Molecular Detection of Microsporidia in Rabbits (Oryctolagus cuniculus) in Tenerife, Canary Islands, Spain
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical
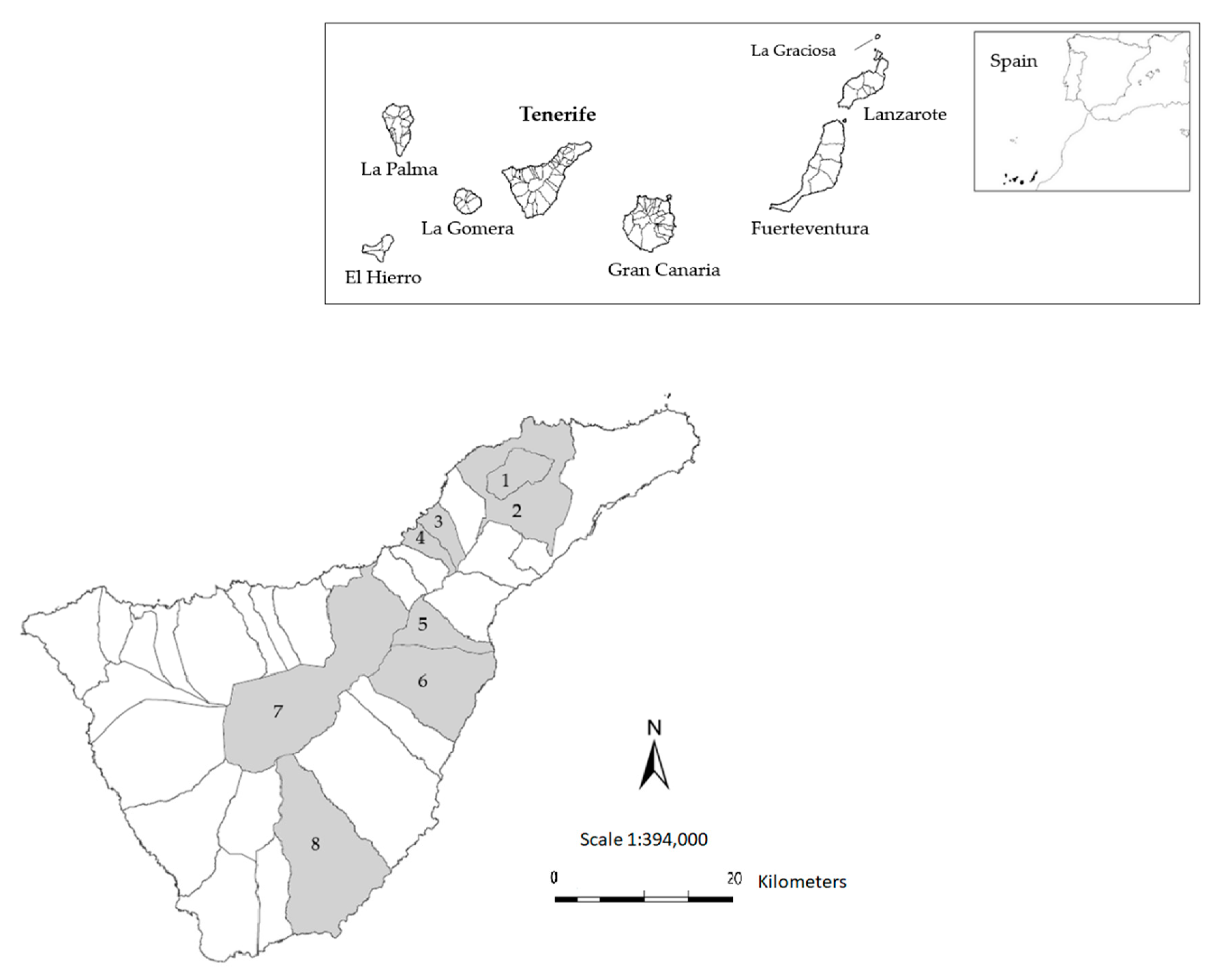
2.2. Study Area, Sample Collection, and Preparation
2.3. DNA Extraction
2.4. PCR Amplification
2.5. Sequencing and Sequencing Data Analysis
3. Results
3.1. Prevalence of Microsporidia in Fecal Samples
3.2. Sequencing and Homology Comparison
4. Discussion
| Wild rabbits | |||||
|---|---|---|---|---|---|
| Europe | |||||
| Country | Diagnostic Method | Prevalence (%) (Positive/Total) | Microsporidia (n) | Genotype (n) | Reference |
| Spain | PCR | 14.3% (1/7) feces | E. bieneusi (1) | – | [6] |
| PCR | 0.8% (3/383) kidney 0.3% (1/383) kidney | E. bieneusi (3) E. intestinalis (1) | – | [19] | |
| Domestic rabbits | |||||
| Europe | |||||
| Austria | PCR 1 | 0% (0/12) CSF 0% (0/32) urine 80% (4/5) lens | E. cuniculi (4) | – | [65] |
| France | Nested-PCR 1 | 80% (4/5) male * (brain, kidneys, liver) 80% (8/10) pregnant * (brain, kidney, lung) 56.5% (13/23) fetuses * (brain, kidney, lung, placenta) | E. cuniculi | I | [66] (P.J.R. Baneux pers. comm.; 2022) |
| Germany | Nested PCR (RFLP) | 0% (0/3) feces | – | – | [62] |
| Nested PCR (RFLP) | 10.5% (2/19) CSF 39.5% (15/38) urine | E. cuniculi (16) | – | [61] | |
| Nested PCR (RFLP) | 48.7% (18/37) urine | E. cuniculi (18) | – | [63] | |
| Real-time PCR 1 | 54.5% (30/55) at least one organ (brain, kidney, lungs, liver, heart, intestine) | E. cuniculi | – | [67] | |
| Real-time PCR 1 | 100% (3/3) CSF 36.7% (18/49) urine | E. cuniculi | – | [68] | |
| Italy | PCR | 36.4% (8/22) kidney | E. cuniculi (8) | – | [64] |
| Poland | Real-time PCR 1 | 26.21% (27/103) urine | E. cuniculi (27) | – | [69] |
| Slovakia | Culture PCR 1 | Case report (brain, kidney, feces) | E. cuniculi | – | [70] |
| Spain | PCR | 25% (3/12) feces | E. bieneusi (3) | – | [6] |
| PCR | 21% (4/19) feces | E. bieneusi (4) | D (1) | [29] | |
| Switzerland | PCR–RFLP 1 WB | 100% (9/9) in all samples (brain, kidney, urine) | E. cuniculi (9) | I | [60] |
| UK | PCR 1 | 33.3% (3/9) lens tissue | E. cuniculi (3) | – | [71] (R.F. Sanchez, pers. comm.; 2022) |
| Asia | |||||
| China | Nested PCR 2 | 10.2% (22/215) feces | E. bieneusi (22) | CHN-RD1 (12) D (3) Type IV (2) Peru6 (1) I (1) CHN-RR1 (1) CHN-RR2 (1) CHN-RR3 (1) | [14] |
| Nested PCR 2 | 0.94% (4/426) feces | E. bieneusi (4) | D (4) | [15] | |
| Nested PCR 2 | 2.8% (9/321) feces | E. bieneusi (9) | J (5) BEB8 (3) Type IV (1) | [16] | |
| Nested PCR | 15.4% (90/584) feces 5.8% (34/584) feces 2.7% (16/584) feces 0.9% (5/584) feces | E. bieneusi (90) E. cuniculi (34) E. intestinalis (16) Co-infection ** (5) | SC02 (39) I (21), N (13), J (6), CHY1 (1), SCR01 (1) SCR02 (1) SCR04 (1) SCR05 (2) SCR06 (2) SCR07 (3) I (19), II (15) SC02 + I (3) J + II (1) J + I (1) | [17] | |
| Nested PCR 2 | Case report (feces) | E. bieneusi | I (2) Peru6 (4) | [18] | |
| Iran | Nested PCR | 10% (1/10) feces 10% (1/10) feces | E. bieneusi (1) E. cuniculi (1) | – | [13] |
| Nested PCR 1 | 3.3% (2/60) brain | E. cuniculi | – | [72] | |
| Nested PCR 1 | 59.6% (34/57) brain | E. cuniculi | – | [72] | |
| Semi-nested PCR 1 | 32% (16/50) urine | E. cuniculi (16) | I (13) | [73] | |
| Japan | Culture PCR 1 | Case report (kidney) | E. cuniculi | I | [74,75] |
| Nested PCR 1 | 7.78% (20/257) urine 0% (0/314) feces | E. cuniculi | I | [76] | |
| Nested PCR 1 | 33.3% (1/3) urine 5.6% (6/107) feces | E. cuniculi | I | [76] | |
| Turkey | PCR 1 | Case report (eye) | E. cuniculi | I | [77] |
| PCR 1 | Case report (brain) | E. cuniculi | I | [78] | |
| PCR 1 | 63% (19/30) eye 0% (0/25) blood 0% (0/24) kidney 0% (0/24) brain 0% (0/9) lung 0% (0/7) placenta 0% (0/2) liver 0% (0/2) heart | E. cuniculi | I | [79] | |
| America | |||||
| Brazil | PCR | 2.56% (11/429) feces | Encephalitozoon spp. | – | [80] |
| Canada | Real-time PCR 1 | 32.4% (11/34) urine | E. cuniculi | – | [81] |
| USA | PCR–RFLP 1 | Case report (kidney) | E. cuniculi | Ia | [82] |
| PCR 1 HMA | Case report (lens tissue) | E. cuniculi | I | [83] | |
| PCR 1 | Case report (lens tissue) | E. cuniculi | Ia | [82] | |
| PCR 1 | Case report (lens tissue) | E. cuniculi | – | [84] | |
| Oceania | |||||
| Australia | PCR–RFLP 1 | Case report (urine) | E. cuniculi | I | [5,85] |
| Africa | |||||
| Egypt | PCR 1 | 2.85% (1/35) urine | E. cuniculi (1) | – | [86] |
| PCR | 30.8% (4/13) feces 7.7% (1/13) feces | E. bieneusi (4) E. intestinalis (1) | – | [7] | |
| PCR 1 | 100% (150/150) in all samples (brain, eyeball, liver, kidney) | E. cuniculi (10) | – | [21] | |
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lees, A.C.; Bell, D.J. A conservation paradox for the 21st century: The European wild rabbit Oryctolagus cuniculus, an invasive alien and an endangered native species. Mamm. Rev. 2008, 38, 304–320. [Google Scholar] [CrossRef]
- Banco de Datos de Biodiversidad de Canarias (EXOS). Available online: https://www.biodiversidadcanarias.es/exos/especie/V00215 (accessed on 26 September 2022).
- Bello-Rodríguez, V.; Mateo, R.G.; Pellissier, L.; Cubas, J.; Cooke, B.; González-Mancebo, J.M. Forecast increase in invasive rabbit spread into ecosystems of an oceanic island (Tenerife) under climate change. Ecol. Appl. 2021, 31, e02206. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Takvorian, P.M.; Weiss, L.M. The Function and Structure of the Microsporidia Polar Tube. Exp. Suppl. 2022, 114, 179–213. [Google Scholar] [PubMed]
- Didier, E.S.; Vossbrinck, C.R.; Baker, M.D.; Rogers, L.B.; Bertucci, D.C.; Shadduck, J.A. Identification and characterization of three Encephalitozoon cuniculi strains. Parasitology 1995, 111, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Del Águila, C.; Izquierdo, F.; Navajas, R.; Pieniazek, N.J.; Miró, G.; Alonso, A.I.; Da Silva, A.J.; Fenoy, S. Enterocytozoon bieneusi in animals: Rabbit and dogs as new hosts. J. Eukaryot. Microbiol. 1999, 46, 8S–9S. [Google Scholar]
- Al-Herrawy, A.; Gad, M.A. Microsporidial Spores in Fecal Samples of Some Domesticated Animals Living in Giza, Egypt. Iran J. Parasitol. 2016, 11, 195–203. [Google Scholar]
- Didier, E.S.; Weiss, L.M. Microsporidiosis: Current status. Curr. Opin. Infect. Dis. 2006, 19, 485–492. [Google Scholar] [CrossRef]
- Valenčáková, A.; Sučik, M. Alternatives in Molecular Diagnostics of Encephalitozoon and Enterocytozoon Infections. J. Fungi 2020, 6, 114. [Google Scholar] [CrossRef]
- Kotková, M.; Sak, B.; Hlásková, L.; Květoňová, D.; Kváč, M. Evidence of transplacental transmission of Encephalitozoon cuniculi genotype II in murine model. Exp. Parasitol. 2018, 193, 51–57. [Google Scholar] [CrossRef]
- Magalhães, T.R.; Pinto, F.F.; Queiroga, F.L. A multidisciplinary review about Encephalitozoon cuniculi in a One Health perspective. Parasitol. Res. 2022, 121, 2463–2479. [Google Scholar] [CrossRef]
- Wright, J.H.; Craighead, E.M. Infectious Motor Paralysis in Young Rabbits. J. Exp. Med. 1922, 36, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Askari, Z.; Mirjalali, H.; Mohebali, M.; Zarei, Z.; Shojaei, S.; Rezaeian, T.; Rezaeian, M. Molecular Detection and Identification of Zoonotic Microsporidia Spore in Fecal Samples of Some Animals with Close-Contact to Human. Iran J. Parasitol. 2015, 10, 381–388. [Google Scholar] [PubMed]
- Yang, Z.; Zhao, W.; Shen, Y.; Zhang, W.; Shi, Y.; Ren, G.; Yang, D.; Ling, H.; Yang, F.; Liu, A.; et al. Subtyping of Cryptosporidium cuniculus and genotyping of Enterocytozoon bieneusi in rabbits in two farms in Heilongjiang Province, China. Parasite 2016, 23, 52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Jiang, J.; Cai, Y.N.; Wang, C.F.; Xu, P.; Yang, G.L.; Zhao, Q. Molecular Characterization of Enterocytozoon bieneusi in Domestic Rabbits (Oryctolagus cuniculus) in Northeastern China. Korean J. Parasitol. 2016, 54, 81–85. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, M.; Jing, B.; Yu, F.; Wu, Y.; Chang, Y.; Zhao, A.; Wei, Z.; Dong, H.; Zhang, L. Molecular Characterization of Cryptosporidium spp., Giardia duodenalis, and Enterocytozoon bieneusi in Rabbits in Xinjiang, China. J. Eukaryot. Microbiol. 2018, 65, 854–859. [Google Scholar] [CrossRef]
- Deng, L.; Chai, Y.; Xiang, L.; Wang, W.; Zhou, Z.; Liu, H.; Zhong, Z.; Fu, H.; Peng, G. First identification and genotyping of Enterocytozoon bieneusi and Encephalitozoon spp. in pet rabbits in China. BMC. Vet. Res. 2020, 16, 212. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Y.; Yang, F.; Gong, B.; Jiang, Y.; Zhou, K.; Cao, J.; Zhang, W.; Liu, A.; Shen, Y. Multilocus Sequence Typing of Enterocytozoon bieneusi Isolates From Various Mammal and Bird Species and Assessment of Population Structure and Substructure. Front. Microbiol. 2020, 11, 1406. [Google Scholar] [CrossRef]
- Martínez-Padilla, A.; Caballero-Gómez, J.; Magnet, A.; Gómez-Guillamón, F.; Izquierdo, F.; Camacho-Sillero, L.; Jiménez-Ruiz, S.; del Águila, C.; García-Bocanegra, I. Zoonotic Microsporidia in Wild Lagomorphs in Southern Spain. Animals 2020, 10, 2218. [Google Scholar] [CrossRef]
- Garcia, L.S. Laboratory identification of the microsporidia. J. Clin. Microbiol. 2002, 40, 1892–1901. [Google Scholar] [CrossRef]
- Morsy, E.A.; Salem, H.M.; Khattab, M.S.; Hamza, D.A.; Abuowarda, M.M. Encephalitozoon cuniculi infection in farmed rabbits in Egypt. Acta. Vet. Scand. 2020, 62, 11. [Google Scholar] [CrossRef]
- Csokai, J.; Joachim, A.; Gruber, A.; Tichy, A.; Pakozdy, A.; Künzel, F. Diagnostic markers for encephalitozoonosis in pet rabbits. Vet. Parasitol. 2009, 163, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Santaniello, A.; Cimmino, I.; Dipineto, L.; Agognon, A.L.; Beguinot, F.; Formisano, P.; Fioretti, A.; Menna, L.F.; Oriente, F. Zoonotic Risk of Encephalitozoon cuniculi in Animal-Assisted Interventions: Laboratory Strategies for the Diagnosis of Infections in Humans and Animals. Int. J. Environ. Res. Public Health 2021, 18, 9333. [Google Scholar] [CrossRef]
- Hinney, B.; Sak, B.; Joachim, A.; Kváč, M. More than a rabbit’s tale–Encephalitozoon spp. in wild mammals and birds. Int. J. Parasitol. Parasites. Wildl. 2016, 5, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Reetz, J.; Nöckler, K.; Reckinger, S.; Vargas, M.M.; Weiske, W.; Broglia, A. Identification of Encephalitozoon cuniculi genotype III and two novel genotypes of Enterocytozoon bieneusi in swine. Parasitol. Int. 2009, 58, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Sak, B.; Holubová, N.; Květoňová, D.; Hlásková, L.; Tinavská, J.; Kicia, M.; Zajączkowska, Z.; Kváč, M. Comparison of the Concentration of Encephalitozoon cuniculi Genotype I and III in Inflammatory Foci under Experimental Conditions. J. Inflamm. Res. 2022, 15, 2721–2730. [Google Scholar] [CrossRef] [PubMed]
- Maestrini, G.; Ricci, E.; Cantile, C.; Mannella, R.; Mancianti, F.; Paci, G.; D’Ascenzi, C.; Perrucci, S. Encephalitozoon cuniculi in rabbits: Serological screening and histopathological findings. Comp. Immunol. Microbiol. Infect. Dis. 2017, 50, 54–57. [Google Scholar] [CrossRef]
- Valencakova, A.; Balent, P.; Ravaszova, P.; Horak, A.; Obornik, M.; Halanova, M.; Malcekova, B.; Novotny, F.; Goldova, M. Molecular identification and genotyping of Microsporidia in selected hosts. Parasitol. Res. 2012, 110, 689–693. [Google Scholar] [CrossRef]
- Galván-Díaz, A.L.; Magnet, A.; Fenoy, S.; Henriques-Gil, N.; Haro, M.; Ponce-Gordo, F.; Millán, J.; Miró, G.; del Águila, C.; Izquierdo, F. Microsporidia detection and genotyping study of human pathogenic E. bieneusi in animals from Spain. PLoS ONE 2014, 9, e92289. [Google Scholar] [CrossRef]
- Harcourt-Brown, F.M. Encephalitozoon cuniculi infection in rabbits. Semin. Avian. Exot. Pet. Med. 2004, 13, 86–93. [Google Scholar] [CrossRef]
- Katzwinkel-Wladarsch, S.; Lieb, M.; Helse, W.; Löscher, T.; Rinder, H. Direct amplification and species determination of microsporidian DNA from stool specimens. Trop. Med. Int. Health 1996, 1, 373–378. [Google Scholar] [CrossRef]
- Sak, B.; Kváč, M.; Květoňová, D.; Albrecht, T.; Piálek, J. The first report on natural Enterocytozoon bieneusi and Encephalitozoon spp. infections in wild East-European House Mice (Mus musculus musculus) and West-European House Mice (M. m. domesticus) in a hybrid zone across the Czech Republic-Germany border. Vet. Parasitol. 2011, 178, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Abreu-Acosta, N.; Lorenzo-Morales, J.; Leal-Guio, Y.; Coronado-Alvarez, N.; Foronda, P.; Alcoba-Florez, J.; Izquierdo, F.; Batista-Díaz, N.; del Águila, C.; Valladares, B. Enterocytozoon bieneusi (microsporidia) in clinical samples from immunocompetent individuals in Tenerife, Canary Islands, Spain. Trans. R. Soc. Trop. Med. Hyg. 2005, 99, 848–855. [Google Scholar] [CrossRef] [PubMed]
- del Aguila, C.; Moura, H.; Fenoy, S.; Navajas, R.; Lopez-Velez, R.; Li, L.; Xiao, L.; Leitch, G.J.; da Silva, A.; Pieniazek, N.J.; et al. In vitro culture, ultrastructure, antigenic, and molecular characterization of Encephalitozoon cuniculi isolated from urine and sputum samples from a Spanish patient with AIDS. J. Clin. Microbiol. 2001, 39, 1105–1108. [Google Scholar] [CrossRef][Green Version]
- Lores, B.; del Águila, C.; Arias, C. Enterocytozoon bieneusi (microsporidia) in faecal samples from domestic animals from Galicia, Spain. Mem. Inst. Oswaldo. Cruz. 2002, 97, 941–945. [Google Scholar] [CrossRef]
- Izquierdo, F.; Castro-Hermida, J.A.; Fenoy, S.; Mezo, M.; González-Warleta, M.; del Águila, C. Detection of microsporidia in drinking water, wastewater and recreational rivers. Water. Res. 2011, 45, 4837–4843. [Google Scholar] [CrossRef]
- Galván, A.L.; Magnet, A.; Izquierdo, F.; Fenoy, S.; Rueda, C.; Fernández-Vadillo, C.; Henriques-Gil, N.; del Águila, C. Molecular characterization of human-pathogenic microsporidia and Cyclospora cayetanensis isolated from various water sources in Spain: A year-long longitudinal study. Appl. Environ. Microbiol. 2013, 79, 449–459. [Google Scholar] [CrossRef]
- Andreu-Ballester, J.C.; Garcia-Ballestero, C.; Amigo, V.; Ballester, F.; Gil-Borrás, R.; Catalán-Serra, I.; Magnet, A.; Fenoy, S.; del Águila, C.; Ferrando-Marco, J.; et al. Microsporidia and its relation to Crohn’s disease. A retrospective study. PLoS ONE 2013, 8, e62107. [Google Scholar] [CrossRef]
- Izquierdo, F.; Ollero, D.; Magnet, A.; Galván-Díaz, A.L.; Llorens, S.; Vaccaro, L.; Hurtado-Marcos, C.; Valdivieso, E.; Miró, G.; Hernández, L.; et al. Microsporidia as a Potential Threat to the Iberian Lynx (Lynx pardinus). Animals 2022, 12, 2507. [Google Scholar] [CrossRef]
- Sak, B.; Kvác, M.; Petrzelková, K.; Kvetonová, D.; Pomajbíková, K.; Mulama, M.; Liyang, J.; Modrý, D. Diversity of microsporidia (Fungi: Microsporidia) among captive great apes in European zoos and African sanctuaries: Evidence for zoonotic transmission? Folia. Parasitol. 2011, 58, 81–86. [Google Scholar] [CrossRef]
- Menéndez, L.C.; Mayayo, T.M. Cuadro clínico y lesional asociado a Encephalitozoon cuniculi, en una explotación industrial de conejos para carne. In XII Symposium de Cunicultura; Asociación Española de Cunicultura, Guadalajara Asociación Española de Cunicultura: Guadalajara, Spain, 1987; pp. 321–324. [Google Scholar]
- Espinosa, J.; Ferreras, M.C.; Benavides, J.; Cuesta, N.; Pérez, C.; García-Iglesias, M.J.; García-Marín, J.F.; Pérez, V. Causes of Mortality and Disease in Rabbits and Hares: A Retrospective Study. Animals 2020, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- Gallego, M.; Avedillo, L. Case Report of Bilateral 3-4 Metatarsal Syndactyly in a Pet Rabbit. Case. Rep. Vet. Med. 2016, 2016, 6957101. [Google Scholar] [CrossRef] [PubMed]
- Martorell, J.; Bailon, D.; Majó, N.; Andaluz, A. Lateral approach to nephrotomy in the management of unilateral renal calculi in a rabbit (Oryctolagus cuniculus). J. Am. Vet. Med. Assoc. 2012, 240, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Morera, N.; Molina, L. Seroprevalencia de Encephalitozoon cuniculi en Conejos asintomáticos del área metropolitana de Barcelona. In Proceedings of the Asociación Veterinarios Españoles Especialistas Pequeños Animales (Avepa), Barcelona, Spain, 18–20 October 2012. [Google Scholar]
- Ardíaca, M.; Montesinos, M. Prevalencia de infección por E. cuniculi en Conejos en una clínica veterinaria de Madrid. In Proceedings of the Asociación Veterinarios Españoles Especialistas Pequeños Animales (Avepa), Madrid, Spain, 27–29 October 2006. [Google Scholar]
- Gallego, M. Urinary calcium assessment and its relation with age, sex and Encephalitozoon cuniculi serological status in otherwise healthy pet rabbits. Vet. Rec. Open. 2019, 6, e000251. [Google Scholar] [CrossRef]
- Gallego-Agúndez, M.; Blanco-García, A.; Martínez-Del Peso, M. Seroprevalencia de Encephalitozoon cuniculi en Conejos de Madrid. Preliminar de 350 muestras. In Proceedings of the XXXXI Congreso Anual de AMVAC, Madrid, Spain, 13–15 March 2014. [Google Scholar]
- Villa-Espinosa, A.; Pueyo-Pintor, R.; Navarro-Serrano, A.; Baselga, J.M.; Baselga, R. El examen serológico con muestras de sangre obtenidas en papel de filtro. XXXV Symp. Cunicult. ASESCU 2011, 19, 25. [Google Scholar]
- Wilson, J.M. Encephalitozoon cuniculi in wild European rabbits and a fox. Res. Vet. Sci. 1979, 26, 114. [Google Scholar] [CrossRef]
- Chalupský, J.; Vávra, J.; Gaudin, J.C.; Vandewalle, P.; Arthur, C.P. Mise en évidence sérologique de la présence d’encéphalitozoonose et de toxoplasmose chez le lapin de Garenne (Oryctolagus cuniculus) en France. Bull. Société Française Parasitol. 1990, 8, 91–95. [Google Scholar]
- Bálent, P.; Halánová, M.; Sedláková, T.; Valencáková, A.; Cisláková, L. Encephalitozoon cuniculi infection in rabbits and laboratory mice in Eastern Slovakia. Bull. Vet. Inst. Pulawy 2004, 48, 113–116. [Google Scholar]
- Thomas, C.; Finn, M.; Twigg, L.; Deplazes, P.; Thompson, R.C. Microsporidia (Encephalitozoon cuniculi) in wild rabbits in Australia. Aust. Vet. J. 1997, 75, 808–810. [Google Scholar] [CrossRef]
- Cox, J.C.; Ross, J. A serological survey of Encephalitozoon cuniculi infection in the wild rabbit in England and Scotland. Res. Vet. Sci. 1980, 28, 396. [Google Scholar] [CrossRef]
- Blevins, M. Prevalence of Encephalitozoon cuniculi in a population of wild rabbits in Norfolk, England. ZooMed 2007, 7, 28–36. [Google Scholar]
- Bose, H.M.; Woodhouse, M.A.; Powell, R. Absence of Encephalitozoon cuniculi antibodies in wild rabbits in England. Vet. Rec. 2015, 177, 48. [Google Scholar] [CrossRef] [PubMed]
- Ferrazzi, V.; Poloni, R.; Lavazza, A.; Gallazzi, D.; Grilli, G. Mixomatosi, Malattia Emorragica Virale ed encefalitozoonosi: Indagine sierologica in conigli (Oryctolagus cuniculus) e silvilaghi (Sylvilagus floridanus) a vita libera. In Proceedings of the Giornate di Coniglicoltura Associazione Scientifica Italiana di Coniglicoltura, Forlì, Italy, 1 October 2005. [Google Scholar]
- Cox, J.C.; Pye, D.; Edmonds, J.W.; Shepherd, R. An investigation of Encephalitozoon cuniculi in the wild rabbits Oryctolagus cuniculus in Victoria, Australia. Epidemiol. Infect. 1980, 84, 295–300. [Google Scholar]
- Mathis, A.; Akerstedt, J.; Tharaldsen, J.; Odegaard, O.; Deplazes, P. Isolates of Encephalitozoon cuniculi from farmed blue foxes (Alopex lagopus) from Norway differ from isolates from Swiss domestic rabbits (Oryctolagus cuniculus). Parasitol. Res. 1996, 82, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Jass, A. Evaluierung von Liquorpunktion und PCR zur klinischen Diagnose der Enzephalitozoonose beim Kaninchen. Doctoral Dissertation, Ludwig Maximilians University Munich, Munich, Germany, July 2004. [Google Scholar]
- Dengjel, B.; Zahler, M.; Hermanns, W.; Heinritzi, K.; Spillmann, T.; Thomschke, A.; Löscher, T.; Gothe, R.; Rinder, H. Zoonotic potential of Enterocytozoon bieneusi. J. Clin. Microbiol. 2001, 39, 4495–4499. [Google Scholar] [CrossRef] [PubMed]
- Sieg, J.; Hein, J.; Jass, A.; Sauter-Louis, C.; Hartmann, K.; Fischer, A. Clinical evaluation of therapeutic success in rabbits with suspected encephalitozoonosis. Vet. Parasitol. 2012, 187, 328–332. [Google Scholar] [CrossRef]
- Pipia, A.P.; Giobbe, M.; Mula, P.; Varcasia, A.; Sanna, G.; Walochnik, J.; Lavazza, A.; Scala, A. Epidemiological and Biomolecular Updates on Encephalitozoon cuniculi in Lagomorpha of Sardinia (Italy). In Veterinary Science Current Aspects in Biology, Animal Pathology, Clinic, and Food Hygiene; Pugliese, A., Gaiti, A., Bioti, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 47–50. [Google Scholar]
- Künzel, F.; Gruber, A.; Tichy, A.; Edelhofer, R.; Nell, B.; Hassan, J.; Leschnik, M.; Thalhammer, J.G.; Joachim, A. Clinical symptoms and diagnosis of encephalitozoonosis in pet rabbits. Vet. Parasitol. 2008, 151, 115–124. [Google Scholar] [CrossRef]
- Baneux, P.J.R.; Pognan, F. In utero transmission of Encephalitozoon cuniculi strain type I in rabbits. Lab. Anim. 2003, 37, 132–138. [Google Scholar] [CrossRef]
- Leipig, M.; Matiasek, K.; Rinder, H.; Janik, D.; Emrich, D.; Baiker, K.; Hermanns, W. Value of histopathology, immunohistochemistry, and real-time polymerase chain reaction in the confirmatory diagnosis of Encephalitozoon cuniculi infection in rabbits. J. Vet. Diagn. Investig. 2013, 25, 16–26. [Google Scholar] [CrossRef]
- Hein, J.; Flock, U.; Sauter-Louis, C.; Hartmann, K. Encephalitozoon cuniculi in rabbits in Germany: Prevalence and sensitivity of antibody testing. Vet. Rec. 2014, 174, 350. [Google Scholar] [CrossRef]
- Zietek, J.; Adaszek, Ł.; Dziegiel, B.; Kalinowski, M.; Kalinowska, A.; Jarosz, Ł.; Winiarczyk, S. Diagnosis of the Encephalitozoonosis cuniculi infections in pet rabbits with neurological symptoms. Pol. J. Vet. Sci. 2014, 17, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Valencakova, A.; Balent, P.; Petrovova, E.; Novotny, F.; Luptakova, L. Encephalitozoonosis in household pet Nederland Dwarf rabbits (Oryctolagus cuniculus). Vet. Parasitol. 2008, 153, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, R.F.; Everson, R.; Hedley, J.; Dawson, C.; Lam, R.; Priestnall, S.L.; Garcia-de Carellan, A.; de Miguel, C.; Seymour, C. Rabbits with naturally occurring cataracts referred for phacoemulsification and intraocular lens implantation: A preliminary study of 12 cases. Vet. Ophthalmol. 2018, 21, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi-Dehkordi, Z.; Norouzi, E.; Rezaeian, H.; Nourian, A.; Noaman, V.; Sazmand, A. First insight into Encephalitozoon cuniculi infection in laboratory and pet rabbits in Iran. Comp. Immunol. Microbiol. Infect. Dis. 2019, 65, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Javadzade, R.; Rostami, A.; Arabkhazaeli, F.; Bahonar, A.; Mohammad-Rahimi, H.; Mirjalali, H. Molecular detection and genotype identification of E. cuniculi from pet rabbits. Comp. Immunol. Microbiol. Infect. Dis. 2021, 75, 101616. [Google Scholar] [CrossRef] [PubMed]
- Furuya, K.; Fukui, D.; Yamaguchi, M.; Nakaoka, Y.; Bando, G.; Kosuge, M. Isolation of Encephalitozoon cuniculi using primary tissue culture techniques from a rabbit in a colony showing encephalitozoonosis. J. Vet. Med. Sci. 2001, 63, 203–206. [Google Scholar] [CrossRef][Green Version]
- Furuya, K. Genotyping of Encephalitozoon cuniculi isolates found in Hokkaido. Jpn. J. Infect. Dis. 2002, 55, 128–130. [Google Scholar]
- Kimura, M.; Aoki, M.; Ichikawa-Seki, M.; Matsuo, K.; Yagita, K.; Itagaki, T. Detection and genotype of Encephalitozoon cuniculi DNA from urine and feces of pet rabbits in Japan. J. Vet. Med. Sci. 2013, 75, 1017–1020. [Google Scholar] [CrossRef]
- Ozkan, O.; Karagoz, A.; Kocak, N.; Alcigir, M.E. The first molecular detection and genotyping of Encephalitozoon cuniculi in rabbit’s eye in Turkey. Kafkas. Univ. Vet. Fak. Derg. 2018, 24, 607–611. [Google Scholar]
- Ozkan, O.; Alcigir, M.E. Encephalitozoonosis infection in a traditional rabbit farm with neurological manifestations. Vet. Parasitol. 2018, 262, 26–29. [Google Scholar] [CrossRef]
- Ozkan, O.; Karagoz, A.; Kocak, N. First molecular evidence of ocular transmission of Encephalitozoonosis during the intrauterine period in rabbits. Parasitol. Int. 2019, 71, 1–4. [Google Scholar] [CrossRef]
- Freitas, S.P.B. Ocorrência de Infecções por Encephalitozoon spp. em Coelhos do Estado de São Paulo, Brasil. Doctoral Dissertation, Universidade Estadual Paulista “Júlio De Mesquita Filho”, São Paulo, Brazil, August 2017. [Google Scholar]
- Reabel, S. Molecular diagnostic methods for detection of Encephalitozoon cuniculi in pet rabbits. Doctoral Dissertation, University of Guelph, Guelph, ON, Canada, December 2012. [Google Scholar]
- Xiao, L.; Li, L.; Visvesvara, G.S.; Moura, H.; Didier, E.S.; Lal, A.A. Genotyping Encephalitozoon cuniculi by multilocus analyses of genes with repetitive sequences. J. Clin. Microbiol. 2001, 39, 2248–2253. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stiles, J.; Didier, E.; Ritchie, B.; Greenacre, C.; Willis, M.; Martin, C. Encephalitozoon cuniculi in the lens of a rabbit with phacoclastic uveitis: Confirmation and treatment. Vet. Comp. Ophthalmol. 1997, 7, 233–238. [Google Scholar]
- Felchle, L.M.; Sigler, R.L. Phacoemulsification for the management of Encephalitozoon cuniculi-induced phacoclastic uveitis in a rabbit. Vet. Ophthalmol. 2002, 5, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.C.; Pye, D. Serodiagnosis of nosematosis by immunofluorescence using cell-culture-grown organisms. Lab. Animal. 1975, 9, 297–304. [Google Scholar] [CrossRef]
- Abu-Akkada, S.; Ashmawy, K.I.; Dweir, A.W. First detection of an ignored parasite, Encephalitozoon cuniculi, in different animal hosts in Egypt. Parasitol. Res. 2015, 114, 843–850. [Google Scholar] [CrossRef]
- Lonardi, C.; Grilli, G.; Ferrazzi, V.; Dal Cin, M.; Rigolin, D.; Piccirillo, A. Serological survey of Encephalitozoon cuniculi infection in commercially reared rabbit does in Northern Italy. Res. Vet. Sci. 2013, 94, 295–298. [Google Scholar] [CrossRef]
- Sokolova, O.I.; Demyanov, A.V.; Bowers, L.C.; Didier, E.S.; Yakovlev, A.V.; Skarlato, S.O.; Sokolova, Y.Y. Emerging Microsporidian Infections in Russian HIV-Infected Patients. J. Clin. Microbiol. 2011, 49, 2102–2108. [Google Scholar] [CrossRef]
- Malysh, J.M.; Tokarev, Y.S.; Sitnicova, N.V.; Martemyanov, V.V.; Frolov, A.N.; Issi, I.V. Tubulinosema loxostegi sp. n. (Microsporidia: Tubulinosematidae) from the Beet Webworm Loxostege sticticalis L. (Lepidoptera: Crambidae) in Western Siberia. Acta Protozool. 2013, 52, 299–308. [Google Scholar]
- Plischuk, S.; Sanscrainte, N.D.; Becnel, J.J.; Estep, A.S.; Lange, C.E. Tubulinosema pampeana sp. n. (Microsporidia, Tubulinosematidae), a pathogen of the South American bumble bee Bombus atratus. J. Invertebr. Pahtol. 2015, 126, 31–42. [Google Scholar] [CrossRef]
- Biganski, S.; Wennmann, J.T.; Vossbrinck, C.R.; Kaur, R.; Jehle, J.A.; Kleespies, R.G. Molecular and morphological characterization of a novel microsporidian species, Tubulinosema suzukii, infecting Drosophila suzukii (Diptera: Drosophilidae). J. Invertebr. Pathol. 2020, 174, 107440. [Google Scholar] [CrossRef]
- Franzen, C.; Fischer, S.; Schroeder, J.; Schölmerich, J.; Schneuwly, S. Morphological and molecular investigations of Tubulinosema ratisbonensis gen. nov., sp. nov. (Microsporidia: Tubulinosematidae fam. Nov.), a parasite infecting a laboratory colony of Drosophila melanogaster (Diptera: Drosophilidae). J. Eukaryot. Microbiol. 2005, 52, 141–152. [Google Scholar] [CrossRef]
- Dubuffet, A.; Chauvet, M.; Moné, A.; Debroas, D.; Lepère, C. A phylogenetic framework to investigate the microsporidian communities through metabarcoding and its application to lake ecosystems. Environ. Microbiol. 2021, 23, 4344–4359. [Google Scholar] [CrossRef] [PubMed]
- Canning, E.U.; Refardt, D.; Vossbrinck, C.R.; Okamura, B.; Curry, A. New diplokaryotic microsporidia (Phylum Microsporidia) from freshwater bryozoans (Bryozoa, Phylactolaemata). Eur. J. Protistol. 2002, 38, 247–265. [Google Scholar] [CrossRef]
- Morris, D.J.; Terry, R.S.; Adams, A. Development and Molecular Characterisation of the Microsporidian Schroedera airthreyi n. sp. in a Freshwater Bryozoan Plumatella sp. (Bryozoa: Phylactolaemata). J. Eukaryot. Microbiol. 2005, 52, 31–37. [Google Scholar] [CrossRef]
- Mathis, A.; Weber, R.; Deplazes, P. Zoonotic potential of the microsporidia. J. Clin. Microbiol. Rev. 2005, 18, 423–445. [Google Scholar] [CrossRef] [PubMed]
- Sak, B.; Brady, D.; Pelikánová, M.; Květoňová, D.; Rost, M.; Kostka, M.; Tolarová, V.; Hůzová, Z.; Kváč, M. Unapparent Microsporidial Infection among Immunocompetent Humans in the Czech Republic. J. Clin. Microbiol. 2011, 49, 1064–1070. [Google Scholar] [CrossRef]
| Sample ID | Microsporidia | Location | Management |
|---|---|---|---|
| MicC28 | Undetermined | La Orotava | Environmental |
| MicC30 | Undetermined | La Orotava | Environmental |
| MicC41 | Encephalitozoon cuniculi | Granadilla de Abona | Farm |
| MicC43 | Encephalitozoon cuniculi | Granadilla de Abona | Farm |
| MicC60 | Undetermined | La Orotava | Environmental |
| MicC66 | Undetermined | El Sauzal | Hunted rabbit |
| MicC80 | Undetermined | San Cristóbal de La Laguna | Found dead rabbit |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baz-González, E.; Martin-Carrillo, N.; García-Livia, K.; Abreu-Acosta, N.; Foronda, P. Molecular Detection of Microsporidia in Rabbits (Oryctolagus cuniculus) in Tenerife, Canary Islands, Spain. Biology 2022, 11, 1796. https://doi.org/10.3390/biology11121796
Baz-González E, Martin-Carrillo N, García-Livia K, Abreu-Acosta N, Foronda P. Molecular Detection of Microsporidia in Rabbits (Oryctolagus cuniculus) in Tenerife, Canary Islands, Spain. Biology. 2022; 11(12):1796. https://doi.org/10.3390/biology11121796
Chicago/Turabian StyleBaz-González, Edgar, Natalia Martin-Carrillo, Katherine García-Livia, Néstor Abreu-Acosta, and Pilar Foronda. 2022. "Molecular Detection of Microsporidia in Rabbits (Oryctolagus cuniculus) in Tenerife, Canary Islands, Spain" Biology 11, no. 12: 1796. https://doi.org/10.3390/biology11121796
APA StyleBaz-González, E., Martin-Carrillo, N., García-Livia, K., Abreu-Acosta, N., & Foronda, P. (2022). Molecular Detection of Microsporidia in Rabbits (Oryctolagus cuniculus) in Tenerife, Canary Islands, Spain. Biology, 11(12), 1796. https://doi.org/10.3390/biology11121796






