Shedding Light on the Dentition and Venom Delivery System of the Rear-Fanged Snake, Galvarinus chilensis chilensis (Serpentes: Dipsadidae: Tachymenini) from Chile
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Specimens
2.2. Scanning Electron Microscopy (SEM)
2.3. Micro-Computed Tomography (Microct) Analysis
2.4. Venom Gland Histology and Histochemistry
2.5. Statistical Analysis
3. Results
3.1. G. ch. chilensis Has No Ontogenetic Differences in the Number of Teeth
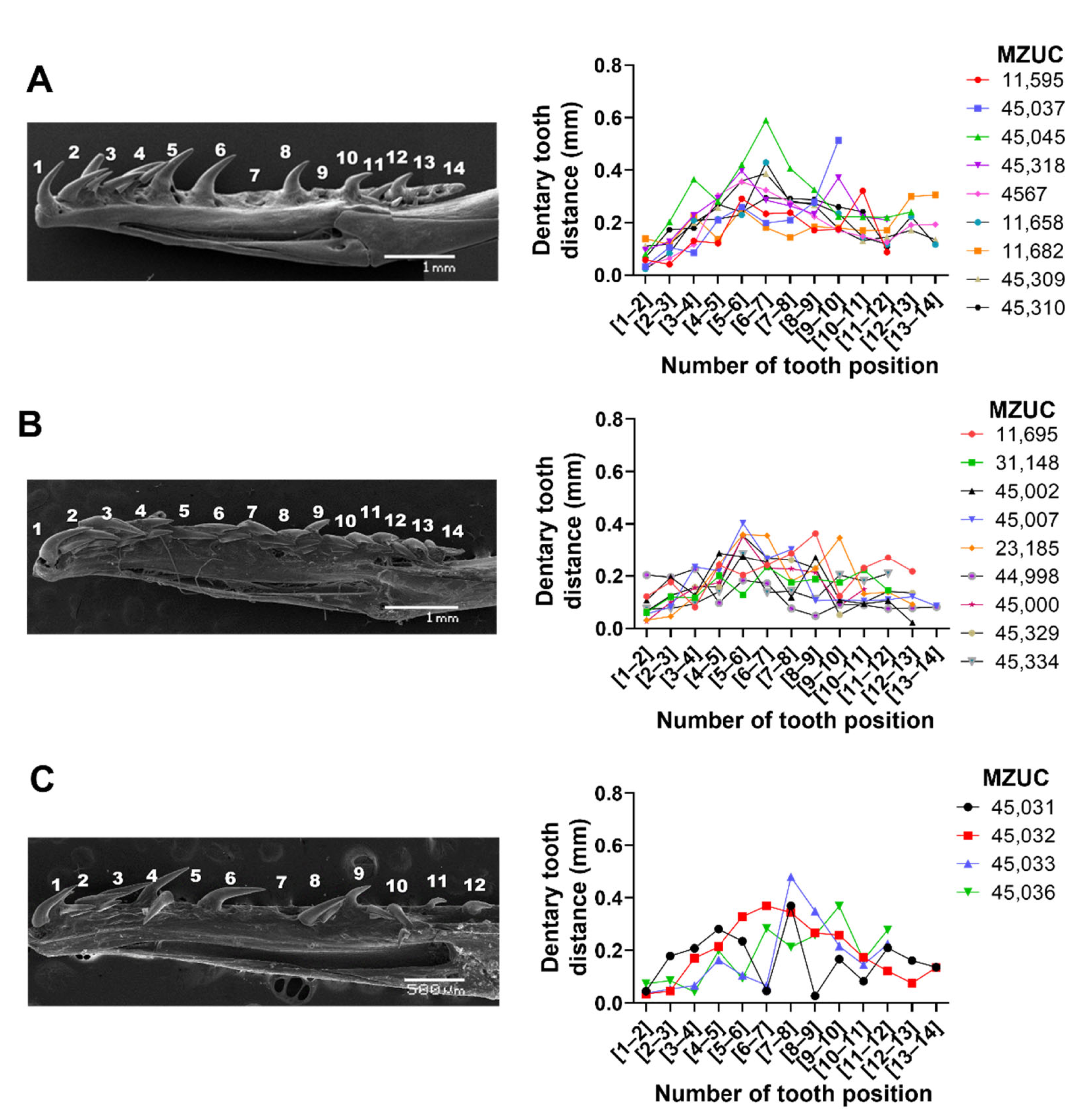
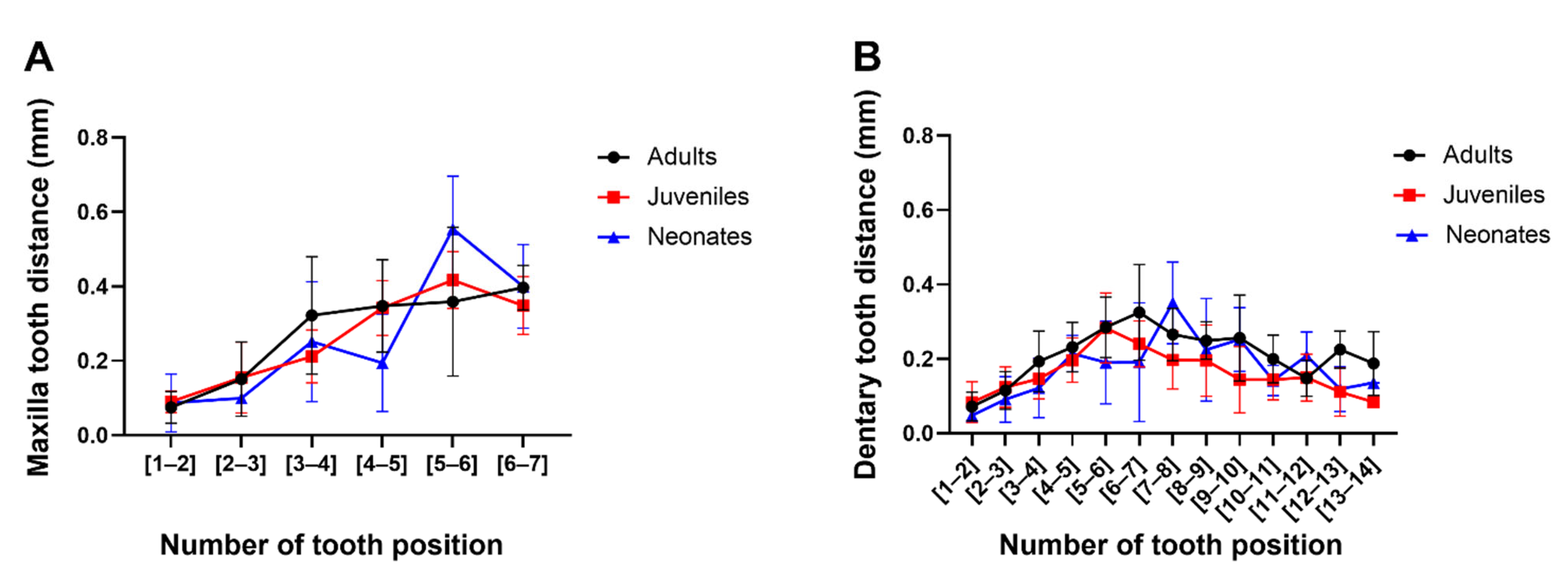
3.2. Tooth Types and Distances between the Dentary and Maxilla Have Unique Patterns and Are Independent of Ontogenetic Status in G. ch. chilensis
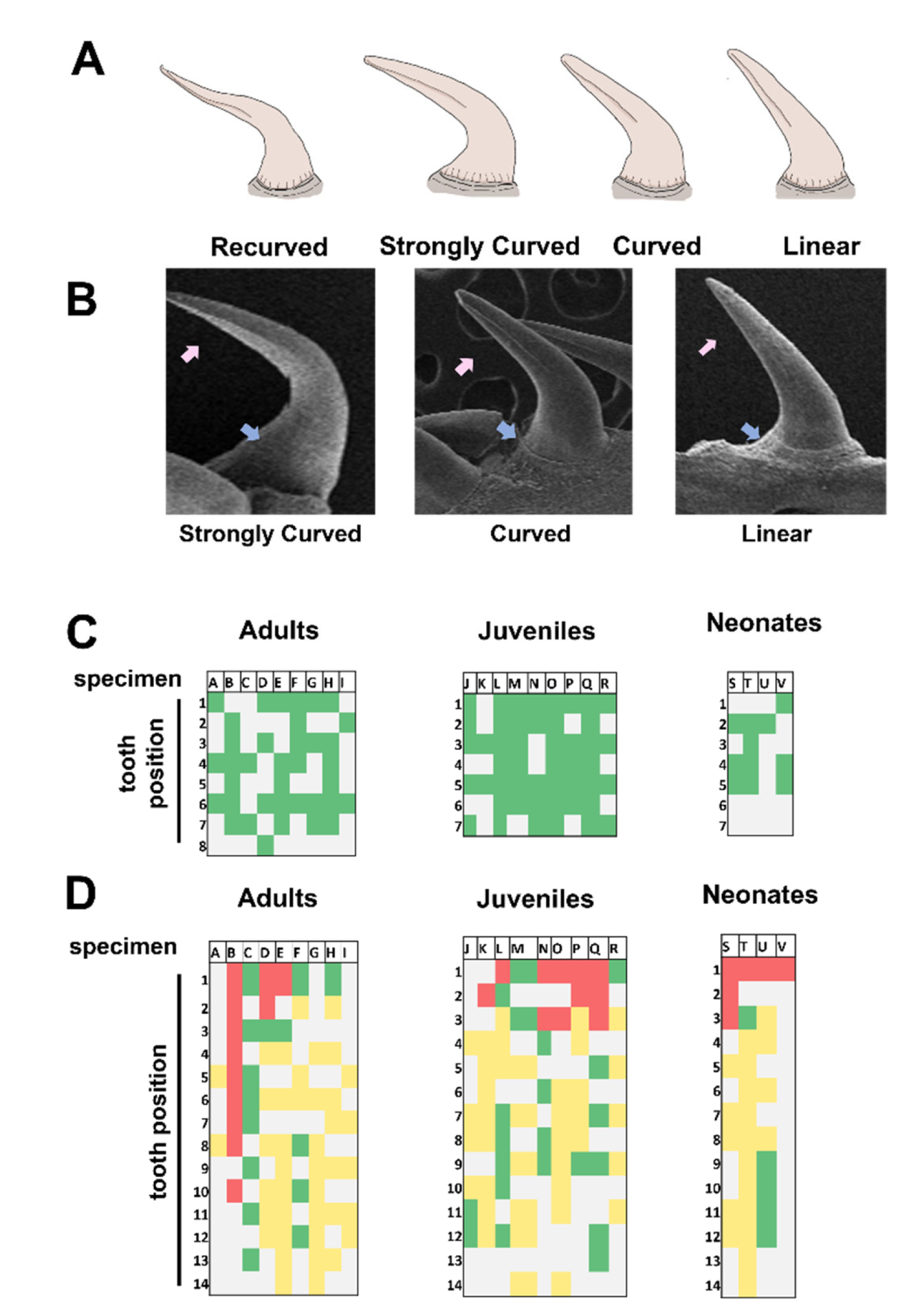
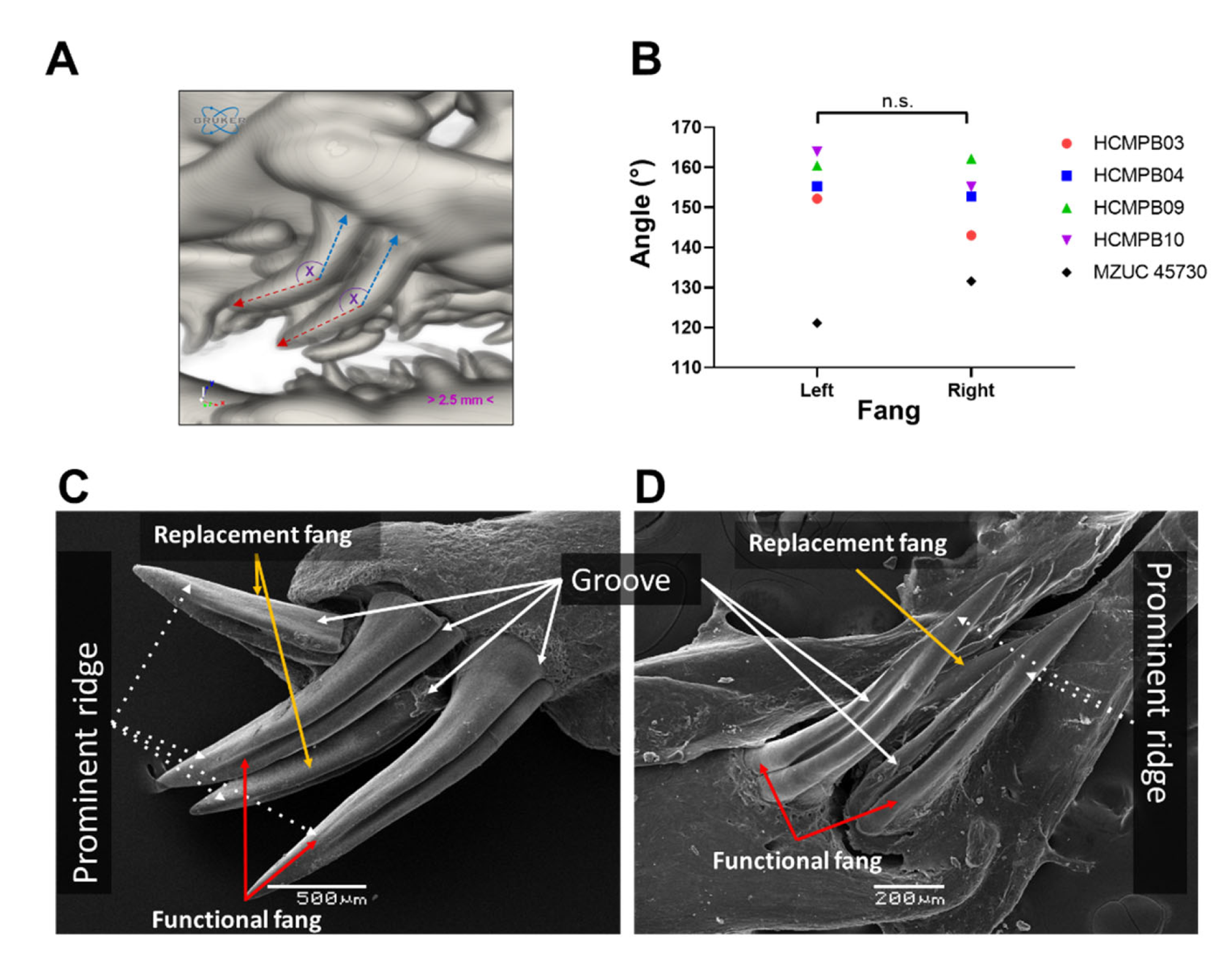
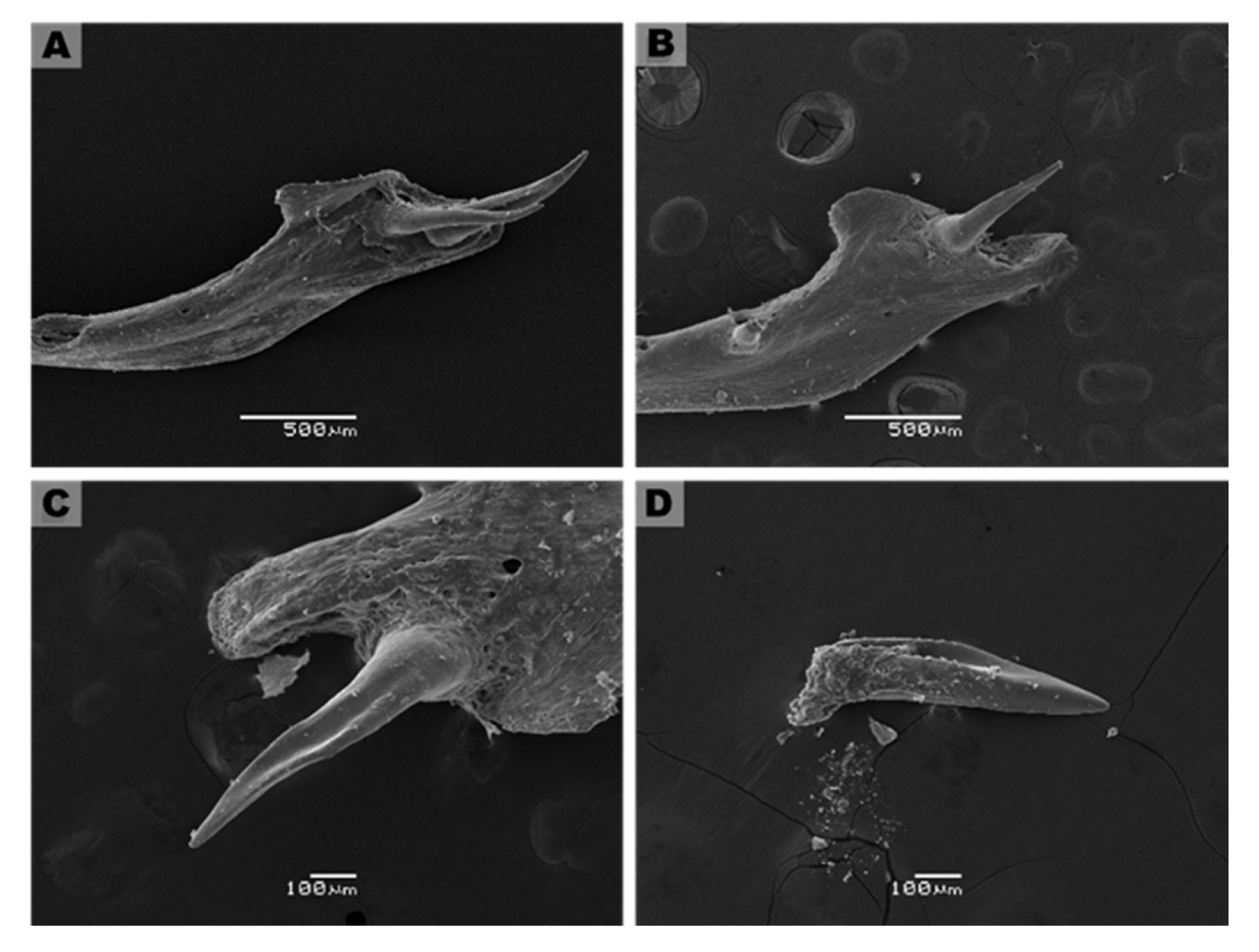
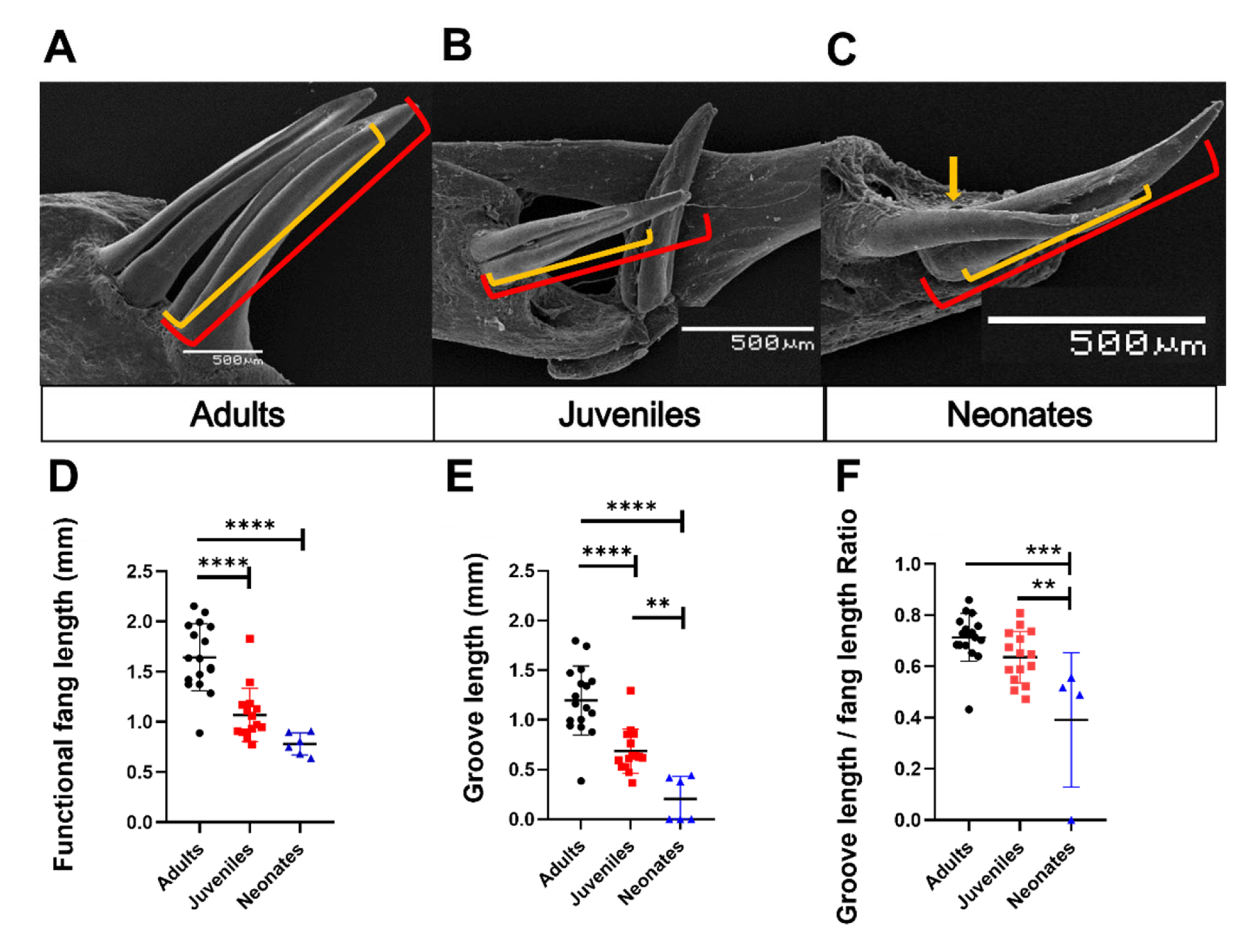
3.3. Description of Fang Morphology of G. ch. chilensis
3.4. Histological Characterization of Duvernoy’s Gland of G. ch. chilensis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Knox, A.; Jackson, K. Ecological and phylogenetic influences on maxillary dentition in snakes. Phyllomedusa J. Herpetol. 2010, 9, 121–131. [Google Scholar] [CrossRef]
- Kardong, K.V. The evolution of the venom apparatus in snakes from colubrids to viperids and elapids. Mem. Inst. Butantan 1982, 46, 105–118. [Google Scholar]
- Budney, L.; Caldwell, M.; Albino, A. Tooth Socket Histology in the Cretaceous Snake Dinilysia, with a Review of Amniote Dental Attachment Tissues. J. Vertebr. Paleontol. 2006, 26, 138–145. [Google Scholar] [CrossRef]
- Kardong, K.V.; Young, B.A. Dentitional surface features in snakes (Reptilia: Serpentes). Amphib.-Reptil. 1996, 17, 261–276. [Google Scholar] [CrossRef][Green Version]
- Wright, D.L.; Kardong, K.V.; Bentley, D.L. The Functional Anatomy of the Teeth of the Western Terrestrial Garter Snake, Thamnophis elegans. Herpetologica 1979, 35, 223–228. [Google Scholar]
- Evans, A.M.; Choiniere, J.N.; Alexander, G.J. The cutting-edge morphology of the mole snake’s dental apparatus. PeerJ 2019, 7, e6943. [Google Scholar] [CrossRef]
- Edmund, A.G. Dentition. Biology of the Reptilia. In Biology of the Reptilian; Bellairs, A., Parson, T., Eds.; Academic Press: Cambridge, MA, USA, 1969; pp. 115–200. [Google Scholar]
- Vonk, F.J.; Admiraal, J.F.; Jackson, K.; Reshef, R.; de Bakker, M.A.G.; Vanderschoot, K.; van den Berge, I.; van Atten, M.; Burgerhout, E.; Beck, A.; et al. Evolutionary origin and development of snake fangs. Nature 2008, 454, 630–633. [Google Scholar] [CrossRef]
- Peichoto, M.E.; Tavares, F.L.; Santoro, M.L.; Mackessy, S.P. Venom proteomes of South and North American opisthoglyphous (Colubridae and Dipsadidae) snake species: A preliminary approach to understanding their biological roles. Comp. Biochem. Physiol. Part D Genom. Proteom. 2012, 7, 361–369. [Google Scholar] [CrossRef]
- Sánchez, M.N.; Timoniuk, A.; Maruñak, S.; Teibler, P.; Acosta, O.; Peichoto, M.E. Biochemical and biological analysis of Philodryas baroni (Baron’s Green Racer; Dipsadidae) venom: Relevance to the findings of human risk assessment. Hum. Exp. Toxicol. 2013, 33, 22–31. [Google Scholar] [CrossRef]
- Sánchez, M.N.; Teibler, G.P.; López, C.A.; Mackessy, S.P.; Peichoto, M.E. Assessment of the potential toxicological hazard of the Green Parrot Snake (Leptophis ahaetulla marginatus): Characterization of its venom and venom-delivery system. Toxicon 2018, 148, 202–212. [Google Scholar] [CrossRef]
- Sánchez, M.N.; Teibler, G.P.; Sciani, J.M.; Casafús, M.G.; Maruñak, S.L.; Mackessy, S.P.; Peichoto, M.E. Unveiling toxicological aspects of venom from the Aesculapian False Coral Snake Erythrolamprus aesculapii. Toxicon 2019, 164, 71–81. [Google Scholar] [CrossRef]
- Sánchez, M.N.; Gonzalez, K.Y.; Sciani, J.M.; Gritti, M.A.; Maruñak, S.L.; Tavares, F.L.; Teibler, G.P.; Peichoto, M.E. First insights into the biochemical and toxicological characterization of venom from the Banded Cat-eyed Snake Leptodeira annulata pulchriceps. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 239, 108897. [Google Scholar] [CrossRef]
- Urra, F.A.; Pulgar, R.; Gutiérrez, R.; Hodar, C.; Cambiazo, V.; Labra, A. Identification and molecular characterization of five putative toxins from the venom gland of the snake Philodryas chamissonis (Serpentes: Dipsadidae). Toxicon 2015, 108, 19–31. [Google Scholar] [CrossRef]
- Chippaux, J.-P. Incidence and mortality due to snakebite in the Americas. PLoS Negl. Trop. Dis. 2017, 11, e0005662. [Google Scholar] [CrossRef]
- Neira, P.; Jofré, L.; Oschilewski, D.; Subercaseaux, B.; Muñoz, N.J.R.c.d.i. Mordedura por Philodryas chamissonis: Presentación de un caso y revisión de la literatura. Rev. Chil. Infectol. 2007, 24, 236–241. [Google Scholar] [CrossRef][Green Version]
- Longbottom, J.; Shearer, F.; Devine, M.; Alcoba, G.; Chappuis, F.; Weiss, D.; Ray, S.; Ray, N.; Warrell, D.; Ruiz de Castaneda, R.; et al. Vulnerability to snakebite envenoming: A global mapping of hotspots. Lancet 2018, 392, 673–684. [Google Scholar] [CrossRef]
- Urra, F.; Miranda-Calle, A.; Araya-Maturana, R. Philodryas (Serpentes: Dipsadidae) Envenomation, a Neglected Issue in Chile. Toxins 2019, 11, 697. [Google Scholar] [CrossRef]
- Ruiz De Gamboa, M. Estados de conservación y lista actualizada de los reptiles nativos de Chile. Bol. Chil. Herpetol. 2020, 7, 1–11. [Google Scholar]
- Trevine, V.C.; Grazziotin, F.G.; Giraudo, A.; Sallesbery-Pinchera, N.; Vianna, J.A.; Zaher, H. The systematics of Tachymenini (Serpentes, Dipsadidae): An updated classification based on molecular and morphological evidence. Zool. Scr. 2022, 51, 643–663. [Google Scholar] [CrossRef]
- Urra, F.; Zúñiga, A.; Melero, N.; Reyes, N.; Herrera, Y.; Miranda-Calle, A.; Ortiz, J. Leucism and albinism in the rear-fanged snakes Tachymenis chilensis chilensis (Schlegel, 1837) and Tachymenis chilensis coronellina Werner, 1898 (Serpentes, Dipsadidae). Herpetozoa 2021, 34, 125–129. [Google Scholar] [CrossRef]
- Valenzuela-Dellarossa, G.; Núñez, H.; Heibl, C.; Ortiz, J.C. Reptilia, Serpentes, Colubridae, Tachymenis Wiegmann, 1836: Latitudinal and altitudinal distribution extension in Chile. Check List 2010, 6, 5–6. [Google Scholar] [CrossRef]
- Vellard, J. Propriétés venimeuses de Tachymenis peruviana Wiegm. Folia Biol. Andin. Pars II–Zool. 1955, 1, 1–14. [Google Scholar]
- Strozzi, L. Study of the morphology of the venom gland of Tachymenis peruviana assimilis (Jan) (Ophidia)]. Bol. Chil. Parasitol. 1965, 20, 98–103. [Google Scholar] [PubMed]
- Troncoso-Palacios, J.; Labra, A. Is the exploratory behavior of Liolaemus nitidus modulated by sex? Acta Herpetol. 2012, 7, 69–80. [Google Scholar] [CrossRef]
- Vaeth, R.H.; Rossman, D.A.; Shoop, W. Observations of Tooth Surface Morphology in Snakes. J. Herpetol. 1985, 19, 20–26. [Google Scholar] [CrossRef]
- Britt, E.J.; Clark, A.J.; Bennett, A.F. Dental Morphologies in Gartersnakes (Thamnophis) and Their Connection to Dietary Preferences. J. Herpetol. 2009, 43, 252–259. [Google Scholar] [CrossRef]
- Ortiz, J.C. Étude sur le status taxonomique de Tachymenis peruviana Wiegmann et Tachymenis chilensis (Schlegel)(Serpentes: Colubridae). Bull. Mus. D’hist. Nat. 1973, 110, 1021–1039. [Google Scholar]
- Greene, H.W.; Jaksic, F. The feeding behavior and natural history of two Chilean snakes, Philodryas chamissonis and Tachymenis chilensis (Colubridae). Rev. Chil. Hist. Nat. 1992, 65, 485–493. [Google Scholar]
- Habit, E.; Ortiz, J.C.; Victoriano, P. Osteología Craneana de Philodryas chamissonis (Wiegmann, 1834) (Colubridae, Serpentes). Bol. Soc. Biol. Concepc. 1992, 63, 83–92. [Google Scholar]
- Smith, J.; Dodson, P. A proposal for a standard terminology of anatomical notation and orientation in fossil vertebrate dentitions. J. Vertebr. Paleontol. 2003, 23, 1–12. [Google Scholar] [CrossRef]
- Suvarna, K.; Layton, C.; Bancroft, J. Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Elsevier: Amsterdam, The Netherladns, 2019; p. 557. [Google Scholar]
- Arteaga, A.; Salazar-Valenzuela, D.; Mebert, K.; Peñafiel, N.; Aguiar, G.; Sánchez-Nivicela, J.; Pyron, R.; Colston, T.; Cisneros-Heredia, D.; Yánez-Muñoz, M.; et al. Systematics of South American snail-eating snakes (Serpentes, Dipsadini), with the description of five new species from Ecuador and Peru. ZooKeys 2018, 766, 79–147. [Google Scholar] [CrossRef]
- Zañartu, N.; Urra, F. Nocturnal activity of a rear-fanged Chilean snake, Tachymenis chilensis coronellina (Serpentes: Dipsadidae). Herpetol. Notes 2021, 14, 1365–1366. [Google Scholar]
- Contreras, J.; Urra, F.; Porras-Rojas, N. First record of Pompilocalus sp. ROIG ALSINA, 1989 (Hymenoptera: Pompilidae) preying on Tachymenis chilensis coronellina (WERNER, 1898)(Serpentes: Dipsadidae) from Central Chile. Herpetol. Notes 2019, 12, 931–932. [Google Scholar]
- Donoso-Barros, R. Reptiles of Chile; Universidad de Chile: Santiago, Chile, 1966; p. 458. [Google Scholar]
- Gajardo-Tobar, R. Los ofidios chilenos son capaces de envenenar? Bol. Hosp. Viña Mar 1947, 3, 43–51. [Google Scholar]
- Gajardo-Tobar, R. Cinco casos de ofidismo. Bol. Hosp. Viña Mar 1958, 14, 172–184. [Google Scholar]
- Savitzky, A.H. Hinged Teeth in Snakes: An Adaptation for Swallowing Hard-Bodied Prey. Science 1981, 212, 346–349. [Google Scholar] [CrossRef]
- Cooney, C.R.; Bright, J.A.; Capp, E.J.R.; Chira, A.M.; Hughes, E.C.; Moody, C.J.A.; Nouri, L.O.; Varley, Z.K.; Thomas, G.H. Mega-evolutionary dynamics of the adaptive radiation of birds. Nature 2017, 542, 344–347. [Google Scholar] [CrossRef]
- McGee, M.D.; Borstein, S.R.; Neches, R.Y.; Buescher, H.H.; Seehausen, O.; Wainwright, P.C. A pharyngeal jaw evolutionary innovation facilitated extinction in Lake Victoria cichlids. Science 2015, 350, 1077–1079. [Google Scholar] [CrossRef]
- Branch, W.R. The Wolf Snakes Lycophidion capense and Lycophidion variegatum (Reptilia, Serpentes, Colubridae) in South Africa. J. Herpetol. 1976, 10, 1–11. [Google Scholar] [CrossRef]
- Carpenter, C.C. Communication and Displays of Snakes. Am. Zool. 1977, 17, 217–223. [Google Scholar] [CrossRef]
- Shaw, C.E. Male Combat in American Colubrid Snakes with Remarks on Combat in Other Colubrid and Elapid Snakes. Herpetologica 1951, 7, 149–168. [Google Scholar]
- Cleuren, S.G.C.; Hocking, D.P.; Evans, A.R. Fang evolution in venomous snakes: Adaptation of 3D tooth shape to the biomechanical properties of their prey. Evolution 2021, 75, 1377–1394. [Google Scholar] [CrossRef] [PubMed]
- Walker, W. A Study of the Snake, Tachymenis peruviana Wiegmann and Its Allies; Museum of Comparative Zoology: Cambridge, MA, USA, 1945; Volume 96. [Google Scholar]
- Cleuren, S.G.C.; Patterson, M.B.; Hocking, D.P.; Warburton, N.M.; Evans, A.R. Fang shape varies with ontogeny and sex in the venomous elapid snake Pseudonaja affinis. J. Morphol. 2022, 283, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Donoso-Barros, R.; Cardenas, S. El veneno de las culebras chilenas. Not. Mens. Mus. Nac. Hist. Nat. 1962, 74, 2–4. [Google Scholar]
- Giraudo, A.; Vidoz, F.; Arzamendia, V.; Nenda, S. Distribution and natural history notes on Tachymenis chilensis chilensis (Schlegel, 1837) (Reptilia: Serpentes: Dipsadidae) in Argentina. Check List. J. Species List. Distrib. 2012, 8, 919–923. [Google Scholar] [CrossRef][Green Version]
- Serapicos, E.; Merusse, J. Morfologia e histoquímica das glândulas de Duvernoy e supralabial de seis espécies de colubrídeos opistoglifodontes (serpentes, Colubridae). Pap. Avulsos Zool. 2006, 46, 187–195. [Google Scholar] [CrossRef]
- Oliveira, L.; Scartozzoni, R.; Almeida-Santos, S.; Jared, C.; Antoniazzi, M.; Salomão, M. Morphology of Duvernoy’s Glands and Maxillary Teeth and a Possible Function of the Duvernoy’s Gland Secretion in Helicops modestus Günther, 1861 (Serpentes: Xenodontinae). S. Am. J. Herpetol. 2016, 11, 54–65. [Google Scholar] [CrossRef]
- Torres-Bonilla, K.A.; Panunto, P.C.; Pereira, B.B.; Zambrano, D.F.; Herrán-Medina, J.; Bernal, M.H.; Hyslop, S. Toxinological characterization of venom from Leptodeira annulata (Banded cat-eyed snake; Dipsadidae, Imantodini). Biochimie 2020, 174, 171–188. [Google Scholar] [CrossRef]
- Taub, A. Ophidian cephalic glands. J. Morphol. 1966, 118, 529–541. [Google Scholar] [CrossRef]
- de Oliveira, L.; Jared, C.; da Costa Prudente, A.L.; Zaher, H.; Antoniazzi, M.M. Oral glands in dipsadine “goo-eater” snakes: Morphology and histochemistry of the infralabial glands in Atractus reticulatus, Dipsas indica, and Sibynomorphus mikanii. Toxicon 2008, 51, 898–913. [Google Scholar] [CrossRef]
- Renner, M.; Sabóia-Morais, S. Estudo histológico e histoquímico da glândula de Duvernoy de Clelia plumbea (Wied) (Serpentes, Colubridae, Xenodontinae). Rev. Bras. Zool. 2000, 17, 583–588. [Google Scholar] [CrossRef]
- Santiago, F.; Xavier Júnior, F.; Carvalho, I.; Braga, R.; Morais, G.; Souza, J.; Borges-Nojosa, D.; Evangelista, J. Caracterização histológica da glândula de Duvernoy da serpente Philodryas nattereri Steindachner, 1870. Braz. J. Dev. 2020, 6, 55661–55672. [Google Scholar] [CrossRef]
- Pough, F.H.; Kwiecinski, G.; Bemis, W. Melanin deposits associated with the venom glands of snakes. J. Morphol. 1978, 155, 63–71. [Google Scholar] [CrossRef]
- Vanzolini, P. Coleodactylus septentrionalis, sp. n., with notes on the distribution of the genus (Sauria, Gekkonidae). Pascal Fr. Bibliogr. Databases 1980, 14, 1–9. [Google Scholar]
- Westeen, E.P.; Durso, A.M.; Grundler, M.C.; Rabosky, D.L.; Davis Rabosky, A.R. What makes a fang? Phylogenetic and ecological controls on tooth evolution in rear-fanged snakes. BMC Evol. Biol. 2020, 20, 80. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera, Y.; Fuentes-Retamal, S.; Kemmerling, U.; Peichoto, M.E.; Ortiz, J.C.; Urra, F.A. Shedding Light on the Dentition and Venom Delivery System of the Rear-Fanged Snake, Galvarinus chilensis chilensis (Serpentes: Dipsadidae: Tachymenini) from Chile. Biology 2022, 11, 1788. https://doi.org/10.3390/biology11121788
Herrera Y, Fuentes-Retamal S, Kemmerling U, Peichoto ME, Ortiz JC, Urra FA. Shedding Light on the Dentition and Venom Delivery System of the Rear-Fanged Snake, Galvarinus chilensis chilensis (Serpentes: Dipsadidae: Tachymenini) from Chile. Biology. 2022; 11(12):1788. https://doi.org/10.3390/biology11121788
Chicago/Turabian StyleHerrera, Yarela, Sebastián Fuentes-Retamal, Ulrike Kemmerling, María Elisa Peichoto, Juan Carlos Ortiz, and Félix A. Urra. 2022. "Shedding Light on the Dentition and Venom Delivery System of the Rear-Fanged Snake, Galvarinus chilensis chilensis (Serpentes: Dipsadidae: Tachymenini) from Chile" Biology 11, no. 12: 1788. https://doi.org/10.3390/biology11121788
APA StyleHerrera, Y., Fuentes-Retamal, S., Kemmerling, U., Peichoto, M. E., Ortiz, J. C., & Urra, F. A. (2022). Shedding Light on the Dentition and Venom Delivery System of the Rear-Fanged Snake, Galvarinus chilensis chilensis (Serpentes: Dipsadidae: Tachymenini) from Chile. Biology, 11(12), 1788. https://doi.org/10.3390/biology11121788




