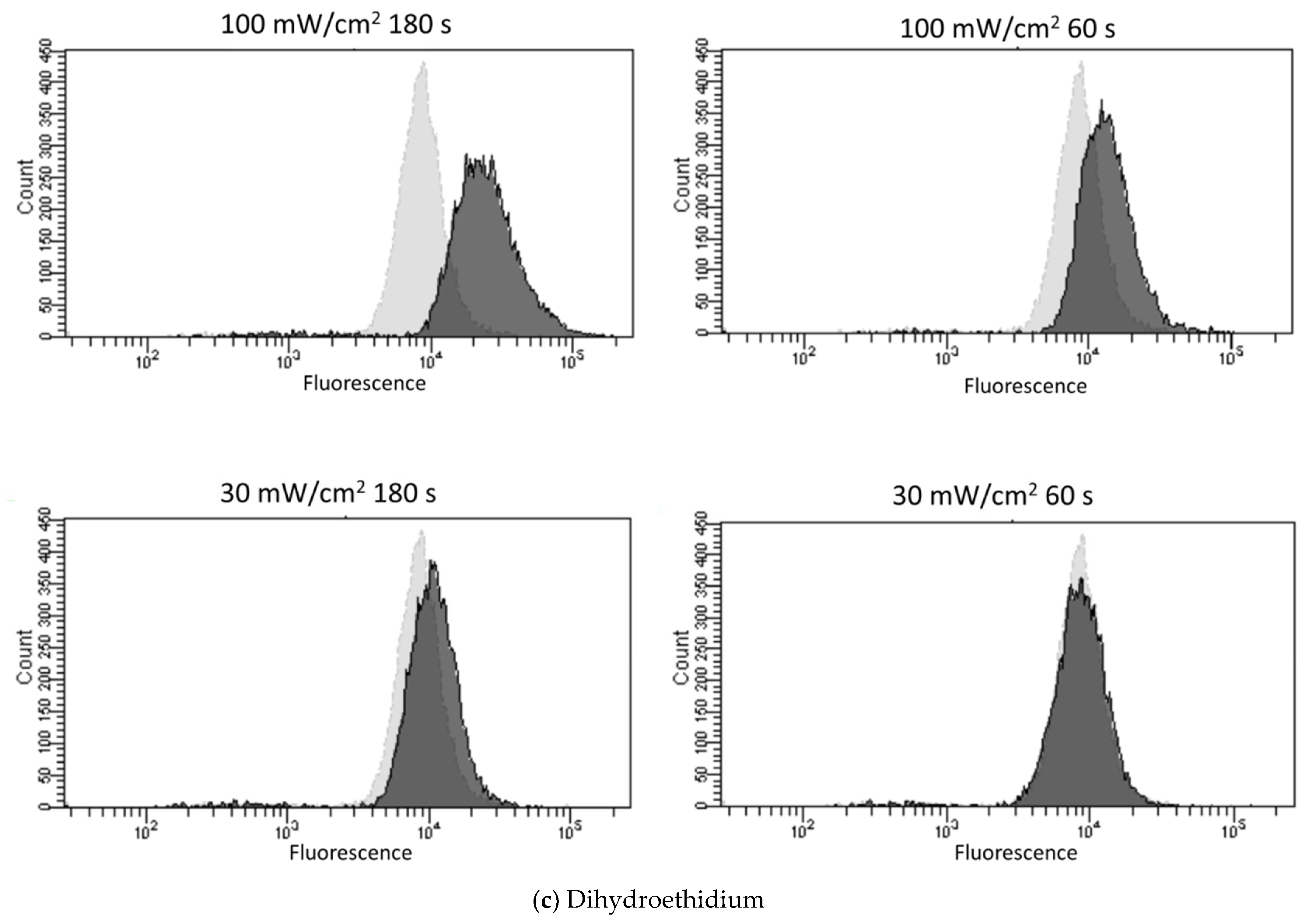
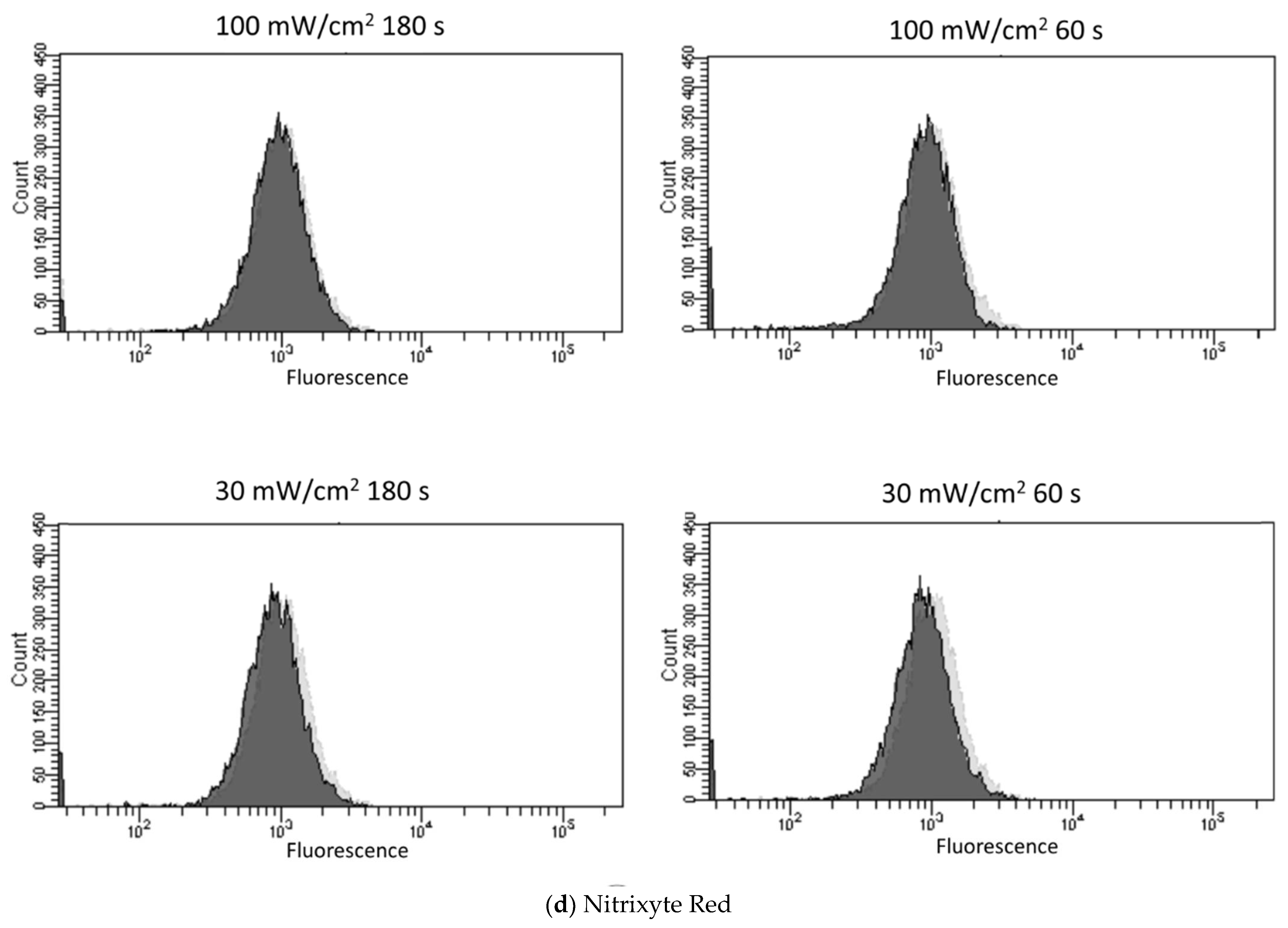
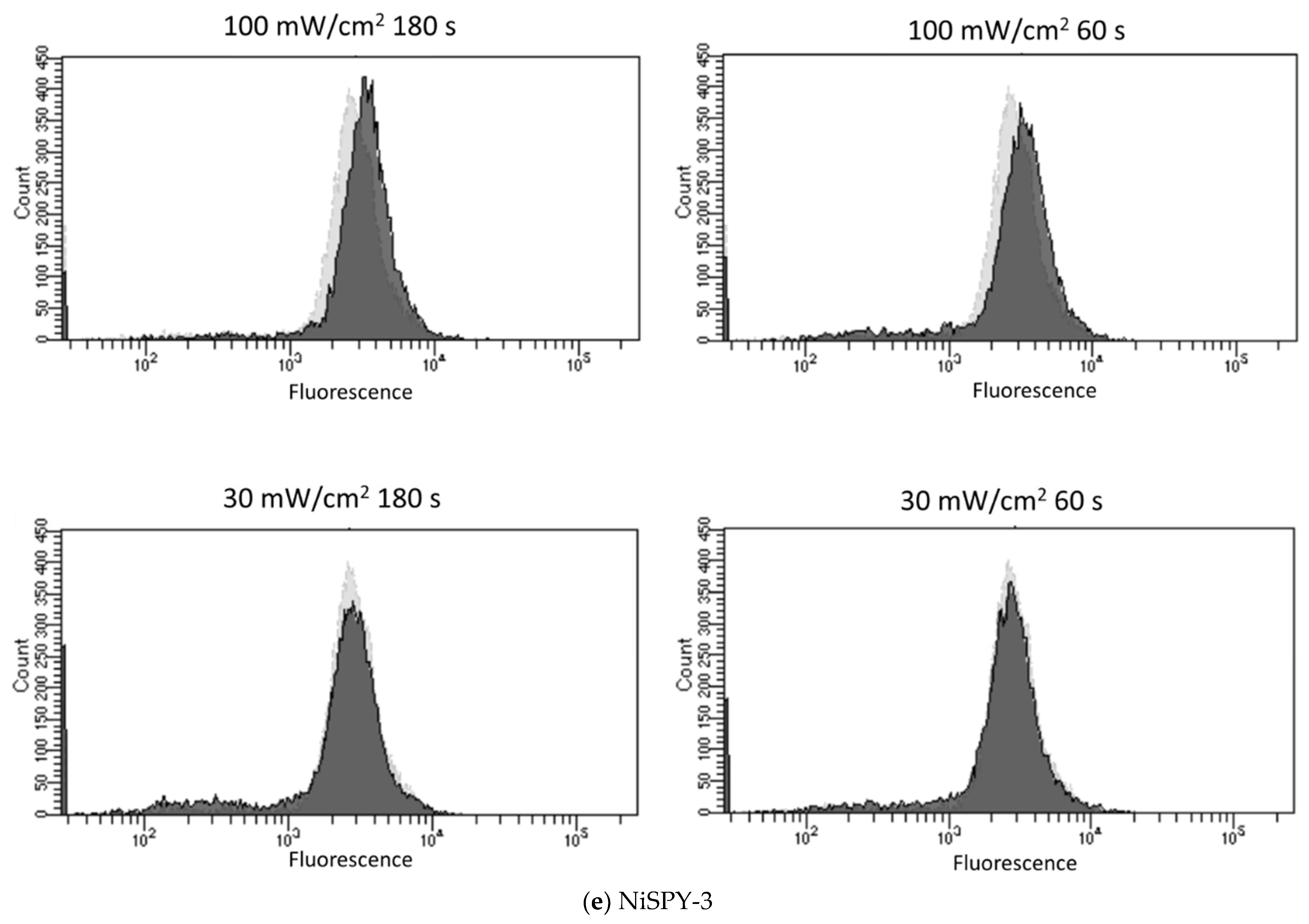
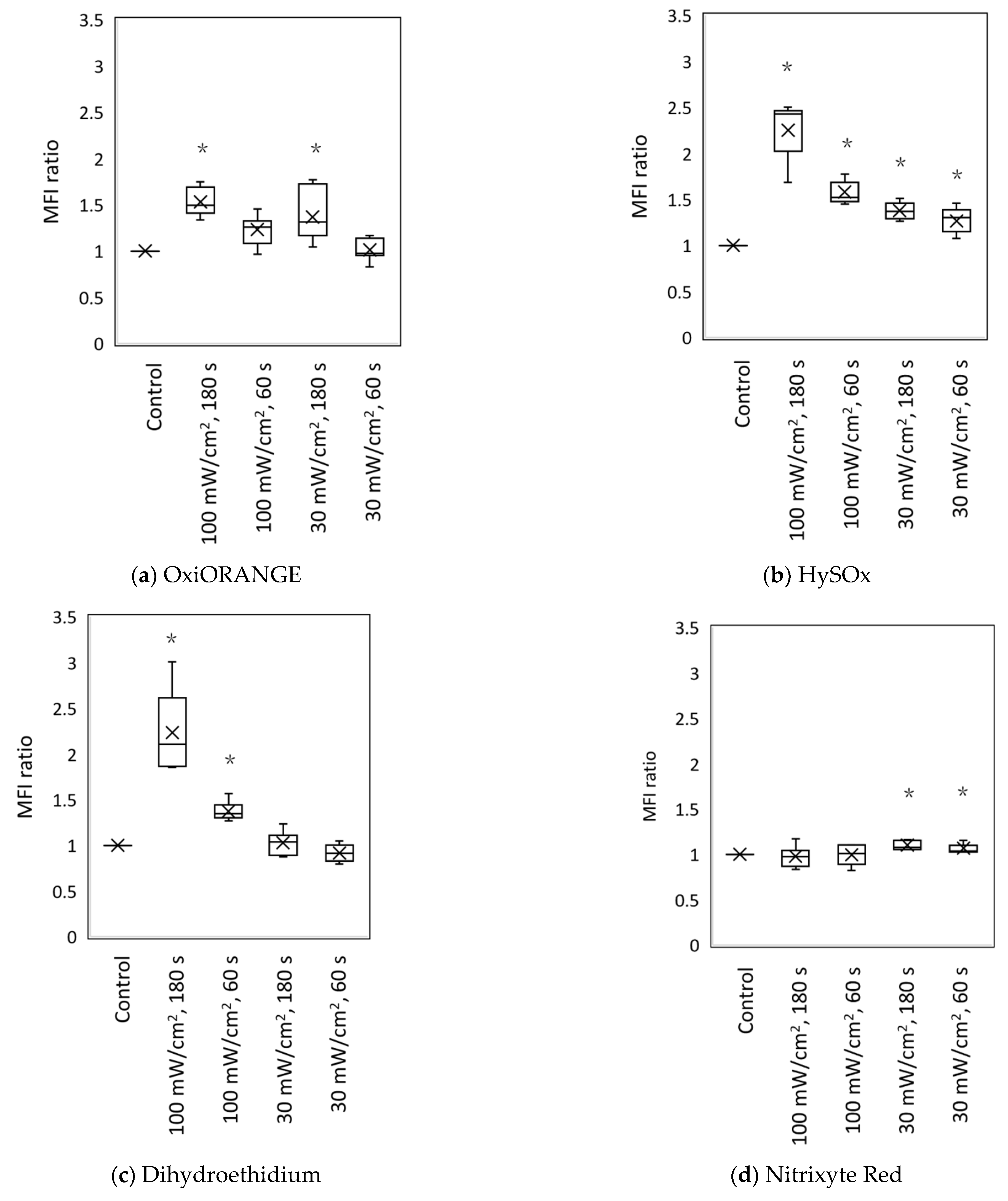
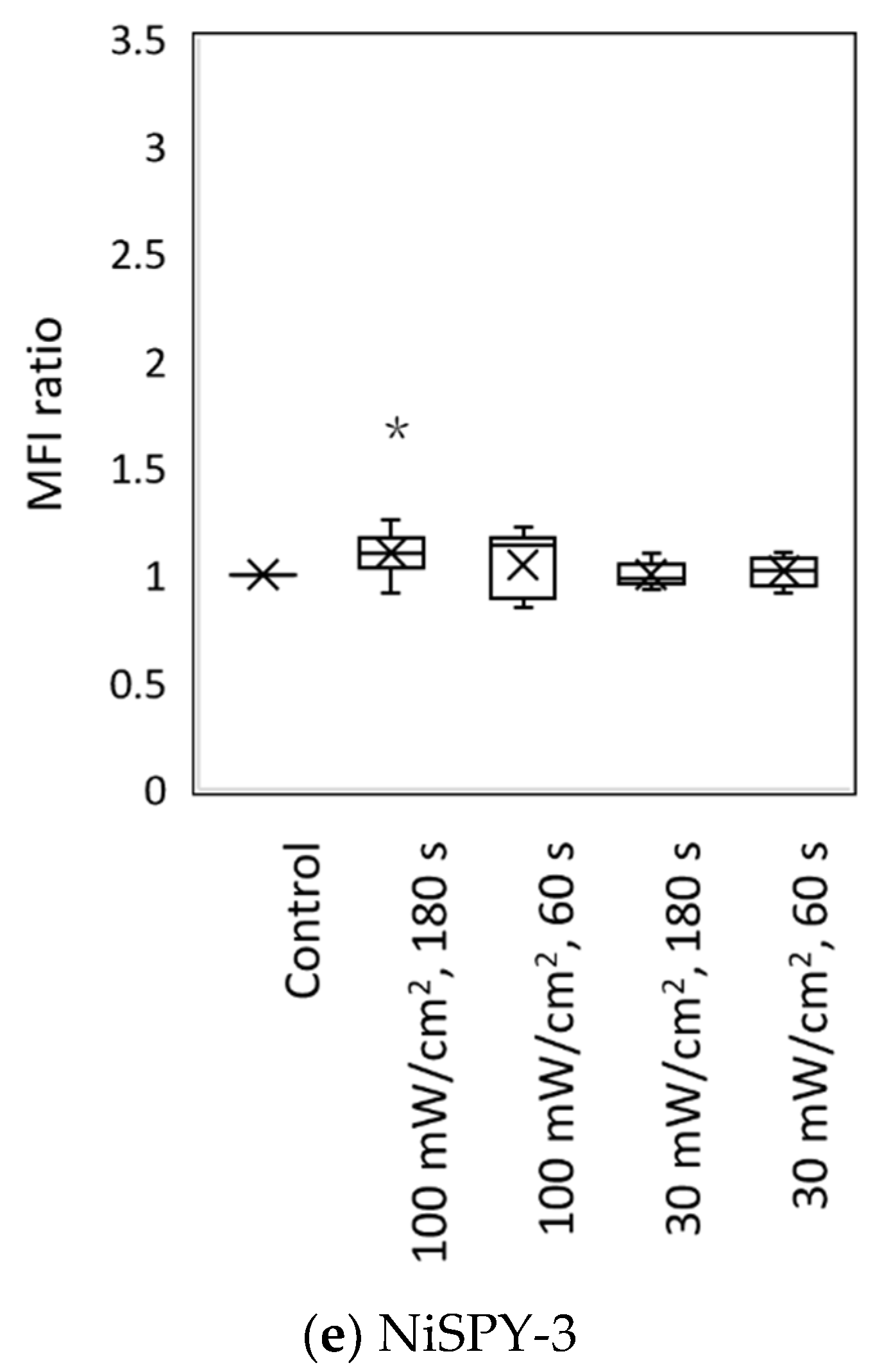
| Fluorescent Probe | Excitation (nm)/Emission (nm) | Detectable ROS and RNS a | Final Concentration | Incubation Time (min) | Positive Control Concentration | Supplier |
|---|---|---|---|---|---|---|
| OxiORANGE | 543/577 | •OH, HClO | 1 μM | 20 | H2O2 500 μM | Goryo Chemical, Hokkaido, Japan |
| HySOx | 555/575 | HClO | 5 μM | 30 | NaOCl 5 μM | Goryo Chemical |
| Dihydroethidium | 518/606 | O2−• | 10 μM | 30 | Antimycin A 10 mM | Thermo Fisher Scientific |
| Nitrixyte Red | 630/660 | NO• | 1× b | 30 | NONOate 50 mM | AAT Bioquest, Sunnyvale, CA, USA |
| NiSPY-3 | 490/515 | ONOO− | 10 μM | 30 | Peroxynitrite 20 µM | Goryo Chemical |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakayama, E.; Kushibiki, T.; Mayumi, Y.; Azuma, R.; Ishihara, M.; Kiyosawa, T. Blue Laser Irradiation Decreases the ATP Level in Mouse Skin and Increases the Production of Superoxide Anion and Hypochlorous Acid in Mouse Fibroblasts. Biology 2022, 11, 301. https://doi.org/10.3390/biology11020301
Nakayama E, Kushibiki T, Mayumi Y, Azuma R, Ishihara M, Kiyosawa T. Blue Laser Irradiation Decreases the ATP Level in Mouse Skin and Increases the Production of Superoxide Anion and Hypochlorous Acid in Mouse Fibroblasts. Biology. 2022; 11(2):301. https://doi.org/10.3390/biology11020301
Chicago/Turabian StyleNakayama, Eiko, Toshihiro Kushibiki, Yoshine Mayumi, Ryuichi Azuma, Miya Ishihara, and Tomoharu Kiyosawa. 2022. "Blue Laser Irradiation Decreases the ATP Level in Mouse Skin and Increases the Production of Superoxide Anion and Hypochlorous Acid in Mouse Fibroblasts" Biology 11, no. 2: 301. https://doi.org/10.3390/biology11020301
APA StyleNakayama, E., Kushibiki, T., Mayumi, Y., Azuma, R., Ishihara, M., & Kiyosawa, T. (2022). Blue Laser Irradiation Decreases the ATP Level in Mouse Skin and Increases the Production of Superoxide Anion and Hypochlorous Acid in Mouse Fibroblasts. Biology, 11(2), 301. https://doi.org/10.3390/biology11020301






