The Role of TLR2 in Infectious Diseases Caused by Mycobacteria: From Cell Biology to Therapeutic Target
Abstract
Simple Summary
Abstract
1. Introduction
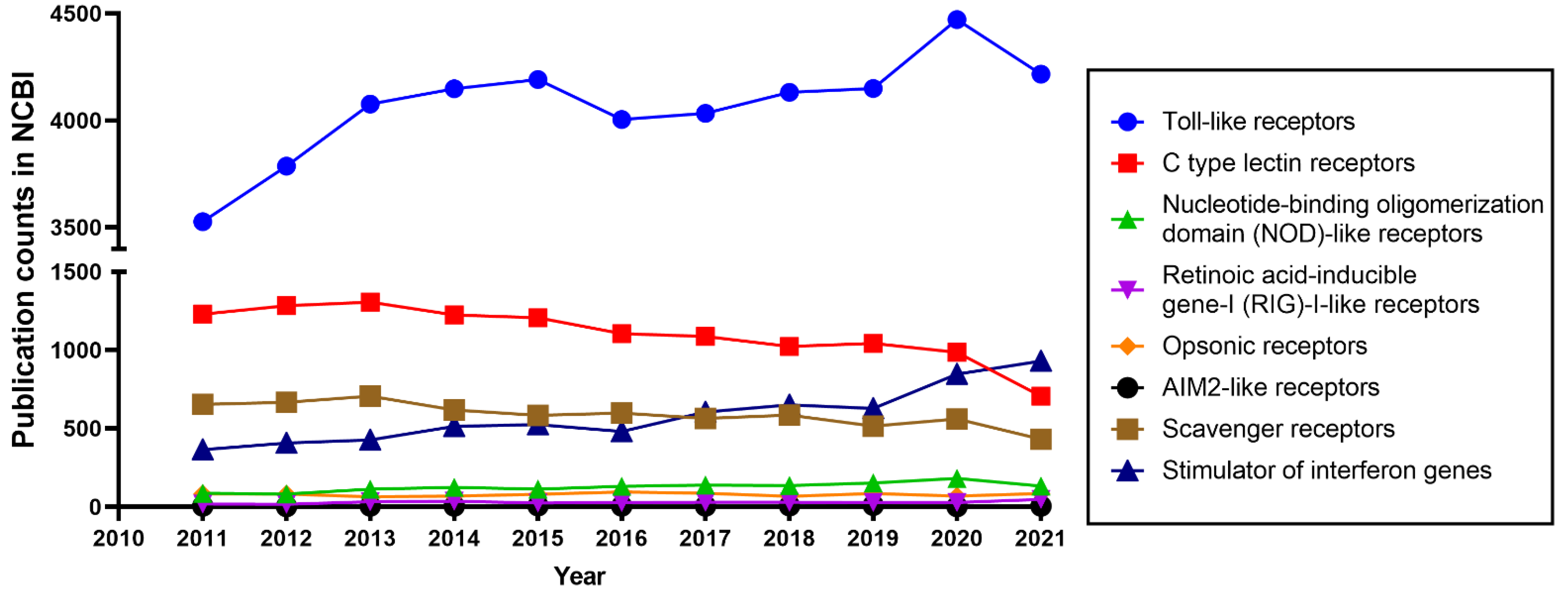
1.1. Innate Immunity and Toll-like Receptors
1.2. The Diversity of TLRs
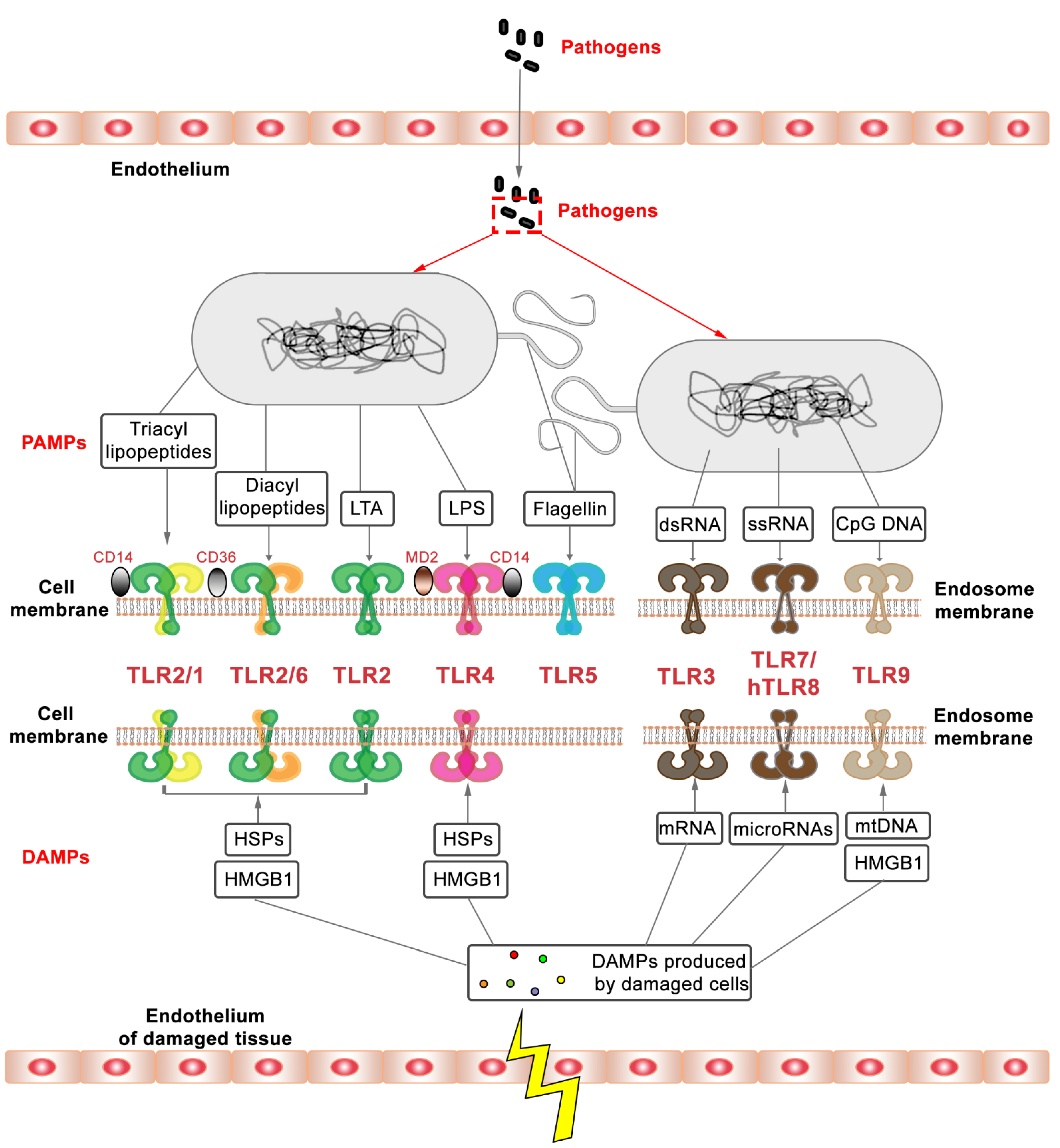
1.3. TLR2, an Important Member of the TLR Family
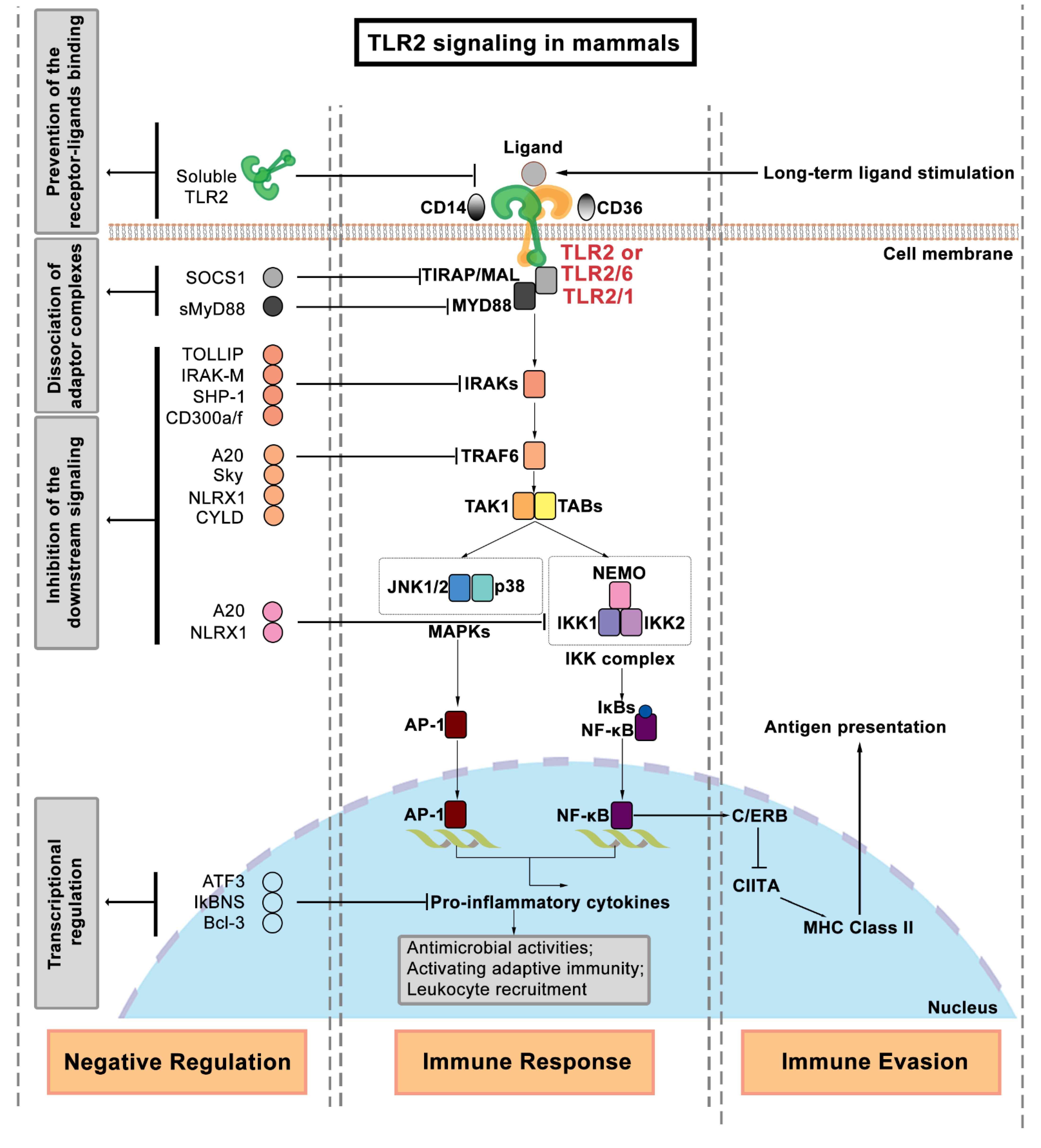
2. Regulation of TLR2 Signaling
3. TLR2 Function in Immune Responses to Mycobacterial Infection
3.1. Tuberculosis and Non-Tuberculosis Diseases Caused by Mycobacteria
3.2. Which TLRs Play a Role in Defense against Mycobacteria?
3.3. TLR2 Recognizes Mycobacterial Components
3.4. TLR2 Is Associated with the Susceptibility to Infection by Various Mycobacteria
4. TLR2 Function in Mediating the Host–Mycobacterial Interaction
4.1. Macrophage–Mycobacterial Interactions
4.2. Neutrophil–Mycobacterial Interactions
5. Therapeutic Targeting of TLR2 Signaling in Diseases
5.1. The Application of TLR2 Ligands in Mycobacterial Infectious Diseases
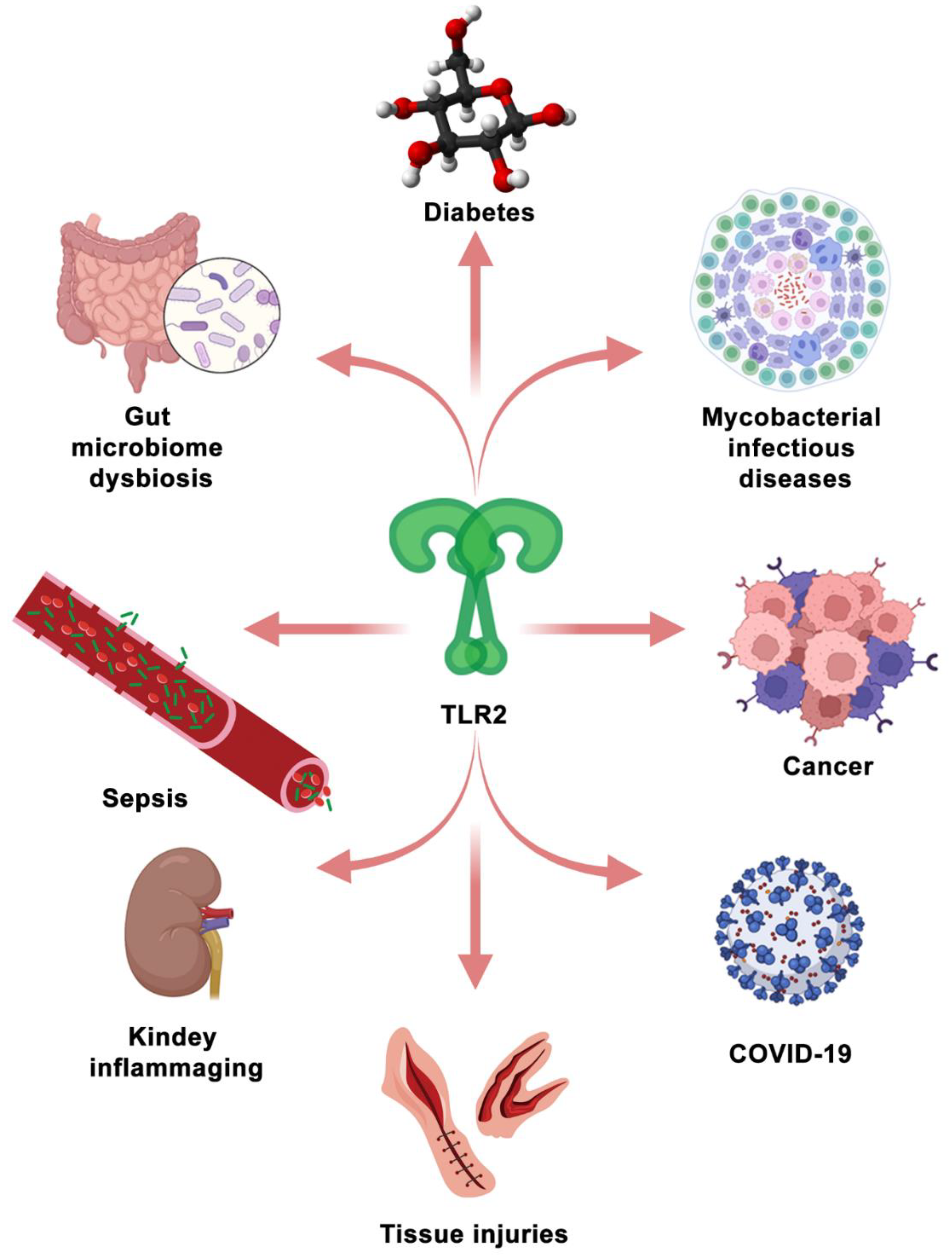
5.2. TLR2 as a Therapeutic Target in the Other Diseases
6. Zebrafish as a Model to Investigate TLR2 as a Therapeutic Target
6.1. General Advantages of the Zebrafish Larval Model
6.2. New Insights of the Tlr2 Function Learned from the Zebrafish Model
7. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hato, T.; Dagher, P.C. How the innate immune system senses trouble and causes trouble. Clin. J. Am. Soc. Nephrol. 2015, 10, 1459–1469. [Google Scholar] [CrossRef]
- Dube, J.Y.; Fava, V.M.; Schurr, E.; Behr, M.A. Underwhelming or misunderstood? Genetic variability of pattern recognition receptors in immune responses and resistance to Mycobacterium tuberculosis. Front. Immunol. 2021, 12, 714808. [Google Scholar] [CrossRef]
- Kumar, H.; Kawai, T.; Akira, S. Pathogen recognition by the innate immune system. Int. Rev. Immunol. 2011, 30, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Y.; Cao, X.; Jin, X.; Jin, T. Pattern recognition receptors in zebrafish provide functional and evolutionary insight into innate immune signaling pathways. Cell. Mol. Immunol. 2017, 14, 80–89. [Google Scholar] [CrossRef]
- Fu, Y.L.; Harrison, R.E. Microbial phagocytic receptors and their potential involvement in cytokine induction in macrophages. Front. Immunol. 2021, 12, 662063. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like receptors and the control of immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef]
- Simpson, M.E.; Petri, W.A. TLR2 as a therapeutic target in bacterial infection. Trends Mol. Med. 2020, 26, 715–717. [Google Scholar] [CrossRef]
- Caplan, I.F.; Maguire-Zeiss, K.A. Toll-like receptor 2 signaling and current approaches for therapeutic modulation in synucleinopathies. Front. Pharmacol. 2018, 9, 417. [Google Scholar] [CrossRef]
- Aghamiri, S.H.; Komlakh, K.; Ghaffari, M. Toll-like receptors (TLRs) and their potential therapeutic applications in diabetic neuropathy. Int. Immunopharmacol. 2022, 102, 108398. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R.; Preston-Hurlburt, P.; Janeway, C.A., Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 1997, 388, 394–397. [Google Scholar] [CrossRef]
- Hashimoto, C.; Hudson, K.L.; Anderson, K.V. The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell 1988, 52, 269–279. [Google Scholar] [CrossRef]
- Lemaitre, B.; Nicolas, E.; Michaut, L.; Reichhart, J.M.; Hoffmann, J.A. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 1996, 86, 973–983. [Google Scholar] [CrossRef]
- Sameer, A.S.; Nissar, S. Toll-like receptors (TLRs): Structure, functions, signaling, and role of their polymorphisms in colorectal cancer susceptibility. Biomed. Res. Int. 2021, 2021, 1157023. [Google Scholar] [CrossRef]
- Behzadi, P.; Garcia-Perdomo, H.A.; Karpinski, T.M. Toll-like receptors: General molecular and structural biology. J. Immunol. Res. 2021, 2021, 9914854. [Google Scholar] [CrossRef]
- Sahoo, B.R. Structure of fish Toll-like receptors (TLR) and NOD-like receptors (NLR). Int. J. Biol. Macromol. 2020, 161, 1602–1617. [Google Scholar] [CrossRef]
- Matsushima, N.; Miyashita, H.; Enkhbayar, P.; Kretsinger, R.H. Comparative geometrical analysis of leucine-rich repeat structures in the Nod-like and Toll-like receptors in vertebrate innate immunity. Biomolecules 2015, 5, 1955–1978. [Google Scholar] [CrossRef]
- Xia, P.; Wu, Y.; Lian, S.; Yan, L.; Meng, X.; Duan, Q.; Zhu, G. Research progress on Toll-like receptor signal transduction and its roles in antimicrobial immune responses. Appl. Microbiol. Biotechnol. 2021, 105, 5341–5355. [Google Scholar] [CrossRef]
- Mielcarska, M.B.; Bossowska-Nowicka, M.; Toka, F.N. Cell surface expression of endosomal Toll-like receptors—A necessity or a superfluous duplication? Front. Immunol. 2020, 11, 620972. [Google Scholar] [CrossRef]
- Meijer, A.H.; Gabby Krens, S.F.; Medina Rodriguez, I.A.; He, S.; Bitter, W.; Ewa Snaar-Jagalska, B.; Spaink, H. Expression analysis of the Toll-like receptor and TIR domain adaptor families of zebrafish. Mol. Immunol. 2004, 40, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.Y.; Yu, G.Y.; Luo, Y.; Xiang, R.; Chuang, T.H. Immunostimulatory activities of CpG-Oligodeoxynucleotides in teleosts: Toll-like receptors 9 and 21. Front. Immunol. 2019, 10, 179. [Google Scholar] [CrossRef] [PubMed]
- Raetz, M.; Kibardin, A.; Sturge, C.R.; Pifer, R.; Li, H.; Burstein, E.; Ozato, K.; Larin, S.; Yarovinsky, F. Cooperation of TLR12 and TLR11 in the IRF8-dependent IL-12 response to Toxoplasma gondii profilin. J. Immunol. 2013, 191, 4818–4827. [Google Scholar] [CrossRef]
- Shukla, D.; Chandel, H.S.; Srivastava, S.; Chauhan, P.; Pandey, S.P.; Patidar, A.; Banerjee, R.; Chattopadhyay, D.; Saha, B. TLR11 or TLR12 silencing reduces Leishmania major infection. Cytokine 2018, 104, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Hatai, H.; Lepelley, A.; Zeng, W.; Hayden, M.S.; Ghosh, S. Toll-like receptor 11 (TLR11) interacts with flagellin and profilin through disparate mechanisms. PLoS ONE 2016, 11, e0148987. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wilhelm, C.L.; Wangdi, T.; Maira-Litran, T.; Lee, S.J.; Raetz, M.; Sturge, C.R.; Mirpuri, J.; Pei, J.; Grishin, N.V.; et al. Absence of TLR11 in mice does not confer susceptibility to Salmonella typhi. Cell 2016, 164, 827–828. [Google Scholar] [CrossRef][Green Version]
- Mathur, R.; Zeng, W.; Hayden, M.S.; Ghosh, S. Mice lacking TLR11 exhibit variable Salmonella typhi susceptibility. Cell 2016, 164, 829–830. [Google Scholar] [CrossRef]
- Kolter, J.; Feuerstein, R.; Spoeri, E.; Gharun, K.; Elling, R.; Trieu-Cuot, P.; Goldmann, T.; Waskow, C.; Chen, Z.J.; Kirschning, C.J.; et al. Streptococci engage TLR13 on myeloid cells in a site-specific fashion. J. Immunol. 2016, 196, 2733–2741. [Google Scholar] [CrossRef]
- Asami, J.; Shimizu, T. Structural and functional understanding of the Toll-like receptors. Protein Sci. 2021, 30, 761–772. [Google Scholar] [CrossRef]
- Ishida, H.; Asami, J.; Zhang, Z.; Nishizawa, T.; Shigematsu, H.; Ohto, U.; Shimizu, T. Cryo-EM structures of Toll-like receptors in complex with UNC93B1. Nat. Struct. Mol. Biol. 2021, 28, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Ishida, H.; Ohto, U.; Shibata, T.; Miyake, K.; Shimizu, T. Structural basis for species-specific activation of mouse Toll-like receptor 9. FEBS Lett. 2018, 592, 2636–2646. [Google Scholar] [CrossRef]
- Jin, M.S.; Kim, S.E.; Heo, J.Y.; Lee, M.E.; Kim, H.M.; Paik, S.G.; Lee, H.; Lee, J.-O. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell 2007, 130, 1071–1082. [Google Scholar] [CrossRef]
- Manuja, A.; Manuja, B.K.; Singha, H. Sequence and functional variability of Toll-like receptor 9 gene in equines. Mol. Immunol. 2019, 105, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Qian, J.; Wang, Y.; Zhang, M.; Chu, Y.; Li, Y. Identification and immunological evaluation of novel TLR2 agonists through structure optimization of Pam3CSK4. Bioorgan. Med. Chem. 2019, 27, 2784–2800. [Google Scholar] [CrossRef] [PubMed]
- Natala, S.R.; Habas, A.; Stocking, E.M.; Orry, A.; Price, D.L.; Gill, M.B.; Bonhaus, D.W.; Abagyan, R.; Wrasidlo, W. Structure based design and synthesis of novel Toll-like receptor 2 (TLR 2) lipid antagonists. Bioorgan. Med. Chem. Lett. 2021, 40, 127861. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef]
- Mukherjee, S.; Karmakar, S.; Babu, S.P. TLR2 and TLR4 mediated host immune responses in major infectious diseases: A review. Braz. J. Infect. Dis. 2016, 20, 193–204. [Google Scholar] [CrossRef]
- Oliveira-Nascimento, L.; Massari, P.; Wetzler, L.M. The role of TLR2 in infection and immunity. Front. Immunol. 2012, 3, 79. [Google Scholar] [CrossRef]
- Jimenez-Dalmaroni, M.J.; Xiao, N.; Corper, A.L.; Verdino, P.; Ainge, G.D.; Larsen, D.S.; Painter, G.F.; Rudd, P.M.; Dwek, R.A.; Hoebe, K.; et al. Soluble CD36 ectodomain binds negatively charged diacylglycerol ligands and acts as a co-receptor for TLR2. PLoS ONE 2009, 4, e7411. [Google Scholar] [CrossRef]
- Uematsu, S.; Akira, S. Toll-Like receptors (TLRs) and their ligands. Handb. Exp. Pharmacol. 2008, 183, 1–20. [Google Scholar] [CrossRef]
- Gong, T.; Liu, L.; Jiang, W.; Zhou, R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 2020, 20, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wang, L.; Chen, S. Endogenous Toll-like receptor ligands and their biological significance. J. Cell. Mol. Med. 2010, 14, 2592–2603. [Google Scholar] [CrossRef] [PubMed]
- Soehnlein, O.; Lindbom, L. Phagocyte partnership during the onset and resolution of inflammation. Nat. Rev. Immunol. 2010, 10, 427–439. [Google Scholar] [CrossRef]
- Bianchi, M.E. HMGB1 loves company. J. Leukoc. Biol. 2009, 86, 573–576. [Google Scholar] [CrossRef]
- Kaur, A.; Kaushik, D.; Piplani, S.; Mehta, S.K.; Petrovsky, N.; Salunke, D.B. TLR2 agonistic small molecules: Detailed structure-activity relationship, applications, and future prospects. J. Med. Chem. 2021, 64, 233–278. [Google Scholar] [CrossRef]
- Basto, A.P.; Leitao, A. Targeting TLR2 for vaccine development. J. Immunol. Res. 2014, 2014, 619410. [Google Scholar] [CrossRef]
- Gopalakrishnan, A.; Salgame, P. Toll-like receptor 2 in host defense against Mycobacterium tuberculosis: To be or not to be—That is the question. Curr. Opin. Immunol. 2016, 42, 76–82. [Google Scholar] [CrossRef]
- Piermattei, A.; Migliara, G.; Di Sante, G.; Foti, M.; Hayrabedyan, S.B.; Papagna, A.; Geloso, M.C.; Corbi, M.; Valentini, M.; Sgambato, A.; et al. Toll-like receptor 2 mediates in vivo pro- and anti-inflammatory effects of Mycobacterium tuberculosis and modulates autoimmune encephalomyelitis. Front. Immunol. 2016, 7, 191. [Google Scholar] [CrossRef]
- Tjarnlund, A.; Guirado, E.; Julian, E.; Cardona, P.J.; Fernandez, C. Determinant role for Toll-like receptor signalling in acute mycobacterial infection in the respiratory tract. Microbes Infect. 2006, 8, 1790–1800. [Google Scholar] [CrossRef]
- Thoma-Uszynski, S.; Stenger, S.; Takeuchi, O.; Ochoa, M.T.; Engele, M.; Sieling, P.A.; Barnes, P.F.; Röllinghoff, M.; Bölcskei, P.L.; Wagner, M.; et al. Induction of direct antimicrobial activity through mammalian toll-like receptors. Science 2001, 291, 1544–1547. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; DiPietro, L.A. Toll-like receptor function in acute wounds. Adv. Wound Care 2017, 6, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, J.L. MyD88 in Mycobacterium tuberculosis infection. Med. Microbiol. Immunol. 2017, 206, 187–193. [Google Scholar] [CrossRef]
- Rajpoot, S.; Wary, K.K.; Ibbott, R.; Liu, D.; Saqib, U.; Thurston, T.L.M.; Baig, M. TIRAP in the mechanism of inflammation. Front. Immunol. 2021, 12, 697588. [Google Scholar] [CrossRef]
- Belhaouane, I.; Hoffmann, E.; Chamaillard, M.; Brodin, P.; Machelart, A. Paradoxical roles of the MAL/Tirap adaptor in pathologies. Front. Immunol. 2020, 11, 569127. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.O.; Sweet, M.J.; Mansell, A.; Kellie, S.; Kobe, B. TRIF-dependent TLR signaling, its functions in host defense and inflammation, and its potential as a therapeutic target. J. Leukoc. Biol. 2016, 100, 27–45. [Google Scholar] [CrossRef]
- Funami, K.; Matsumoto, M.; Oshiumi, H.; Inagaki, F.; Seya, T. Functional interfaces between TICAM-2/TRAM and TICAM-1/TRIF in TLR4 signaling. Biochem. Soc. Trans. 2017, 45, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Carty, M.; Bowie, A.G. SARM: From immune regulator to cell executioner. Biochem. Pharmacol. 2019, 161, 52–62. [Google Scholar] [CrossRef]
- McClure, R.; Massari, P. TLR-dependent human mucosal epithelial cell responses to microbial pathogens. Front. Immunol. 2014, 5, 386. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011, 34, 637–650. [Google Scholar] [CrossRef]
- Walsh, M.C.; Lee, J.; Choi, Y. Tumor necrosis factor receptor- associated factor 6 (TRAF6) regulation of development, function, and homeostasis of the immune system. Immunol. Rev. 2015, 266, 72–92. [Google Scholar] [CrossRef]
- Kang, S.S.; Sim, J.R.; Yun, C.H.; Han, S.H. Lipoteichoic acids as a major virulence factor causing inflammatory responses via Toll-like receptor 2. Arch. Pharm. Res. 2016, 39, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Brightbill, H.D.; Libraty, D.H.; Krutzik, S.R.; Yang, R.B.; Belisle, J.T.; Bleharski, J.R.; Maitland, M.; Norgard, M.V.; Plevy, S.E.; Smale, S.T.; et al. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science 1999, 285, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef]
- Hossain, M.M.; Norazmi, M.N. Pattern recognition receptors and cytokines in Mycobacterium tuberculosis infection—The double-edged sword? Biomed. Res. Int. 2013, 2013, 179174. [Google Scholar] [CrossRef]
- Cook, D.N.; Pisetsky, D.S.; Schwartz, D.A. Toll-like receptors in the pathogenesis of human disease. Nat. Immunol. 2004, 5, 975–979. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, V.D.; Das, S.; Surve, P.; Ghosh, K. Toll-like receptors in autoimmunity with special reference to systemic lupus erythematosus. Indian J. Hum. Genet. 2012, 18, 155–160. [Google Scholar] [CrossRef][Green Version]
- Netea, M.G.; Van der Meer, J.W.; Kullberg, B.J. Toll-like receptors as an escape mechanism from the host defense. Trends Microbiol. 2004, 12, 484–488. [Google Scholar] [CrossRef]
- Mojumdar, K.; Giordano, C.; Lemaire, C.; Liang, F.; Divangahi, M.; Qureshi, S.T.; Petrof, B. Divergent impact of Toll-like receptor 2 deficiency on repair mechanisms in healthy muscle versus Duchenne muscular dystrophy. J. Pathol. 2016, 239, 10–22. [Google Scholar] [CrossRef]
- Moles, A.; Murphy, L.; Wilson, C.L.; Chakraborty, J.B.; Fox, C.; Park, E.J.; Mann, J.; Oakley, F.; Howarth, R.; Brain, J.; et al. A TLR2/S100A9/CXCL-2 signaling network is necessary for neutrophil recruitment in acute and chronic liver injury in the mouse. J. Hepatol. 2014, 60, 782–791. [Google Scholar] [CrossRef]
- Hu, W.; van Steijn, L.; Li, C.; Verbeek, F.J.; Cao, L.; Merks, R.M.H.; Spaink, H.P. A novel function of TLR2 and MyD88 in the regulation of leukocyte cell migration behavior during wounding in zebrafish larvae. Front. Cell Dev. Biol. 2021, 9, 624571. [Google Scholar] [CrossRef]
- Dasu, M.R.; Thangappan, R.K.; Bourgette, A.; DiPietro, L.A.; Isseroff, R.; Jialal, I. TLR2 expression and signaling-dependent inflammation impair wound healing in diabetic mice. Lab. Investig. 2010, 90, 1628–1636. [Google Scholar] [CrossRef]
- Landen, N.X.; Li, D.; Stahle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef]
- Kondo, T.; Kawai, T.; Akira, S. Dissecting negative regulation of Toll-like receptor signaling. Trends Immunol. 2012, 33, 449–458. [Google Scholar] [CrossRef]
- Yang, L.; Seki, E. Toll-like receptors in liver fibrosis: Cellular crosstalk and mechanisms. Front. Physiol. 2012, 3, 138. [Google Scholar] [CrossRef]
- LeBouder, E.; Rey-Nores, J.E.; Rushmere, N.K.; Grigorov, M.; Lawn, S.D.; Affolter, M.; Griffin, G.E.; Ferrara, P.; Schiffrin, E.J.; Morgan, P.; et al. Soluble forms of Toll-like receptor (TLR)2 capable of modulating TLR2 signaling are present in human plasma and breast milk. J. Immunol. 2003, 171, 6680–6689. [Google Scholar] [CrossRef]
- Iwami, K.I.; Matsuguchi, T.; Masuda, A.; Kikuchi, T.; Musikacharoen, T.; Yoshikai, Y. Cutting edge: Naturally occurring soluble form of mouse Toll-like receptor 4 inhibits lipopolysaccharide signaling. J. Immunol. 2000, 165, 6682–6686. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.; Lee, J.Y. Intrinsic and extrinsic regulation of innate immune receptors. Yonsei Med. J. 2011, 52, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Trengove, M.C.; Ward, A.C. SOCS proteins in development and disease. Am. J. Clin. Exp. Immunol. 2013, 2, 1–29. [Google Scholar] [PubMed]
- Mukherjee, S.; Biswas, T. Activation of TOLLIP by porin prevents TLR2-associated IFN-γ and TNF-α-induced apoptosis of intestinal epithelial cells. Cell. Signal. 2014, 26, 2674–2682. [Google Scholar] [CrossRef] [PubMed]
- Capelluto, D.G. Tollip: A multitasking protein in innate immunity and protein trafficking. Microbes Infect. 2012, 14, 140–147. [Google Scholar] [CrossRef]
- Kobayashi, K.; Hernandez, L.D.; Galan, J.E.; Janeway, C.A., Jr.; Medzhitov, R.; Flavell, R.A. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell 2002, 110, 191–202. [Google Scholar] [CrossRef]
- So, T. The immunological significance of tumor necrosis factor receptor-associated factors (TRAFs). Int. Immunol. 2022, 34, 7–20. [Google Scholar] [CrossRef]
- Jang, J.H.; Kim, H.; Jung, I.Y.; Cho, J.H. A20 inhibits LPS-induced inflammation by regulating TRAF6 polyubiquitination in rainbow trout. Int. J. Mol. Sci. 2021, 22, 9801. [Google Scholar] [CrossRef] [PubMed]
- Fekete, T.; Bencze, D.; Biro, E.; Benko, S.; Pazmandi, K. Focusing on the cell type specific regulatory actions of NLRX1. Int. J. Mol. Sci. 2021, 22, 1316. [Google Scholar] [CrossRef]
- Nagai-Singer, M.A.; Morrison, H.A.; Allen, I.C. NLRX1 is a multifaceted and enigmatic regulator of immune system function. Front. Immunol. 2019, 10, 2419. [Google Scholar] [CrossRef]
- Zhang, J.; Ou, J.; Wan, X. LRRC62 attenuates Toll-like receptor signaling by deubiquitinating TAK1 via CYLD. Exp. Cell Res. 2019, 383, 111497. [Google Scholar] [CrossRef]
- Zilberman-Rudenko, J.; Shawver, L.M.; Wessel, A.W.; Luo, Y.; Pelletier, M.; Tsai, W.L.; Lee, Y.; Vonortas, S.; Cheng, L.; Ashwell, J.D.; et al. Recruitment of A20 by the C-terminal domain of NEMO suppresses NF-κB activation and autoinflammatory disease. Proc. Natl. Acad. Sci. USA 2016, 113, 1612–1617. [Google Scholar] [CrossRef]
- Skaug, B.; Chen, J.; Du, F.; He, J.; Ma, A.; Chen, Z.J. Direct, noncatalytic mechanism of IKK inhibition by A20. Mol. Cell 2011, 44, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Whitmore, M.M.; Iparraguirre, A.; Kubelka, L.; Weninger, W.; Hai, T.; Williams, B.R. Negative regulation of TLR-signaling pathways by activating transcription factor-3. J. Immunol. 2007, 179, 3622–3630. [Google Scholar] [CrossRef] [PubMed]
- Kuwata, H.; Matsumoto, M.; Atarashi, K.; Morishita, H.; Hirotani, T.; Koga, R.; Takeda, K. IκBNS inhibits induction of a subset of Toll-like receptor-dependent genes and limits inflammation. Immunity 2006, 24, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Carmody, R.J.; Ruan, Q.; Palmer, S.; Hilliard, B.; Chen, Y.H. Negative regulation of Toll-like receptor signaling by NF-κB p50 ubiquitination blockade. Science 2007, 317, 675–678. [Google Scholar] [CrossRef]
- Koch, B.E.V.; Yang, S.; Lamers, G.; Stougaard, J.; Spaink, H.P. Intestinal microbiome adjusts the innate immune setpoint during colonization through negative regulation of MyD88. Nat. Commun. 2018, 9, 4099. [Google Scholar] [CrossRef] [PubMed]
- Hoefsloot, W.; van Ingen, J.; Andrejak, C.; Angeby, K.; Bauriaud, R.; Bemer, P.; Beylis, N.; Boeree, M.J.; Cacho, J.; Chihota, V.; et al. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: An NTM-NET collaborative study. Eur. Respir. J. 2013, 42, 1604–1613. [Google Scholar] [CrossRef] [PubMed]
- Prevots, D.R.; Marras, T.K. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: A review. Clin. Chest Med. 2015, 36, 13–34. [Google Scholar] [CrossRef]
- Saxena, S.; Spaink, H.P.; Forn-Cuni, G. Drug resistance in nontuberculous mycobacteria: Mechanisms and models. Biology 2021, 10, 96. [Google Scholar] [CrossRef]
- Aman, J.; Duijvelaar, E.; Botros, L.; Kianzad, A.; Schippers, J.R.; Smeele, P.J.; Azhang, S.; Bartelink, I.H.; Bayoumy, A.A.; Bet, P.M.; et al. Imatinib in patients with severe COVID-19: A randomised, double-blind, placebo-controlled, clinical trial. Lancet Respir. Med. 2021, 9, 957–968, Erratum in Lancet Respir. Med. 2021, 9, e84. [Google Scholar] [CrossRef]
- Kilinc, G.; Saris, A.; Ottenhoff, T.H.M.; Haks, M.C. Host-directed therapy to combat mycobacterial infections. Immunol. Rev. 2021, 301, 62–83. [Google Scholar] [CrossRef]
- Mehta, P.; Ray, A.; Mazumder, S. TLRs in mycobacterial pathogenesis: Black and white or shades of gray. Curr. Microbiol. 2021, 78, 2183–2193. [Google Scholar] [CrossRef]
- Stocks, C.J.; Schembri, M.A.; Sweet, M.J.; Kapetanovic, R. For when bacterial infections persist: Toll-like receptor-inducible direct antimicrobial pathways in macrophages. J. Leukoc. Biol. 2018, 103, 35–51. [Google Scholar] [CrossRef]
- Faridgohar, M.; Nikoueinejad, H. New findings of Toll-like receptors involved in Mycobacterium tuberculosis infection. Pathog. Glob. Health 2017, 111, 256–264. [Google Scholar] [CrossRef]
- Sharma, N.; Shariq, M.; Quadir, N.; Singh, J.; Sheikh, J.A.; Hasnain, S.E.; Ehtesham, N.Z. Mycobacterium tuberculosis protein PE6 (Rv0335c), a novel TLR4 agonist, evokes an inflammatory response and modulates the cell death pathways in macrophages to enhance intracellular survival. Front. Immunol. 2021, 12, 696491. [Google Scholar] [CrossRef] [PubMed]
- Thada, S.; Horvath, G.L.; Muller, M.M.; Dittrich, N.; Conrad, M.L.; Sur, S.; Hussain, A.; Pelka, K.; Gaddam, S.; Latz, E.; et al. Interaction of TLR4 and TLR8 in the innate immune response against Mycobacterium tuberculosis. Int. J. Mol. Sci. 2021, 22, 1560. [Google Scholar] [CrossRef] [PubMed]
- Abel, B.; Thieblemont, N.; Quesniaux, V.J.; Brown, N.; Mpagi, J.; Miyake, K.; Bihl, F.; Ryffel, B. Toll-like receptor 4 expression is required to control chronic Mycobacterium tuberculosis infection in mice. J. Immunol. 2002, 169, 3155–3162. [Google Scholar] [CrossRef]
- Reiling, N.; Holscher, C.; Fehrenbach, A.; Kroger, S.; Kirschning, C.J.; Goyert, S.; Ehlers, S. Cutting edge: Toll-like receptor (TLR)2- and TLR4-mediated pathogen recognition in resistance to airborne infection with Mycobacterium tuberculosis. J. Immunol. 2002, 169, 3480–3484. [Google Scholar] [CrossRef]
- Bafica, A.; Scanga, C.A.; Feng, C.G.; Leifer, C.; Cheever, A.; Sher, A. TLR9 regulates Th1 responses and cooperates with TLR2 in mediating optimal resistance to Mycobacterium tuberculosis. J. Exp. Med. 2005, 202, 1715–1724. [Google Scholar] [CrossRef]
- Marinho, F.A.; de Paula, R.R.; Mendes, A.C.; de Almeida, L.A.; Gomes, M.T.; Carvalho, N.B.; Oliveira, F.S.; Caliari, M.V.; Oliveira, S.C. Toll-like receptor 6 senses Mycobacterium avium and is required for efficient control of mycobacterial infection. Eur. J. Immunol. 2013, 43, 2373–2385. [Google Scholar] [CrossRef]
- Carvalho, N.B.; Oliveira, F.S.; Duraes, F.V.; de Almeida, L.A.; Florido, M.; Prata, L.O.; Caliari, M.V.; Appelberg, R.; Oliveira, S.C. Toll-like receptor 9 is required for full host resistance to Mycobacterium avium infection but plays no role in induction of Th1 responses. Infect. Immun. 2011, 79, 1638–1646. [Google Scholar] [CrossRef][Green Version]
- Hu, W.; Yang, S.; Shimada, Y.; Munch, M.; Marin-Juez, R.; Meijer, A.H.; Spaink, H.P. Infection and RNA-seq analysis of a zebrafish tlr2 mutant shows a broad function of this toll-like receptor in transcriptional and metabolic control and defense to Mycobacterium marinum infection. BMC Genom. 2019, 20, 878. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.I.; Kurnasov, O.; Natarajan, V.; Hong, M.; Gudkov, A.V.; Osterman, A.L.; Wilson, I.A. Structural basis of TLR5-flagellin recognition and signaling. Science 2012, 335, 859–864. [Google Scholar] [CrossRef]
- Harding, C.V.; Boom, W.H. Regulation of antigen presentation by Mycobacterium tuberculosis: A role for Toll-like receptors. Nat. Rev. Microbiol. 2010, 8, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.S.; Sundling, C.; Folkesson, E.; Froberg, G.; Nobrega, C.; Canto-Gomes, J.; Chambers, B.J.; Lakshmikanth, T.; Brodin, P.; Bruchfeld, J.; et al. High dimensional immune profiling reveals different response patterns in active and latent tuberculosis following stimulation with mycobacterial glycolipids. Front. Immunol. 2021, 12, 727300. [Google Scholar] [CrossRef]
- Kumar, A.; Saini, V.; Kumar, A.; Kaur, J.; Kaur, J. Modulation of trehalose dimycolate and immune system by Rv0774c protein enhanced the intracellular survival of Mycobacterium smegmatis in human macrophages cell line. Front. Cell. Infect. Microbiol. 2017, 7, 289. [Google Scholar] [CrossRef]
- Saraav, I.; Singh, S.; Sharma, S. Outcome of Mycobacterium tuberculosis and Toll-like receptor interaction: Immune response or immune evasion? Immunol. Cell Biol. 2014, 92, 741–746. [Google Scholar] [CrossRef]
- Pennini, M.E.; Pai, R.K.; Schultz, D.C.; Boom, W.H.; Harding, C.V. Mycobacterium tuberculosis 19-kDa lipoprotein inhibits IFN-γ-induced chromatin remodeling of MHC2TA by TLR2 and MAPK signaling. J. Immunol. 2006, 176, 4323–4330. [Google Scholar] [CrossRef] [PubMed]
- Pai, R.K.; Convery, M.; Hamilton, T.A.; Boom, W.H.; Harding, C.V. Inhibition of IFN-γ-induced class II transactivator expression by a 19-kDa lipoprotein from Mycobacterium tuberculosis: A potential mechanism for immune evasion. J. Immunol. 2003, 171, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Drage, M.G.; Pecora, N.D.; Hise, A.G.; Febbraio, M.; Silverstein, R.L.; Golenbock, D.T.; Boom, W.H.; Harding, C.V. TLR2 and its co-receptors determine responses of macrophages and dendritic cells to lipoproteins of Mycobacterium tuberculosis. Cell. Immunol. 2009, 258, 29–37. [Google Scholar] [CrossRef]
- Gehring, A.J.; Dobos, K.M.; Belisle, J.T.; Harding, C.V.; Boom, W.H. Mycobacterium tuberculosis LprG (Rv1411c): A novel TLR-2 ligand that inhibits human macrophage class II MHC antigen processing. J. Immunol. 2004, 173, 2660–2668. [Google Scholar] [CrossRef]
- Pecora, N.D.; Gehring, A.J.; Canaday, D.H.; Boom, W.H.; Harding, C.V. Mycobacterium tuberculosis LprA is a lipoprotein agonist of TLR2 that regulates innate immunity and APC function. J. Immunol. 2006, 177, 422–429. [Google Scholar] [CrossRef]
- Su, H.; Zhu, S.; Zhu, L.; Huang, W.; Wang, H.; Zhang, Z.; Xu, Y. Recombinant lipoprotein Rv1016c Derived from Mycobacterium tuberculosis is a TLR-2 ligand that induces macrophages apoptosis and inhibits MHC II antigen processing. Front. Cell. Infect. Microbiol. 2016, 6, 147. [Google Scholar] [CrossRef]
- Jung, S.B.; Yang, C.S.; Lee, J.S.; Shin, A.R.; Jung, S.S.; Son, J.W.; Harding, C.V.; Kim, H.-J.; Park, J.-K.; Paik, T.-H.; et al. The mycobacterial 38-kilodalton glycolipoprotein antigen activates the mitogen-activated protein kinase pathway and release of proinflammatory cytokines through Toll-like receptors 2 and 4 in human monocytes. Infect. Immun. 2006, 74, 2686–2696. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.T.; Li, J.Y.; Zhang, Y.; Gao, X.; Cai, H. Recombinant MPT83 derived from Mycobacterium tuberculosis induces cytokine production and upregulates the function of mouse macrophages through TLR2. J. Immunol. 2012, 188, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Hook, J.S.; Cao, M.; Weng, K.; Kinnare, N.; Moreland, J.G. Mycobacterium tuberculosis lipoarabinomannan activates human neutrophils via a TLR2/1 mechanism distinct from Pam3CSK4. J. Immunol. 2020, 204, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Bhattacharjee, O.; Goswami, A.; Pal, N.K.; Majumdar, S. Arabinosylated lipoarabinomannan (Ara-LAM) mediated intracellular mechanisms against tuberculosis infection: Involvement of protein kinase C (PKC) mediated signaling. Tuberculosis 2015, 95, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Richardson, E.T.; Drage, M.G.; Boom, W.H.; Harding, C.V. Mycobacterium tuberculosis lipoprotein and lipoglycan binding to Toll-like receptor 2 correlates with agonist activity and functional outcomes. Infect. Immun. 2018, 86, e00450-18. [Google Scholar] [CrossRef] [PubMed]
- Gilleron, M.; Nigou, J.; Nicolle, D.; Quesniaux, V.; Puzo, G. The acylation state of mycobacterial lipomannans modulates innate immunity response through Toll-like receptor 2. Chem. Biol. 2006, 13, 39–47. [Google Scholar] [CrossRef]
- Gilleron, M.; Quesniaux, V.F.; Puzo, G. Acylation state of the phosphatidylinositol hexamannosides from Mycobacterium bovis bacillus Calmette Guerin and Mycobacterium tuberculosis H37Rv and its implication in Toll-like receptor response. J. Biol. Chem. 2003, 278, 29880–29889. [Google Scholar] [CrossRef]
- Bowdish, D.M.; Sakamoto, K.; Kim, M.J.; Kroos, M.; Mukhopadhyay, S.; Leifer, C.A.; Tryggvason, K.; Gordon, S.; Russell, D.G. MARCO, TLR2, and CD14 are required for macrophage cytokine responses to mycobacterial trehalose dimycolate and Mycobacterium tuberculosis. PLoS Pathog. 2009, 5, e1000474. [Google Scholar] [CrossRef]
- Bulut, Y.; Michelsen, K.S.; Hayrapetian, L.; Naiki, Y.; Spallek, R.; Singh, M.; Arditi, M. Mycobacterium tuberculosis heat shock proteins use diverse Toll-like receptor pathways to activate pro-inflammatory signals. J. Biol. Chem. 2005, 280, 20961–20967. [Google Scholar] [CrossRef]
- Saraav, I.; Singh, S.; Pandey, K.; Sharma, M.; Sharma, S. Mycobacterium tuberculosis MymA is a TLR2 agonist that activate macrophages and a TH1 response. Tuberculosis 2017, 106, 16–24. [Google Scholar] [CrossRef]
- Palucci, I.; Camassa, S.; Cascioferro, A.; Sali, M.; Anoosheh, S.; Zumbo, A.; Minerva, M.; Iantomasi, R.; De Maio, F.; Di Sante, G.; et al. PE_PGRS33 contributes to Mycobacterium tuberculosis entry in macrophages through interaction with TLR2. PLoS ONE 2016, 11, e0150800. [Google Scholar] [CrossRef]
- Zumbo, A.; Palucci, I.; Cascioferro, A.; Sali, M.; Ventura, M.; D’Alfonso, P.; Iantomasi, R.; Di Sante, G.; Ria, F.; Sanguinetti, M.; et al. Functional dissection of protein domains involved in the immunomodulatory properties of PE_PGRS33 of Mycobacterium tuberculosis. Pathog. Dis. 2013, 69, 232–239. [Google Scholar] [CrossRef]
- Pattanaik, K.P.; Ganguli, G.; Naik, S.K.; Sonawane, A. Mycobacterium tuberculosis EsxL induces TNF-α secretion through activation of TLR2 dependent MAPK and NF-κB pathways. Mol. Immunol. 2021, 130, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Pandey, A.D.; Mukhopadhyay, S. The PPE18 protein of Mycobacterium tuberculosis inhibits NF-κB/rel-mediated proinflammatory cytokine production by upregulating and phosphorylating suppressor of cytokine signaling 3 protein. J. Immunol. 2011, 186, 5413–5424. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Ramaswamy, P.A.; Ghosh, S.; Joshi, D.C.; Pathak, N.; Siddiqui, I.; Sharma, P.; Hasnain, S.; Mande, S.C.; Mukhopadhyay, S. The PPE18 of Mycobacterium tuberculosis interacts with TLR2 and activates IL-10 induction in macrophage. J. Immunol. 2009, 183, 6269–6281. [Google Scholar] [CrossRef]
- Su, H.; Kong, C.; Zhu, L.; Huang, Q.; Luo, L.; Wang, H.; Xu, Y. PPE26 induces TLR2-dependent activation of macrophages and drives Th1-type T-cell immunity by triggering the cross-talk of multiple pathways involved in the host response. Oncotarget 2015, 6, 38517–38537. [Google Scholar] [CrossRef]
- Deng, W.; Li, W.; Zeng, J.; Zhao, Q.; Li, C.; Zhao, Y.; Xie, J. Mycobacterium tuberculosis PPE family protein Rv1808 manipulates cytokines profile via co-activation of MAPK and NF-κB signaling pathways. Cell. Physiol. Biochem. 2014, 33, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yang, E.; Huang, Q.; Ni, W.; Kong, C.; Liu, G.; Li, G.; Su, H.; Wang, H. PPE57 induces activation of macrophages and drives Th1-type immune responses through TLR2. J. Mol. Med. 2015, 93, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, J.Y.; Chen, S.T.; Huang, H.R.; Cai, H. The rLrp of Mycobacterium tuberculosis inhibits proinflammatory cytokine production and downregulates APC function in mouse macrophages via a TLR2-mediated PI3K/Akt pathway activation-dependent mechanism. Cell. Mol. Immunol. 2016, 13, 729–746. [Google Scholar] [CrossRef]
- Sweet, L.; Zhang, W.; Torres-Fewell, H.; Serianni, A.; Boggess, W.; Schorey, J. Mycobacterium avium glycopeptidolipids require specific acetylation and methylation patterns for signaling through Toll-like receptor 2. J. Biol. Chem. 2008, 283, 33221–33231. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Schorey, J.S. Exosomes released from infected macrophages contain Mycobacterium avium glycopeptidolipids and are proinflammatory. J. Biol. Chem. 2007, 282, 25779–25789. [Google Scholar] [CrossRef] [PubMed]
- Sweet, L.; Schorey, J.S. Glycopeptidolipids from Mycobacterium avium promote macrophage activation in a TLR2- and MyD88-dependent manner. J. Leukoc. Biol. 2006, 80, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Roux, A.L.; Ray, A.; Pawlik, A.; Medjahed, H.; Etienne, G.; Rottman, M.; Catherinot, E.; Coppée, J.-Y.; Chaoui, K.; Monsarrat, B.; et al. Overexpression of proinflammatory TLR-2-signalling lipoproteins in hypervirulent mycobacterial variants. Cell. Microbiol. 2011, 13, 692–704. [Google Scholar] [CrossRef]
- Briken, V.; Porcelli, S.A.; Besra, G.S.; Kremer, L. Mycobacterial lipoarabinomannan and related lipoglycans: From biogenesis to modulation of the immune response. Mol. Microbiol. 2004, 53, 391–403. [Google Scholar] [CrossRef]
- Wieland, C.W.; Knapp, S.; Florquin, S.; de Vos, A.F.; Takeda, K.; Akira, S.; Golenbock, D.T.; Verbon, A.; Van Der Poll, T. Non-mannose-capped lipoarabinomannan induces lung inflammation via Toll-like receptor 2. Am. J. Respir. Crit. Care Med. 2004, 170, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Texereau, J.; Chiche, J.D.; Taylor, W.; Choukroun, G.; Comba, B.; Mira, J.P. The importance of Toll-like receptor 2 polymorphisms in severe infections. Clin. Infect. Dis. 2005, 41, S408–S415. [Google Scholar] [CrossRef]
- Ogus, A.C.; Yoldas, B.; Ozdemir, T.; Uguz, A.; Olcen, S.; Keser, I.; Coskun, M.; Cilli, A.; Yegin, O. The Arg753GLn polymorphism of the human Toll-like receptor 2 gene in tuberculosis disease. Eur. Respir. J. 2004, 23, 219–223. [Google Scholar] [CrossRef]
- Pattabiraman, G.; Panchal, R.; Medvedev, A.E. The R753Q polymorphism in Toll-like receptor 2 (TLR2) attenuates innate immune responses to mycobacteria and impairs MyD88 adapter recruitment to TLR2. J. Biol. Chem. 2017, 292, 10685–10695. [Google Scholar] [CrossRef]
- Sanchez, D.; Lefebvre, C.; Rioux, J.; Garcia, L.F.; Barrera, L.F. Evaluation of Toll-like receptor and adaptor molecule polymorphisms for susceptibility to tuberculosis in a Colombian population. Int. J. Immunogenet. 2012, 39, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.; Nasiri, M.R.; Sanaei, R.; Anoosheh, S.; Farnia, P.; Sepanjnia, A.; Tajik, N. The NRAMP1, VDR, TNF-α, ICAM1, TLR2 and TLR4 gene polymorphisms in Iranian patients with pulmonary tuberculosis: A case-control study. Infect. Genet. Evol. 2016, 39, 92–98. [Google Scholar] [CrossRef]
- Yim, J.J.; Kim, H.J.; Kwon, O.J.; Koh, W.J. Association between microsatellite polymorphisms in intron II of the human Toll-like receptor 2 gene and nontuberculous mycobacterial lung disease in a Korean population. Hum. Immunol. 2008, 69, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.G.; Scanga, C.A.; Collazo-Custodio, C.M.; Cheever, A.W.; Hieny, S.; Caspar, P.; Sher, A. Mice lacking myeloid differentiation factor 88 display profound defects in host resistance and immune responses to Mycobacterium avium infection not exhibited by Toll-like receptor 2 (TLR2)- and TLR4-deficient animals. J. Immunol. 2003, 171, 4758–4764. [Google Scholar] [CrossRef] [PubMed]
- Drennan, M.B.; Nicolle, D.; Quesniaux, V.J.; Jacobs, M.; Allie, N.; Mpagi, J.; Frémond, C.; Wagner, H.; Kirschning, C.; Ryffel, B. Toll-like receptor 2-deficient mice succumb to Mycobacterium tuberculosis infection. Am. J. Pathol. 2004, 164, 49–57. [Google Scholar] [CrossRef]
- Carlos, D.; Frantz, F.G.; Souza-Junior, D.A.; Jamur, M.C.; Oliver, C.; Ramos, S.G.; Quesniaux, V.F.; Ryffel, B.; Silva, C.L.; Bozza, M.T.; et al. TLR2-dependent mast cell activation contributes to the control of Mycobacterium tuberculosis infection. Microbes Infect. 2009, 11, 770–778. [Google Scholar] [CrossRef]
- McBride, A.; Konowich, J.; Salgame, P. Host defense and recruitment of Foxp3(+) T regulatory cells to the lungs in chronic Mycobacterium tuberculosis infection requires Toll-like receptor 2. PLoS Pathog. 2013, 9, e1003397. [Google Scholar] [CrossRef]
- Konowich, J.; Gopalakrishnan, A.; Dietzold, J.; Verma, S.; Bhatt, K.; Rafi, W.; Salgame, P. Divergent functions of TLR2 on hematopoietic and nonhematopoietic cells during chronic Mycobacterium tuberculosis infection. J. Immunol. 2017, 198, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, I.; Yamada, H.; Li, C.; Mizuno, S.; Takeuchi, O.; Akira, S. Mycobacterial infection in TLR2 and TLR6 knockout mice. Microbiol. Immunol. 2003, 47, 327–336. [Google Scholar] [CrossRef]
- Cooper, A.M. Mouse model of tuberculosis. Cold Spring Harb. Perspect. Med. 2014, 5, a018556. [Google Scholar] [CrossRef]
- Hu, W. The Function of Toll-Like Receptor 2 in Infection and Inflammation. Ph.D. Thesis, Leiden University, Leiden, The Netherlands, 2021. [Google Scholar]
- BoseDasgupta, S.; Pieters, J. Macrophage-microbe interaction: Lessons learned from the pathogen Mycobacterium tuberculosis. Semin. Immunopathol. 2018, 40, 577–591. [Google Scholar] [CrossRef]
- Kramarska, E.; Squeglia, F.; De Maio, F.; Delogu, G.; Berisio, R. PE_PGRS33, an important virulence factor of Mycobacterium tuberculosis and potential target of host humoral immune response. Cells 2021, 10, 161. [Google Scholar] [CrossRef]
- Bocchino, M.; Galati, D.; Sanduzzi, A.; Colizzi, V.; Brunetti, E.; Mancino, G. Role of mycobacteria-induced monocyte/macrophage apoptosis in the pathogenesis of human tuberculosis. Int. J. Tuberc. Lung Dis. 2005, 9, 375–383. [Google Scholar] [PubMed]
- Sanchez, D.; Rojas, M.; Hernandez, I.; Radzioch, D.; Garcia, L.F.; Barrera, L.F. Role of TLR2- and TLR4-mediated signaling in Mycobacterium tuberculosis-induced macrophage death. Cell. Immunol. 2010, 260, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Chang, Q.; Dai, X.; Liu, D.; Jiang, Y.; Dai, Y. Early secreted antigenic target of 6-kDa of Mycobacterium tuberculosis promotes caspase-9/caspase-3-mediated apoptosis in macrophages. Mol. Cell. Biochem. 2019, 457, 179–189. [Google Scholar] [CrossRef]
- Ghorpade, D.S.; Leyland, R.; Kurowska-Stolarska, M.; Patil, S.A.; Balaji, K.N. MicroRNA-155 is required for Mycobacterium bovis BCG-mediated apoptosis of macrophages. Mol. Cell. Biol. 2012, 32, 2239–2253. [Google Scholar] [CrossRef]
- Ayelign, B.; Workneh, M.; Molla, M.D.; Dessie, G. Role of vitamin-D supplementation in TB/HIV co-infected patients. Infect. Drug Resist. 2020, 13, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Krutzik, S.R.; Hewison, M.; Liu, P.T.; Robles, J.A.; Stenger, S.; Adams, J.S.; Modlin, R.L. IL-15 links TLR2/1-induced macrophage differentiation to the vitamin D-dependent antimicrobial pathway. J. Immunol. 2008, 181, 7115–7120. [Google Scholar] [CrossRef]
- Rivas-Santiago, B.; Hernandez-Pando, R.; Carranza, C.; Juarez, E.; Contreras, J.L.; Aguilar-Leon, D.; Torres, M.; Sada, E. Expression of cathelicidin LL-37 during Mycobacterium tuberculosis infection in human alveolar macrophages, monocytes, neutrophils, and epithelial cells. Infect. Immun. 2008, 76, 935–941. [Google Scholar] [CrossRef]
- Hinman, A.E.; Jani, C.; Pringle, S.C.; Zhang, W.R.; Jain, N.; Martinot, A.J.; Barczak, A.K. Mycobacterium tuberculosis canonical virulence factors interfere with a late component of the TLR2 response. eLife 2021, 10, e73984. [Google Scholar] [CrossRef]
- Lasunskaia, E.B.; Campos, M.N.; de Andrade, M.R.; Damatta, R.A.; Kipnis, T.L.; Einicker-Lamas, M.; Da Silva, W.D. Mycobacteria directly induce cytoskeletal rearrangements for macrophage spreading and polarization through TLR2-dependent PI3K signaling. J. Leukoc. Biol. 2006, 80, 1480–1490. [Google Scholar] [CrossRef]
- Eum, S.Y.; Kong, J.H.; Hong, M.S.; Lee, Y.J.; Kim, J.H.; Hwang, S.H.; Cho, S.-N.; Via, L.; Barry, C.E. Neutrophils are the predominant infected phagocytic cells in the airways of patients with active pulmonary TB. Chest 2010, 137, 122–128. [Google Scholar] [CrossRef]
- Borkute, R.R.; Woelke, S.; Pei, G.; Dorhoi, A. Neutrophils in tuberculosis: Cell biology, cellular networking and multitasking in host defense. Int. J. Mol. Sci. 2021, 22, 4801. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, A.; Dietzold, J.; Verma, S.; Bhagavathula, M.; Salgame, P. Toll-like receptor 2 prevents neutrophil-driven immunopathology during infection with Mycobacterium tuberculosis by curtailing CXCL5 production. Infect Immun. 2019, 87, e00760-18. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Lutay, N.; Hallgren, O.; Westergren-Thorsson, G.; Svensson, M.; Godaly, G. Mycobacterium bovis bacilli Calmette-Guerin regulates leukocyte recruitment by modulating alveolar inflammatory responses. Innate Immun. 2012, 18, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Tyne, A.S.; Chan, J.G.Y.; Shanahan, E.R.; Atmosukarto, I.; Chan, H.K.; Britton, W.J.; West, N.P. TLR2-targeted secreted proteins from Mycobacterium tuberculosis are protective as powdered pulmonary vaccines. Vaccine 2013, 31, 4322–4329. [Google Scholar] [CrossRef] [PubMed]
- Mangtani, P.; Abubakar, I.; Ariti, C.; Beynon, R.; Pimpin, L.; Fine, P.E.M.; Rodrigues, L.C.; Smith, P.; Lipman, M.; Whiting, P.; et al. Protection by BCG vaccine against tuberculosis: A systematic review of randomized controlled trials. Clin. Infect. Dis. 2014, 58, 470–480. [Google Scholar] [CrossRef]
- Tran, V.; Liu, J.; Behr, M.A. BCG vaccines. Microbiol. Spectr. 2014, 2, 1–11. [Google Scholar] [CrossRef]
- Yang, Q.; Liao, M.; Wang, W.; Zhang, M.; Chen, Q.; Guo, J.; Peng, B.; Huang, J.; Liu, H.; Yahagi, A.; et al. CD157 confers host resistance to Mycobacterium tuberculosis via TLR2-CD157-PKCzeta-induced reactive oxygen species production. mBio 2019, 10, e01949-19. [Google Scholar] [CrossRef]
- Ahmed, A.; Dolasia, K.; Mukhopadhyay, S. Mycobacterium tuberculosis PPE18 protein reduces inflammation and increases survival in animal model of sepsis. J. Immunol. 2018, 200, 3587–3598. [Google Scholar] [CrossRef]
- Khan, S.; Shafiei, M.; Longoria, C.; Schoggins, J.W.; Savani, R.; Zaki, H. SARS-CoV-2 spike protein induces inflammation via TLR2-dependent activation of the NF-kB pathway. eLife 2021, 10, e68563. [Google Scholar] [CrossRef]
- Proud, P.C.; Tsitoura, D.; Watson, R.J.; Chua, B.Y.; Aram, M.J.; Bewley, K.R.; Cavell, B.E.; Cobb, R.; Dowall, S.; Fotheringham, S.A.; et al. Prophylactic intranasal administration of a TLR2/6 agonist reduces upper respiratory tract viral shedding in a SARS-CoV-2 challenge ferret model. EBioMedicine 2021, 63, 103153. [Google Scholar] [CrossRef]
- Zheng, M.; Karki, R.; Williams, E.P.; Yang, D.; Fitzpatrick, E.; Vogel, P.; Jonsson, C.B.; Kanneganti, T.-D. TLR2 senses the SARS-CoV-2 envelope protein to produce inflammatory cytokines. Nat. Immunol. 2021, 22, 829–838. [Google Scholar] [CrossRef]
- Medha; Bhatt, P.; Priyanka; Sharma, M.; Sharma, S. Prediction and identification of T cell epitopes of COVID-19 with balanced cytokine response for the development of peptide based vaccines. Silico Pharm. 2021, 9, 40. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Marks, K.E.; Cho, K.; Stickling, C.; Reynolds, J.M. Toll-like receptor 2 in autoimmune inflammation. Immune Netw. 2021, 21, e18. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yin, H.; Zhao, M.; Lu, Q. TLR2 and TLR4 in autoimmune diseases: A comprehensive review. Clin. Rev. Allergy Immunol. 2014, 47, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Sepe, V.; Libetta, C.; Gregorini, M.; Rampino, T. The innate immune system in human kidney inflammaging. J. Nephrol. 2021, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Arslan, F.; Keogh, B.; McGuirk, P.; Parker, A.E. TLR2 and TLR4 in ischemia reperfusion injury. Mediat. Inflamm. 2010, 2010, 704202. [Google Scholar] [CrossRef] [PubMed]
- Qasem, A.; Naser, A.E.; Naser, S.A. Enteropathogenic infections modulate intestinal serotonin transporter (SERT) function by activating Toll-like receptor 2 (TLR-2) in Crohn’s disease. Sci. Rep. 2021, 11, 22624. [Google Scholar] [CrossRef] [PubMed]
- Gruijs, M.; Ganzevles, S.H.; Stigter-van Walsum, M.; van der Mast, R.; van Ostaijen-Ten Dam, M.M.; Tuk, C.W.; Schilham, M.W.; Leemans, C.R.; Brakenhoff, R.H.; van Egmond, M.; et al. NK cell-dependent antibody-mediated immunotherapy is improved in vitro and in vivo when combined with agonists for Toll-like receptor 2 in head and neck cancer models. Int. J. Mol. Sci. 2021, 22, 11057. [Google Scholar] [CrossRef]
- Beilmann-Lehtonen, I.; Bockelman, C.; Mustonen, H.; Koskensalo, S.; Hagstrom, J.; Haglund, C. The prognostic role of tissue TLR2 and TLR4 in colorectal cancer. Virchows Arch. 2020, 477, 705–715. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, Y.Y.; Zhang, Y.L. High expression of TLR2 in the serum of patients with tuberculosis and lung cancer, and can promote the progression of lung cancer. Math. Biosci. Eng. 2019, 17, 1959–1972. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, A.; Bolli, E.; Tarone, L.; Cavallo, F.; Conti, L. Toll-like receptor 2 at the crossroad between cancer cells, the immune system, and the microbiota. Int. J. Mol. Sci. 2020, 21, 9418. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Chiba, A.; Fukumoto, M.; Morooka, N.; Mochizuki, N. Zebrafish vascular development: General and tissue-specific regulation. J. Lipid Atheroscler. 2021, 10, 145–159. [Google Scholar] [CrossRef]
- van der Vaart, M.; Spaink, H.P.; Meijer, A.H. Pathogen recognition and activation of the innate immune response in zebrafish. Adv. Hematol. 2012, 2012, 159807. [Google Scholar] [CrossRef]
- Li, S.; Yeo, K.S.; Levee, T.M.; Howe, C.J.; Her, Z.P.; Zhu, S. Zebrafish as a neuroblastoma model: Progress made, promise for the future. Cells 2021, 10, 580. [Google Scholar] [CrossRef]
- Torraca, V.; Mostowy, S. Zebrafish infection: From pathogenesis to cell biology. Trends Cell Biol. 2018, 28, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Patton, E.E.; Zon, L.I.; Langenau, D.M. Zebrafish disease models in drug discovery: From preclinical modelling to clinical trials. Nat. Rev. Drug Discov. 2021, 20, 611–628. [Google Scholar] [CrossRef] [PubMed]
- Sieber, S.; Grossen, P.; Bussmann, J.; Campbell, F.; Kros, A.; Witzigmann, D.; Huwyler, J. Zebrafish as a preclinical in vivo screening model for nanomedicines. Adv. Drug Deliv. Rev. 2019, 151–152, 152–168. [Google Scholar] [CrossRef]
- Meijer, A.H.; Spaink, H.P. Host-pathogen interactions made transparent with the zebrafish model. Curr. Drug Targets 2011, 12, 1000–1017. [Google Scholar] [CrossRef]
- Lachmandas, E.; Beigier-Bompadre, M.; Cheng, S.C.; Kumar, V.; van Laarhoven, A.; Wang, X.; Ammerdorffer, A.; Boutens, L.; de Jong, D.; Kanneganti, T.-D.; et al. Rewiring cellular metabolism via the AKT/mTOR pathway contributes to host defence against Mycobacterium tuberculosis in human and murine cells. Eur. J. Immunol. 2016, 46, 2574–2586. [Google Scholar] [CrossRef]
- Liu, P.T.; Krutzik, S.R.; Modlin, R.L. Therapeutic implications of the TLR and VDR partnership. Trends Mol. Med. 2007, 13, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Rougeot, J.; Torraca, V.; Zakrzewska, A.; Kanwal, Z.; Jansen, H.J.; Sommer, F.; Spaink, H.P.; Meijer, A.H. RNAseq profiling of leukocyte populations in zebrafish larvae reveals a cxcl11 chemokine gene as a marker of macrophage polarization during mycobacterial infection. Front. Immunol. 2019, 10, 832. [Google Scholar] [CrossRef]
- Torraca, V.; Cui, C.; Boland, R.; Bebelman, J.P.; van der Sar, A.M.; Smit, M.J.; Siderius, M.; Spaink, H.; Meijer, A.H. The CXCR3-CXCL11 signaling axis mediates macrophage recruitment and dissemination of mycobacterial infection. Dis. Model. Mech. 2015, 8, 253–269. [Google Scholar] [CrossRef]
- Dona, E.; Barry, J.D.; Valentin, G.; Quirin, C.; Khmelinskii, A.; Kunze, A.; Durdu, S.; Newton, L.R.; Fernandez-Minan, A.; Huber, W.; et al. Directional tissue migration through a self-generated chemokine gradient. Nature 2013, 503, 285–289. [Google Scholar] [CrossRef]
- Tweedy, L.; Knecht, D.A.; Mackay, G.M.; Insall, R.H. Self-generated chemoattractant gradients: Attractant depletion extends the range and robustness of chemotaxis. PLoS Biol. 2016, 14, e1002404. [Google Scholar] [CrossRef] [PubMed]
- Manolopoulou, I.; Matheu, M.P.; Cahalan, M.D.; West, M.; Kepler, T.B. Bayesian spatio-dynamic modeling in cell motility studies: Learning nonlinear taxic fields guiding the immune response. J. Am. Stat. Assoc. 2012, 107, 855–865. [Google Scholar] [CrossRef]
- Yiu, J.H.; Dorweiler, B.; Woo, C.W. Interaction between gut microbiota and Toll-like receptor: From immunity to metabolism. J. Mol. Med. 2017, 95, 13–20. [Google Scholar] [CrossRef]
- Olona, A.; Hateley, C.; Muralidharan, S.; Wenk, M.R.; Torta, F.; Behmoaras, J. Sphingolipid metabolism during Toll-like receptor 4 (TLR4)-mediated macrophage activation. Br. J. Pharmacol. 2021, 178, 4575–4587. [Google Scholar] [CrossRef]
- van Steijn, L.; Verbeek, F.J.; Spaink, H.P.; Merks, R.M.H. Predicting metabolism from gene expression in an improved whole-genome metabolic network model of Danio rerio. Zebrafish 2019, 16, 348–362. [Google Scholar] [CrossRef]
- Rada, I.; Deldicque, L.; Francaux, M.; Zbinden-Foncea, H. Toll like receptor expression induced by exercise in obesity and metabolic syndrome: A systematic review. Exerc. Immunol. Rev. 2018, 24, 60–71. [Google Scholar]
- Fan, X.L.; Song, Y.; Qin, D.X.; Lin, P.Y. Regulatory effects of Clock and Bmal1 on circadian rhythmic TLR expression. Int. Rev. Immunol. 2021, 1–12. [Google Scholar] [CrossRef]
| Spp | Ligand(s) | Abbreviation | PRRs | Accessory Molecules | Observations | Ref. |
|---|---|---|---|---|---|---|
| M. tuberculosis | Lipoproteins | |||||
| 19-kDa lipoprotein (Rv3763) | LpqH | TLR2/1 | CD14 | Inhibits MHC expression and antigen processing; IFN-γ-induced genes are inhibited by prolonged LpqH stimulation | [115,116,117] | |
| 24-kDa lipoprotein (Rv1270c) | LprA | TLR2/1 | CD14/CD36 | Induces cytokine response and regulates antigen presenting cell functions | [117,119] | |
| 24-kDa lipoprotein (Rv1411c) | LprG | TLR2/1; TLR2 | CD14 | Long-term exposure of LprG inhibits the processing of MHC-II antigen; Short-term exposure of LprG induces the production of TNF-α | [117,118] | |
| 24-kDa lipoprotein (Rv1016c) | LpqT | TLR2 | Unknown | Induces TLR2-dependent apoptosis in macrophages and inhibits MHC expression and antigen processing | [120] | |
| 38-kDa glycolipoprotein | PhoS1 | TLR2/1, TLR4 | Unknown | Activates the ERK1/2 and p38 MAPK signaling, which in turn induces TNF-α and IL-6 expression | [117,121] | |
| Lipoylated and glycosylated Mtb lipoprotein (Rv2873) | MPT83 | TLR2 | unknown | MPT83-induced cytokine production is decreased in TLR2 defective mice | [122] | |
| Lipoglycans/Glycolipids | ||||||
| Lipoarabinomannan | LAM | TLR2/1; TLR2 | CD14 | Mtb LAM induces the production of pro- and anti-inflammatory cytokines to activate neutrophils | [111,123] | |
| Arabinosylated lipoarabinomannan | AraLAM | TLR2 | Unknown | Induces pro-inflammatory responses | [124] | |
| Lipomannans | LM | TLR2/1; TLR2; | CD40/CD86 | Induces TNF-α and NO secretion to activate macrophages | [125,126] | |
| phosphatidylinositol dimannoside | PIM2/6 | TLR2 | Unknown | Induces the expression of TNF-α to activate macrophages | [111,127] | |
| Trehalose dimycolate | TDM | TLR2 | CD14/MARCO | Induces NF-κB signaling | [128] | |
| Others | ||||||
| Heat shock protein 70 | HSP70 | TLR2 | Unknown | Inhibits the secretion of IL-6 in TLR2-deficient macrophages | [129] | |
| 55-kDa flavin containing monooxygenase (Rv3083) | MymA | TLR2 | CD40/CD80/ CD86/HLA-DR | Upregulates the expression of TLR2 and its co-simulatory molecules Activates macrophages by inducing TNF- α and IL-12 | [130] | |
| PE_PGRS proteins (Rv1818c) | PE_PGRS33 | TLR2 | CD14 | Contributes to Mtb entering macrophages by interacting with TLR2 | [131,132] | |
| Secreted antigenic targets of 6-kDa (ESAT-6)family proteins (Rv1198) | EsxL | TLR2 | Unknown | Induces TNF-α and IL-6 through TLR2-dependent NF-κB and MAPK signaling | [133] | |
| PE/PPE protein (Rv1196) | PPE18 | TLR2 | Unknown | Interacts with TLR2 to produce IL-10 and SOCS3 to in turn inhibit TLR2 signaling | [134,135] | |
| PE/PPE protein (Rv1789) | PPE26 | TLR2 | CD80/CD86 | Activates macrophages by inducing pro-inflammatory cytokines TNF-α, IL-6, and IL-12 | [136] | |
| PE/PPE protein (Rv1808) | PPE32 | TLR2 | Unknown | Induces both anti-inflammatory cytokine IL-10 and pro-inflammatory cytokines TNF-α and IL-6 | [137] | |
| PE/PPE protein (Rv3425) | PPE57 | TLR2 | CD40/CD80/ CD86 | Activates macrophages by inducing pro-inflammatory cytokines TNF-α, IL-6, and IL-12 | [138] | |
| Leucine-responsive regulatory protein | Lrp | TLR2 | Unknown | Inhibits LPS-induced pro-inflammatory cytokine IL-12 and TNF-α production | [139] | |
| M. avium | Glycopeptidolipids | GPLs | TLR2, TLR4 | Unknown | Promotes the activation of macrophages dependent on TLR2 and MYD88 TLR2 recognition of GPLs is dependent on specific acetylation and methylation patterns | [140,141,142] |
| M. abscessus | Glycopeptidolipids | GPLs | TLR2 | Unknown | The switch of Mab from the smooth to the rough morphotype depends on the presence of bacterial surface GPLs | [143] |
| M. smegmatis | Phosphoinositol-capped LAM | PILAM | TLR2/ TLR1 | Unknown | High affinity binding to TLR2 and strong pro-inflammatory responses | [125,144] |
| Arabinosylated lipoarabinomannan | AraLAM | TLR2 | CD14? | The lung inflammation induced by AraLAM is diminished in TLR2-deficient mice | [145] | |
| Dimannoside hosphatidyl-myo-inositol mannosides | PIM2/6 | TLR2 | Unknown | Induces the expression of TNF to activate primary macrophages | [127] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, W.; Spaink, H.P. The Role of TLR2 in Infectious Diseases Caused by Mycobacteria: From Cell Biology to Therapeutic Target. Biology 2022, 11, 246. https://doi.org/10.3390/biology11020246
Hu W, Spaink HP. The Role of TLR2 in Infectious Diseases Caused by Mycobacteria: From Cell Biology to Therapeutic Target. Biology. 2022; 11(2):246. https://doi.org/10.3390/biology11020246
Chicago/Turabian StyleHu, Wanbin, and Herman P. Spaink. 2022. "The Role of TLR2 in Infectious Diseases Caused by Mycobacteria: From Cell Biology to Therapeutic Target" Biology 11, no. 2: 246. https://doi.org/10.3390/biology11020246
APA StyleHu, W., & Spaink, H. P. (2022). The Role of TLR2 in Infectious Diseases Caused by Mycobacteria: From Cell Biology to Therapeutic Target. Biology, 11(2), 246. https://doi.org/10.3390/biology11020246







