The Effect of Short-Wavelength White LED Illumination throughout the Night on the Milk Fatty Acid Profile of High-Yielding Dairy Cows
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Housing Conditions, and the Experimental Protocol
2.2. Sampling of the Milk Fat and Analysis of Its Fatty Acid Profile
2.3. Statistical Analysis
3. Results
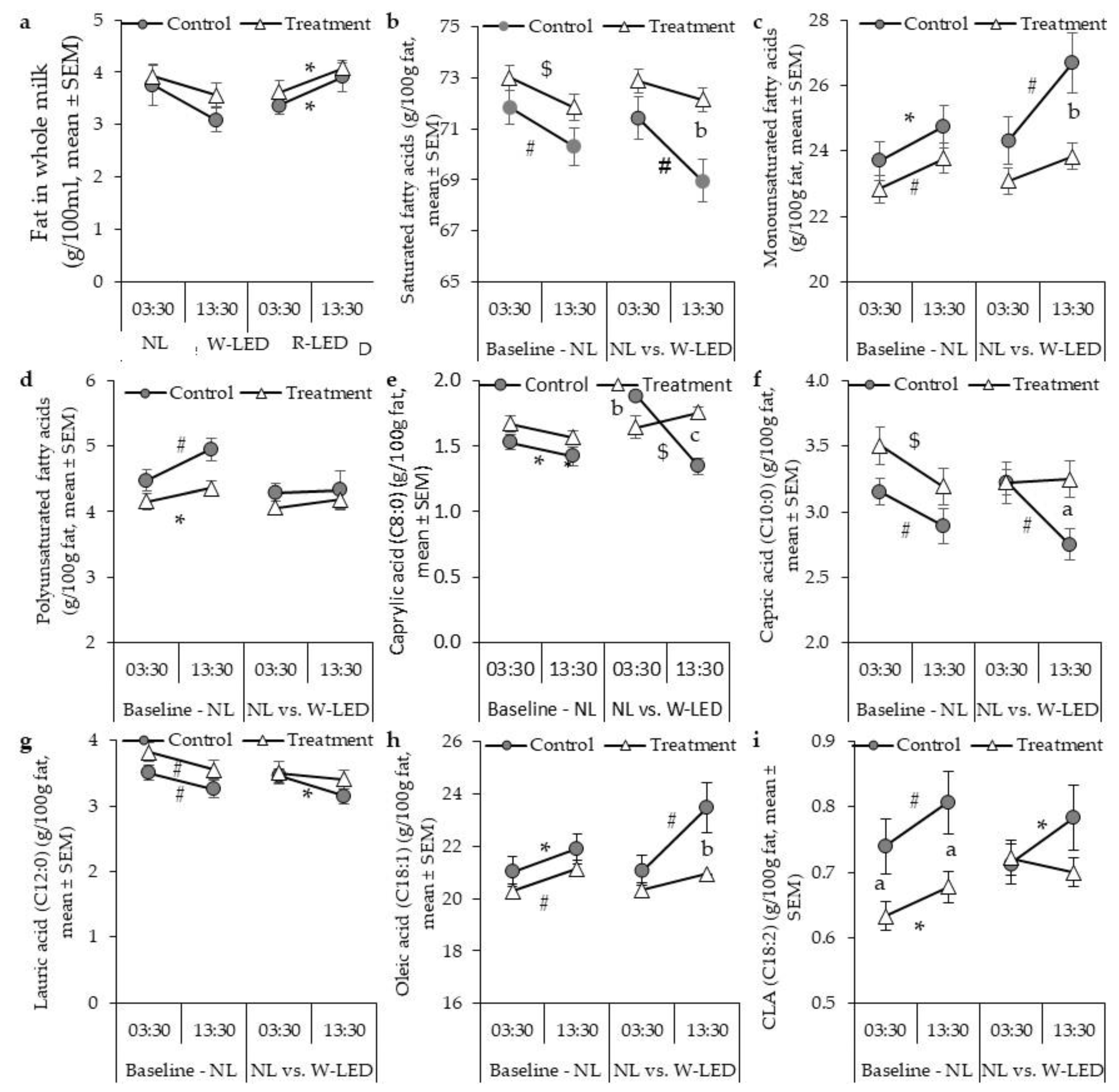
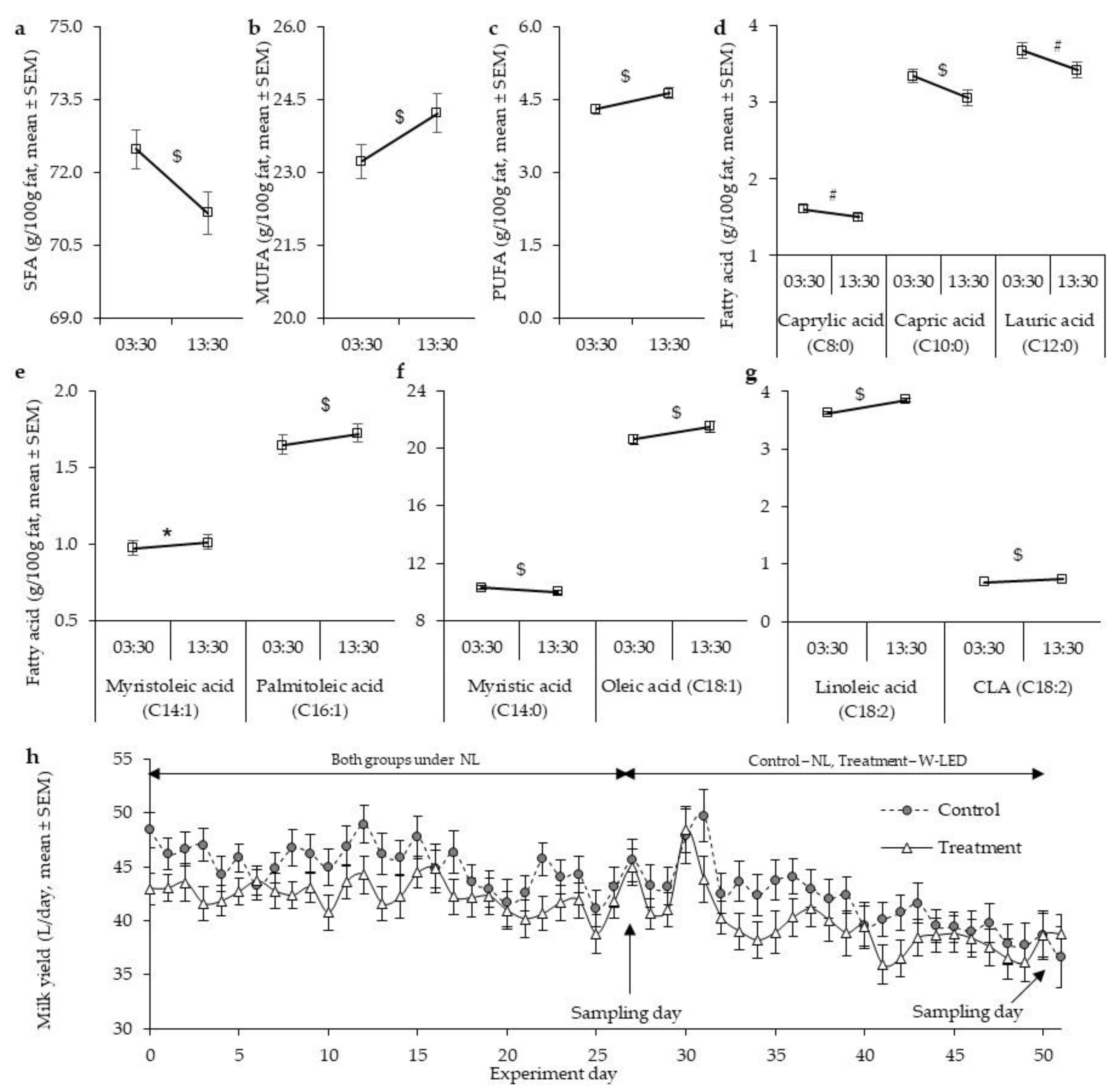
3.1. Milk Fat Composition under the Natural Illumination Regime Is Time-Dependent, Showing Higher Mono- and Polyunsaturated Fatty Acid Levels at Day Milking
3.2. Whole-Night White LED Illumination Results in a Higher Level of SFA at DM and Abolishes the between-Milking-Time Variation in Milk Fat Composition Found under the Natural Light Regime
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Ingredients | Amount, % of Dry Matter |
|---|---|
| Corn, ground | 25.1 |
| Wheat silage | 22.8 |
| Wheat hay | 12.9 |
| Gluten feed | 10.2 |
| Dried distillers grain | 7.1 |
| Soybean meal | 4.8 |
| Rapeseed meal | 4.2 |
| Lactose water | 2.6 |
| Wheat grain, rolled | 2.4 |
| Protected fat | 1.9 |
| Cottonseed | 1.5 |
| Barley grain, rolled | 1.1 |
| Clover hay | 0.9 |
| Calcium salts | 0.9 |
| Bicarbonate | 0.7 |
| Limestone | 0.5 |
| Urea | 0.3 |
| Vitamins and minerals 1 | |
| Chemical composition | |
| Dry matter (%) | 64.6 |
| Ether extracts (%) | 4.4 |
| Crude protein (%) Crude NDF (%) 2 | 16.5 28.7 |
| NDF–forage (%) 3 | 17.5 |
| NEL (Mcal/gDM) 4 | 1.78 |
| Fatty Acid Standard | Fatty Acid Group | Status |
|---|---|---|
| Butyric Acid (C4:0) | Saturated | Quantified |
| Caproic Acid (C6:0) | Saturated | Quantified |
| Caprylic Acid (C8:0) | Saturated | Quantified |
| Capric Acid (C10:0) | Saturated | Quantified |
| Lauric Acid (C12:0) | Saturated | Quantified |
| Myristic Acid (C14:0) | Saturated | Quantified |
| Pentadecylic Acid (C15:0) | Saturated | Quantified |
| Palmitic Acid (C16:0) | Saturated | Quantified |
| Margaric Acid (C17:0) | Saturated | Quantified |
| Stearic Acid (C18:0) | Saturated | Quantified |
| Myristoleic Acid (C14:1) | Monounsaturated | Quantified |
| Palmitoleic Acid (C16:1) | Monounsaturated | Quantified |
| Oleic Acid (C18:1) | Monounsaturated | Quantified |
| Conjugated Linoleic Acid, CLA (C18:2) | Polyunsaturated | Quantified |
| Linoleic Acid (C18:2) | Polyunsaturated | Quantified |
| Undecanoic Acid (C11:0) | Saturated | Undetected |
| Tridecanoic Acid (C13:0) | Saturated | Undetected |
| Arachidic Acid (C20:0) | Saturated | Undetected |
| Heneicosanoic Acid (C21:0) | Saturated | Undetected |
| Behenic Acid (C22:0) | Saturated | Undetected |
| Tricosanoic Acid (C23:0) | Saturated | Undetected |
| Lignoceric Acid (C24:0) | Saturated | Undetected |
| Cis-10-Pentadecenoic Acid (C15:1) | Monounsaturated | Undetected |
| Cis-10-Heptadecenoic Acid (C17:1) | Monounsaturated | Undetected |
| Cis-9-Oleic Acid (C18:1) | Monounsaturated | Undetected |
| Cis-11-Eicosenoic Acid (C20:1) | Monounsaturated | Undetected |
| Erucic Acid (C22:1) | Monounsaturated | Undetected |
| Nervonic Acid (C24:1) | Monounsaturated | Undetected |
| Linolelaidic Acid (C18:2) | Polyunsaturated | Undetected |
| Gamma-Linolenic Acid (C18:3) | Polyunsaturated | Undetected |
| Linolenic Acid (C18:3) | Polyunsaturated | Undetected |
| Cis-11,14,17-Eicosatrienoic Acid (C20:3) | Polyunsaturated | Undetected |
| Cis-11,14-Eicosadienoic Acid (C20:2) | Polyunsaturated | Undetected |
| Cis-8,11,14-Eicosatrienoic Acid (C20:3) | Polyunsaturated | Undetected |
| Arachidonic Acid (C20:4) | Polyunsaturated | Undetected |
| Cis-5,8,11,14,17-Eicosapentaenoic Acid (C20:5) | Polyunsaturated | Undetected |
| Cis-13,16-Docosadienoic Acid (C22:2) | Polyunsaturated | Undetected |
| Cis-4,7,10,13,16,19-Docosahexaenoic Acid (C22:6) | Polyunsaturated | Undetected |
| Baseline—Natural Light | Treatment—White LED Illumination vs. Natural Light | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Treatment | Control | Treatment | |||||||||
| 03:30 | 13:30 | p | 03:30 | 13:30 | p | 03:30 | 13:30 | p | 03:30 | 13:30 | p | |
| Fat | 3.8 ± 0.4 | 3.1 ± 0.2 | 0.164 | 3.9 ± 0.2 | 3.6 ± 0.2 | 0.043 | 3.4 ± 0.2 | 3.9 ± 0.3 | 0.041 | 3.6 ± 0.2 | 4.1 ± 0.1 | 0.005 |
| Saturated fatty acids | 72 ± 0.7 | 70.3 ± 1 | 0.003 | 73 ± 1 | 72 ± 0.5 | <0.001 | 71 ± 0.8 | 69 ± 0.8 | 0.003 | 73 ± 0.5 | 72 ± 0.5 # | 0.206 |
| Butyric Acid (C4:0) | 6.2 ± 0.2 | 6.3 ± 0.2 | 0.842 | 6.4 ± 0.3 | 6.1 ± 0.2 | 0.522 | 7 ± 0.2 # | 5.8 ± 0.2 | 0.001 | 6.2 ± 0.2 | 6.6 ± 0.1 $ | 0.083 |
| Caproic Acid (C6:0) | 3.0 ± 0.1 | 2.9 ± 0.1 | 0.663 | 3.2 ± 0.1 | 3.0 ± 0.1 | 0.377 | 3.5 ± 0.1 # | 2.7 ± 0.1 | <0.001 | 3.1 ± 0.1 | 3.3 ± 0.1 $ | 0.058 |
| Caprylic Acid (C8:0) | 1.5 ± 0.1 | 1.4 ± 0.1 | 0.02 | 1.7 ± 0.1 | 1.6 ± 0.1 | 0.074 | 1.9 ± 0.1 # | 1.4 ± 0.1 | <0.001 | 1.6 ± 0.1 | 1.8 ± 0.0 $ | 0.145 |
| Capric Acid (C10:0) | 3.2 ± 0.1 | 2.9 ± 0.1 | 0.004 | 3.5 ± 0.1 | 3.2 ± 0.1 | <0.001 | 3.2 ± 0.1 | 2.8 ± 0.1 | 0.001 | 3.2 ± 0.2 | 3.3 ± 0.1 * | 0.549 |
| Lauric Acid (C12:0) | 3.5 ± 0.1 | 3.3 ± 0.1 | 0.004 | 3.8 ± 0.1 | 3.6 ± 0.2 | 0.003 | 3.4 ± 0.1 | 3.2 ± 0.1 | 0.011 | 3.5 ± 0.2 | 3.4 ± 0.2 | 0.139 |
| Myristic Acid (C14:0) | 10 ± 0.1 | 10 ± 0.2 | 0.022 | 10 ± 0.2 | 10 ± 0.2 | 1.0 | 9.8 ± 0.2 | 9.6 ± 0.3 | 0.850 | 10 ± 0.2 | 9.8 ± 0.2 | 0.246 |
| Pentadecylic Acid (C15:0) | 1.1 ± 0.1 | 1.1 ± 0.1 | 0.058 | 1.0 ± 0.1 | 1 ± 0.1 | 0.874 | 1.1 ± 0.0 | 1.0 ± 0.1 | 0.112 | 1.0 ± 0.0 | 1.0 ± 0.0 | 0.34 |
| Palmitic Acid (C16:0) | 32.2 ± 0.5 | 31.0 ± 0.4 | 0.008 | 32 ± 0.5 | 32 ± 0.5 | 0.046 | 31.8 ± 0.5 | 32.2 ± 0.5 | 0.43 | 33 ± 0.5 # | 32.5 ± 0.4 | 0.015 |
| Margaric Acid (C17:0) | 0.6 ± 0.0 | 0.6 ± 0.0 | 0.166 | 0.7 ± 0.1 | 0.6 ± 0.0 | 0.666 | 0.7 ± 0.0 | 0.6 ± 0.0 | 0.003 | 0.7 ± 0.1 | 0.6 ± 0.0 | 0.957 |
| Stearic Acid (C18:0) | 10.2 ± 0.3 | 10.0 ± 0.2 | 0.141 | 11 ± 0.3 | 11 ± 0.3 | 0.812 | 9.1 ± 0.2 | 9.8 ± 0.3 | 0.001 | 10 ± 0.3 # | 9.8 ± 0.3 | 0.08 |
| Monounsaturated fatty acids | 23.7 ± 0.6 | 25 ± 0.7 | 0.031 | 23 ± 0.4 | 24 ± 1 | 0.001 | 24.3 ± 0.7 * | 26.7 ± 0.9 # | 0.003 | 23 ± 0.4 | 23.8 ± 0.4 | 0.122 |
| Myristoleic Acid (C14:1) | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.009 | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.02 | 1.1 ± 0.1 | 1.2 ± 0.1 | 0.325 | 1.1 ± 0.1 | 1.1 ± 0.1 | 0.222 |
| Palmitoleic Acid (C16:1) | 1.7 ± 0.1 | 1.8 ± 0.1 | 0.394 | 1.6 ± 0.1 | 1.6 ± 0.1 | 0.831 | 2.0 ± 0.2 | 2.0 ± 0.2 | 0.430 | 1.7 ± 0.1 | 1.8 ± 0.1 | 0.186 |
| Oleic Acid (C18:1) | 21.1 ± 0.6 | 22 ± 0.6 | 0.05 | 20 ± 0.4 | 21 ± 0.4 | 0.003 | 21.1 ± 0.6 | 2.4 ± 1 # | 0.003 | 20 ± 0.4 | 20.9 ± 0.4 | 0.199 |
| Polyunsaturated fatty acids | 4.5 ± 0.2 | 5 ± 0.2 | 0.002 | 4.2 ± 0.1 | 4.4 ± 0.1 | 0.011 | 4.3 ± 0.1 | 4.3 ± 0.3 | 0.21 | 4.1 ± 0.1 | 4.2 ± 0.1 | 0.185 |
| Linoleic Acid (C18:2) | 3.7 ± 0.1 * | 4.1 ± 0.2 * | 0.002 | 3.5 ± 0.1 | 3.6 ± 0.1 | 0.03 | 3.6 ± 0.1 | 3.6 ± 0.3 | 0.258 | 3.4 ± 0.1 | 3.5 ± 0.1 | 0.166 |
| Conjugated Linoleic Acid, (C18:2) | 0.7 ± 0.0 | 0.8 ± 0.1 * | 0.004 | 0.6 ± 0.0 | 0.7 ± 0 | 0.011 | 0.7 ± 0.0 | 0.8 ± 0.1 | 0.043 | 0.7 ± 0.0 | 0.7 ± 0.0 | 0.366 |
References
- Salfer, I.J.; Bartell, P.A.; Dechow, C.D.; Harvatine, K.J. Annual Rhythms of Milk Synthesis in Dairy Herds in 4 Regions of the United States and Their Relationships to Environmental Indicators. J. Dairy Sci. 2020, 103, 3696–3707. [Google Scholar] [CrossRef] [PubMed]
- Salfer, I.J.; Dechow, C.D.; Harvatine, K.J. Annual Rhythms of Milk and Milk Fat and Protein Production in Dairy Cattle in the United States. J. Dairy Sci. 2019, 102, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Dahl, G.E.; Tao, S.; Thompson, I.M. Lactation Biology Symposium: Effects of Photoperiod on Mammary Gland Development and Lactation. J. Anim. Sci. 2012, 90, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Dahl, G.E.; Buchanan, B.A.; Tucker, H.A. Photoperiodic Effects on Dairy Cattle: A Review. J. Dairy Sci. 2000, 83, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.R.E.; Stanisiewski, E.P.; Erdman, R.A.; Douglass, L.W.; Dahl, G.E. Effects of Long Daily Photoperiod and Bovine Somatotropin (Trobest®) on Milk Yield in Cows. J. Dairy Sci. 1999, 82, 1716–1722. [Google Scholar] [CrossRef]
- Peters, R.R.; Chapin, L.T.; Leining, K.B.; Tucker, H.A. Supplemental Lighting Stimulates Growth and Lactation in Cattle. Science 1978, 199, 911–912. [Google Scholar] [CrossRef]
- Phillips, C.J.C.; Schofield, S.A. The Effect of Supplementary Light on the Production and Behaviour of Dairy Cows. Anim. Prod. 1989, 48, 293–303. [Google Scholar] [CrossRef]
- Marcek, J.M.; Swanson, L.V. Effect of Photoperiod on Milk Production and Prolactin of Holstein Dairy Cows. J. Dairy Sci. 1984, 67, 2380–2388. [Google Scholar] [CrossRef]
- Son, J.; Park, J.; Kang, D.; Belal, S.A.; Cha, J.; Shim, K. Effects of White, Yellow, and Blue Colored LEDs on Milk Production, Milk Composition, and Physiological Responses in Dairy Cattle. Anim. Sci. J. 2020, 91, e13337. [Google Scholar] [CrossRef]
- Peters, R.R.; Chapin, L.T.; Emery, R.S.; Tucker, H.A. Growth and Hormonal Response of Heifers to Various Photoperiods. J. Anim. Sci. 1980, 51, 1148–1153. [Google Scholar] [CrossRef]
- Fonken, L.K.; Nelson, R.J. The Effects of Light at Night on Circadian Clocks and Metabolism. Endocr. Rev. 2014, 35, 648–670. [Google Scholar] [CrossRef] [PubMed]
- Touitou, Y.; Reinberg, A.; Touitou, D. Association between Light at Night, Melatonin Secretion, Sleep Deprivation, and the Internal Clock: Health Impacts and Mechanisms of Circadian Disruption. Life Sci. 2017, 173, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Tähkämö, L.; Partonen, T.; Pesonen, A.K. Systematic Review of Light Exposure Impact on Human Circadian Rhythm. Chronobiol. Int. 2019, 36, 151–170. [Google Scholar] [CrossRef] [PubMed]
- IARC Monographs Vol 124 Group. Carcinogenicity of Night Shift Work. Lancet. Oncol. 2019, 20, 1058–1059. [Google Scholar] [CrossRef]
- Giannetto, C.; Piccione, G. Daily Rhythms of 25 Physiological Variables in Bos Taurus Maintained under Natural Conditions. J. Appl. Biomed. 2009, 7, 55–61. [Google Scholar] [CrossRef]
- Plaut, K.; Casey, T. Does the Circadian System Regulate Lactation? In Proceedings of the Animal; Cambridge University Press: Cambridge, UK, 2012; Volume 6, pp. 394–402. [Google Scholar]
- Asher, A.; Shabtay, A.; Brosh, A.; Eitam, H.; Agmon, R.; Cohen-Zinder, M.; Zubidat, A.E.; Haim, A. “Chrono-Functional Milk”: The Difference between Melatonin Concentrations in Night-Milk versus Day-Milk under Different Night Illumination Conditions. Chronobiol. Int. 2015, 32, 1409–1416. [Google Scholar] [CrossRef]
- Pattison, P.M.; Tsao, J.Y.; Brainard, G.C.; Bugbee, B. LEDs for Photons, Physiology and Food. Nature 2018, 563, 493–500. [Google Scholar] [CrossRef]
- Thomas, A.; Thelen, J.; Go, A.; Surbrook, T.; Vanee, M.; Althouse, J. A Dairy Long Day Lighting Success Story: MI Dairy Increases Production and Cuts Costs. In Proceedings of the 2017 ASABE Annual International Meeting, Spokane, WA, USA, 16–19 July 2017; p. 1. [Google Scholar]
- Lindkvist, S.; Ternman, E.; Ferneborg, S.; Bånkestad, D.; Lindqvist, J.; Ekesten, B.; Agenäs, S. Effects of Achromatic and Chromatic Lights on Pupillary Response, Endocrinology, Activity, and Milk Production in Dairy Cows. PLoS ONE 2021, 16, e0253776. [Google Scholar] [CrossRef]
- Elsabagh, M.; Mon, M.; Takao, Y.; Shinoda, A.; Watanabe, T.; Kushibiki, S.; Obitsu, T.; Sugino, T. Exposure to Blue LED Light before the Onset of Darkness under a Long-Day Photoperiod Alters Melatonin Secretion, Feeding Behaviour and Growth in Female Dairy Calves. Anim. Sci. J. 2020, 91, e13353. [Google Scholar] [CrossRef]
- Murphy, B.A.; Herlihy, M.M.; Nolan, M.B.; O’Brien, C.; Furlong, J.G.; Butler, S.T. Identification of the Blue Light Intensity Administered to One Eye Required to Suppress Bovine Plasma Melatonin and Investigation into Effects on Milk Production in Grazing Dairy Cows. J. Dairy Sci. 2021, 104, 12127–12138. [Google Scholar] [CrossRef]
- Poulsen, N.A.; Hein, L.; Kargo, M.; Buitenhuis, A.J. Realization of Breeding Values for Milk Fatty Acids in Relation to Seasonal Variation in Organic Milk. J. Dairy Sci. 2020, 103, 2434–2441. [Google Scholar] [CrossRef] [PubMed]
- Gottardo, P.; Penasa, M.; Righi, F.; Lopez-Villalobos, N.; Cassandro, M.; De Marchi, M. Fatty Acid Composition of Milk from Holstein-Friesian, Brown Swiss, Simmental and Alpine Grey Cows Predicted by Mid-Infrared Spectroscopy. Ital. J. Anim. Sci. 2017, 16, 380–389. [Google Scholar] [CrossRef]
- Briggs, M.A.; Petersen, K.S.; Kris-Etherton, P.M. Saturated Fatty Acids and Cardiovascular Disease: Replacements for Saturated Fat to Reduce Cardiovascular Risk. Healthcare 2017, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Lovegrove, J.A.; Givens, D.I. Dairy Food Products: Good or Bad for Cardiometabolic Disease? Nutr. Res. Rev. 2016, 29, 249–267. [Google Scholar] [CrossRef]
- Vasilopoulou, D.; Markey, O.; Kliem, K.E.; Fagan, C.C.; Grandison, A.S.; Humphries, D.J.; Todd, S.; Jackson, K.G.; Givens, D.I.; Lovegrove, J.A. Reformulation Initiative for Partial Replacement of Saturated with Unsaturated Fats in Dairy Foods Attenuates the Increase in LDL Cholesterol and Improves Flow-Mediated Dilatation Compared with Conventional Dairy: The Randomized, Controlled REplacement Of. Am. J. Clin. Nutr. 2020, 111, 739–748. [Google Scholar] [CrossRef]
- Livingstone, K.M.; Lovegrove, J.A.; Givens, D.I. The Impact of Substituting SFA in Dairy Products with MUFA or PUFA on CVD Risk: Evidence from Human Intervention Studies. Nutr. Res. Rev. 2012, 25, 193–206. [Google Scholar] [CrossRef]
- Teng, Z.W.; Yang, G.Q.; Wang, L.F.; Fu, T.; Lian, H.X.; Sun, Y.; Han, L.Q.; Zhang, L.Y.; Gao, T.Y. Effects of the Circadian Rhythm on Milk Composition in Dairy Cows: Does Day Milk Differ from Night Milk? J. Dairy Sci. 2021, 104, 8301–8313. [Google Scholar] [CrossRef]
- Quist, M.A.; LeBlanc, S.J.; Hand, K.J.; Lazenby, D.; Miglior, F.; Kelton, D.F. Milking-to-Milking Variability for Milk Yield, Fat and Protein Percentage, and Somatic Cell Count. J. Dairy Sci. 2008, 91, 3412–3423. [Google Scholar] [CrossRef]
- Rottman, L.W.; Ying, Y.; Zhou, K.; Bartell, P.A.; Harvatine, K.J. The Daily Rhythm of Milk Synthesis Is Dependent on the Timing of Feed Intake in Dairy Cows. Physiol. Rep. 2014, 2, e12049. [Google Scholar] [CrossRef]
- Niu, M.; Ying, Y.; Bartell, P.A.; Harvatine, K.J. The Effects of Feeding Rations That Differ in Fiber and Fermentable Starch within a Day on Milk Production and the Daily Rhythm of Feed Intake and Plasma Hormones and Metabolites in Dairy Cows. J. Dairy Sci. 2017, 100, 187–198. [Google Scholar] [CrossRef]
- Ben Meir, Y.A.; Nikbachat, M.; Jacoby, S.; Portnik, Y.; Levit, H.; Elazary, A.K.; Gershon, E.; Adin, G.; Zinder, M.C.; Shabtay, A.; et al. Effect of Lactation Trimester and Parity on Eating Behavior, Milk Production and Efficiency Traits of Dairy Cows. Animal 2019, 13, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Vandehaar, M.J.; Spurlock, D.M.; Weigel, K.A.; Armentano, L.E.; Staples, C.R.; Connor, E.E.; Wang, Z.; Coffey, M.; Veerkamp, R.F.; et al. Modeling Genetic and Nongenetic Variation of Feed Efficiency and Its Partial Relationships between Component Traits as a Function of Management and Environmental Factors. J. Dairy Sci. 2017, 100, 412–427. [Google Scholar] [CrossRef] [PubMed]
- Asher, A.; Shabtay, A.; Haim, A.; Aharoni, Y.; Miron, J.; Adin, G.; Tamir, A.; Arieli, A.; Halachmi, I.; Moallem, U.; et al. Time Required to Determine Performance Variables and Production Efficiency of Lactating Dairy Cows. J. Dairy Sci. 2014, 97, 4340–4353. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhao, S. Determination of Fatty Acid Methyl Esters (FAMEs) in Milk Matrix Using an Agilent 5977E GC/MS. Agilent Technologies Application Note, Publication Number 5991–4867EN. Available online: https://www.agilent.com/cs/library/applications/5991-4867EN-D2.pdf (accessed on 10 December 2022).
- Sander, L.C. Principles of Quantitation: Chromatography. J. Res. Natl. Inst. Stand. Technol. 2017, 122, 5. [Google Scholar] [CrossRef]
- Van Der Lest, R.; Hillerton, J.E. Short-Term Effects of Frequent Milking of Dairy Cows. J. Dairy Res. 1989, 56, 587–592. [Google Scholar] [CrossRef]
- Hanus, O.; Samkova, E.; Křížova, L.; Hasoňova, L.; Kala, R. Role of Fatty Acids in Milk Fat and the Influence of Selected Factors on Their Variability—A Review. Molecules 2018, 23, 1636. [Google Scholar] [CrossRef]
- Salfer, I.J.; Harvatine, K.J. Night-Restricted Feeding of Dairy Cows Modifies Daily Rhythms of Feed Intake, Milk Synthesis and Plasma Metabolites Compared with Day-Restricted Feeding. Br. J. Nutr. 2020, 123, 849–858. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations; OECD/FAO. OECD-FAO Agricultural Outlook 2018–2027; FAO, Food and Agriculture Organization of the United Nations: Rome, Italy, 2018; Volume 181. [Google Scholar]
- Astrup, A.; Dyerberg, J.; Elwood, P.; Hermansen, K.; Hu, F.B.; Jakobsen, M.U.; Kok, F.J.; Krauss, R.M.; Lecerf, J.M.; LeGrand, P.; et al. The Role of Reducing Intakes of Saturated Fat in the Prevention of Cardiovascular Disease: Where Does the Evidence Stand in 2010? Am. J. Clin. Nutr. 2011, 93, 684–688. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Innis, S.; Ammerican Dietetic Assocition. Dietitians of Canada Position of the American Dietetic Association and Dietitians of Canada: Dietary Fatty Acids. J. Am. Diet. Assoc. 2007, 107, 1599–1611. [Google Scholar] [CrossRef]
- Drewnowski, A. The Contribution of Milk and Milk Products to Micronutrient Density and Affordability of the U.S. Diet. J. Am. Coll. Nutr. 2011, 30, 422S–428S. [Google Scholar] [CrossRef]
- Lindmark Månsson, H. Fatty Acids in Bovine Milk Fat. Food Nutr. Res. 2008, 52, 1821. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Pickova, J.; Vázquez-Gutiérrez, J.L.; Langton, M. Influence of Seasonal Variation and Ultra High Temperature Processing on Lipid Profile and Fat Globule Structure of Swedish Cow Milk. Food Chem. 2018, 239, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Heck, J.M.L.; van Valenberg, H.J.F.; Dijkstra, J.; van Hooijdonk, A.C.M. Seasonal Variation in the Dutch Bovine Raw Milk Composition. J. Dairy Sci. 2009, 92, 4745–4755. [Google Scholar] [CrossRef] [PubMed]
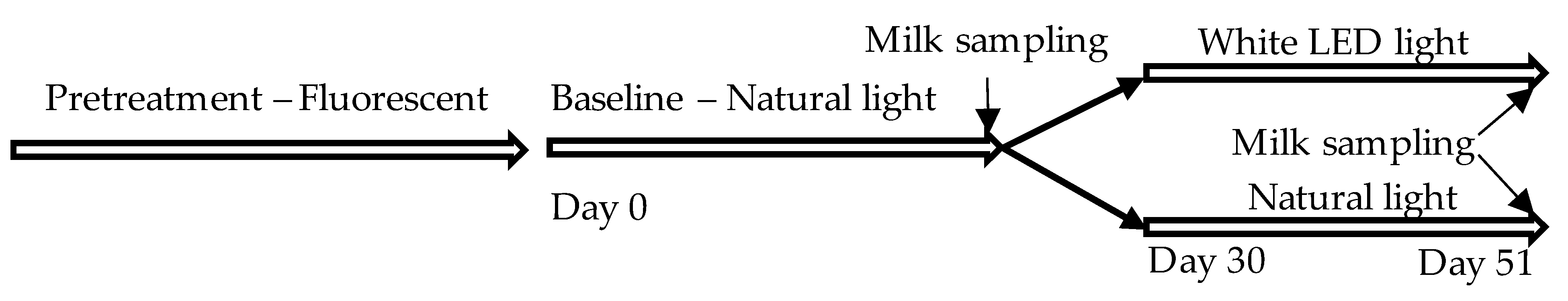
| Variable/Phase | White Fluorescent (Pretreatment) | Natural Light (Baseline) | White LED vs. Natural Light (Mid-phase) | ||||
|---|---|---|---|---|---|---|---|
| Control (n = 16) | Treatment (n = 18) | Control (n = 16) | Treatment (n = 18) | Control (n = 16) | Treatment (n = 18) | ||
| Body weight (kg) | 692.7 ± 67.9 | 654.5 ± 42.1 | 714.2 ± 73.2 | 670.3 ± 44.8 | 716.6 ± 80 | 676.0 ± 49.9 | |
| Milk yield (L/d) | 48.4 ± 6.7 | 43 ± 6.3 | 47.8 ± 10 | 48.7 ± 9.1 | 38.8 ± 11 | 38.8 ± 7.6 | |
| Experimental Period | 26 September 2019 | 27 September 2019–28 October 2019 | 29 October 2019–17 November 2019 | ||||
| Sunrise 1 | 05:32 | 05:52 | 06:12 | ||||
| Sunset 1 | 17:32 | 16:58 | 16:40 | ||||
| Natural LD ratio | 12:12 | 11:13 | 10.5:13.5 | ||||
| Cowshed | Illumination type | White fluorescent | Natural light | Natural light | White LED | ||
| Dominant wavelength (nm) | 545 | 670 | 670 | 462 | |||
| Intensity (Lux) | 140 | 1.7 | 1.7 | 125.4 | |||
| Illumination on–off (h) | 16:30–05:30 | - | - | 16:30–05:30 | |||
| LD ratio | 24:0 | 11:13 | 10.5:13.5 | 24:0 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asher, A.; Fialko, M.; Fares, F.; Moallem, U.; Yaacoby, S.; Gutman, R. The Effect of Short-Wavelength White LED Illumination throughout the Night on the Milk Fatty Acid Profile of High-Yielding Dairy Cows. Biology 2022, 11, 1799. https://doi.org/10.3390/biology11121799
Asher A, Fialko M, Fares F, Moallem U, Yaacoby S, Gutman R. The Effect of Short-Wavelength White LED Illumination throughout the Night on the Milk Fatty Acid Profile of High-Yielding Dairy Cows. Biology. 2022; 11(12):1799. https://doi.org/10.3390/biology11121799
Chicago/Turabian StyleAsher, Aviv, Matan Fialko, Florin Fares, Uzi Moallem, Shamai Yaacoby, and Roee Gutman. 2022. "The Effect of Short-Wavelength White LED Illumination throughout the Night on the Milk Fatty Acid Profile of High-Yielding Dairy Cows" Biology 11, no. 12: 1799. https://doi.org/10.3390/biology11121799
APA StyleAsher, A., Fialko, M., Fares, F., Moallem, U., Yaacoby, S., & Gutman, R. (2022). The Effect of Short-Wavelength White LED Illumination throughout the Night on the Milk Fatty Acid Profile of High-Yielding Dairy Cows. Biology, 11(12), 1799. https://doi.org/10.3390/biology11121799






