Isolation of Endogenous TGF-β1 from Root Canals for Pulp Tissue Engineering: A Translational Study
Abstract
:Simple Summary
Abstract
1. Introduction
- How do disinfecting agents affect the amounts of growth factors, and can the irrigation and disinfection protocol be adapted to a subsequent regenerative treatment?
- Is it possible to process growth factors in a chairside protocol after collection from root dentin such that these can be mixed with a biomaterial and injected back into the canal at a sufficiently high concentration?
- Is it feasible to collect sufficient amounts of growth factors during conventional root canal treatment?
2. Materials and Methods
2.1. Optimization of Release Parameters Ex Vivo
2.1.1. Step 1: Irrigation
- 2% sodium hypochlorite (Hypochlorit-SPEIKO®, Dr. Speier, Bielefeld, Germany)
- 0.2% chlorhexidine (Chlorhexidine digluconate solution, Sigma-Aldrich, St. Louis, MO, USA)
- 0.9% saline (control)
2.1.2. Step 2: Root Canal Enlargement
2.1.3. Step 3: Intracanal Dressing with Calcium Hydroxide
2.2. Concentration and Preparation Ex Vivo
2.3. Cytotoxic Effects of EDTA and Neutralization
2.4. In Vivo Availability of TGF-β1
2.5. Statistical Analysis
3. Results
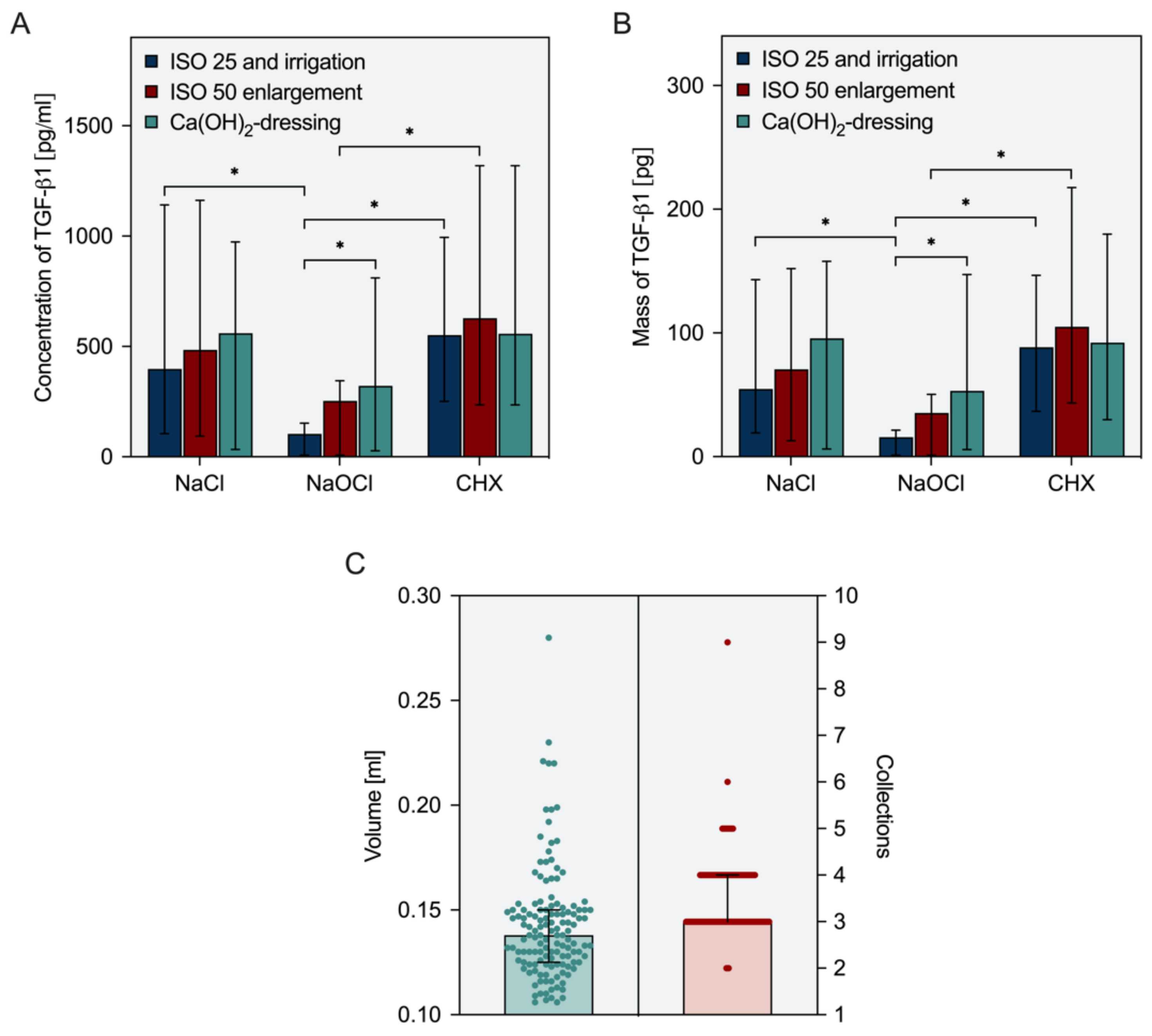
3.1. Release Parameters Ex Vivo
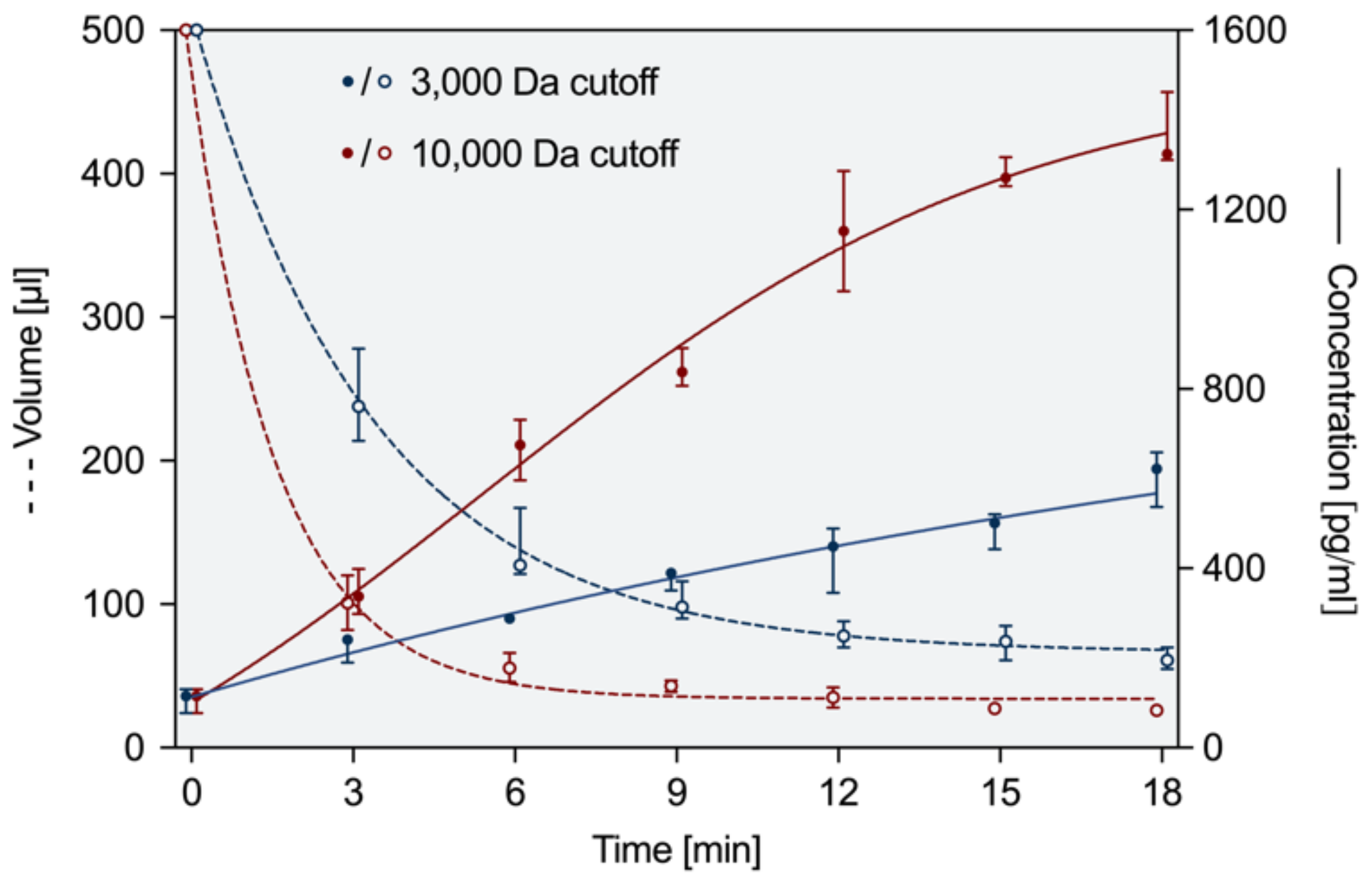
3.2. Concentration and Preparation Ex Vivo
3.3. Cytotoxic Effects of EDTA and Neutralization
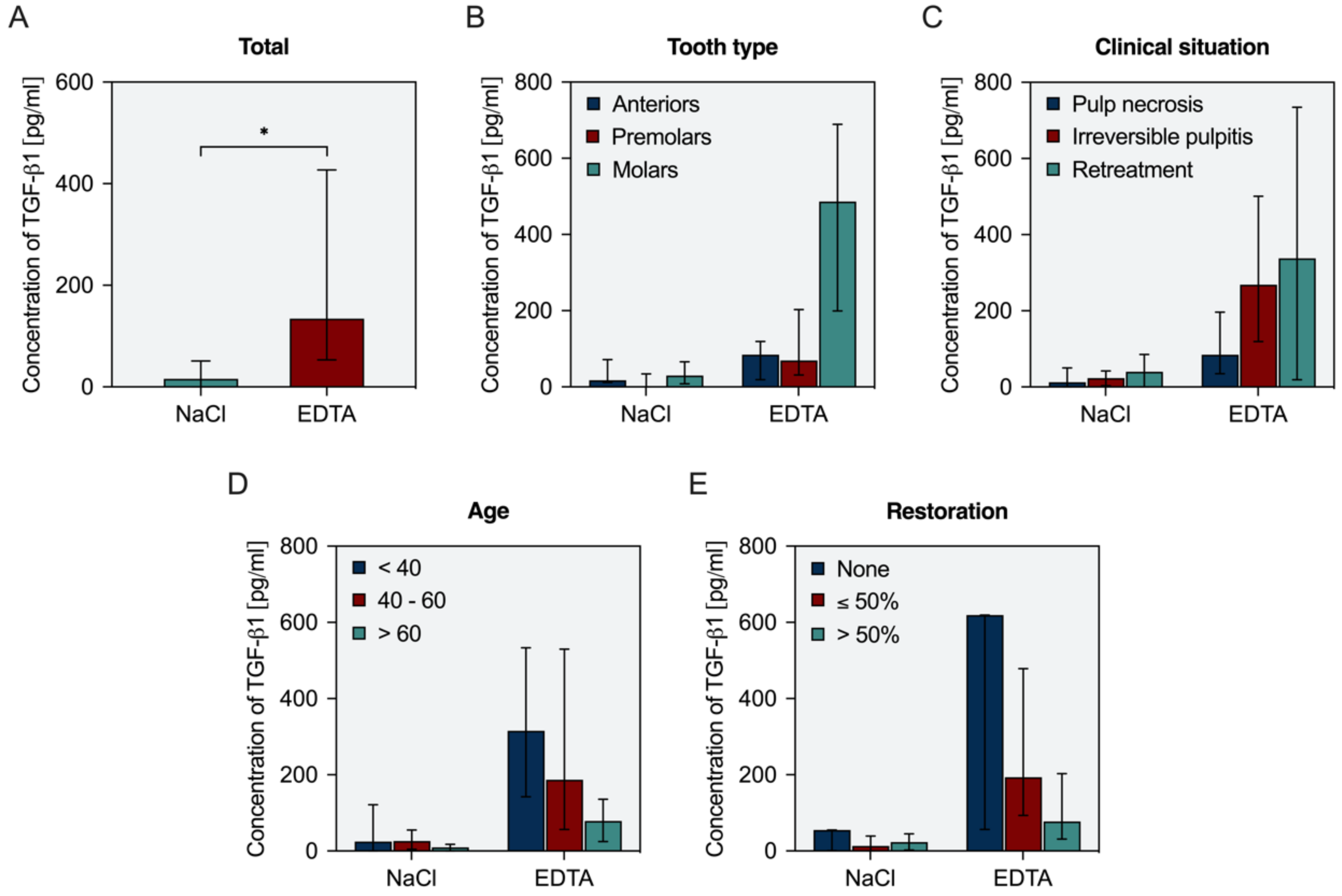
3.4. In Vivo Availability of TGF-β1
4. Discussion
4.1. Release Parameters Ex Vivo
4.2. Concentration and Neutralization
4.3. In Vivo Availability of TGF-β1
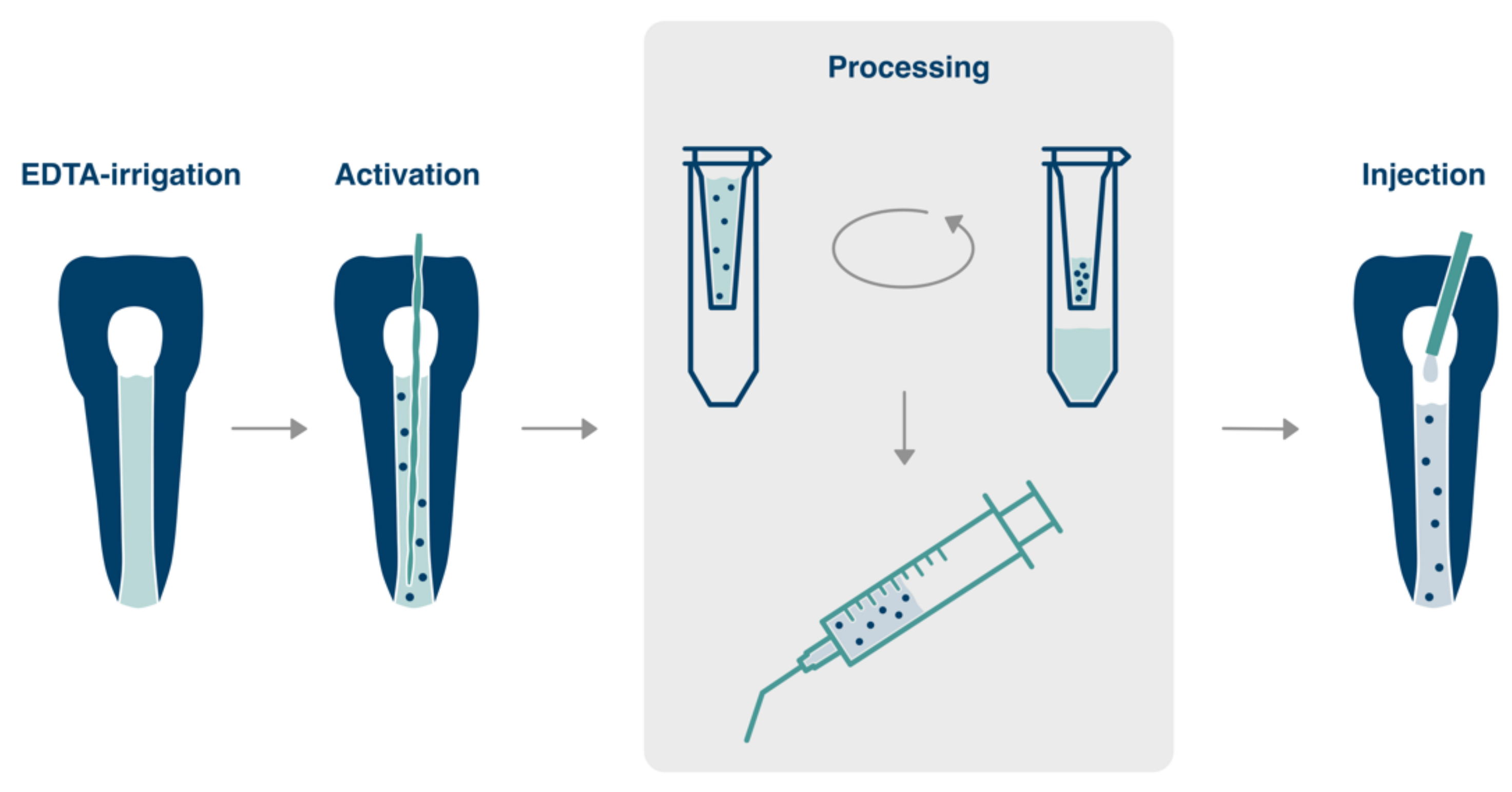
- First visit:
- Initiation of root canal preparation under copious irrigation with sodium hypochlorite at 2%,
- further canal enlargement and use of saline (or chlorhexidine),
- intracanal dressing with calcium hydroxide and temporary seal (2 to 4 weeks).
- Second visit:
- 4.
- Removal of calcium hydroxide by irrigation with EDTA,
- 5.
- refreshment of EDTA inside the canal and ultrasonic activation for 30 s,
- 6.
- collection of EDTA solution from the canal and repetition until 100 µL of solution are available (3 to 4 times),
- 7.
- transfer of the solution to a filter with a molecular weight cut-off of 10,000 Da, addition of ultrapure water to a volume of 500 µL (1:4 dilution), spin for 6 min,
- 8.
- mix with a fibrin-based biomaterial,
- 9.
- re-insert into the root canal in contact with the periapical tissues,
- 10.
- cover the material, e.g., with a hydraulic calcium silicate cement, and place an adhesive seal.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nakashima, M.; Iohara, K.; Murakami, M.; Nakamura, H.; Sato, Y.; Ariji, Y.; Matsushita, K. Pulp regeneration by transplantation of dental pulp stem cells in pulpitis: A pilot clinical study. Stem Cell Res. Ther. 2017, 8, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xuan, K.; Li, B.; Guo, H.; Sun, W.; Kou, X.; He, X.; Zhang, Y.; Sun, J.; Liu, A.; Liao, L.; et al. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci. Transl. Med. 2018, 10, eaaf3227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galler, K.M.; Widbiller, M. Cell-free approaches for dental pulp tissue engineering. J. Endod. 2020, 46, S143–S149. [Google Scholar] [CrossRef] [PubMed]
- Galler, K.M.; Widbiller, M. Perspectives for cell-homing approaches to engineer dental pulp. J. Endod. 2017, 43, S40–S45. [Google Scholar] [CrossRef]
- Widbiller, M.; Schmalz, G. Endodontic regeneration: Hard shell, soft core. Odontology 2021, 109, 303–312. [Google Scholar] [CrossRef]
- Chen, F.-M.; Wu, L.-A.; Zhang, M.; Zhang, R.; Sun, H.-H. Homing of endogenous stem/progenitor cells for in situ tissue regeneration: Promises, strategies, and translational perspectives. Biomaterials 2011, 32, 3189–3209. [Google Scholar] [CrossRef]
- Chrepa, V.; Henry, M.A.; Daniel, B.J.; Diogenes, A.R. Delivery of apical mesenchymal stem cells into root canals of mature teeth. J. Dent. Res. 2015, 94, 1653–1659. [Google Scholar] [CrossRef]
- Ducret, M.; Costantini, A.; Gobert, S.; Farges, J.-C.; Bekhouche, M.; Kocourek, T. Fibrin-based scaffolds for dental pulp regeneration: From biology to nanotherapeutics. Eur. Cells Mater. 2021, 41, 1–14. [Google Scholar] [CrossRef]
- Galler, K.M.; Brandl, F.P.; Kirchhof, S.; Widbiller, M.; Eidt, A.; Buchalla, W.; Göpferich, A.; Schmalz, G. Suitability of different natural and synthetic biomaterials for dental pulp tissue engineering. Tissue Eng. Part A 2018, 24, 234–244. [Google Scholar] [CrossRef]
- Ruangsawasdi, N.; Zehnder, M.; Weber, F.E. Fibrin gel improves tissue ingrowth and cell differentiation in human immature premolars implanted in rats. J. Endod. 2014, 40, 246–250. [Google Scholar] [CrossRef] [Green Version]
- Galler, K.M.; Widbiller, M.; Buchalla, W.; Eidt, A.; Hiller, K.-A.; Hoffer, P.C.; Schmalz, G. EDTA conditioning of dentine promotes adhesion, migration and differentiation of dental pulp stem cells. Int. Endod. J. 2016, 49, 581–590. [Google Scholar] [CrossRef]
- Smith, A.J.; Duncan, H.F.; Diogenes, A.R.; Simon, S.; Cooper, P.R. Exploiting the bioactive properties of the dentin-pulp complex in regenerative endodontics. J. Endod. 2016, 42, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Scheven, B.A.; Takahashi, Y.; Ferracane, J.L.; Shelton, R.M.; Cooper, P.R. Dentine as a bioactive extracellular matrix. Arch. Oral Biol. 2012, 57, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Widbiller, M.; Eidt, A.; Lindner, S.R.; Hiller, K.-A.; Schweikl, H.; Buchalla, W.; Galler, K.M. Dentine matrix proteins: Isolation and effects on human pulp cells. Int. Endod. J. 2018, 51 (Suppl. S4), e278–e290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widbiller, M.; Schweikl, H.; Bruckmann, A.; Rosendahl, A.; Hochmuth, E.; Lindner, S.R.; Buchalla, W.; Galler, K.M. Shotgun proteomics of human dentin with different prefractionation methods. Sci. Rep. 2019, 9, 4457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widbiller, M.; Austah, O.; Lindner, S.R.; Sun, J.; Diogenes, A.R. Neurotrophic proteins in dentin and their effect on trigeminal sensory neurons. J. Endod. 2019, 45, 729–735. [Google Scholar] [CrossRef]
- Bègue-Kirn, C.; Smith, A.J.; Ruch, J.V.; Wozney, J.M.; Purchio, A.; Hartmann, D.; Lesot, H. Effects of dentin proteins, transforming growth factor beta 1 (TGF beta 1) and bone morphogenetic protein 2 (BMP2) on the differentiation of odontoblast in vitro. Int. J. Dev. Biol. 1992, 36, 491–503. [Google Scholar]
- Widbiller, M.; Eidt, A.; Hiller, K.-A.; Buchalla, W.; Schmalz, G.; Galler, K.M. Ultrasonic activation of irrigants increases growth factor release from human dentine. Clin. Oral Investig. 2017, 21, 879–888. [Google Scholar] [CrossRef]
- Sadaghiani, L.; Gleeson, H.B.; Youde, S.; Waddington, R.J.; Lynch, C.D.; Sloan, A.J. Growth factor liberation and dpsc response following dentine conditioning. J. Dent. Res. 2016, 95, 1298–1307. [Google Scholar] [CrossRef] [Green Version]
- Arslan, H.; Ahmed, H.M.A.; Şahin, Y.; Yıldız, E.D.; Gündoğdu, E.C.; Güven, Y.; Khalilov, R. Regenerative endodontic procedures in necrotic mature teeth with periapical radiolucencies: A preliminary randomized clinical study. J. Endod. 2019, 45, 863–872. [Google Scholar] [CrossRef]
- Glynis, A.; Foschi, F.; Kefalou, I.; Koletsi, D.; Tzanetakis, G.N. Regenerative endodontic procedures for the treatment of necrotic mature teeth with apical periodontitis. A systematic review and meta-analysis of randomized controlled trials. J. Endod. 2021, 47, 873–882. [Google Scholar] [CrossRef]
- Widbiller, M.; Driesen, R.B.; Eidt, A.; Lambrichts, I.; Hiller, K.-A.; Buchalla, W.; Schmalz, G.; Galler, K.M. Cell homing for pulp tissue engineering with endogenous dentin matrix proteins. J. Endod. 2018, 44, 956–962.e2. [Google Scholar] [CrossRef]
- Galler, K.M.; Krastl, G.; Simon, S.; Gorp, G.V.; Meschi, N.; Vahedi, B.; Lambrechts, P. European Society of Endodontology position statement: Revitalization procedures. Int. Endod. J. 2016, 49, 717–723. [Google Scholar] [CrossRef]
- American Association of Endodontists (AAE). Clinical Considerations for a Regenerative Procedure. Available online: https://www.aae.org/specialty/wp-content/uploads/sites/2/2017/06/currentregenerativeendodonticconsiderations.pdf (accessed on 10 December 2021).
- Torabinejad, M.; Nosrat, A.; Verma, P.; Udochukwu, O. Regenerative endodontic treatment or mineral trioxide aggregate apical plug in teeth with necrotic pulps and open apices: A systematic review and meta-analysis. J. Endod. 2017, 43, 1806–1820. [Google Scholar] [CrossRef] [PubMed]
- Bucchi, C.; Marcé-Nogué, J.; Galler, K.M.; Widbiller, M. Biomechanical performance of an immature maxillary central incisor after revitalization: A finite element analysis. Int. Endod. J. 2019, 52, 1508–1518. [Google Scholar] [CrossRef] [PubMed]
- Cvek, M. Prognosis of luxated non-vital maxillary incisors treated with calcium hydroxide and filled with gutta-percha. A retrospective clinical study. Dent. Traumatol. 1992, 8, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Lolato, A.; Bucchi, C.; Taschieri, S.; Kabbaney, A.E.; Fabbro, M.D. Platelet concentrates for revitalization of immature necrotic teeth: A systematic review of the clinical studies. Platelets 2016, 27, 383–392. [Google Scholar] [CrossRef]
- Shivashankar, V.Y.; Johns, D.A.; Maroli, R.K.; Sekar, M.; Chandrasekaran, R.; Karthikeyan, S.; Renganathan, S.K. Comparison of the effect of PRP, PRF and induced bleeding in the revascularization of teeth with necrotic pulp and open apex: A triple blind randomized clinical trial. J. Clin. Diagn. Res. 2017, 11, ZC34–ZC39. [Google Scholar] [CrossRef]
- Brizuela, C.; Meza, G.; Urrejola, D.; Quezada, M.A.; Concha, G.; Ramírez, V.; Angelopoulos, I.; Cadiz, M.I.; Tapia-Limonchi, R.; Khoury, M. Cell-based regenerative endodontics for treatment of periapical lesions: A randomized, controlled phase I/II clinical trial. J. Dent. Res. 2020, 99, 523–529. [Google Scholar] [CrossRef]
- Galler, K.M.; Buchalla, W.; Hiller, K.-A.; Federlin, M.; Eidt, A.; Schiefersteiner, M.; Schmalz, G. Influence of root canal disinfectants on growth factor release from dentin. J. Endod. 2015, 41, 363–368. [Google Scholar] [CrossRef]
- Diogenes, A.R.; Ruparel, N.B.; Teixeira, F.B.; Hargreaves, K.M. Translational science in disinfection for regenerative endodontics. J. Endod. 2014, 40, S52–S57. [Google Scholar] [CrossRef]
- Hecker, S.; Hiller, K.-A.; Galler, K.M.; Erb, S.; Mader, T.; Schmalz, G. Establishment of an optimized ex vivo system for artificial root canal infection evaluated by use of sodium hypochlorite and the photodynamic therapy. Int. Endod. J. 2013, 46, 449–457. [Google Scholar] [CrossRef]
- Vianna, M.E.; Gomes, B.P.; Berber, V.B.; Zaia, A.A.; Ferraz, C.C.R.; de Souza-Filho, F.J. In vitro evaluation of the antimicrobial activity of chlorhexidine and sodium hypochlorite. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 97, 79–84. [Google Scholar] [CrossRef]
- Gomes, B.P.; Vianna, M.E.; Zaia, A.A.; Almeida, J.F.A.; Souza-Filho, F.J.; Ferraz, C.C.R. Chlorhexidine in endodontics. Braz. Dent. J. 2013, 24, 89–102. [Google Scholar] [CrossRef]
- Zandi, H.; Petronijevic, N.; Mdala, I.; Kristoffersen, A.K.; Enersen, M.; Rôças, I.N.; Siqueira, J.F.; Ørstavik, D. Outcome of endodontic retreatment using 2 root canal irrigants and influence of infection on healing as determined by a molecular method: A randomized clinical trial. J. Endod. 2019, 45, 1089–1098.e5. [Google Scholar] [CrossRef] [PubMed]
- Widbiller, M.; Althumairy, R.I.; Diogenes, A.R. Direct and indirect effect of chlorhexidine on survival of stem cells from the apical papilla and its neutralization. J. Endod. 2019, 45, 156–160. [Google Scholar] [CrossRef]
- Ryan, E.A.; Mockros, L.F.; Weisel, J.W.; Lorand, L. Structural origins of fibrin clot rheology. Biophys. J. 1999, 77, 2813–2826. [Google Scholar] [CrossRef] [Green Version]
- Wong, J.; Manoil, D.; Näsman, P.; Belibasakis, G.N.; Neelakantan, P. Microbiological aspects of root canal infections and disinfection strategies: An update review on the current knowledge and challenges. Front. Oral Health 2021, 2, 672887. [Google Scholar] [CrossRef]
- Murray, P.E.; Stanley, H.R.; Matthews, J.B.; Sloan, A.J.; Smith, A.J. Age-related odontometric changes of human teeth. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2002, 93, 474–482. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Widbiller, M.; Rosendahl, A.; Wölflick, M.; Linnebank, M.; Welzenbach, B.; Hiller, K.-A.; Buchalla, W.; Galler, K.M. Isolation of Endogenous TGF-β1 from Root Canals for Pulp Tissue Engineering: A Translational Study. Biology 2022, 11, 227. https://doi.org/10.3390/biology11020227
Widbiller M, Rosendahl A, Wölflick M, Linnebank M, Welzenbach B, Hiller K-A, Buchalla W, Galler KM. Isolation of Endogenous TGF-β1 from Root Canals for Pulp Tissue Engineering: A Translational Study. Biology. 2022; 11(2):227. https://doi.org/10.3390/biology11020227
Chicago/Turabian StyleWidbiller, Matthias, Andreas Rosendahl, Melanie Wölflick, Moritz Linnebank, Benedikt Welzenbach, Karl-Anton Hiller, Wolfgang Buchalla, and Kerstin M. Galler. 2022. "Isolation of Endogenous TGF-β1 from Root Canals for Pulp Tissue Engineering: A Translational Study" Biology 11, no. 2: 227. https://doi.org/10.3390/biology11020227
APA StyleWidbiller, M., Rosendahl, A., Wölflick, M., Linnebank, M., Welzenbach, B., Hiller, K.-A., Buchalla, W., & Galler, K. M. (2022). Isolation of Endogenous TGF-β1 from Root Canals for Pulp Tissue Engineering: A Translational Study. Biology, 11(2), 227. https://doi.org/10.3390/biology11020227





