Evaluation of Age-Dependent Changes in the Coloration of Male Killifish Nothobranchius Guentheri Using New Photoprocessing Methods
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Housing
2.2. Anesthesia of Fish
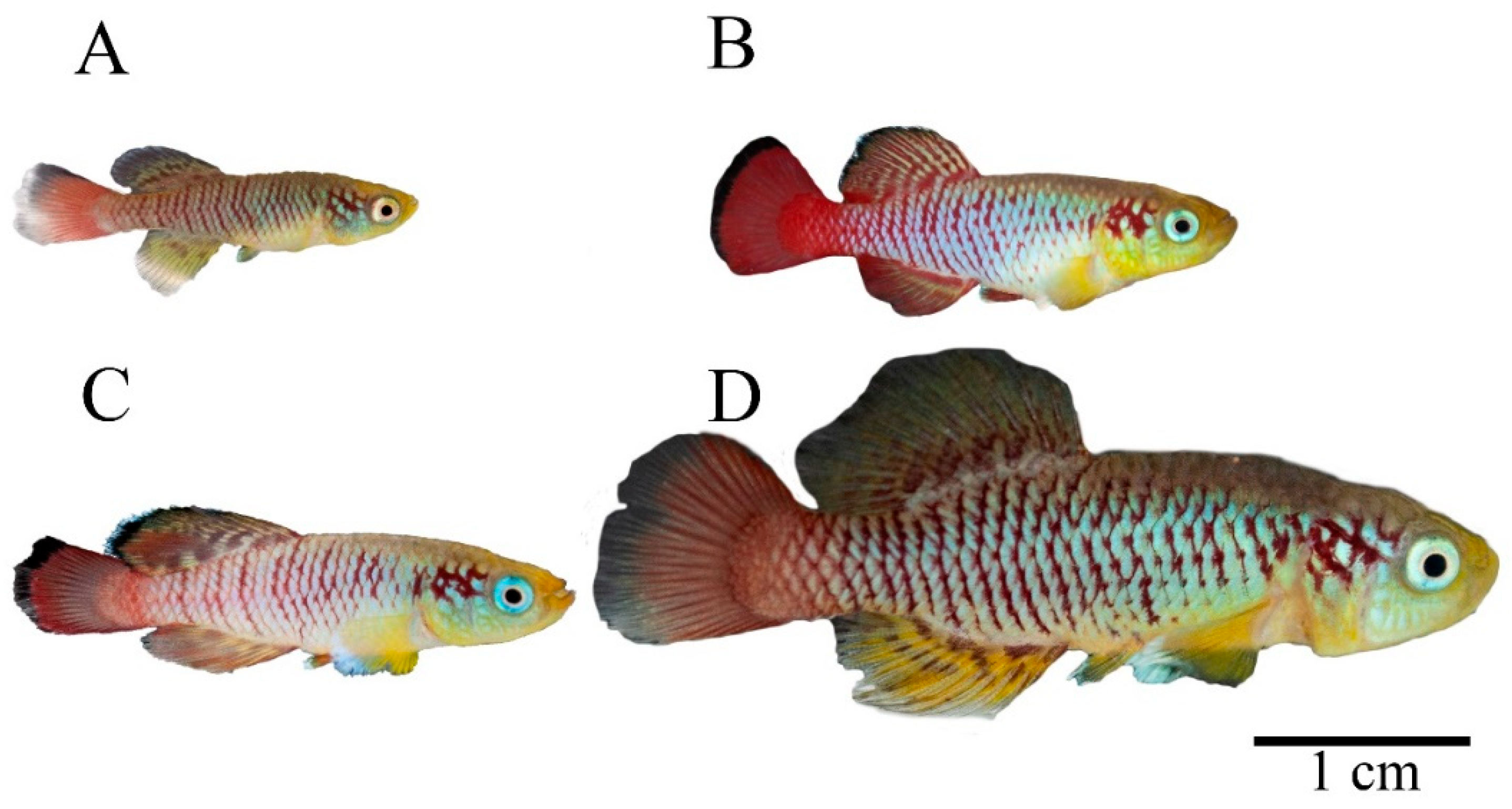
2.3. Imaging
2.4. Photographic Imaging Conditions
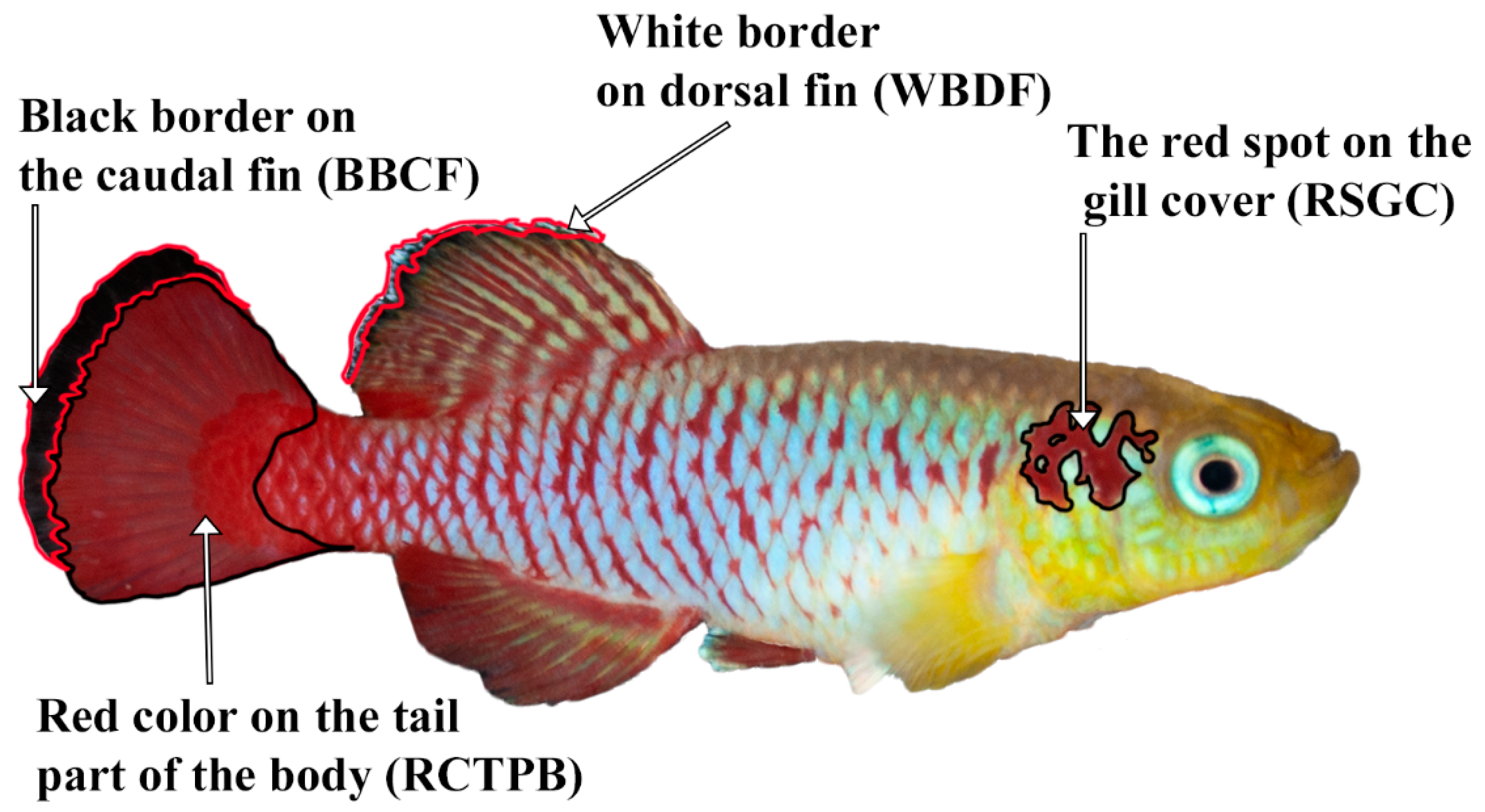
2.5. Image Processing
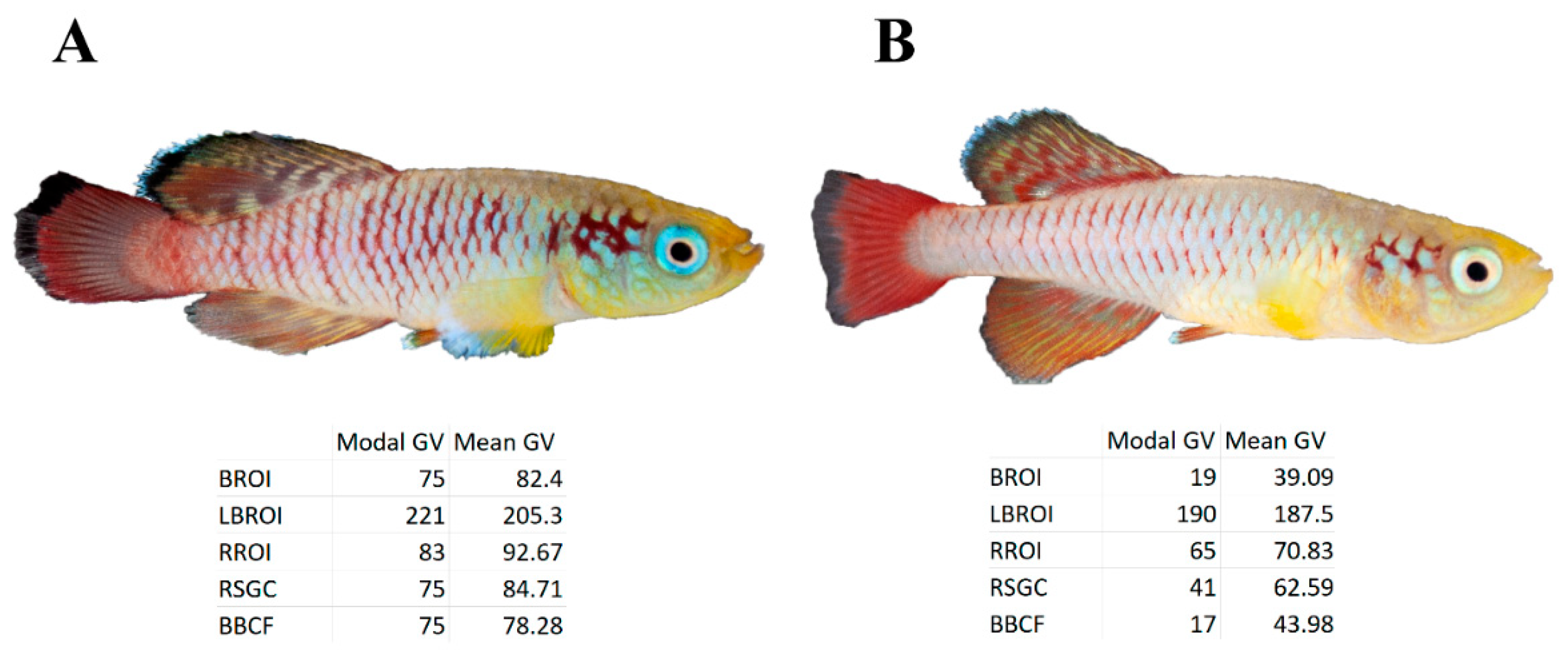
- Red spot on the gill cover (RSGC);
- Black border on the caudal fin (BBCF);
- White border on the dorsal fin (WBDF).
- Zone of black zones on the dorsal and caudal fins, black region of interest (BROI) (Figure S2c);
- Zone of red zones on the entire lateral part, red region of interest (RROI) (Figure S2b);
- Zone of light blue areas on the entire lateral part, light blue region of interest (LBROI) (Figure S2a).
2.6. Statistical Analysis
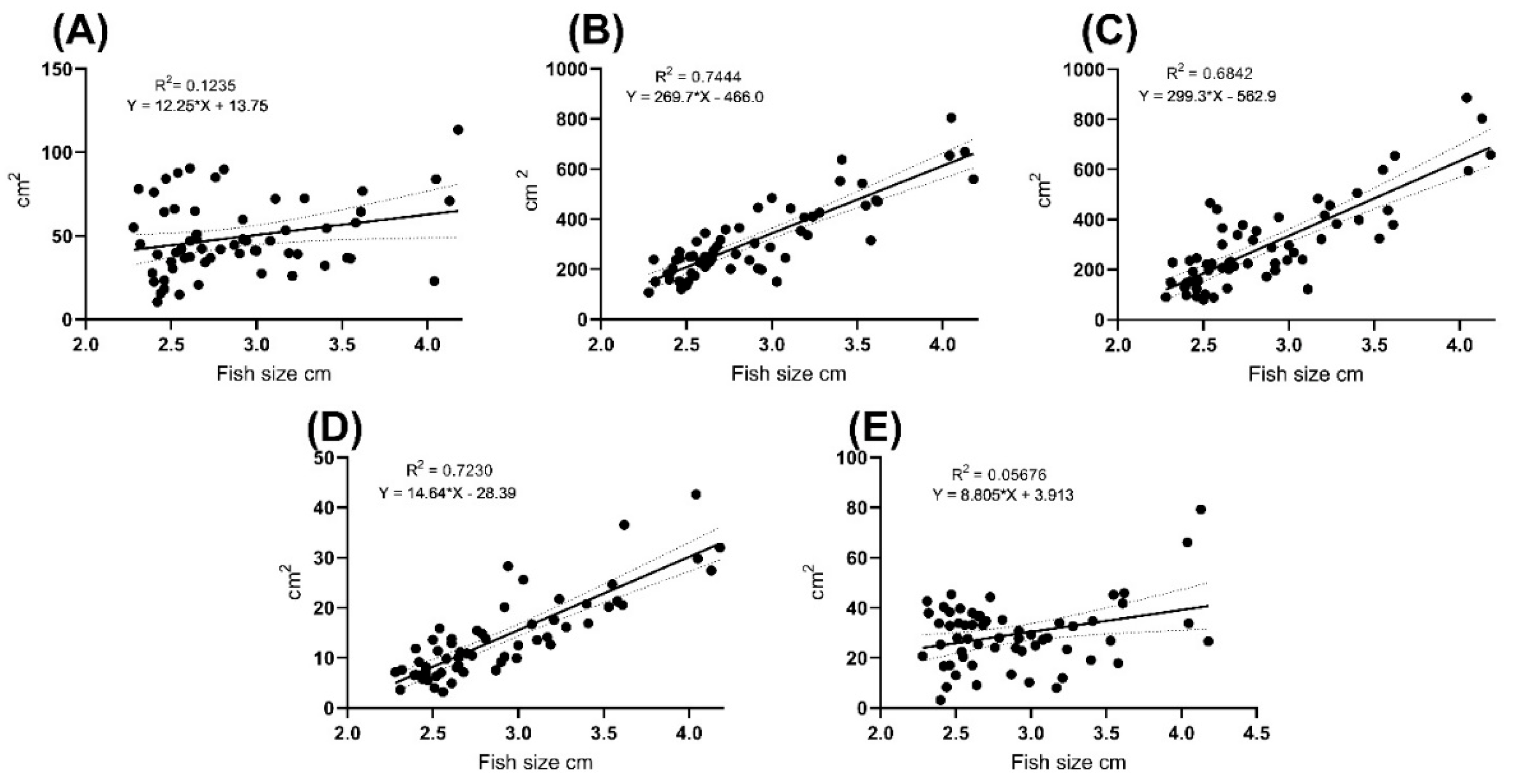
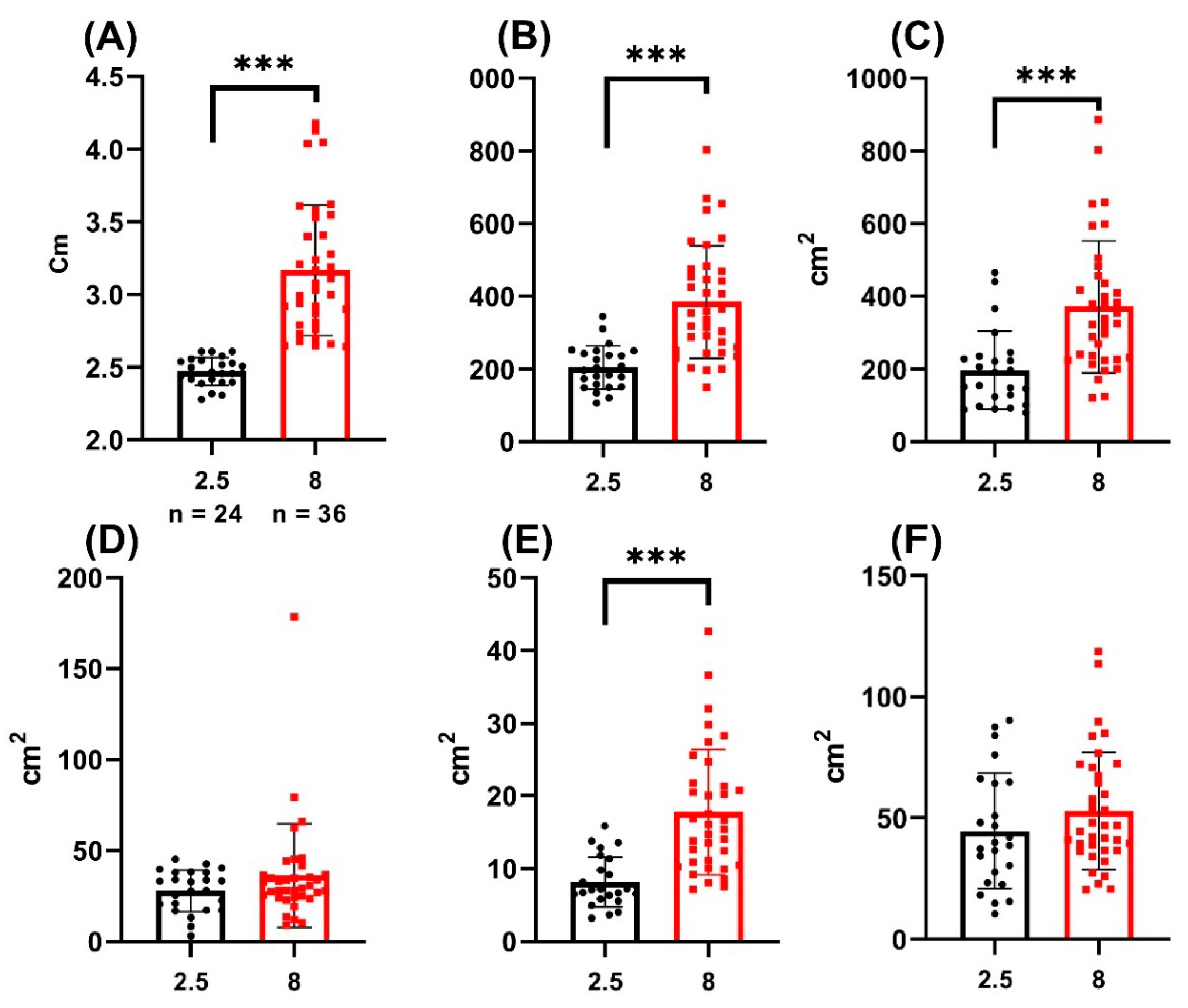
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rahman Khan, F.; Sulaiman Alhewairini, S. Zebrafish (Danio rerio) as a Model Organism. Curr. Trends Cancer Manag. 2019, 2019, 81517. [Google Scholar]
- Naruse, K.; Sakaizumi, M.; Shima, A. Medaka as a model organism for research in experimental biology. Fish Biol. J. Medaka 1994, 6, 47–52. [Google Scholar]
- Nüsslein-Volhard, C.; Singh, A.P. How fish color their skin: A paradigm for development and evolution of adult patterns: Multipotency, plasticity, and cell competition regulate proliferation and spreading of pigment cells in zebrafish coloration. BioEssays 2017, 39, 1600231. [Google Scholar] [CrossRef] [PubMed]
- Furness, A.I. The evolution of an annual life cycle in killifish: Adaptation to ephemeral aquatic environments through embryonic diapause. Biol. Rev. 2016, 91, 796–812. [Google Scholar] [CrossRef] [PubMed]
- Cellerino, C. Annual fishes of the genus Nothobranchius as an experimental model. Annu. Fishes Life Hist. Strategy Divers. Evol. 2016, 1, 93–98. [Google Scholar]
- Lucas-Sánchez, A.; Almaida-Pagán, P.F.; Mendiola, P.; de Costa, J. Nothobranchius as a model for aging studies. A review. Aging Dis. 2014, 5, 281–291. [Google Scholar] [CrossRef]
- Herrera, M.; Jagadeeswaran, P. Annual fish as a genetic model for aging. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2004, 59, B101–B107. [Google Scholar] [CrossRef]
- Nikiforov-Nikishin, D.L.; Irkha, V.A.; Kochetkov, N.I.; Kalita, T.L.; Nikiforov-Nikishin, A.L.; Blokhin, E.E.; Antipov, S.S.; Makarenkov, D.A.; Zhavnerov, A.N.; Glebova, I.A.; et al. Some Aspects of Development and Histological Structure of the Visual System of Nothobranchius Guentheri. Animals 2021, 11, 2755. [Google Scholar] [CrossRef]
- Guvatova, Z.G.; Fedorova, M.S.; Vershinina, Y.S.; Pudova, E.A.; Lipatova, A.V.; Volodin, V.V.; Gladysh, N.; Tokarev, A.; Kornev, A.; Pavlov, V.; et al. De Novo Transcriptome Profiling of Brain Tissue from the Annual Killifish Nothobranchius guentheri. Life 2021, 11, 137. [Google Scholar] [CrossRef]
- Aragona, M.; Porcino, C.; Guerrera, M.C.; Montalbano, G.; Levanti, M.; Abbate, F.; Laurà, R.; Germanà, A. Localization of Neurotrophin Specific Trk Receptors in Mechanosensory Systems of Killifish (Nothobranchius guentheri). Int. J. Mol. Sci. 2021, 22, 10411. [Google Scholar] [CrossRef]
- Maan, M.E.; Sefc, K.M. Colour variation in cichlid fish: Developmental mechanisms, selective pressures and evolutionary consequences. Semin. Cell Dev. Biol. Acad. Press 2013, 24, 516–528. [Google Scholar] [CrossRef] [PubMed]
- Sabaj, M.H.; Maurakis, E.G.; Woolcott, W.S. Spawning behaviors in the bluehead chub, Nocomis leptocephalus, river chub, N. micropogon and central stoneroller, Campostoma anomalum. Am. Midl. Nat. 2000, 144, 187–201. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Chiu, Y.-C.; Hsu, W.-L.; Chan, Y.-P. Age-related markers assayed at different developmental stages of the annual fish Nothobranchius rachovii. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2008, 63, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Valdesalici, S.; Cellerino, A. Extremely short lifespan in the annual fish Nothobranchius furzeri. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270 (Suppl. S2), S189–S191. [Google Scholar] [CrossRef]
- Haas, R. Sexual selection in Nothobranchius guentheri (Pisces: Cyprinodontidae). Evolution 1976, 30, 614–622. [Google Scholar] [CrossRef]
- Krysanov, E.; Demidova, T. Extensive karyotype variability of African fish genus Nothobranchius (Cyprinodontiformes). Comp. Cytogenet. 2018, 12, 387–402. [Google Scholar] [CrossRef]
- Krysanov, E.; Demidova, T.; Nagy, B. Divergent karyotypes of the annual killifish genus Nothobranchius (Cyprinodontiformes, Nothobranchiidae). Comp. Cytogenet. 2016, 10, 439. [Google Scholar] [CrossRef]
- Passos, C.; Tassino, B.; Rosenthal, G.G.; Reichard, M. Reproductive Behavior and Sexual Selection in Annual Fishes; CRC Press: Boca Raton, FL, USA, 2015; pp. 207–230. [Google Scholar]
- Yu, X.; Li, G. Effects of resveratrol on longevity, cognitive ability and aging-related histological markers in the annual fish Nothobranchius guentheri. Exp. Gerontol. 2012, 47, 940–949. [Google Scholar] [CrossRef]
- Sedlácek, O.; Baciaková, B.; Kratochvíl, L. Evolution of body colouration in killifishes (Cyprinodontiformes: Aplocheilidae, Nothobranchiidae, Rivulidae): Is male ornamentation constrained by intersexual genetic correlation? Zool. Anz. J. Comp. Zool. 2014, 253, 207–215. [Google Scholar] [CrossRef]
- Vrtílek, M.; Reichard, M. Highly plastic resource allocation to growth and reproduction in females of an A frican annual fish. Ecol. Freshw. Fish 2015, 24, 616–628. [Google Scholar] [CrossRef]
- Reichard, M.; Polačik, M. Reproductive isolating barriers between colour-differentiated populations of an African annual killifish, Nothobranchius korthausae (Cyprinodontiformes). Biol. J. Linn. Soc. 2010, 100, 62–72. [Google Scholar] [CrossRef]
- Proulx, R.; St-Laurent, M.H.; Parrott, L. Emergent phenotypes: Association between morphology and coloration in fish. Evol. Ecol. Res. 2008, 1, 1037–1050. [Google Scholar]
- Alphen, J.J.M.V. Evolution of colour patterns in East African cichlid fish. J. Evol. Biol. 1999, 12, 514–534. [Google Scholar]
- Reichard, M.; Polačik, M.; Sedláček, O. Distribution, colour polymorphism and habitat use of the African killifish Nothobranchius furzeri, the vertebrate with the shortest life span. J. Fish Biol. 2003, 74, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, P.D.; Maguire, S.M.; Harris, R.M.; Rodriguez, A.A.; DeAngelis, R.S.; Flores, S.A.; Hofmann, H.A. The melanocortin system regulates body pigmentation and social behaviour in a colour polymorphic cichlid fish. Proc. R. Soc. B Biol. Sci. 2017, 284, 20162838. [Google Scholar] [CrossRef] [PubMed]
- Moretz, J.A.; Morris, M.R. Evolutionarily labile responses to a signal of aggressive intent. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, 2271–2277. [Google Scholar] [CrossRef]
- Kalinowski, C.T.; Robaina, L.; Fernández-Palacios, H.; Schuchardt, D.; Izquierdo, M. Effect of different carotenoid sources and their dietary levels on red porgy (Pagrus pagrus) growth and skin colour. Aquaculture 2005, 244, 223–231. [Google Scholar] [CrossRef]
- de Sá, R.O.; Berois, N.; García, G. Overview, Future Challenges, and Evolution of Annualism. Annual Fishes: Life History Strategy, Diversity, and Evolution, 1st ed.; Press Taylor & Francis Group: Boca Raton, FL, USA, 2015; pp. 309–318. [Google Scholar]
- Kittilsen, S.; Schjolden, J.; Beitnes-Johansen, I.; Shaw, J.; Pottinger, T.; Sørensen, C.; Braastad, B.; Bakken, M.; Overli, O. Melanin-based skin spots reflect stress responsiveness in salmonid fish. Horm. Behav. 2009, 56, 292–298. [Google Scholar] [CrossRef]
- Brown, L.A. Anesthesia in fish. Vet. Clin. N. Am. Small Anim. Pract. 1988, 18, 317–330. [Google Scholar] [CrossRef]
- Neiffer, D.L.; Stamper, M.A. Fish sedation, anesthesia, analgesia, and euthanasia: Considerations, methods, and types of drugs. ILAR J. 2009, 50, 343–360. [Google Scholar] [CrossRef]
- Chervova, L.S.; Lapshin, D.N. Behavioral control of the efficiency of pharmacological anesthesia in fish. J. Ichthyol. 2011, 51, 1126–1132. [Google Scholar] [CrossRef]
- García-Melo, J.E.; García-Melo, L.J.; García-Melo, J.D.; Rojas-Briñez, D.K.; Guevara, G.; Maldonado-Ocampo, J.A. Photafish system: An affordable device for fish photography in the wild. Zootaxa 2019, 4554, 141–172. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kweon, H.S.; Park, E.H. Improved photographic technique for enhancing the morphological resolution of small juvenile fishes. Ichthyol. Res. 1999, 46, 423–425. [Google Scholar] [CrossRef]
- Stevens, M.; Kweon, H.-S.; Park, E.-H. Using digital photography to study animal coloration. Biol. J. Linn. Soc. 2007, 90, 211–237. [Google Scholar] [CrossRef]
- Steinke, D.; Hanner, R.; Hebert, P.D.N. Rapid high-quality imaging of fishes using a flat-bed scanner. Ichthyol. Res. 2009, 56, 210–211. [Google Scholar] [CrossRef]
- Steinke, D.; Hanner, R. The FISH-BOL collaborators’ protocol. Mitochondrial DNA 2011, 22 (Suppl. S1), 10–14. [Google Scholar] [CrossRef]
- Jacquin, L.; Gauthey, Z.; Roussille, V.; Le Hénaff, M.; Tentelier, C.; Labonne, J. Melanin in a changing world: Brown trout coloration reflects alternative reproductive strategies in variable environments. Behav. Ecol. 2017, 28, 1423–1434. [Google Scholar] [CrossRef]
- Selz, O.M.; Thommen, R.; Pierotti, M.E.R.; Anaya-Rojas, J.M.; Seehausen, O. Differences in male coloration are predicted by divergent sexual selection between populations of a cichlid fish. Proc. R. Soc. B Biol. Sci. 2016, 283, 20160172. [Google Scholar] [CrossRef]
- Wright, D.S.; Rietveld, E.; Maan, M.E. Developmental effects of environmental light on male nuptial coloration in Lake Victoria cichlid fish. PeerJ 2018, 6, e4209. [Google Scholar] [CrossRef]
- Endler, J.A. On the measurement and classification of colour in studies of animal colour patterns. Biol. J. Linn. Soc. 1990, 41, 315–352. [Google Scholar] [CrossRef]
- Culumber, Z.W.; Monks, S. Does fin coloration signal social status in a dominance hierarchy of the livebearing fish Xiphophorus variatus? Behav. Process. 2014, 107, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Atsumi, K.; Koizumi, I. Web image search revealed large-scale variations in breeding season and nuptial coloration in a mutually ornamented fish, Tribolodon hakonensis. Ecol. Res. 2017, 32, 567–578. [Google Scholar] [CrossRef]
- Endler, J.A.; Mielke, P.W., Jr. Comparing entire colour patterns as birds see them. Biol. J. Linn. Soc. 2005, 86, 405–431. [Google Scholar] [CrossRef]
- Gerald, M.S.; Bernstein, J.; Hinkson, R.; Fosbury, R.A. Formal method for objective assessment of primate color. Am. J. Primatol. Off. J. Am. Soc. Primatol. 2001, 53, 79–85. [Google Scholar] [CrossRef]
- Svensson, P.A.; Nilsson Sköld, H. Skin biopsies as tools to measure fish coloration and colour change. Ski. Biopsy Perspect. Croat. Intech. Open 2011, 299–316. [Google Scholar] [CrossRef][Green Version]
- Fujii, R. The regulation of motile activity in fish chromatophores. Pigment. Cell Res. 2000, 13, 300–319. [Google Scholar] [CrossRef]
- Korzan, W.J.; Fernald, R.D. Territorial male color predicts agonistic behavior of conspecifics in a color polymorphic species. Behav. Ecol. 2007, 18, 318–323. [Google Scholar] [CrossRef]
- Plenderleith, M.; van Oosterhout, C.; Robinson, R.L.; Turner, G.F. Female preference for conspecific males based on olfactory cues in a Lake Malawi cichlid fish. Biol. Lett. 2005, 1, 411–414. [Google Scholar] [CrossRef]
- Yamamoto, T. Sex Differentiation. In Fish Physiology; Hoar, W.S., Randall, D., Eds.; Academic Press: New York, NY, USA, 1976; Volume 3, pp. 117–175. [Google Scholar]
- Strüssmann, C.A.; Nakamura, N. Morphology, endocrinology, and environmental modulation of gonadal sex differentiation in teleost fishes. Fish Physiol. Biochem. 2002, 26, 13–29. [Google Scholar] [CrossRef]
- Beeching, S.C. Colour pattern and inhibition of aggression in the cichlid fish Astronotus ocellatus. J. Fish Biol. 1995, 47, 50–58. [Google Scholar] [CrossRef]
- Fisher, H.S.; Rosenthal, G.G. Male swordtails court with an audience in mind. Biol. Lett. 2007, 3, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, E.; Taylor, J.F.; Migaud, H. Morphological skin colour changes in teleosts. Fish Fish. 2010, 11, 159–193. [Google Scholar] [CrossRef]
- Dickman, M.C.; Schliwa, M.; Barlow, G.W. Melanophore death and disappearance produces color metamorphosis in the polychromatic Midas cichlid (Cichlasoma citrinellum). Cell Tissue Res. 1988, 253, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Quigley, I.K.; Turner, J.M.; Nuckels, R.J.; Manuel, J.L.; Budi, E.H.; MacDonald, E.L.; Parichy, D.M. Pigment pattern evolution by differential deployment of neural crest and post-embryonic melanophore lineages in Danio fishes. Development 2004, 131, 6053–6069. [Google Scholar] [CrossRef]
- Bagnara, J.T.; Taylor, J.D.; Hadley, M.E. The dermal chromatophore unit. J. Cell Biol. 1968, 38, 67–79. [Google Scholar] [CrossRef]
- Takahashi, D. Female mate choice based on male nuptial coloration in pale chub Zacco Platypus. Zool. Sci. 2018, 35, 23–27. [Google Scholar] [CrossRef]
- Ansai, S.; Mochida, K.; Fujimoto, S.; Mokodongan, D.F.; Sumarto, B.K.A.; Masengi, K.W.A.; Hadiaty, R.K.; Nagano, A.J.; Toyoda, A.; Naruse, K.; et al. Genome editing reveals fitness effects of a gene for sexual dichromatism in Sulawesian fishes. Nat. Commun. 2021, 12, 1350. [Google Scholar] [CrossRef] [PubMed]
- Grether, G.F. Carotenoid limitation and mate preference evolution: A test of the indicator hypothesis in guppies (Poecilia reticulata). Evol. Int. J. Org. Evol. 2000, 54, 1712–1724. [Google Scholar] [CrossRef] [PubMed]
- Godin, J.G.J.; McDonough, H.E. Predator preference for brightly colored males in the Guppy: A viability cost for a sexually selected trait. Behav. Ecol. 2003, 14, 194–200. [Google Scholar] [CrossRef]
- Johnson, S.; Candolin, U. Predation cost of a sexual signal in the threespine stickleback. Behav. Ecol. 2017, 28, 1160–1165. [Google Scholar] [CrossRef]
- Di Girolamo, N.; Coroneo, M.; Wakefield, D. Epidermal Growth Factor Receptor Signaling Is Partially Responsible for the Increased Matrix Metalloproteinase-1 Expression in Ocular Epithelial Cells after UVB Radiation. Am. J. Pathol. 2005, 167, 489–503. [Google Scholar] [CrossRef][Green Version]
- Kodric-Brown, A. Sexual dichromatism and temporary colour changes in the reproduction of shes. Am. Zool. 1998, 38, 70–81. [Google Scholar] [CrossRef]
- Ng’oma, E.; Groth, M.; Ripa, R.; Platzer, M.; Cellerino, A. Transcriptome pro-ling of natural dichromatism in the annualshes Nothobranchius furzeri and Nothobranchius kadleci. BMC Genom. 2014, 15, 754. [Google Scholar] [CrossRef]
- Nelissen, M.H.J. Communication. In Cichlid Fishes: Behaviour, Ecology and Evolution; Keenleyside, M.H.A., Ed.; Chapman Hall: London, UK, 1991; pp. 225–240. [Google Scholar]
- Hengartner, M.O. The biochemistry of apoptosis. Nature 2000, 407, 770–776. [Google Scholar] [CrossRef]
- Sugimoto, M. Morphological color changes in fish: Regulation of pigment cell density and morphology. Microsc. Res. Tech. 2002, 58, 496–503. [Google Scholar] [CrossRef]
- Irion, U.; Nüsslein-Volhard, C. The identification of genes involved in the evolution of color patterns in fish. Curr. Opin. Genet. Dev. 2019, 57, 31–38. [Google Scholar] [CrossRef]
- Desjardins, J.K.; Fernald, R.D. How do social dominance and social information inuence reproduction and the brain? Integr. Comp. Biol. 2008, 48, 596–603. [Google Scholar] [CrossRef]
- Fernald, R.D. Social regulation of reproduction: What changes and why? Horm. Brain Behav. 2009, 1, 683–691. [Google Scholar]
- Mikulin, A.E. Functional Significance of Pigments and Pigmentation in Fish Ontogenesis; VNIRO Publishing House: Moscow, Russia, 2000; p. 231. (In Russian) [Google Scholar]


| 8 MPH | 2.5 MPH | p-Value | Whole Sample | |
|---|---|---|---|---|
| Fish length | 2.47 ± 0.09 | 3.16 ± 0.44 | ≤0.0001 | 2.89 ± 0.49 |
| BROI MeGV | 45.44 ± 9.42 | 59.11 ± 15.27 | 0.0145 | 50.91 ± 13.75 |
| RROI MeGV | 75.49 ± 12.45 | 78.62 ± 13.84 | 0.8733 | 76.74 ± 13 |
| LBROI MeGV | 183 ± 27.63 | 171.9 ± 20.66 | 0.2528 | 176.9 ± 19.16 |
| BBCF MeGV | 50.03 ± 14.65 | 62.62 ± 15.77 | 0.0153 | 55.06 ± 16.22 |
| RSGC MeGV | 78.98 ± 13.46 | 80.49 ± 11.72 | 0.9978 | 79.58 ± 12.71 |
| BROI MoGV | 46.06 ± 21.06 | 57.46 ± 20.23 | 0.9525 | 50.62 ± 21.31 |
| RROI MoGV | 71.72 ± 18 | 78.58 ± 17.96 | 0.5817 | 74.47 ± 18.15 |
| LBROI MoGV | 180.2 ± 17.62 | 172.5 ± 32.45 | 0.8143 | 178.5 ± 29.76 |
| BBCF MoGV | 38.19 ± 14.34 | 55.50 ± 18.92 | 0.0003 | 45.12 ± 18.29 |
| RSGC MoGV | 64.97 ± 13.56 | 67.42 ± 14.07 | 0.8563 | 65.95 ± 13.70 |
| BROI Area | RROI Area | LBROI Area | RSGC Area | BBCF Area | |
|---|---|---|---|---|---|
| 8 MPH | 52.95 ± 24.26 | 371.7 ± 182 | 385.1 ± 155.1 | 17.75 ± 8.62 | 36.38 ± 28.44 |
| 2.5 MPH | 44.67 ± 23.9 | 197.4 ± 106.9 | 205.5 ± 59.21 | 8.13 ± 3.45 | 27.85 ± 11.42 |
| p-value | 0.1975 | <0.0001 | ≤0.001 | ≤0.001 | 0.1686 |
| Whole sample | 49.64 ± 24.46 | 302 ± 177.5 | 313.3 ± 153.3 | 13.91 ± 8.44 | 32.97 ± 23.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikiforov-Nikishin, D.L.; Kochetkov, N.I.; Mikodina, E.V.; Nikiforov-Nikishin, A.L.; Simakov, Y.G.; Golovacheva, N.A.; Gorbunov, A.V.; Chebotarev, S.N.; Kirichenko, E.Y.; Zabiyaka, I.Y.; et al. Evaluation of Age-Dependent Changes in the Coloration of Male Killifish Nothobranchius Guentheri Using New Photoprocessing Methods. Biology 2022, 11, 205. https://doi.org/10.3390/biology11020205
Nikiforov-Nikishin DL, Kochetkov NI, Mikodina EV, Nikiforov-Nikishin AL, Simakov YG, Golovacheva NA, Gorbunov AV, Chebotarev SN, Kirichenko EY, Zabiyaka IY, et al. Evaluation of Age-Dependent Changes in the Coloration of Male Killifish Nothobranchius Guentheri Using New Photoprocessing Methods. Biology. 2022; 11(2):205. https://doi.org/10.3390/biology11020205
Chicago/Turabian StyleNikiforov-Nikishin, Dmitry L., Nikita I. Kochetkov, Ekaterina V. Mikodina, Alexei L. Nikiforov-Nikishin, Yuri G. Simakov, Natalya A. Golovacheva, Alexander V. Gorbunov, Sergei N. Chebotarev, Evgeniya Yu. Kirichenko, Igor Yu. Zabiyaka, and et al. 2022. "Evaluation of Age-Dependent Changes in the Coloration of Male Killifish Nothobranchius Guentheri Using New Photoprocessing Methods" Biology 11, no. 2: 205. https://doi.org/10.3390/biology11020205
APA StyleNikiforov-Nikishin, D. L., Kochetkov, N. I., Mikodina, E. V., Nikiforov-Nikishin, A. L., Simakov, Y. G., Golovacheva, N. A., Gorbunov, A. V., Chebotarev, S. N., Kirichenko, E. Y., Zabiyaka, I. Y., Pastukhov, I. S., & Bren, A. B. (2022). Evaluation of Age-Dependent Changes in the Coloration of Male Killifish Nothobranchius Guentheri Using New Photoprocessing Methods. Biology, 11(2), 205. https://doi.org/10.3390/biology11020205






