Vibrating Exercise Equipment in Middle-Age and Older Women with Chronic Low Back Pain and Effects on Bioelectrical Activity, Range of Motion and Pain Intensity: A Randomized, Single-Blinded Sham Intervention Study
Abstract
:Simple Summary
Abstract
1. Introduction
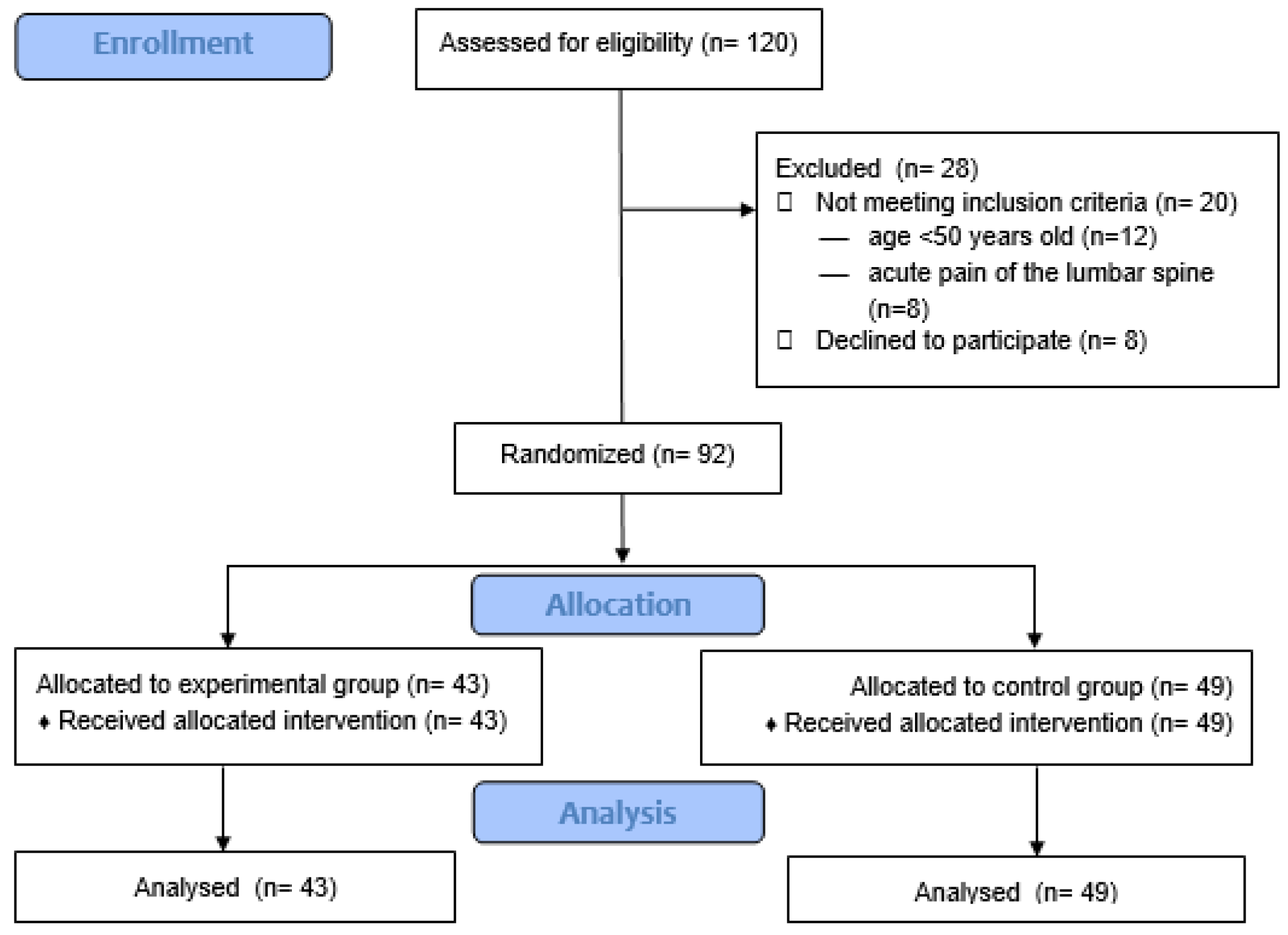
2. Materials and Methods
2.1. Study Methodology
2.2. Study Participants
2.3. Methods
2.4. Intervention
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Participants
3.2. Erector Spinae sEMG Results
3.3. Lumbar ROM Results
3.4. Pain Scores (VAS)
4. Discussion
4.1. Erector Spinae sEMG
4.2. Lumbar ROM
4.3. Pain Scores (VAS)
4.4. Clinical Implications
4.5. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| Experimental Group | Control Group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lumbar ROM Before | Lumbar ROM After | Lumbar ROM Before | Lumbar ROM After | ||||||
| rs | p-Value | rs | p-Value | rs | p-Value | rs | p-Value | ||
| Before | Baseline | 0.239019 | 0.102 | 0.123192 | 0.404 | 0.198600 | 0.2017 | 0.109459 | 0.4847 |
| Functional | 0.106851 | 0.470 | 0.189222 | 0.198 | −0.089447 | 0.5684 | 0.088912 | 0.1571 | |
| After | Baseline | 0.086178 | 0.560 | −0.057862 | 0.696 | 0.190182 | 0.2219 | 0.003511 | 0.9822 |
| Functional | 0.005431 | 0.971 | −0.008733 | 0.953 | 0.011753 | 0.9404 | 0.050751 | 0.7464 | |
| rs = Spearman’s correlation coefficient | |||||||||
| Course of the Class | Name and Description of Exercise | Time (min) |
|---|---|---|
| Welcome and introduction | The group is welcomed and the equipment is distributed. The participants are asked about their wellbeing and pain level, Participants are reminded of the principles of using the equipment, and the importance of maintaining correct and normal breathing during exercising. | 5 |
| Warm-up 5 min | Starting position (SP) = Participants stand with their arms by their sides. Shoulder circles are performed forward and backward. | 1 |
| SP as above, with shoulder circles forward and backward, combined with a light stepping motion of the feet | 1 | |
| SP as above, marching the feet, swinging each alternate arm forward and backward. | 1 | |
| SP as above, marching the feet, with parallel arm movement forward and backward. | 1 | |
| SP with the arms by the sides holding the Smovey in each hand. The hands are moved inward and outward | 1 | |
| Main part 32 min | SP as above, marching (four steps forward, four steps backward) with alternate arm movements and with lifting the knees up on every fourth step. | 2 |
| SP as above, stepping out to the side with alternating abduction of each arm to the side. | 2 | |
| SP as above, raising each alternate leg upward, with alternating arm movement to the side. | 2 | |
| SP as above, with a step with raised knees, with movement of the arms up and down on the outside. | 2 | |
| SP as above, stepping with alternating arm movement front to back. | 2 | |
| SP as above, stepping with alternate arm movement forward and backward. | 2 | |
| SP as above, stepping forward and backward with weight transfer, with arms moving forward and backward, crossing at the front at the height of the chest. | 2 | |
| SP as above, diagonal movement of the legs forward and backward, with parallel shoulder movement sideways. | 2 | |
| SP as above, the Smovey joined together, held behind with both hands at the height of the hips, slight arm movement sideways. | 2 | |
| SP = standing in straddle position, arms at the height of the shoulders, crossing and abduction of the arms | 2 | |
| SP = lying on the side, arm movement above the head and then toward legs. Uppermost leg is lifted upward. Performed both right and left. | 2 | |
| SP as above, arms kept straight along the torso, the Smovey joined together, forward and backward movement of the arm and the leg in opposite directions. Performed right and left. | 2 | |
| SP as above, the knee of the upper leg is bent and moved forward, massage of the buttock with the Smovey. Performed right and left. | 2 | |
| SP = lying on the back, the Smovey is held behind the head with both hands, moving arms forward once to the right and once to the left of the torso. | 2 | |
| SP as above, left leg is bent at the knee, the heel is leaned against the ground, right leg lifted straight up and down with parallel arm movement toward the right leg. Repeated right and left. | 2 | |
| SP as above, legs are bent at an angle of 90°, the Smovey is held with both hands at the height of the chest. Moving legs sideways while moving arms in the opposite direction. | 2 | |
| Cool down 5 min | SP standing with the Smovey held in each hand, bending forward with arms directed forward. | 1 |
| SP = standing in straddle position, the Smovey is held with both hands, lifting the hands up and bending forward, moving the Smovey toward the floor. | 1 | |
| SP as above but bending to the sides. | 1 | |
| SP as above, the Smovey is held on the shoulders, with rocking sideways movements. | 1 | |
| SP as above, raising arms up while breathing in and lowering arms while breathing out. | 1 | |
| Organizational matters | The end of the class, asking the participants about their perceived intensity of exercise using the Borg scale, saying goodbye. | 3 |
References
- Vancampfort, D.; Stubbs, B.; Koyanagi, A. Physical chronic conditions, multimorbidity and sedentary behavior amongst middle-aged and older adults in six low- and middle-income countries. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, W.J.; Johnson, A.; Keldermans, D.; Tang, B. Exercise and Low Back Pain in the Older Adult: Current Recommendations. J. Allied Health 2019, 48, 302–307. [Google Scholar] [PubMed]
- Jesus-Moraleida, F.R.; Silva, J.P.; Pereira, D.S.; Dias, J.M.D.; Dias, R.C.; Ferreira, M.L.; Hayden, J.A.; Pereira, L.S.M. Exercise therapy for older adults with low-back pain. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef]
- de Oliveira, N.T.B.; Ricci, N.A.; Dos Santos Franco, Y.R.; Salvador, E.M.E.S.; Almeida, I.C.B.; Cabral, C.M.N. Effectiveness of the Pilates method versus aerobic exercises in the treatment of older adults with chronic low back pain: A randomized controlled trial protocol. BMC Musculoskelet. Disord. 2019, 20, 250. [Google Scholar] [CrossRef]
- Colgrove, Y.M.; Gravino-Dunn, N.S.; Dinyer, S.C.; Sis, E.A.; Heier, A.C.; Sharma, N.K. Physical and Physiological Effects of Yoga for an Underserved Population with Chronic Low Back Pain. Int. J. Yoga 2019, 12, 252–264. [Google Scholar] [CrossRef]
- Akodu, A.K.; Akindutire, O.M. The effect of stabilization exercise on pain-related disability, sleep disturbance, and psychological status of patients with non-specific chronic low back pain. Korean J. Pain 2018, 31, 199–205. [Google Scholar] [CrossRef]
- Psycharakis, S.G.; Coleman, S.G.S.; Linton, L.; Kaliarntas, K.; Valentin, S. Muscle Activity During Aquatic and Land Exercises in People With and Without Low Back Pain. Phys Ther. 2019, 99, 297–310. [Google Scholar] [CrossRef]
- Geneen, L.J.; Moore, R.A.; Clarke, C.; Martin, D.; Colvin, L.A.; Smith, B.H. Physical activity and exercise for chronic pain in adults: An overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2017. [Google Scholar] [CrossRef] [Green Version]
- Levin, O.; Netz, Y.; Ziv, G. The beneficial effects of different types of exercise interventions on motor and cognitive functions in older age: A systematic review. Eur Rev. Aging Phys. Act. 2017, 14, 20. [Google Scholar] [CrossRef]
- Hides, J.; Hodges, P.; Lambrecht, G. State-of-the-Art Exercise Concepts for Lumbopelvic and Spinal Muscles—Transferability to Microgravity. Front. Physiol. 2019, 10, 837. [Google Scholar] [CrossRef]
- Mora, J.C.; Valencia, W.M. Exercise and Older Adults. Clin. Geriatr. Med. 2018, 34, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G. The Use of Vibration as Physical Exercise and Therapy. J. Funct. Morphol. Kinesiol. 2017, 2, 17. [Google Scholar] [CrossRef] [Green Version]
- Goossens, N.; Janssens, L.; Caeyenberghs, K.; Albouy, G.; Brumagne, S. Differences in brain processing of proprioception related to postural control in patients with recurrent non-specific low back pain and healthy controls. Neuroimage Clin. 2019, 23, 101881. [Google Scholar] [CrossRef]
- Awan, R.; Khan, N.; Malik, S. The Effect of WBV on Balance, Mobility and Strength in Aging Adults: A Systematic Review. Biol. Syst. Open Access 2017, 6, 179. [Google Scholar] [CrossRef] [Green Version]
- Sitjà-Rabert, M.; Martínez-Zapata, M.J.; Fort Vanmeerhaeghe, A.; Rey Abella, F.; Romero-Rodríguez, D.; Bonfill, X. Effects of a whole body vibration (WBV) exercise intervention for institutionalized older people: A randomized, multicentre, parallel, clinical trial. J. Am. Med. Dir. Assoc. 2015, 16, 125–131. [Google Scholar] [CrossRef]
- Saggini, R.; Carmignano, S.M.; Palermo, T.; Bellomo, R.G. Mechanical Vibration in Rehabilitation: State of the Art. J. Nov. Physiother. 2016, 6. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Ning, H.-T.; Xiao, S.-M.; Hu, M.-Y.; Wu, X.-Y.; Deng, H.-W.; Feng, H. Effects of vibration therapy on muscle mass, muscle strength and physical function in older adults with sarcopenia: A systematic review and meta-analysis. Eur. Rev. Aging Phys. Act. 2020, 17, 14. [Google Scholar] [CrossRef]
- Chang, S.-F.; Lin, P.-C.; Yang, R.-S.; Yang, R.-J. The preliminary effect of whole-body vibration intervention on improving the skeletal muscle mass index, physical fitness, and quality of life among older people with sarcopenia. BMC Geriatr. 2018, 18, 17. [Google Scholar] [CrossRef]
- Lai, C.-C.; Tu, Y.-K.; Wang, T.-G.; Huang, Y.-T.; Chien, K.-L. Effects of resistance training, endurance training and whole-body vibration on lean body mass, muscle strength and physical performance in older people: A systematic review and network meta-analysis. Age Ageing 2018, 47, 367–373. [Google Scholar] [CrossRef] [Green Version]
- Zago, M.; Capodaglio, P.; Ferrario, C.; Tarabini, M.; Galli, M. Whole-body vibration training in obese subjects: A systematic review. PLoS ONE 2018, 13, e0202866. [Google Scholar] [CrossRef] [Green Version]
- Choi, W.; Han, D.; Kim, J.; Lee, S. Whole-Body Vibration Combined with Treadmill Training Improves Walking Performance in Post-Stroke Patients: A Randomized Controlled Trial. Med. Sci. Monit. 2017, 23, 4918–4925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celletti, C.; Suppa, A.; Bianchini, E.; Lakin, S.; Toscano, M.; La Torre, G.; Di Piero, V.; Camerota, F. Promoting post-stroke recovery through focal or whole body vibration: Criticisms and prospects from a narrative review. Neurol. Sci. 2020, 41, 11–24. [Google Scholar] [CrossRef] [Green Version]
- Souron, R.; Besson, T.; Millet, G.Y.; Lapole, T. Acute and chronic neuromuscular adaptations to local vibration training. Eur. J. Appl. Physiol. 2017, 117, 1939–1964. [Google Scholar] [CrossRef]
- Taani, M.H.; Siglinsky, E.; Libber, J.; Krueger, D.; Binkley, N.; Kovach, C.R.; Buehring, B. Semi-Recumbent Vibration Exercise in Older Adults: A Pilot Study of Methodology, Feasibility, and Safety. Gerontol. Geriatr. Med. 2019, 5, 2333721419881552. [Google Scholar] [CrossRef]
- Delafontaine, A.; Vialleron, T.; Fischer, M.; Laffaye, G.; Chèze, L.; Artico, R.; Genêt, F.; Fourcade, P.C.; Yiou, E. Acute Effects of Whole-Body Vibration on the Postural Organization of Gait Initiation in Young Adults and Elderly: A Randomized Sham Intervention Study. Front. Neurol. 2019, 10, 1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; King, G.A.; Dillon, L.; Su, X. Controlled whole-body vibration training reduces risk of falls among community-dwelling older adults. J. Biomech. 2015, 48, 3206–3212. [Google Scholar] [CrossRef] [PubMed]
- Leite, H.R.; Camargos, A.C.R.; Mendonça, V.A.; Lacerda, A.C.R.; Soares, B.A.; Oliveira, V.C. Current evidence does not support whole body vibration in clinical practice in children and adolescents with disabilities: A systematic review of randomized controlled trial. Braz. J. Phys. Ther. 2019, 23, 196–211. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, H.; Zhu, Y.; Chen, B.; Zheng, Y.; Liu, X.; Qiao, J.; Wang, X. Effects of whole body vibration exercise on lumbar-abdominal muscles activation for patients with chronic low back pain. BMC Sports Sci. Med. Rehabil. 2020, 12, 78. [Google Scholar] [CrossRef] [PubMed]
- Cenik, F.; Keilani, M.; Galid, A.; Crevenna, R. First exercise group for Turkish breast cancer patients in Vienna—A pilot project to include Turkish migrants. Disabil. Rehabil. 2020, 42, 20–25. [Google Scholar] [CrossRef]
- Crevenna, R.; Cenik, F.; Galle, A.; Komanadj, T.S.; Keilani, M. Feasibility, acceptance and long-term exercise behaviour in cancer patients: An exercise intervention by using a swinging-ring system. Wien. Klin. Wochenschr. 2015, 127, 751–755. [Google Scholar] [CrossRef]
- Germann, D.; El Bouse, A.; Shnier, J.; Abdelkader, N.; Kazemi, M. Effects of local vibration therapy on various performance parameters: A narrative literature review. J. Can. Chiropr. Assoc. 2018, 62, 170–181. [Google Scholar] [PubMed]
- GHO|By Category|Life Expectancy and Healthy Life Expectancy—Data by Country. Available online: https://apps.who.int/gho/data/view.main.SDG2016LEXv?xml:lang=en (accessed on 10 June 2020).
- Gordon, R.; Bloxham, S. A Systematic Review of the Effects of Exercise and Physical Activity on Non-Specific Chronic Low Back Pain. Healthcare 2016, 4, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilke, J.; Krause, F. Myofascial chains of the upper limb: A systematic review of anatomical studies. Clin. Anat. 2019, 32, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Ajimsha, M.S.; Shenoy, P.D.; Gampawar, N. Role of fascial connectivity in musculoskeletal dysfunctions: A narrative review. J. Bodyw. Mov. Ther. 2020, 24, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Dischiavi, S.L.; Wright, A.A.; Hegedus, E.J.; Bleakley, C.M. Biotensegrity and myofascial chains: A global approach to an integrated kinetic chain. Med. Hypotheses 2018, 110, 90–96. [Google Scholar] [CrossRef]
- Scarr, G. Biotensegrity: What is the big deal? J. Bodyw. Mov. Ther. 2020, 24, 134–137. [Google Scholar] [CrossRef] [Green Version]
- Stecco, C.; Pirri, C.; Fede, C.; Yucesoy, C.A.; De Caro, R.; Stecco, A. Fascial or Muscle Stretching? A Narrative Review. Appl. Sci. 2021, 11, 307. [Google Scholar] [CrossRef]
- Fan, C.; Fede, C.; Gaudreault, N.; Porzionato, A.; Macchi, V.; DE Caro, R.; Stecco, C. Anatomical and functional relationships between external abdominal oblique muscle and posterior layer of thoracolumbar fascia. Clin. Anat. 2018, 31, 1092–1098. [Google Scholar] [CrossRef]
- Chen, B.; Dong, Y.; Guo, J.; Zheng, Y.; Zhang, J.; Wang, X. Effects of Whole-Body Vibration on Lumbar-Abdominal Muscles Activation in Healthy Young Adults: A Pilot Study. Med. Sci. Monit. 2019, 25, 1945–1951. [Google Scholar] [CrossRef]
- Zheng, Y.-L.; Wang, X.-F.; Chen, B.-L.; Gu, W.; Wang, X.; Xu, B.; Zhang, J.; Wu, Y.; Chen, C.-C.; Liu, X.-C.; et al. Effect of 12-Week Whole-Body Vibration Exercise on Lumbopelvic Proprioception and Pain Control in Young Adults with Nonspecific Low Back Pain. Med. Sci. Monit. 2019, 25, 443–452. [Google Scholar] [CrossRef]
- Chow, D.H.K.; Lee, T.Y.; Pope, M.H. Effects of whole body vibration on spinal proprioception in healthy individuals. Work 2018, 61, 403–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermens, H.J.; Freriks, B.; Merletti, R.; Stegeman, D.F.; Blok, J.H.; Rau, G.; Klug, C.; Hägg, G.; Blok, W.J. European Recommendations for Surface Electromyography: Results of the SENIAM Project; Roessingh Research and Development b.v.: Enschede, The Netherlands, 1999. [Google Scholar]
- Lima, M.; Ferreira, A.S.; Reis, F.J.J.; Paes, V.; Meziat-Filho, N. Chronic low back pain and back muscle activity during functional tasks. Gait Posture 2018, 61, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, A.; Ergin, O.; Karacan, I.; Oncu, M. Validity and Reliability of the Metric Measurements in the Assessment of Lumbar Spine Motion in Patients With Ankylosing Spondylitis. Spine 2012, 37, E1189–E1196. [Google Scholar] [CrossRef] [PubMed]
- Price, D.D.; Staud, R.; Robinson, M.E. How should we use the visual analogue scale (VAS) in rehabilitation outcomes? II: Visual analogue scales as ratio scales: An alternative to the view of Kersten et al. J. Rehabil. Med. 2012, 44, 800–804. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.; Bil, K. Correlating Heart Rate and Perceived Exertion during Aerobic Exercise in Alzheimer’s Disease. Nurs. Health Sci. 2010, 12, 375–380. [Google Scholar] [CrossRef] [Green Version]
- Becker, S.; Bergamo, F.; Schnake, K.J.; Schreyer, S.; Rembitzki, I.V.; Disselhorst-Klug, C. The relationship between functionality and erector spinae activity in patients with specific low back pain during dynamic and static movements. Gait Posture 2018, 66, 208–213. [Google Scholar] [CrossRef]
- Russo, M.; Deckers, K.; Eldabe, S.; Kiesel, K.; Gilligan, C.; Vieceli, J.; Crosby, P. Muscle Control and Non-specific Chronic Low Back Pain. Neuromodulation 2018, 21, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hemming, R.; Sheeran, L.; van Deursen, R.; Sparkes, V. Investigating differences in trunk muscle activity in non-specific chronic low back pain subgroups and no-low back pain controls during functional tasks: A case-control study. BMC Musculoskelet. Disord. 2019, 20, 459. [Google Scholar] [CrossRef] [Green Version]
- Willard, F.H.; Vleeming, A.; Schuenke, M.D.; Danneels, L.; Schleip, R. The thoracolumbar fascia: Anatomy, function and clinical considerations. J. Anat. 2012, 221, 507–536. [Google Scholar] [CrossRef] [Green Version]
- Nelson-Wong, E.; Glinka, M.; Noguchi, M.; Langevin, H.; Badger, G.J.; Callaghan, J.P. Acute Surgical Injury Alters the Tensile Properties of Thoracolumbar Fascia in a Porcine Model. J. Biomech. Eng. 2018, 140, 1010121–1010127. [Google Scholar] [CrossRef]
- Sharma, S.K.; Saiyad, S.; Bid, D.N. Role of Latissimus Dorsi and Lower Trapezius in Chronic Mechanical Low Back Pain due to Thoraco-lumbar Dysfunction. Indian J. Physiother. Occup. Ther. 2013, 7, 219–224. [Google Scholar] [CrossRef]
- Fan, C.; Guidolin, D.; Ragazzo, S.; Fede, C.; Pirri, C.; Gaudreault, N.; Porzionato, A.; Macchi, V.; De Caro, R.; Stecco, C. Effects of Cesarean Section and Vaginal Delivery on Abdominal Muscles and Fasciae. Medicina 2020, 56, 260. [Google Scholar] [CrossRef] [PubMed]
- Fede, C.; Albertin, G.; Petrelli, L.; Sfriso, M.M.; Biz, C.; De Caro, R.; Stecco, C. Hormone Receptor Expression in Human Fascial Tissue. Eur. J. Histochem. 2016, 60, 2710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowotny, A.H.; Calderon, M.G.; de Souza, P.A.; Aguiar, A.F.; Léonard, G.; Alves, B.M.O.; Amorim, C.F.; da Silva, R.A. Lumbar stabilisation exercises versus back endurance-resistance exercise training in athletes with chronic low back pain: Protocol of a randomised controlled trial. BMJ Open Sport Exerc. Med. 2018, 4, e000452. [Google Scholar] [CrossRef] [PubMed]
- Daneau, C.; Cantin, V.; Descarreaux, M. Effect of Massage on Clinical and Physiological Variables During Muscle Fatigue Task in Participants With Chronic Low Back Pain: A Crossover Study. J. Manip. Physiol. Ther. 2019, 42, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.; Yamasaki, M. Association of lower extremity range of motion and muscle strength with physical performance of community-dwelling older women. J. Physiol Anthr. 2016, 35, 1–9. [Google Scholar] [CrossRef] [Green Version]
- da Silva, R.A.; Vieira, E.R.; Léonard, G.; Beaulieu, L.-D.; Ngomo, S.; Nowotny, A.H.; Amorim, C.F. Age- and low back pain-related differences in trunk muscle activation during one-legged stance balance task. Gait Posture 2019, 69, 25–30. [Google Scholar] [CrossRef]
- Langevin, H.M.; Fox, J.R.; Koptiuch, C.; Badger, G.J.; Greenan- Naumann, A.C.; Bouffard, N.A.; Konofagou, E.E.; Lee, W.-N.; Triano, J.J.; Henry, S.M. Reduced thoracolumbar fascia shear strain in human chronic low back pain. BMC Musculoskelet. Disord. 2011, 12, 203. [Google Scholar] [CrossRef]
- Nuzzo, J.L. The Case for Retiring Flexibility as a Major Component of Physical Fitness. Sports Med. 2020, 50, 853–870. [Google Scholar] [CrossRef]
- Vulfsons, S.; Chervonenko, S.; Haddad, M.; Weisman, M.H.; Lavi, N.; Dar, G. Decreased amplitude of surface electromyo- graphic recordings of muscle activation along the posterior myofascial kinematic chain in subjects with chronic nonspecific low back pain compared to healthy subjects. J. Back Musculoskelet. Rehabil. 2018, 31, 785–793. [Google Scholar] [CrossRef]
- Shamsi, M.; Sarrafzadeh, J.; Jamshidi, A.; Arjmand, N.; Ghezelbash, F. Comparison of spinal stability following motor control and general exercises in nonspecific chronic low back pain patients. Clin. Biomech. 2017, 48, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Arendt-Nielsen, L.; Graven-Nielsen, T.; Svarrer, H.; Svensson, P. The influence of low back pain on muscle activity and coordination during gait: A clinical and experimental study. Pain 1996, 64, 231–240. [Google Scholar] [CrossRef]
- Suh, J.H.; Kim, H.; Jung, G.P.; Ko, J.Y.; Ryu, J.S. The effect of lumbar stabilization and walking exercises on chronic low back pain. Medicine 2019, 98, e16173. [Google Scholar] [CrossRef] [PubMed]
- Sbardelotto, M.L.; Costa, R.R.; Malysz, K.A.; Pedroso, G.S.; Pereira, B.C.; Sorato, H.R.; Silveira, P.C.L.; Nesi, R.T.; Grande, A.J.; Pinho, R.A. Improvement in muscular strength and aerobic capacities in elderly people occurs independently of physical training type or exercise model. Clinics 2019, 74, e833. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.G.; Jackson, E.A.; Richardson, C.R. Exercise Prescriptions in Older Adults. Am. Fam. Physician 2017, 95, 425–432. [Google Scholar] [PubMed]
| VEE (Experimental) Group n = 43 | Sham-VEE (Control) Group n = 49 | p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Me | Min | Max | Q1 | Q3 | Me | Min | Max | Q1 | Q3 | ||
| Age (years) | 66.0 | 50.0 | 76.0 | 63.0 | 69.0 | 66.0 | 56.0 | 80.0 | 64.0 | 69.0 | 0.68 * |
| Height (cm) | 160.0 | 146.0 | 176.0 | 156.0 | 165.0 | 160.0 | 149.0 | 171.0 | 155.0 | 164.0 | 0.89 * |
| Weight (kg) | 75.4 | 47.1 | 107.5 | 64.2 | 85.0 | 70.6 | 49.3 | 177.7 | 63.0 | 77.0 | 0.17 * |
| BMI (kg/m2) | 28.4 | 19.1 | 42.3 | 25.2 | 32.5 | 27.7 | 19.3 | 42.7 | 25.8 | 29.2 | 0.24 * |
| VEE (Experimental) Group | Sham-VEE (Control) Group | p-Value * | p-Value ** | p-Value *** | p-Value **** | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | |||||||||||||||||||||
| Me | Min | Max | Q1 | Q3 | Me | Min | Max | Q1 | Q3 | Me | Min | Max | Q1 | Q3 | Me | Min | Max | Q1 | Q3 | |||||
| Flexion sEMG—left ES | 18.2 | 7.6 | 57.3 | 12 | 19.9 | 14.1 | 4.5 | 49.9 | 8.8 | 18.5 | 14.7 | 8.4 | 33.4 | 11.7 | 17.6 | 13.7 | 7.8 | 28.1 | 10.9 | 17.5 | 0.045 | 0.34 | 0.07 | 0.70 |
| Flexion sEMG—right ES | 15.4 | 6.6 | 39.9 | 12 | 19.8 | 12.6 | 5.6 | 30 | 9.1 | 16.7 | 13.9 | 6.5 | 45.2 | 10.2 | 17.3 | 12.3 | 4.6 | 30.4 | 10 | 16 | 0.010 | 0.22 | 0.30 | 0.87 |
| Rest (in maximum flexion)—left ES | 18.1 | 3.1 | 40.6 | 12 | 20.2 | 12.5 | 2.8 | 53.3 | 8 | 17.5 | 14.7 | 3.5 | 27.9 | 11.4 | 18.9 | 12.2 | 3.5 | 30.8 | 9.2 | 16.8 | 0.038 | 0.10 | 0.28 | 0.73 |
| Rest (in maximum flexion)—right ES | 15.5 | 2.7 | 32.9 | 10.3 | 20.6 | 11.1 | 2.3 | 29.4 | 7.2 | 16.9 | 13 | 3.2 | 46.3 | 9.9 | 17.8 | 10.9 | 3.1 | 30 | 7.7 | 17 | 0.06 | 0.14 | 0.31 | 0.95 |
| Extension—left ES | 22.9 | 10.2 | 47.4 | 17.2 | 31.6 | 20.3 | 4.5 | 54.1 | 13.4 | 28.5 | 18.2 | 3.8 | 48.3 | 14.4 | 25.6 | 18.5 | 8.3 | 58.9 | 14.7 | 25.1 | 0.06 | 0.57 | 0.13 | 0.89 |
| Extension—right ES | 21.8 | 8.5 | 38.9 | 15.3 | 29.1 | 20.2 | 5.9 | 45.4 | 14.2 | 24.1 | 17.7 | 8.1 | 48.6 | 13.9 | 23.6 | 15 | 7.8 | 43.9 | 12.3 | 23.3 | 0.031 | 0.64 | 0.07 | 0.29 |
| Rest—left erector spinae | 5.7 | 2.1 | 19.4 | 4 | 7.1 | 5.3 | 2.3 | 18.1 | 4.4 | 7.1 | 5.7 | 2.3 | 20.1 | 4.6 | 8.3 | 5.3 | 2.4 | 16.1 | 4.3 | 7 | 0.78 | 0.16 | 0.34 | 0.88 |
| Rest—right erector spinae | 3.5 | 1.9 | 16.2 | 2.8 | 4.9 | 3.2 | 1.5 | 7.3 | 2.5 | 4.6 | 3.4 | 1.6 | 18.5 | 2.7 | 4.8 | 3.2 | 1.9 | 11 | 2.5 | 4.4 | 0.049 | 0.37 | 0.52 | 0.88 |
| VEE (Experimental) Group | Sham-VEE (Control) Group | p-Value * | p-Value ** | p-Value *** | p-Value **** | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | |||||||||||||||||||||
| Me | Min | Max | Q1 | Q3 | Me | Min | Max | Q1 | Q3 | Me | Min | Max | Q1 | Q3 | Me | Min | Max | Q1 | Q3 | |||||
| Distance between points in a standing position (cm) | 13.0 | 10.0 | 16.0 | 12.0 | 14.0 | 14.0 | 12.0 | 18.0 | 10.0 | 15.0 | 13.0 | 10.0 | 15.0 | 12.0 | 14.0 | 13.0 | 10.0 | 15.0 | 12.0 | 14.0 | 0.0015 | 0.11 | 0.79 | 0.68 |
| Distance between points in a flexing position (cm) | 17.0 | 13.0 | 21.0 | 15.5 | 19.0 | 18.0 | 15.0 | 23.0 | 17.0 | 19.5 | 17.0 | 13.0 | 22.0 | 16.0 | 19.0 | 18.0 | 14.0 | 22.0 | 16.0 | 19.0 | 0.0017 | 0.09 | 0.88 | 0.29 |
| VEE (Experimental) Group | Sham-VEE (Control) Group | p-Value * | p-Value ** | p-Value *** | p-Value **** | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | |||||||||||||||||||||
| Me | Min | Max | Q1 | Q3 | Me | Min | Max | Q1 | Q3 | Me | Min | Max | Q1 | Q3 | Me | Min | Max | Q1 | Q3 | |||||
| VAS | 4.0 | 1.0 | 10.0 | 2.0 | 5.0 | 1.0 | 0.0 | 8.0 | 0.0 | 4.0 | 5.0 | 1.0 | 10.0 | 3.0 | 8.0 | 1.0 | 0.0 | 9.0 | 0.0 | 5.0 | 0.001 | <0.001 | 0.07 | 0.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zurek, G.; Kasper-Jędrzejewska, M.; Dobrowolska, I.; Mroczek, A.; Delaunay, G.; Ptaszkowski, K.; Halski, T. Vibrating Exercise Equipment in Middle-Age and Older Women with Chronic Low Back Pain and Effects on Bioelectrical Activity, Range of Motion and Pain Intensity: A Randomized, Single-Blinded Sham Intervention Study. Biology 2022, 11, 268. https://doi.org/10.3390/biology11020268
Zurek G, Kasper-Jędrzejewska M, Dobrowolska I, Mroczek A, Delaunay G, Ptaszkowski K, Halski T. Vibrating Exercise Equipment in Middle-Age and Older Women with Chronic Low Back Pain and Effects on Bioelectrical Activity, Range of Motion and Pain Intensity: A Randomized, Single-Blinded Sham Intervention Study. Biology. 2022; 11(2):268. https://doi.org/10.3390/biology11020268
Chicago/Turabian StyleZurek, Grzegorz, Martyna Kasper-Jędrzejewska, Iwona Dobrowolska, Agata Mroczek, Gerda Delaunay, Kuba Ptaszkowski, and Tomasz Halski. 2022. "Vibrating Exercise Equipment in Middle-Age and Older Women with Chronic Low Back Pain and Effects on Bioelectrical Activity, Range of Motion and Pain Intensity: A Randomized, Single-Blinded Sham Intervention Study" Biology 11, no. 2: 268. https://doi.org/10.3390/biology11020268
APA StyleZurek, G., Kasper-Jędrzejewska, M., Dobrowolska, I., Mroczek, A., Delaunay, G., Ptaszkowski, K., & Halski, T. (2022). Vibrating Exercise Equipment in Middle-Age and Older Women with Chronic Low Back Pain and Effects on Bioelectrical Activity, Range of Motion and Pain Intensity: A Randomized, Single-Blinded Sham Intervention Study. Biology, 11(2), 268. https://doi.org/10.3390/biology11020268







