Effect of Gold Nanostars Plus Amikacin against Carbapenem-Resistant Klebsiella pneumoniae Biofilms
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Nanostars
2.2. Microbiological Studies
2.2.1. Minimum Inhibitory Concentration and Minimum Bactericidal Concentration
2.2.2. Minimum Biofilm Inhibitory Concentration and Minimum Biofilm Eradication Concentration
2.2.3. Synergy between Amikacin or Meropenem and GNS against Carbapenem-Resistant K. pneumoniae Biofilm
2.2.4. Antibacterial Mechanism of GNS
2.3. Cell Proliferation
2.4. Galleria Mellonella Model
2.5. Statistical Analysis
3. Results
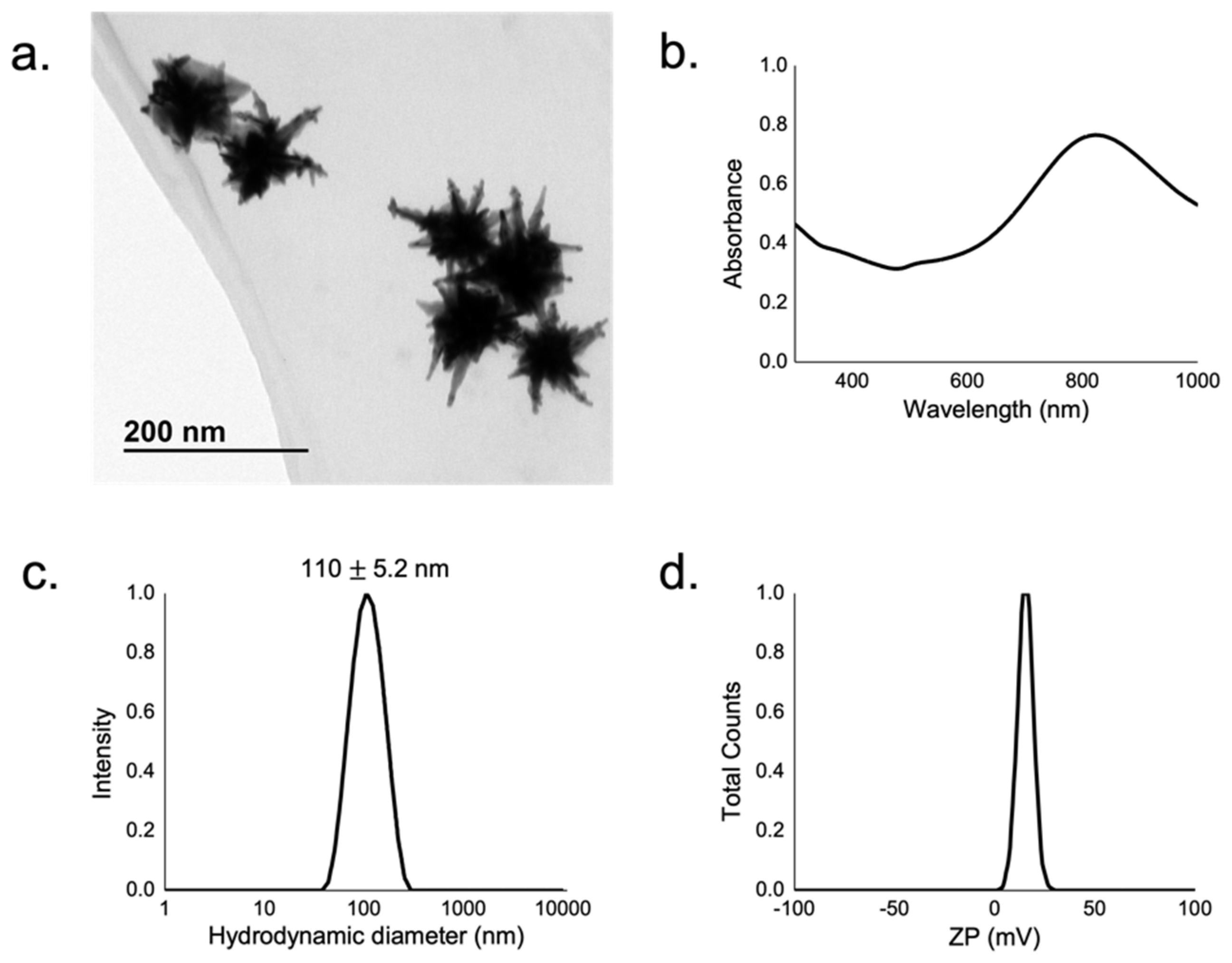
3.1. GNS Characterization
3.2. Microbiological Studies
3.2.1. Minimum Inhibitory Concentration (MIC), Minimum Bactericidal Concentration (MBC), Minimum Biofilm Inhibitory Concentration (MBIC), and Minimum Biofilm Eradication Concentration (MBEC)
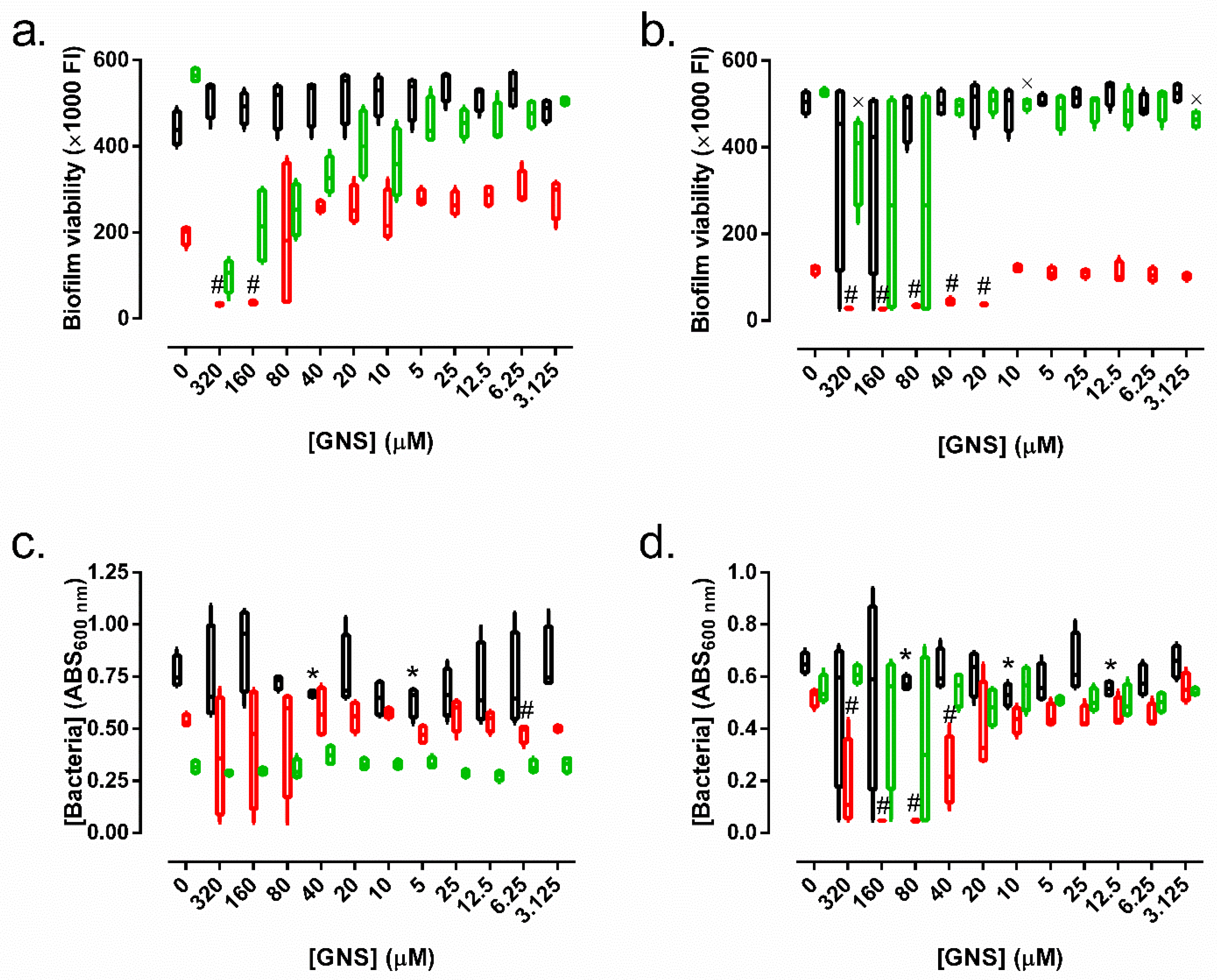
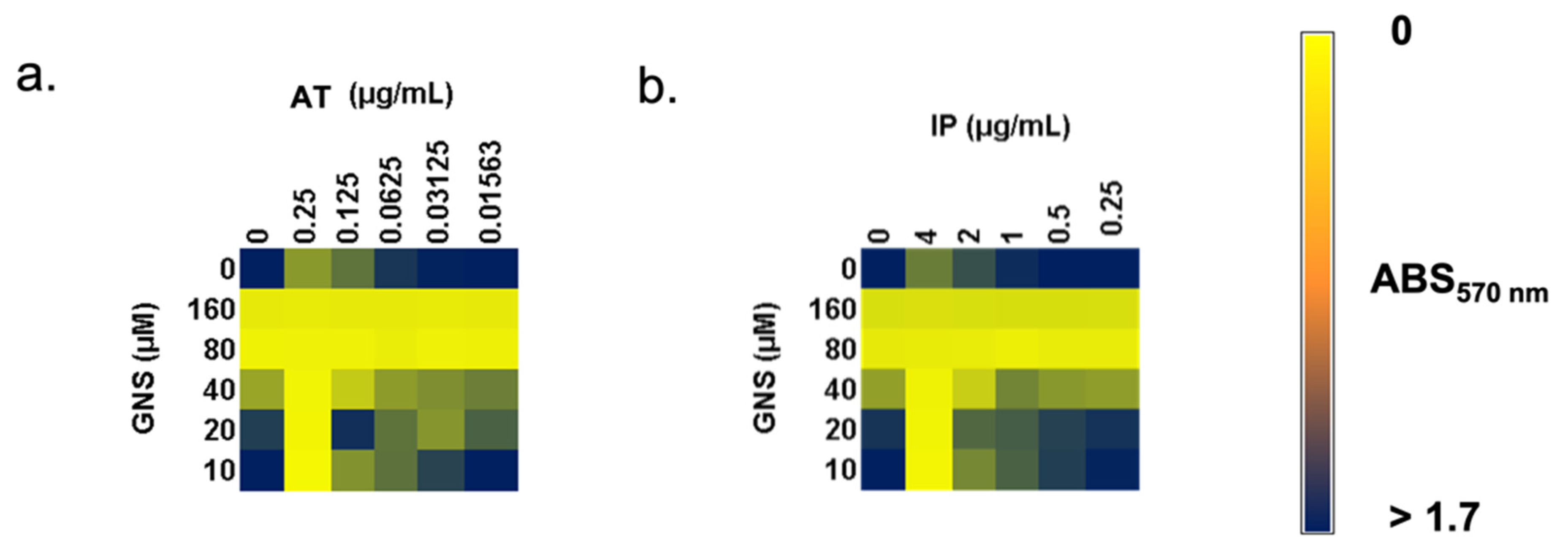
3.2.2. Synergy between Amikacin or Meropenem Plus GNS against Carbapenem-Resistant K. pneumoniae Biofilm
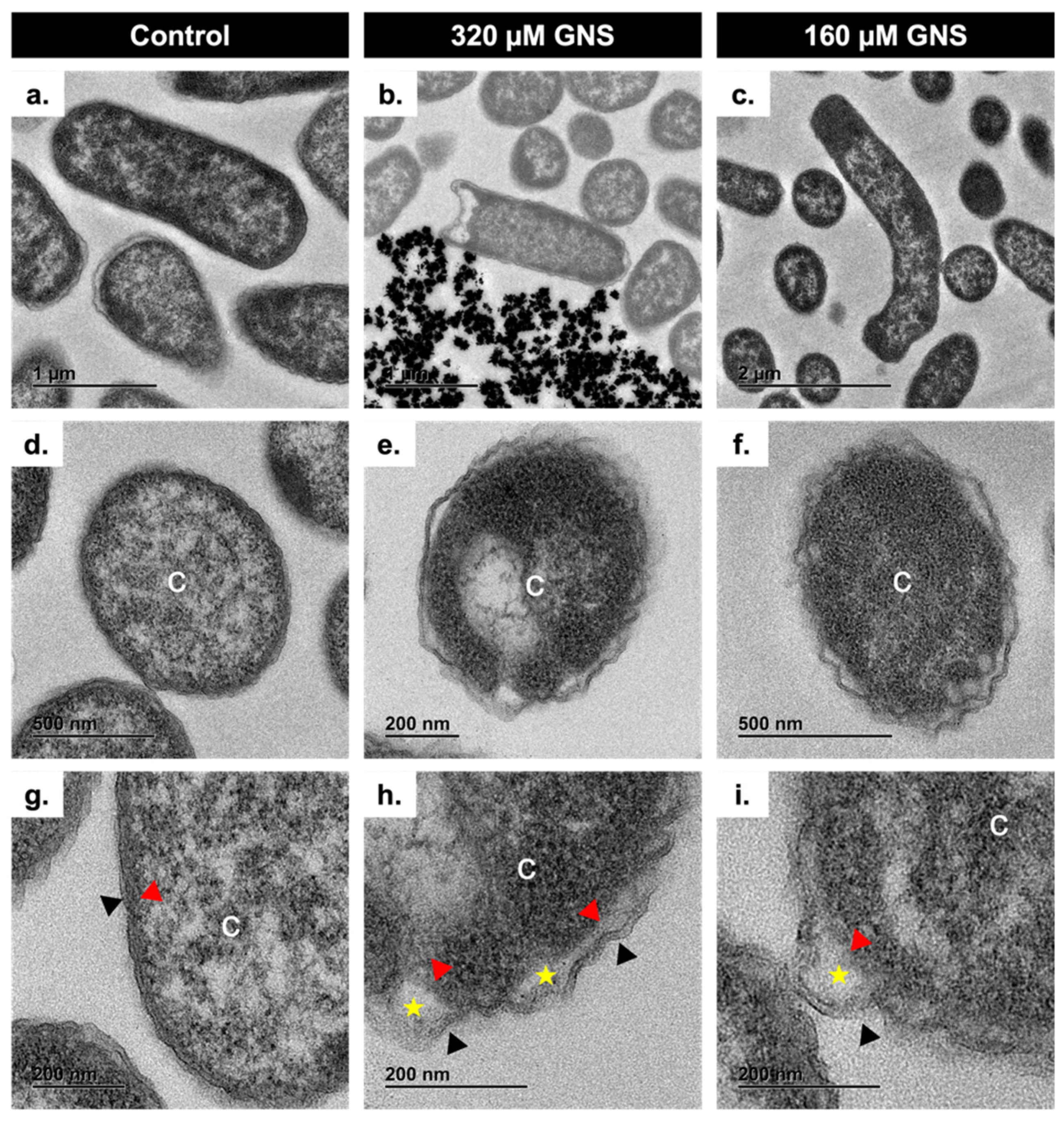
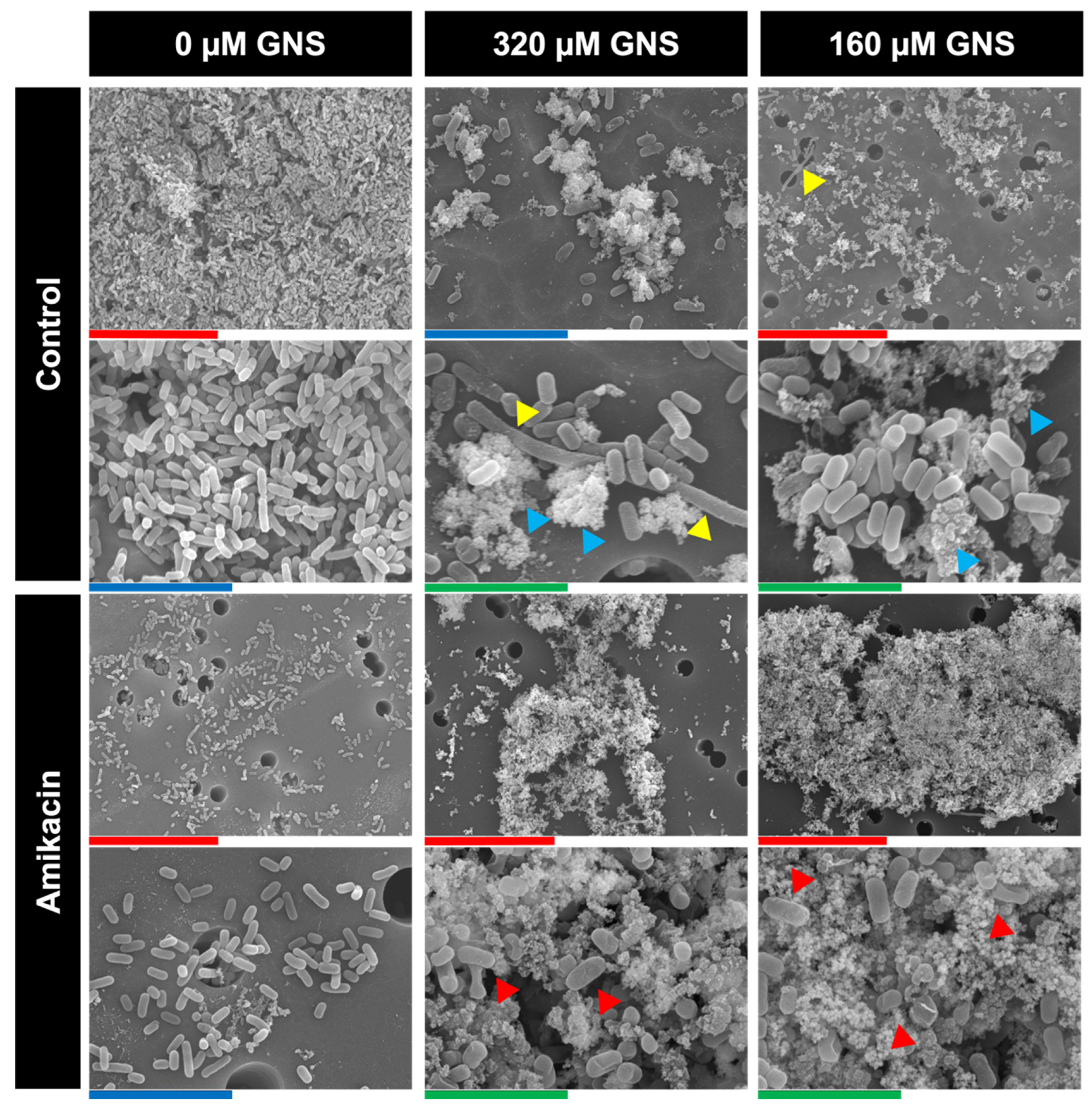
3.2.3. Antibacterial Mechanism of GNS
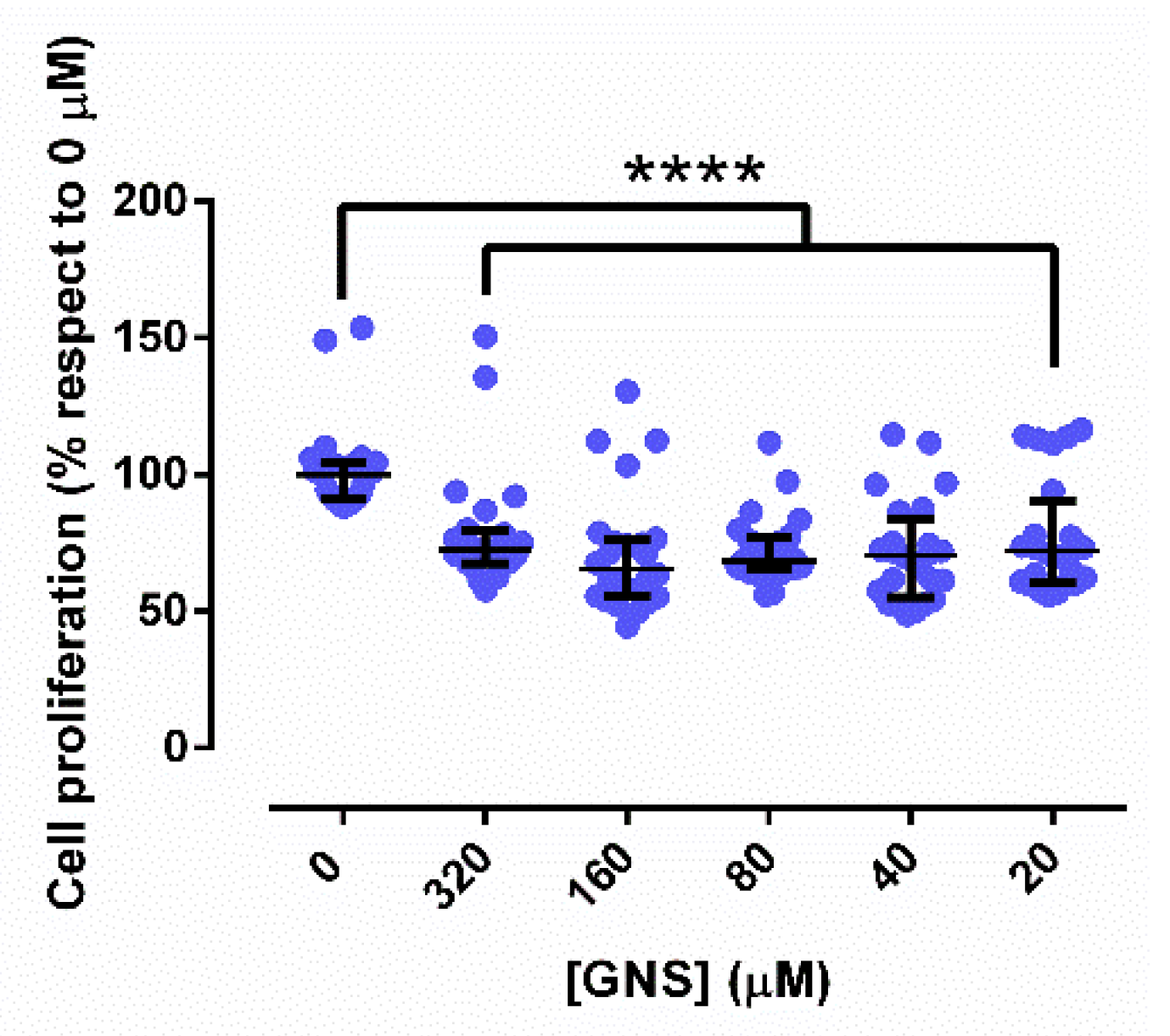
3.3. Cell Proliferation
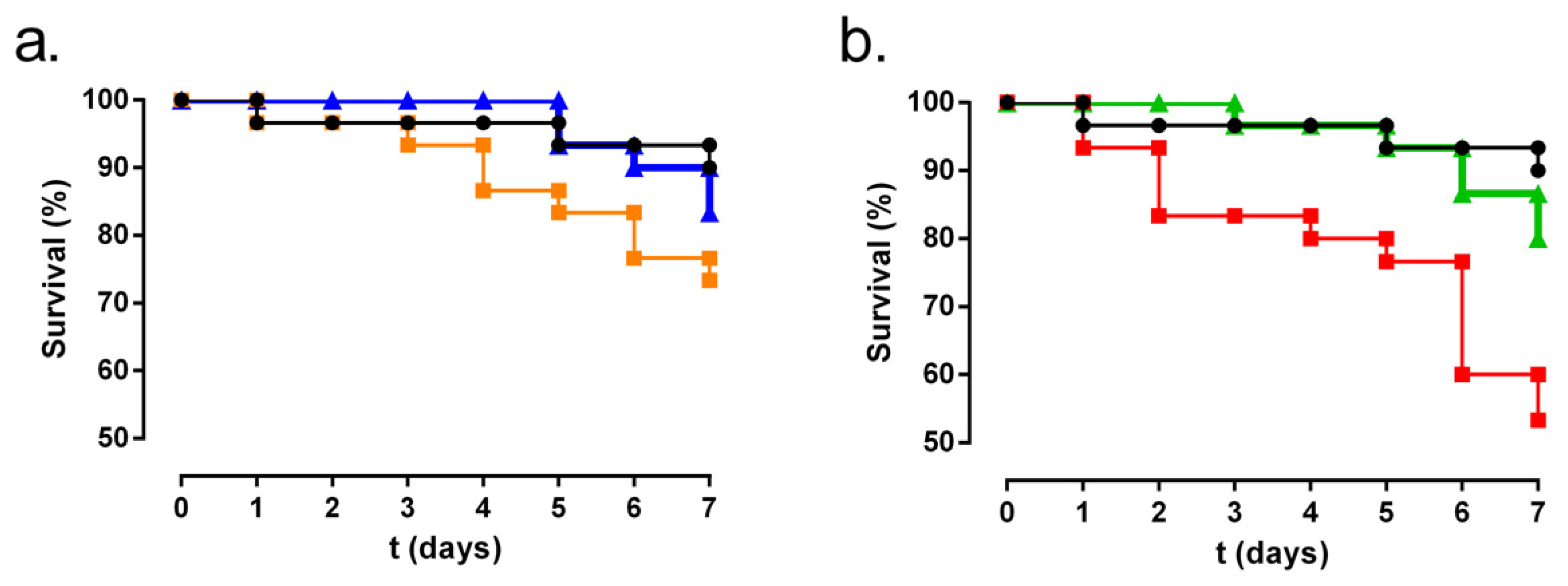
3.4. Galleria Mellonella Larvae Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ashurst, J.V.; Dawson, A. Klebsiella Pneumonia; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Martin, R.M.; Bachman, M.A. Colonization, Infection, and the Accessory Genome of Klebsiella Pneumoniae. Front. Cell. Infect. Microbiol. 2018, 8, 4. [Google Scholar] [CrossRef]
- Bengoechea, J.A.; Sa Pessoa, J. Klebsiella pneumoniae Infection Biology: Living to Counteract Host Defences. FEMS Microbiol. Rev. 2019, 43, 123–144. [Google Scholar] [CrossRef]
- Corrin, B.; Nicholson, A.G. Infectious Diseases. In Pathology of the Lungs; Elsevier: Amsterdam, The Netherlands, 2011; pp. 155–262. ISBN 978-0-7020-3369-8. [Google Scholar]
- Galani, I.; Karaiskos, I.; Giamarellou, H. Multidrug-Resistant Klebsiella Pneumoniae: Mechanisms of Resistance Including Updated Data for Novel β-Lactam-β-Lactamase Inhibitor Combinations. Expert Rev. Anti-Infect. Ther. 2021, 19, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-R.; Lee, J.H.; Park, K.S.; Kim, Y.B.; Jeong, B.C.; Lee, S.H. Global Dissemination of Carbapenemase-Producing Klebsiella Pneumoniae: Epidemiology, Genetic Context, Treatment Options, and Detection Methods. Front. Microbiol. 2016, 7, 895. [Google Scholar] [CrossRef] [PubMed]
- Pitout, J.D.D. Multiresistant Enterobacteriaceae: New Threat of an Old Problem. Expert Rev. Anti-Infect. Ther. 2008, 6, 657–669. [Google Scholar] [CrossRef]
- Moya, C.; Maicas, S. Antimicrobial Resistance in Klebsiella pneumoniae Strains: Mechanisms and Outbreaks. Proceedings 2020, 66, 11. [Google Scholar] [CrossRef]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival Mechanisms of Clinically Relevant Microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef]
- Deva, A.K.; Adams, W.P.; Vickery, K. The Role of Bacterial Biofilms in Device-Associated Infection. Plast. Reconstr. Surg. 2013, 132, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Righi, E.; Carnelutti, A.; Graziano, E.; Russo, A. Multidrug-Resistant Klebsiella Pneumoniae: Challenges for Treatment, Prevention and Infection Control. Expert Rev. Anti-Infect. Ther. 2018, 16, 749–761. [Google Scholar] [CrossRef]
- Le In Vitro Activity of Carbapenems Alone and in Combination With Amikacin Against KPC-Producing Klebsiella Pneumoniae. J. Clin. Med. Res. 2011, 3, 106–110. [CrossRef][Green Version]
- Ota, K.; Kaku, N.; Yanagihara, K. Efficacy of Meropenem and Amikacin Combination Therapy against Carbapenemase-Producing Klebsiella pneumoniae Mouse Model of Pneumonia. J. Infect. Chemother. 2020, 26, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Daikos, G.L.; Markogiannakis, A. Carbapenemase-Producing Klebsiella Pneumoniae: (When) Might We Still Consider Treating with Carbapenems? Clin. Microbiol. Infect. 2011, 17, 1135–1141. [Google Scholar] [CrossRef]
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report: 2021; World Health Organization: Geneva, Switzerland, 2021; ISBN 978-92-4-002733-6.
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Aguilera-Correa, J.J.; Esteban, J.; Vallet-Regí, M. Inorganic and Polymeric Nanoparticles for Human Viral and Bacterial Infections Prevention and Treatment. Nanomaterials 2021, 11, 137. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; González, B.; Izquierdo-Barba, I. Nanomaterials as Promising Alternative in the Infection Treatment. Int. J. Mol. Sci. 2019, 20, 3806. [Google Scholar] [CrossRef]
- Gisbert-Garzarán, M.; Manzano, M.; Vallet-Regí, M. Mesoporous Silica Nanoparticles for the Treatment of Complex Bone Diseases: Bone Cancer, Bone Infection and Osteoporosis. Pharmaceutics 2020, 12, 83. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Colilla, M.; Izquierdo-Barba, I. Bioactive Mesoporous Silicas as Controlled Delivery Systems: Application in Bone Tissue Regeneration. J. Biomed. Nanotechnol. 2008, 4, 1–15. [Google Scholar] [CrossRef]
- Vallet-Regí, M. Our Contributions to Applications of Mesoporous Silica Nanoparticles. Acta Biomater. 2021, 137, 44–52. [Google Scholar] [CrossRef] [PubMed]
- García, A.; González, B.; Harvey, C.; Izquierdo-Barba, I.; Vallet-Regí, M. Effective Reduction of Biofilm through Photothermal Therapy by Gold Core@shell Based Mesoporous Silica Nanoparticles. Microporous Mesoporous Mater. 2021, 328, 111489. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Lozano, D.; González, B.; Izquierdo-Barba, I. Biomaterials against Bone Infection. Adv. Healthc. Mater. 2020, 9, 2000310. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Rámila, A.; del Real, R.P.; Pérez-Pariente, J. A New Property of MCM-41: Drug Delivery System. Chem. Mater. 2001, 13, 308–311. [Google Scholar] [CrossRef]
- Colilla, M.; Izquierdo-Barba, I.; Sánchez-Salcedo, S.; Fierro, J.L.G.; Hueso, J.L.; Vallet-Regí, M. Synthesis and Characterization of Zwitterionic SBA-15 Nanostructured Materials. Chem. Mater. 2010, 22, 6459–6466. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Zarei, M.; Hashemi, S.A.; Ramakrishna, S.; Chiang, W.-H.; Lai, C.W.; Gholami, A. Gold Nanostars-Diagnosis, Bioimaging and Biomedical Applications. Drug Metab. Rev. 2020, 52, 299–318. [Google Scholar] [CrossRef]
- Fabris, L. Gold Nanostars in Biology and Medicine: Understanding Physicochemical Properties to Broaden Applicability. J. Phys. Chem. C 2020, 124, 26540–26553. [Google Scholar] [CrossRef]
- Paris, J.L.; Baeza, A.; Vallet-Regí, M. Overcoming the Stability, Toxicity, and Biodegradation Challenges of Tumor Stimuli-Responsive Inorganic Nanoparticles for Delivery of Cancer Therapeutics. Expert Opin. Drug Deliv. 2019, 16, 1095–1112. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, Q.; Yin, Q.; Pan, G.; Tu, Z.; Liu, L. Reduced Graphene Oxide Functionalized with Gold Nanostar Nanocomposites for Synergistically Killing Bacteria through Intrinsic Antimicrobial Activity and Photothermal Ablation. ACS Appl. Bio Mater. 2019, 2, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A Study of the Nucleation and Growth Processes in the Synthesis of Colloidal Gold. Discuss. Faraday Soc. 1951, 11, 55. [Google Scholar] [CrossRef]
- Yuan, H.; Khoury, C.G.; Hwang, H.; Wilson, C.M.; Grant, G.A.; Vo-Dinh, T. Gold Nanostars: Surfactant-Free Synthesis, 3D Modelling, and Two-Photon Photoluminescence Imaging. Nanotechnology 2012, 23, 075102. [Google Scholar] [CrossRef] [PubMed]
- Waites, K.B.; Bade, D.J.; Bébéar, C.; Brown, S.D.; Davidson, M.K.; Duffy, L.B.; Kenny, G.; Matlow, A.; Shortridge, D.; Talkington, D.; et al. Methods for Antimicrobial Susceptibility Testing for Human Mycoplasmas; Approved Guideline; CLSI Standards: Guidelines for Health Care Excellence; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2011; ISBN 978-1-56238-769-3. [Google Scholar]
- Elshikh, M.; Ahmed, S.; Funston, S.; Dunlop, P.; McGaw, M.; Marchant, R.; Banat, I.M. Resazurin-Based 96-Well Plate Microdilution Method for the Determination of Minimum Inhibitory Concentration of Biosurfactants. Biotechnol. Lett. 2016, 38, 1015–1019. [Google Scholar] [CrossRef]
- Pettit, R.K.; Weber, C.A.; Pettit, G.R. Application of a High Throughput Alamar Blue Biofilm Susceptibility Assay to Staphylococcus Aureus Biofilms. Ann. Clin. Microbiol. Antimicrob. 2009, 8, 28. [Google Scholar] [CrossRef]
- Peeters, E.; Nelis, H.J.; Coenye, T. Comparison of Multiple Methods for Quantification of Microbial Biofilms Grown in Microtiter Plates. J. Microbiol. Methods 2008, 72, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Hernandes, C.; da Silva Coppede, J.; Bertoni, B.W.; de Castro França, S.; Pereira, A.M.S. Flash Microbiocide: A Rapid and Economic Method for Determination of MBC and MFC. Am. J. Plant Sci. 2013, 4, 850–852. [Google Scholar] [CrossRef]
- Limpens, E.; Ivanov, S.; van Esse, W.; Voets, G.; Fedorova, E.; Bisseling, T. Medicago N2 -Fixing Symbiosomes Acquire the Endocytic Identity Marker Rab7 but Delay the Acquisition of Vacuolar Identity. Plant. Cell 2009, 21, 2811–2828. [Google Scholar] [CrossRef]
- Pettit, R.K.; Weber, C.A.; Kean, M.J.; Hoffmann, H.; Pettit, G.R.; Tan, R.; Franks, K.S.; Horton, M.L. Microplate Alamar Blue Assay for Staphylococcus Epidermidis Biofilm Susceptibility Testing. Antimicrob. Agents Chemother. 2005, 49, 2612–2617. [Google Scholar] [CrossRef] [PubMed]
- Okoliegbe, I.N.; Hijazi, K.; Cooper, K.; Ironside, C.; Gould, I.M. Antimicrobial Synergy Testing: Comparing the Tobramycin and Ceftazidime Gradient Diffusion Methodology Used in Assessing Synergy in Cystic Fibrosis-Derived Multidrug-Resistant Pseudomonas Aeruginosa. Antibiotics 2021, 10, 967. [Google Scholar] [CrossRef] [PubMed]
- Moody, J. Synergism Testing: Broth Microdilution Checkerboard and Broth Macrodilution Methods. In Clinical Microbiology Procedures Handbook; Leber, A.L., Ed.; ASM Press: Washington, DC, USA, 2016; pp. 5.16.1–5.16.23. ISBN 978-1-68367-076-6. [Google Scholar]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Dieases (ESCMID). EUCAST Definitive Document, E. Def 1.2, May 2000: Terminology Relating to Methods for the Determination of Susceptibility of Bacteria to Antimicrobial Agents. Clin. Microbiol. Infect. 2000, 6, 503–508. [Google Scholar] [CrossRef]
- Odds, F.C. Synergy, Antagonism, and What the Chequerboard Puts between Them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef] [PubMed]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual; Markossian, S., Grossman, A., Brimacombe, K., Arkin, M., Auld, D., Austin, C.P., Baell, J., Chung, T.D.Y., Coussens, N.P., Dahlin, J.L., et al., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004. [Google Scholar]
- Gu, X.; Xu, Z.; Gu, L.; Xu, H.; Han, F.; Chen, B.; Pan, X. Preparation and Antibacterial Properties of Gold Nanoparticles: A Review. Env. Chem. Lett. 2021, 19, 167–187. [Google Scholar] [CrossRef]
- Cui, Y.; Zhao, Y.; Tian, Y.; Zhang, W.; Lü, X.; Jiang, X. The Molecular Mechanism of Action of Bactericidal Gold Nanoparticles on Escherichia Coli. Biomaterials 2012, 33, 2327–2333. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Florez, V.; Mendez-Sanchez, S.C.; Patrón-Soberano, O.A.; Rodríguez-González, V.; Blach, D.; Martínez, O.F. Gold Nanoparticle-Mediated Generation of Reactive Oxygen Species during Plasmonic Photothermal Therapy: A Comparative Study for Different Particle Sizes, Shapes, and Surface Conjugations. J. Mater. Chem. B 2020, 8, 2862–2875. [Google Scholar] [CrossRef] [PubMed]
- Piktel, E.; Suprewicz, Ł.; Depciuch, J.; Chmielewska, S.; Skłodowski, K.; Daniluk, T.; Król, G.; Kołat-Brodecka, P.; Bijak, P.; Pajor-Świerzy, A.; et al. Varied-Shaped Gold Nanoparticles with Nanogram Killing Efficiency as Potential Antimicrobial Surface Coatings for the Medical Devices. Sci. Rep. 2021, 11, 12546. [Google Scholar] [CrossRef]
- Huynh, P.T.; Nguyen, G.D.; Tran, K.T.L.; Ho, T.M.; Duong, B.T.; Lam, V.Q.; Ngo, T.V.K. One-Pot, Surfactant-Free Synthesis of Gold Nanostars and Evaluation of Their Antibacterial Effects against Propionibacterium acnes. J. Nanomater. 2021, 2021, 6650661. [Google Scholar] [CrossRef]
- Ortiz-Benítez, E.A.; Velázquez-Guadarrama, N.; Durán Figueroa, N.V.; Quezada, H.; Olivares-Trejo, J.D.J. Antibacterial Mechanism of Gold Nanoparticles on Streptococcus pneumoniae. Metallomics 2019, 11, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- De Sousa Oliveira, K.; de Lima, L.A.; Cobacho, N.B.; Dias, S.C.; Franco, O.L. Mechanisms of Antibacterial Resistance. In Antibiotic Resistance; Elsevier: Amsterdam, The Netherlands, 2016; pp. 19–35. ISBN 978-0-12-803642-6. [Google Scholar]
- Weiss, D.S. Bacterial Cell Division and the Septal Ring: The Septal Ring. Mol. Microbiol. 2004, 54, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, S.; DiLuzio, W.R.; Weibel, D.B.; Whitesides, G.M. Controlling the Shape of Filamentous Cells of Escherichia coli. Nano Lett. 2005, 5, 1819–1823. [Google Scholar] [CrossRef]
- Georgopapadakou, N.H.; Smith, S.A.; Sykes, R.B. Mode of Action of Azthreonam. Antimicrob. Agents Chemother. 1982, 21, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, T.; Ishino, F.; Nakagawa, J.-I.; Tamaki, S.; Matsuhashi, M. Studies on the Mechanism of Action of Imipenem (N-Formimidoylthienamycin) in Vitro: Binding to the Penicillin-Binding Proteins (PBPs) in Escherichia Coli and Pseudomonas Aeruginosa, and Inhibition of Enzyme Activities Due to the PBPs in E. Coli. J. Antibiot. 1984, 37, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Odenholt, I.; Löwdin, E.; Cars, O. In Vitro Pharmacodynamic Studies of L-749,345 in Comparison with Imipenem and Ceftriaxone against Gram-Positive and Gram-Negative Bacteria. Antimicrob. Agents Chemother. 1998, 42, 2365–2370. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Scholar, E. Aztreonam. In xPharm: The Comprehensive Pharmacology Reference; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1–5. ISBN 978-0-08-055232-3. [Google Scholar]
- Popham, D.L.; Young, K.D. Role of Penicillin-Binding Proteins in Bacterial Cell Morphogenesis. Curr. Opin. Microbiol. 2003, 6, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Zahir, T.; Camacho, R.; Vitale, R.; Ruckebusch, C.; Hofkens, J.; Fauvart, M.; Michiels, J. High-Throughput Time-Resolved Morphology Screening in Bacteria Reveals Phenotypic Responses to Antibiotics. Commun. Biol. 2019, 2, 269. [Google Scholar] [CrossRef]
- Pruteanu, M.; Baker, T.A. Proteolysis in the SOS Response and Metal Homeostasis in Escherichia Coli. Res. Microbiol. 2009, 160, 677–683. [Google Scholar] [CrossRef]
- Khattar, M.M. Overexpression of the HslVU Operon Suppresses SOS-Mediated Inhibition of Cell Division in Escherichia coli. FEBS Lett. 1997, 414, 402–404. [Google Scholar] [CrossRef] [PubMed]
- Seifi, K.; Kazemian, H.; Heidari, H.; Rezagholizadeh, F.; Saee, Y.; Shirvani, F.; Houri, H. Evaluation of Biofilm Formation Among Klebsiella pneumoniae Isolates and Molecular Characterization by ERIC-PCR. Jundishapur J. Microbiol. 2016, 9, e30682. [Google Scholar] [CrossRef] [PubMed]
- Giri, K.; Rivas Yepes, L.; Duncan, B.; Kolumam Parameswaran, P.; Yan, B.; Jiang, Y.; Bilska, M.; Moyano, D.F.; Thompson, M.A.; Rotello, V.M.; et al. Targeting Bacterial Biofilms via Surface Engineering of Gold Nanoparticles. RSC Adv. 2015, 5, 105551–105559. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.G.; Ansari, M.A.; Alzohairy, M.A.; Alomary, M.N.; AlYahya, S.; Jalal, M.; Khan, H.M.; Asiri, S.M.M.; Ahmad, W.; Mahdi, A.A.; et al. Biogenic Gold Nanoparticles as Potent Antibacterial and Antibiofilm Nano-Antibiotics against Pseudomonas Aeruginosa. Antibiotics 2020, 9, 100. [Google Scholar] [CrossRef]
- Álvarez, E.; Estévez, M.; Jiménez-Jiménez, C.; Colilla, M.; Izquierdo-Barba, I.; González, B.; Vallet-Regí, M. A Versatile Multicomponent Mesoporous Silica Nanosystem with Dual Antimicrobial and Osteogenic Effects. Acta Biomater. 2021, 136, 570–581. [Google Scholar] [CrossRef]
- Arcos, D.; Vallet-Regí, M. Substituted Hydroxyapatite Coatings of Bone Implants. J. Mater. Chem. B 2020, 8, 1781–1800. [Google Scholar] [CrossRef]
- Claudel, M.; Schwarte, J.V.; Fromm, K.M. New Antimicrobial Strategies Based on Metal Complexes. Chemistry 2020, 2, 849–899. [Google Scholar] [CrossRef]
- Mu, H.; Liu, Q.; Niu, H.; Sun, Y.; Duan, J. Gold Nanoparticles Make Chitosan–Streptomycin Conjugates Effective towards Gram-Negative Bacterial Biofilm. RSC Adv. 2016, 6, 8714–8721. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Q.; Ruan, Z.; Yin, Y. Intrinsic Effects of Gold Nanoparticles on Proliferation and Invasion Activity in SGC-7901 Cells. Oncol. Rep. 2016, 35, 1457–1462. [Google Scholar] [CrossRef][Green Version]
- Moya-Andérico, L.; Vukomanovic, M.; del Mar Cendra, M.; Segura-Feliu, M.; Gil, V.; del Río, J.A.; Torrents, E. Utility of Galleria Mellonella Larvae for Evaluating Nanoparticle Toxicology. Chemosphere 2021, 266, 129235. [Google Scholar] [CrossRef]
- Pareek, V.; Devineau, S.; Sivasankaran, S.K.; Bhargava, A.; Panwar, J.; Srikumar, S.; Fanning, S. Silver Nanoparticles Induce a Triclosan-Like Antibacterial Action Mechanism in Multi-Drug Resistant Klebsiella Pneumoniae. Front. Microbiol. 2021, 12, 638640. [Google Scholar] [CrossRef]
- Shaaban, M.I.; Shaker, M.A.; Mady, F.M. Imipenem/Cilastatin Encapsulated Polymeric Nanoparticles for Destroying Carbapenem-Resistant Bacterial Isolates. J. Nanobiotechnol. 2017, 15, 29. [Google Scholar] [CrossRef]
- Wu, G.; Ji, H.; Guo, X.; Li, Y.; Ren, T.; Dong, H.; Liu, J.; Liu, Y.; Shi, X.; He, B. Nanoparticle Reinforced Bacterial Outer-Membrane Vesicles Effectively Prevent Fatal Infection of Carbapenem-Resistant Klebsiella Pneumoniae. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102148. [Google Scholar] [CrossRef]
- Hitt, S.J.; Bishop, B.M.; van Hoek, M.L. Komodo-Dragon Cathelicidin-Inspired Peptides Are Antibacterial against Carbapenem-Resistant Klebsiella Pneumoniae. J. Med. Microbiol. 2020, 69, 1262–1272. [Google Scholar] [CrossRef]
- Mil-Homens, D.; Martins, M.; Barbosa, J.; Serafim, G.; Sarmento, M.J.; Pires, R.F.; Rodrigues, V.; Bonifácio, V.D.B.; Pinto, S.N. Carbapenem-Resistant Klebsiella pneumoniae Clinical Isolates: In Vivo Virulence Assessment in Galleria mellonella and Potential Therapeutics by Polycationic Oligoethyleneimine. Antibiotics 2021, 10, 56. [Google Scholar] [CrossRef]
| Antibiotic | ATCC23357 (MIC) | KP52 (MIC) | KP5 (MIC) |
|---|---|---|---|
| Amikacin * | S (4) | S (2) | S (4) |
| Ampicilin | R (<32) | R (>32) | R (>32) |
| Cefotaxime | S (<0.25) | I (2) | R (64) |
| Cefoxitine | S (<4) | S (8) | S (8) |
| Ceftazidime | S (0.5) | S (0.5) | R (>64) |
| Gentamycin | S (<1) | S (<1) | S (<1) |
| Tobramycin | S (<1) | R (8) | R (8) |
| Imipenem | S (<0.25) | I (8) | R (>16) |
| Cefuroxime | S (8) | R (32) | R (>64) |
| Amoxicilin-clavulanic acid | S (8) | R (>32) | R (>32) |
| Ciprofloxacin | S (<0.25) | R (>4) | R (>4) |
| Fosfomycin | R (64) | R (>256) | R (>256) |
| Co-trimoxazole | S (<20) | R (>320) | R (>320) |
| Cefuroxime-axetil | S (8) | R (32) | R (>64) |
| Cefepime | S (<0.12) | S (0.5) | R (>32) |
| Colistin * | S (0.125) | S (0.019) | S (0.125) |
| Ertapenem | S (<0.12) | R (>8) | R (>8) |
| BSBL | Negative | Negative | Positive |
| Ceftazidime/avibactam | S (0.75) | S (0.5) | S (0.75) |
| Nadilixic acid | S (4) | R (>32) | R (>32) |
| Nitrofurantoin | S (<16) | R (128) | R (128) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilera-Correa, J.J.; García-Álvarez, R.; Mediero, A.; Esteban, J.; Vallet-Regí, M. Effect of Gold Nanostars Plus Amikacin against Carbapenem-Resistant Klebsiella pneumoniae Biofilms. Biology 2022, 11, 162. https://doi.org/10.3390/biology11020162
Aguilera-Correa JJ, García-Álvarez R, Mediero A, Esteban J, Vallet-Regí M. Effect of Gold Nanostars Plus Amikacin against Carbapenem-Resistant Klebsiella pneumoniae Biofilms. Biology. 2022; 11(2):162. https://doi.org/10.3390/biology11020162
Chicago/Turabian StyleAguilera-Correa, John Jairo, Rafaela García-Álvarez, Aranzazu Mediero, Jaime Esteban, and María Vallet-Regí. 2022. "Effect of Gold Nanostars Plus Amikacin against Carbapenem-Resistant Klebsiella pneumoniae Biofilms" Biology 11, no. 2: 162. https://doi.org/10.3390/biology11020162
APA StyleAguilera-Correa, J. J., García-Álvarez, R., Mediero, A., Esteban, J., & Vallet-Regí, M. (2022). Effect of Gold Nanostars Plus Amikacin against Carbapenem-Resistant Klebsiella pneumoniae Biofilms. Biology, 11(2), 162. https://doi.org/10.3390/biology11020162








