Porcine Interleukin-17 and 22 Co-Expressed by Yarrowia lipolytica Enhance Immunity and Increase Protection against Bacterial Challenge in Mice and Piglets
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteria and Plasmids
2.2. Construction of Recombinant Yarrowia lipolytica pINA1297-IL-17/22
2.3. Analysis of rIL-17/22 Expression by ELISA and Western Blotting
2.4. Bioactivity Assay of rIL-17/22 In Vitro
2.5. Preparation of Recombinant Yeast
2.6. Experimental Protocol for Mice
2.7. Animal Experimental Protocol for Piglets
2.8. Evaluation of the Immunoregulator Effect of Po1h-pINA1297-IL-17/22 In Vivo
2.8.1. Assay of Cytokines in the Serum of Mice by ELISA
2.8.2. Cytokine Levels in the Plasma of Piglets by ELISA
2.8.3. Changes of CD4+ and CD8+ T Cells in the Blood by Flow Cytometry (FCM)
2.8.4. Measures of IgG in Serum and SIgA in Small Intestines and Feces by ELISA
2.9. Statistical Analysis
3. Results
3.1. Construction of the Po1h-pINA1297-IL-17/22
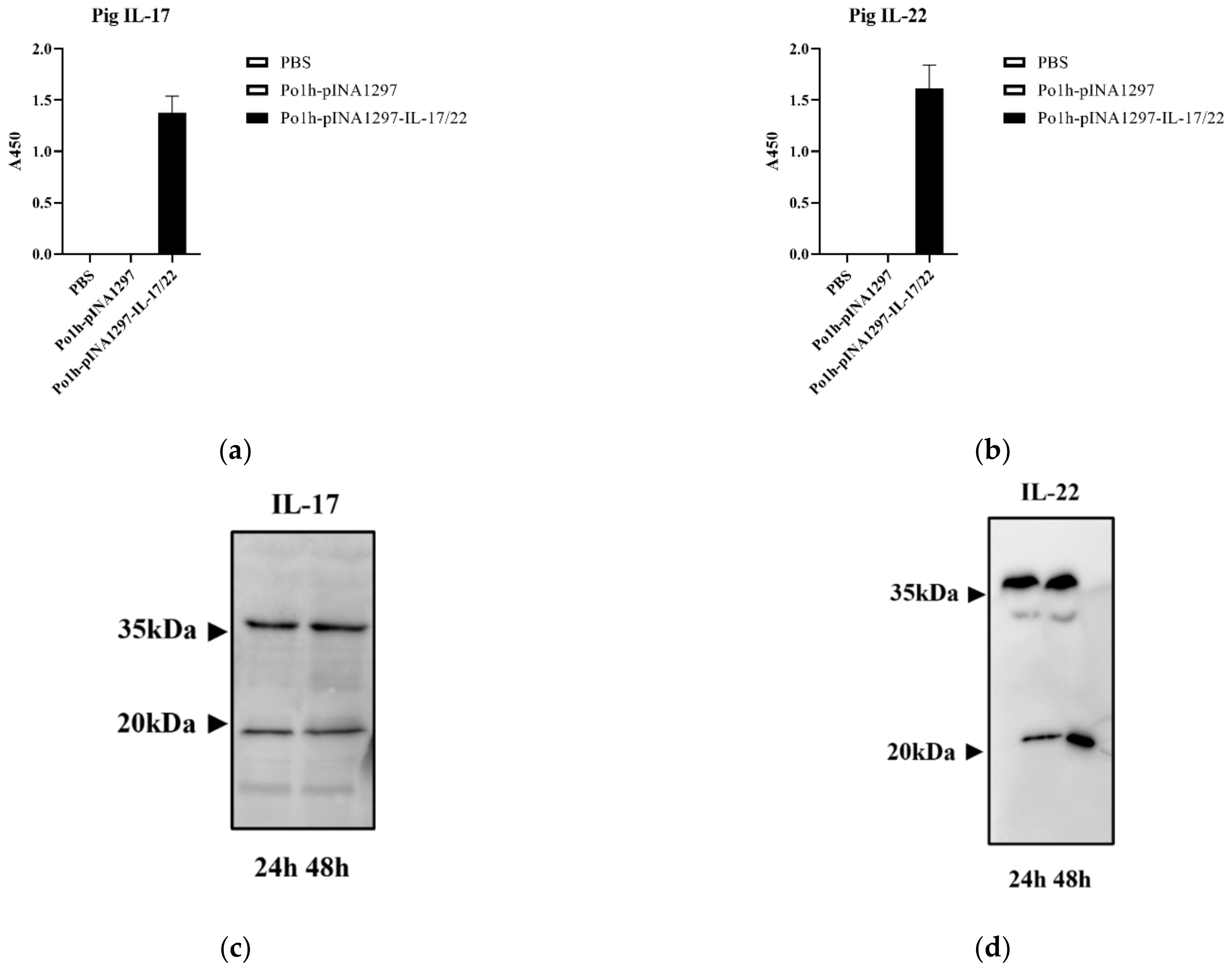
3.2. Expression of the Po1h-pINA1297-IL-17/22
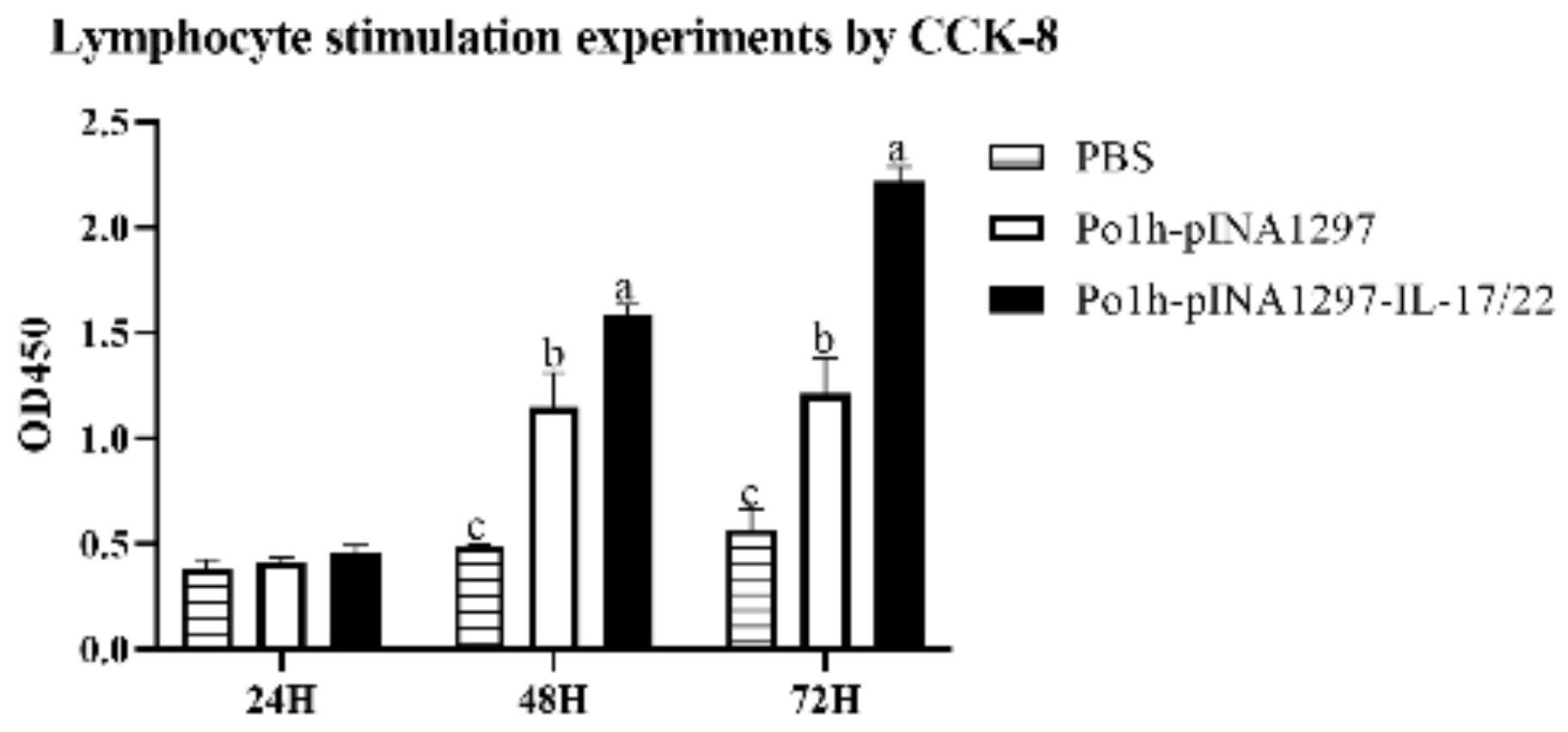
3.3. Bioactivity of the Po1h-pINA1297-IL-17/22
3.4. Weight Change in the Experimental Mice
3.5. Evaluation of the Immunoregulator Activities of Po1h-pINA1297-IL-17/22 in Mice
3.5.1. Analysis of Total IgG
3.5.2. Changes of Cytokines Levels
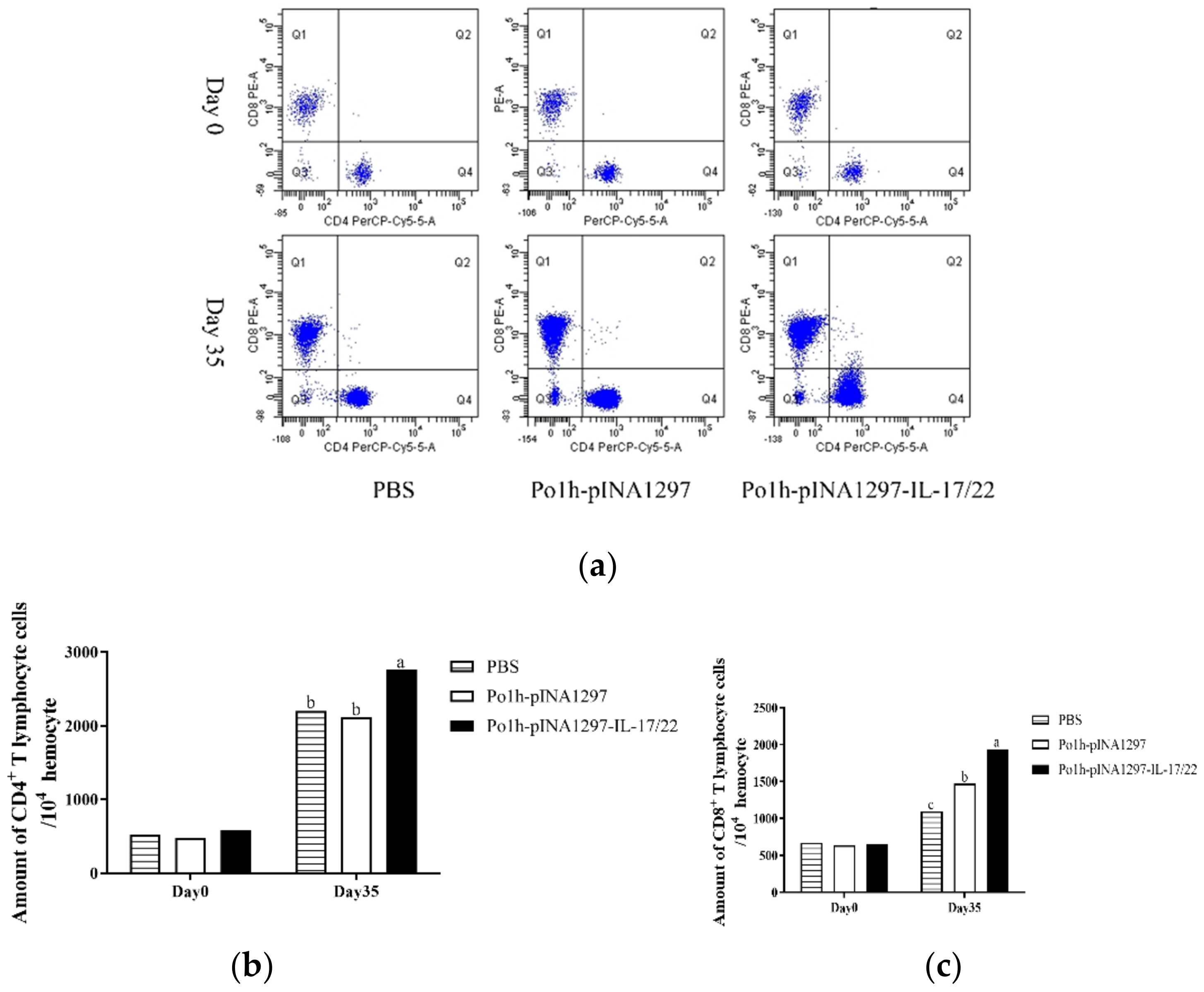
3.5.3. Changes of CD4+ and CD8+ T Cells
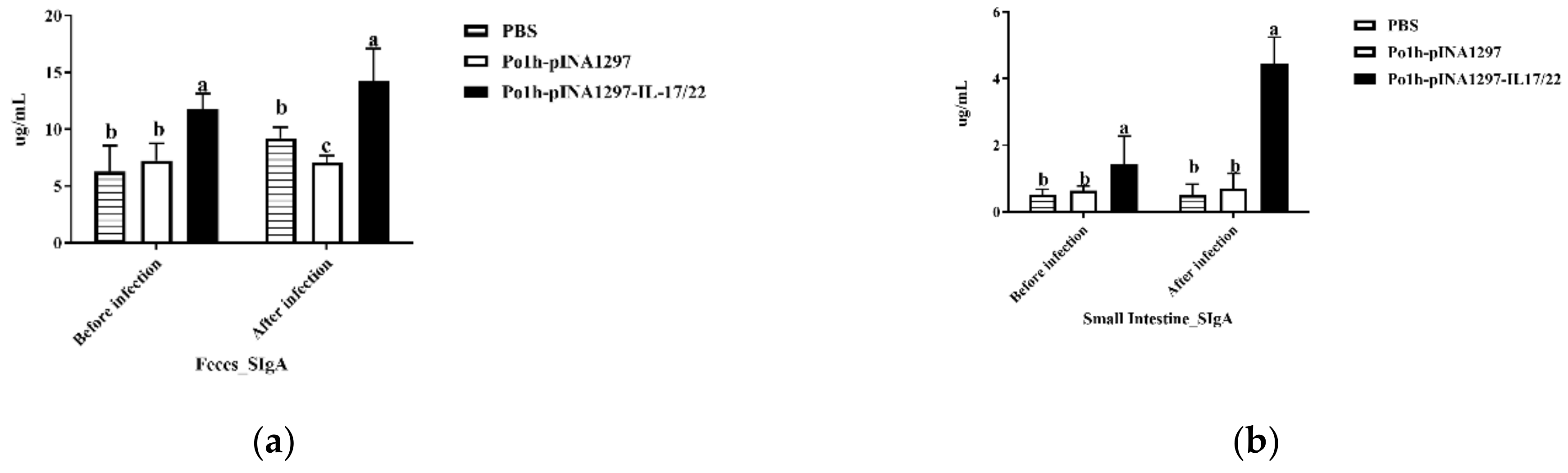
3.5.4. Mucosal SIgA in the Gut
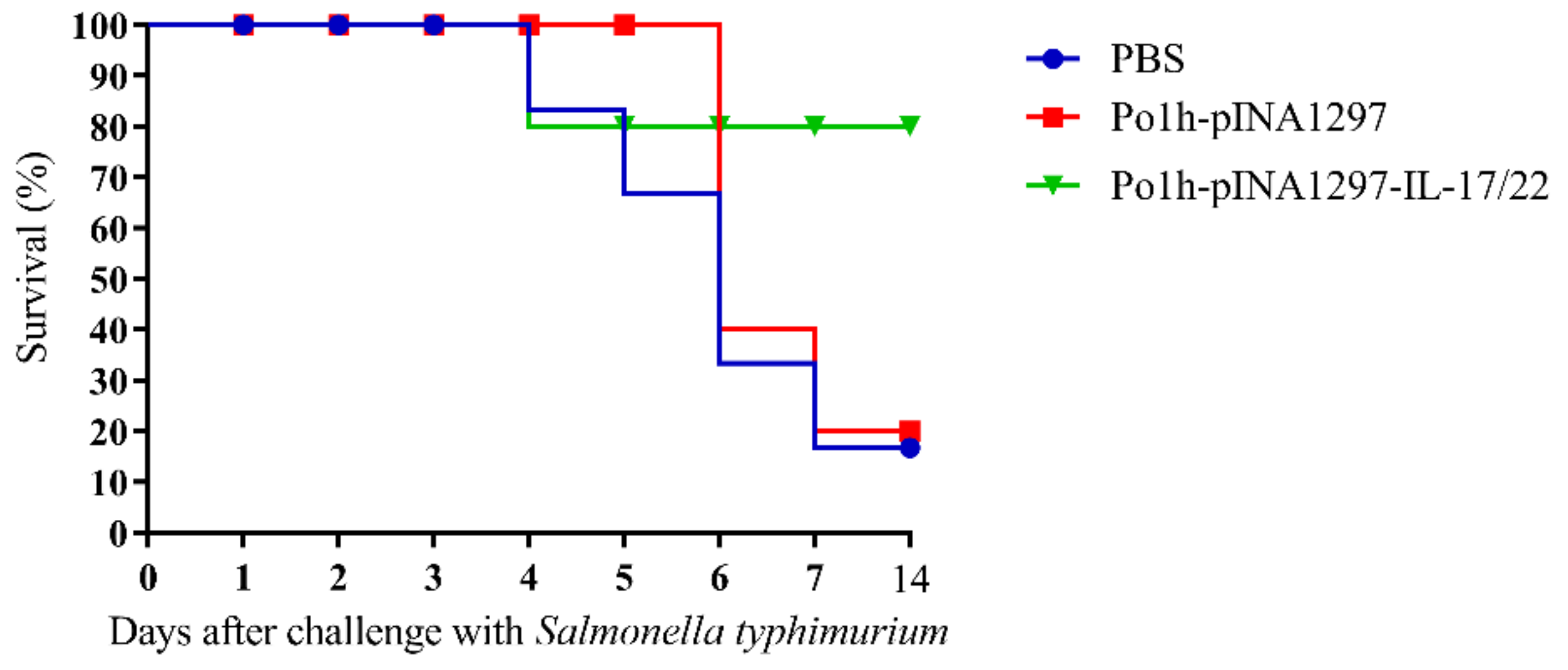
3.5.5. Evaluation of the Protection against Salmonella typhimurium Challenge
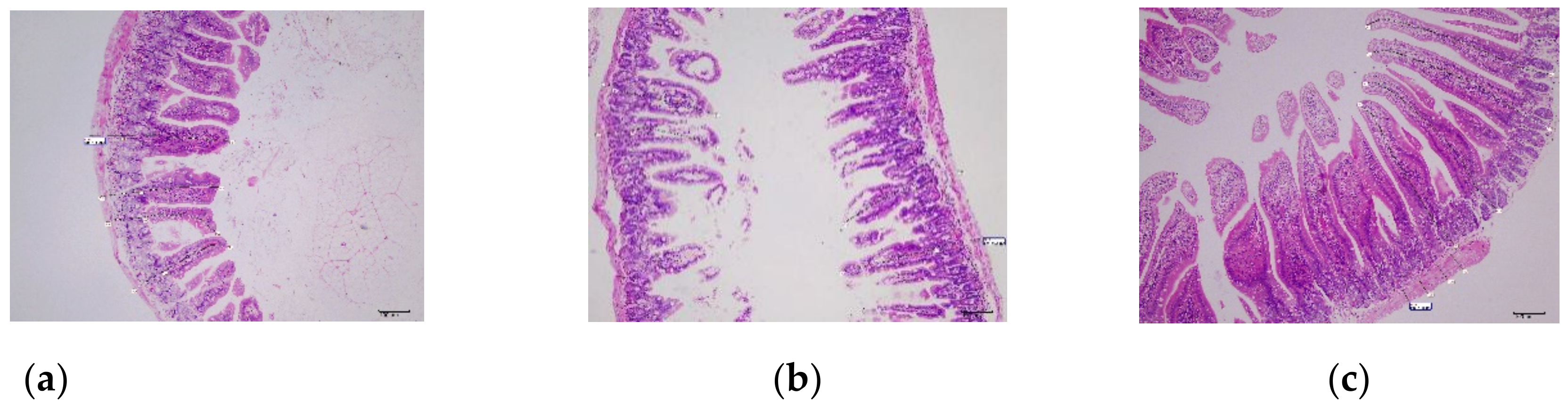
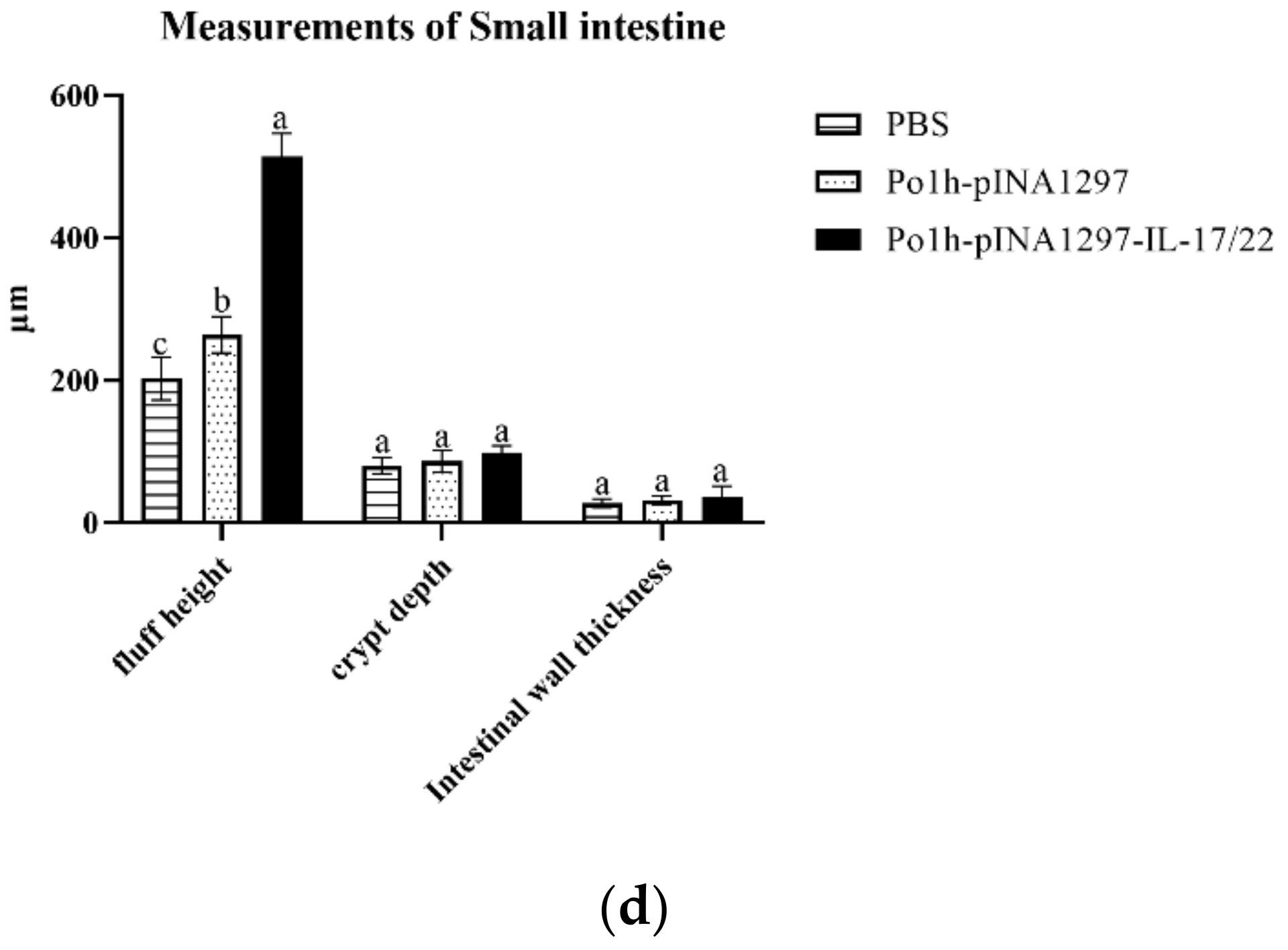
3.5.6. Histological Examination of Small Intestine
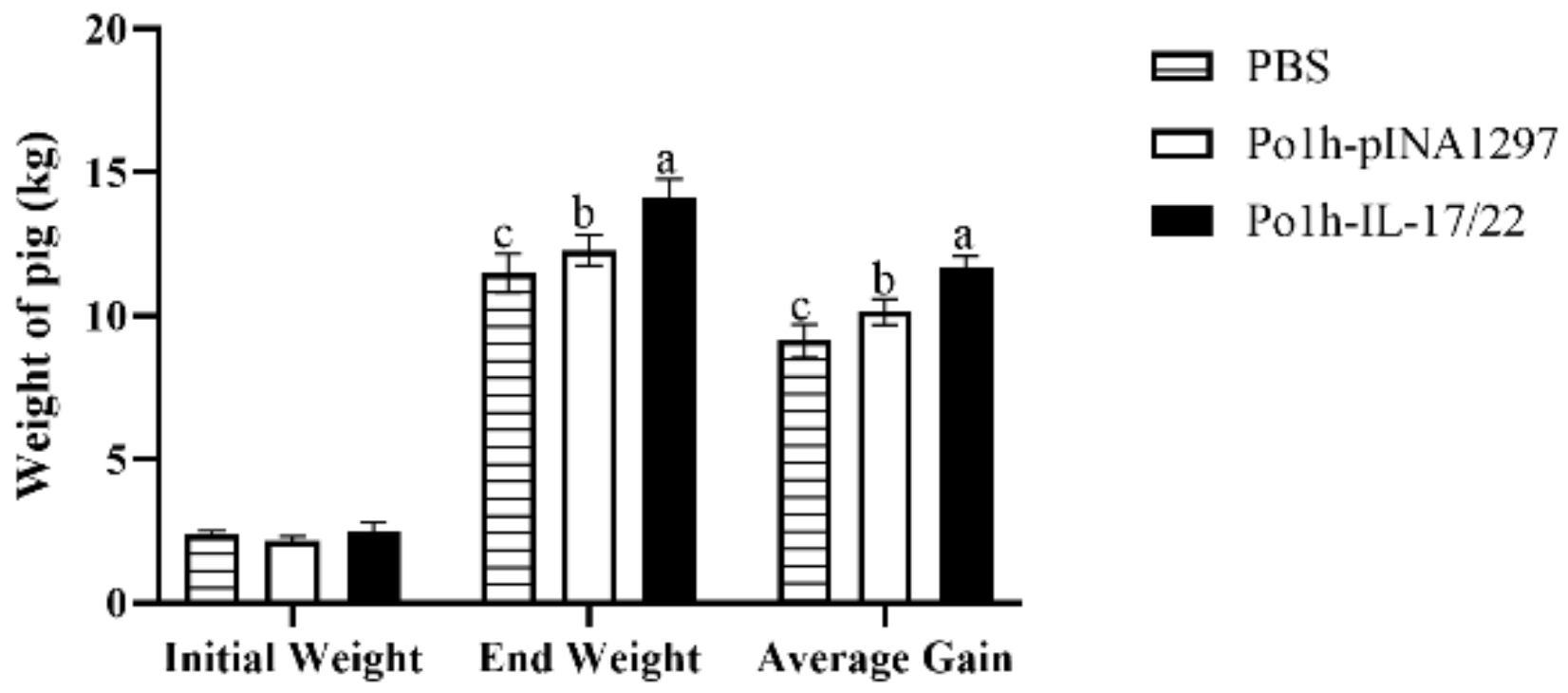
3.6. Weight Change of the Experimental Piglets
3.7. Evaluation of Immunoregulator Activities of Po1h-pINA1297-IL-17/22 in Pig
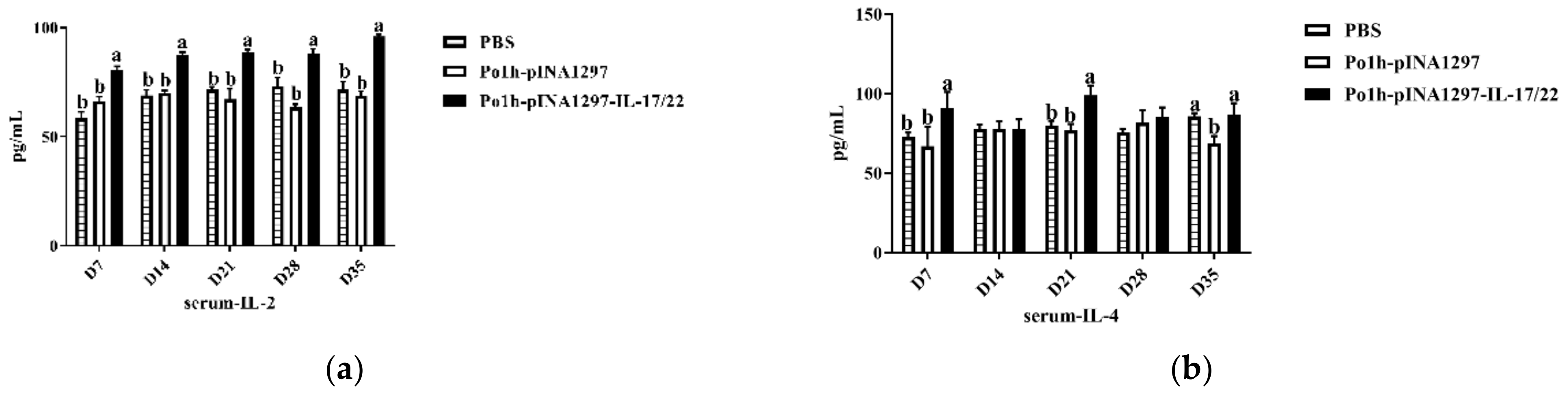
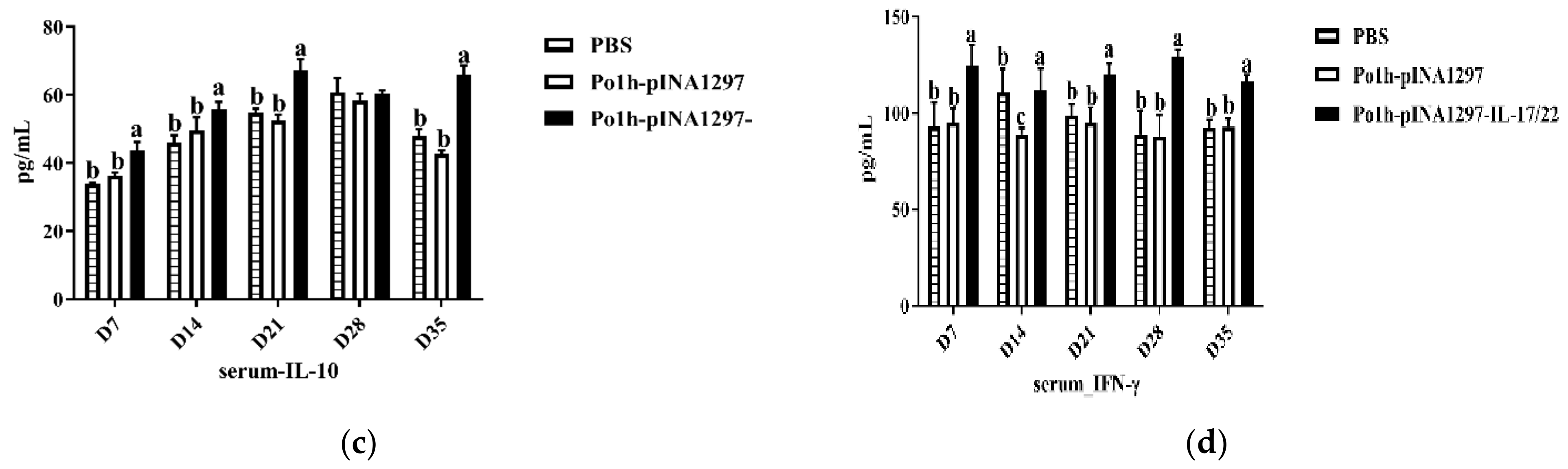
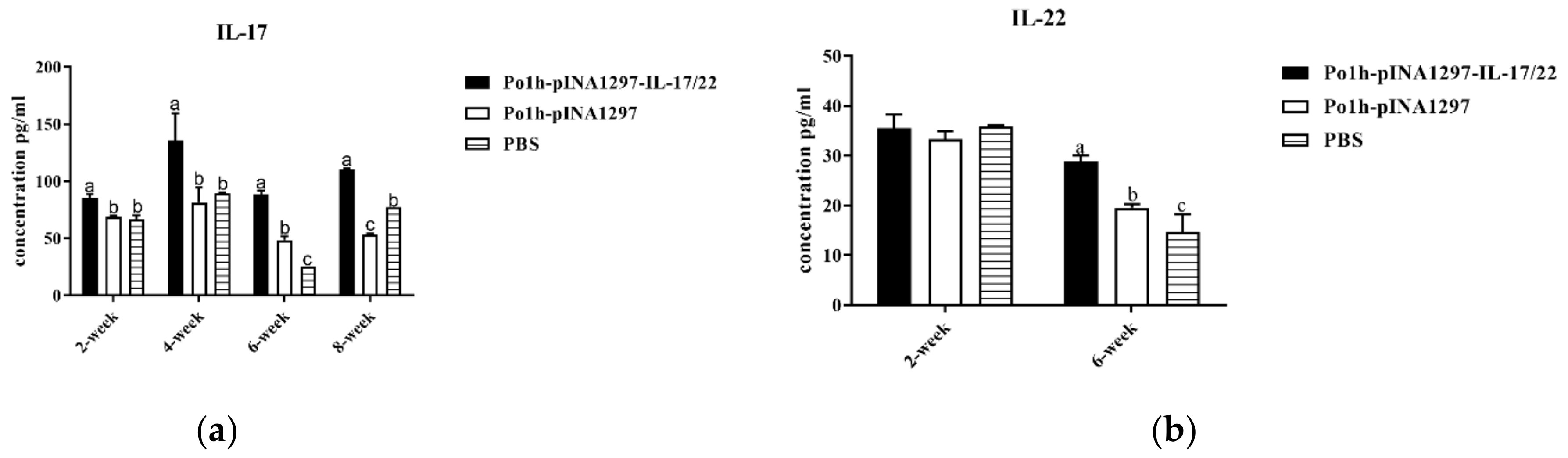
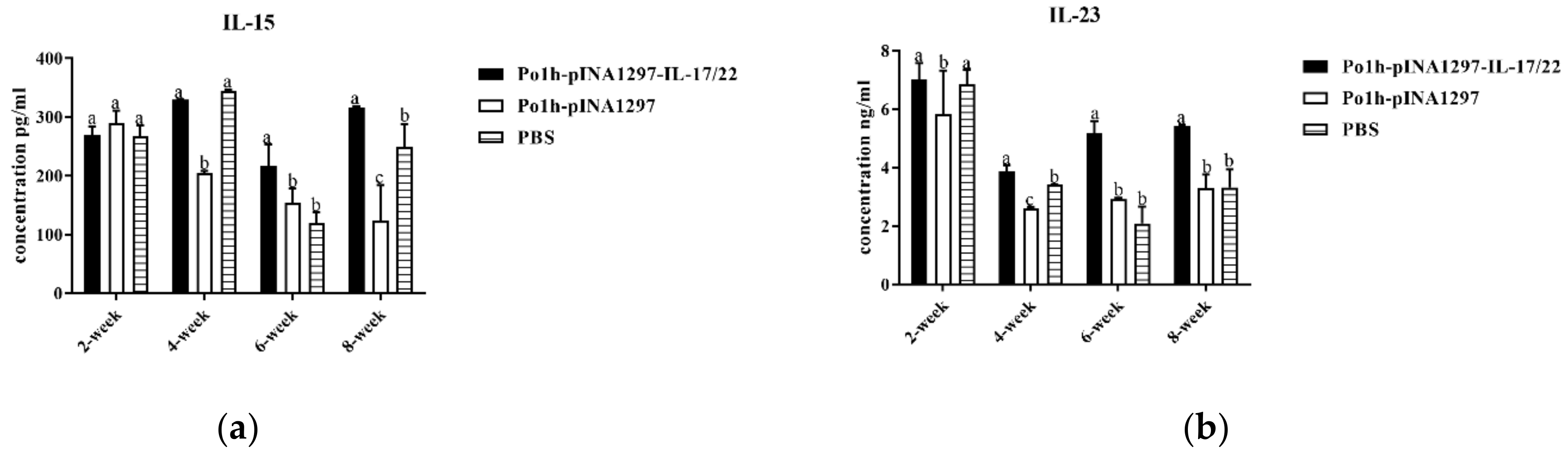
3.7.1. Change of Cytokine Levels in Plasma
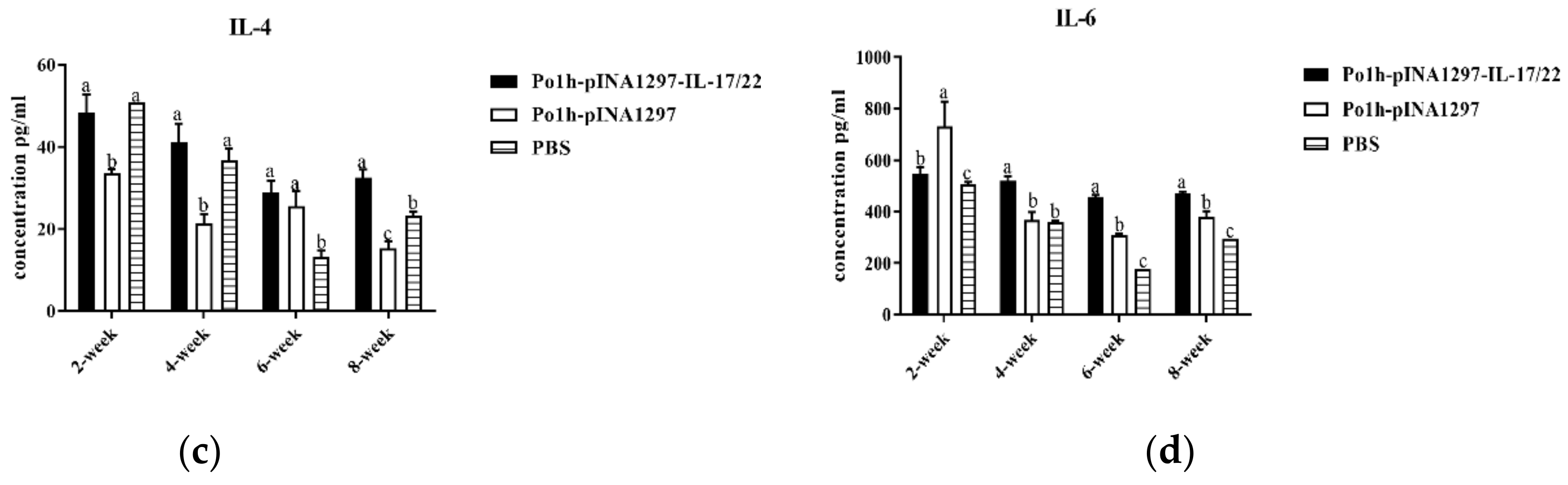
3.7.2. Change in Immune Memory Relative Cytokine Levels in Plasma
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mann, A.; Nehra, K.; Rana, J.; Dahiya, T. Antibiotic resistance in agriculture: Perspectives on upcoming strategies to overcome upsurge in resistance. Curr. Res. Microb. Sci. 2021, 2, 100030. [Google Scholar] [CrossRef] [PubMed]
- Hedman, H.D.; Vasco, K.A.; Zhang, L. A review of antimicrobial resistance in poultry farming within low-resource settings. Animals 2020, 10, 1264. [Google Scholar] [CrossRef] [PubMed]
- Van Boeckel, T.P.; Pires, J.; Silvester, R.; Zhao, C.; Song, J.; Criscuolo, N.G.; Gilbert, M.; Bonhoeffer, S.; Laxminarayan, R. Global trends in antimicrobial resistance in animals in low-and middle-income countries. Science 2019, 365, eaaw1944. [Google Scholar] [CrossRef] [PubMed]
- Agyare, C.; Boamah, V.E.; Zumbi, C.N.; Osei, F.B. Antibiotic use in poultry production and its effects on bacterial resistance. In Antimicrobial Resistance—A Global Threat; Kumar, Y., Ed.; IntechOpen: London, UK, 2018; pp. 33–51. [Google Scholar]
- Hu, Y.J.; Cowling, B.J. Reducing antibiotic use in livestock, China. Bull. World Health Organ. 2020, 98, 360. [Google Scholar] [CrossRef] [PubMed]
- Allen, H.K. Antibiotic resistance gene discovery in food-producing animals. Curr. Opin. Microbiol. 2014, 19, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.G.; Orr, M.T.; Fox, C.B. Key roles of adjuvants in modern vaccines. Nat. Med. 2013, 19, 1597–1608. [Google Scholar] [CrossRef]
- Pires, I.S.; Hammond, P.T.; Irvine, D. Engineering strategies for immunomodulatory cytokine therapies: Challenges and clinical progress. Adv. Ther. 2021, 4, 2100035. [Google Scholar] [CrossRef] [PubMed]
- Katoh, S.; Kitazawa, H.; Shimosato, T.; Tohno, M.; Kawai, Y.; Saito, T. Cloning and characterization of swine interleukin-17, preferentially expressed in the intestines. J. Interferon Cytokine Res. 2004, 24, 553–559. [Google Scholar] [CrossRef]
- McGeachy, M.J.; Cua, D.J.; Gaffen, S.L. The IL-17 family of cytokines in health and disease. Immunity 2019, 50, 892–906. [Google Scholar] [CrossRef]
- Manirarora, J.N.; Walker, K.E.; Patil, V.; Renukaradhya, G.J.; LaBresh, J.; Sullivan, Y.; Francis, O.; Lunney, J.K. Development and Characterization of New Monoclonal Antibodies Against Porcine Interleukin-17A and Interferon-Gamma. Front. Immunol. 2022, 13, 786396. [Google Scholar] [CrossRef]
- Luo, Y.; Van Nguyen, U.; de la Fe Rodriguez, P.Y.; Devriendt, B.; Cox, E. F4+ ETEC infection and oral immunization with F4 fimbriae elicits an IL-17-dominated immune response. Vet. Res. 2015, 46, 121. [Google Scholar] [CrossRef] [PubMed]
- Conti, H.R.; Shen, F.; Nayyar, N.; Stocum, E.; Sun, J.N.; Lindemann, M.J.; Ho, A.W.; Hai, J.H.; Yu, J.J.; Jung, J.W. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J. Exp. Med. 2009, 206, 299–311. [Google Scholar] [CrossRef]
- Khader, S.A.; Gaffen, S.L.; Kolls, J.K. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol. 2009, 2, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Mayuzumi, H.; Inagaki-Ohara, K.; Uyttenhove, C.; Okamoto, Y.; Matsuzaki, G. Interleukin-17A is required to suppress invasion of Salmonella enterica serovar Typhimurium to enteric mucosa. Immunology 2010, 131, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Dreesen, L.; De Bosscher, K.; Grit, G.; Staels, B.; Lubberts, E.; Bauge, E.; Geldhof, P. Giardia muris infection in mice is associated with a protective interleukin 17A response and induction of peroxisome proliferator-activated receptor alpha. Infect. Immun. 2014, 82, 3333–3340. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chan, C.; Yang, M.; Deng, J.; Poon, V.K.; Leung, V.H.; Ko, K.-H.; Zhou, J.; Yung Yuen, K.; Zheng, B.-J. A critical role of IL-17 in modulating the B-cell response during H5N1 influenza virus infection. Cell Mol. Immunol. 2011, 8, 462–468. [Google Scholar] [CrossRef]
- Wei, H.-X.; Wang, B.; Li, B. IL-10 and IL-22 in mucosal immunity: Driving protection and pathology. Front. Immunol. 2020, 11, 1315. [Google Scholar] [CrossRef] [PubMed]
- Layunta, E.; Jäverfelt, S.; Dolan, B.; Arike, L.; Pelaseyed, T. IL-22 promotes the formation of a MUC17 glycocalyx barrier in the postnatal small intestine during weaning. Cell Rep. 2021, 34, 108757. [Google Scholar] [CrossRef]
- Sugimoto, K.; Ogawa, A.; Mizoguchi, E.; Shimomura, Y.; Andoh, A.; Bhan, A.K.; Blumberg, R.S.; Xavier, R.J.; Mizoguchi, A. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J. Clin. Investig. 2008, 118, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.-E.; Stockinger, B.; Helmby, H. IL-22 mediates goblet cell hyperplasia and worm expulsion in intestinal helminth infection. PLoS Pathog. 2013, 9, e1003698. [Google Scholar] [CrossRef]
- Gaudino, S.J.; Beaupre, M.; Lin, X.; Joshi, P.; Rathi, S.; McLaughlin, P.A.; Kempen, C.; Mehta, N.; Eskiocak, O.; Yueh, B. IL-22 receptor signaling in Paneth cells is critical for their maturation, microbiota colonization, Th17-related immune responses, and anti-Salmonella immunity. Mucosal Immunol. 2021, 14, 389–401. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, C.; Zheng, M.; Chen, J.-L.; Meng, M.J.; Zhao, Z.-Z.; Chen, Q.; Xie, Z.; Li, J.L.; Yang, Y. Enhancement of immunity to an Escherichia coli vaccine in mice orally inoculated with a fusion gene encoding porcine interleukin 4 and 6. Vaccine 2007, 25, 7094–7101. [Google Scholar] [CrossRef]
- Peng, J.; Xiao, Y.; Wan, X.; Chen, Q.; Wang, H.; Li, J.; Chen, J.; Gao, R. Enhancement of immune response and anti-infection of mice by porcine antimicrobial peptides and interleukin-4/6 fusion gene encapsulated in chitosan nanoparticles. Vaccines 2020, 8, 552. [Google Scholar] [CrossRef]
- Madzak, C. Yarrowia lipolytica strains and their biotechnological applications: How natural biodiversity and metabolic engineering could contribute to cell factories improvement. J. Fungi 2021, 7, 548. [Google Scholar] [CrossRef] [PubMed]
- Gerdts, V. Adjuvants for veterinary vaccines--types and modes of action. Berl. Munch. Tierarztl. Wochenschr. 2015, 128, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, S.-R.; Li, L.-H.; Park, H.-J.; Park, J.-H.; Lee, K.Y.; Kim, M.-K.; Shin, B.A.; Choi, S.-Y. High cleavage efficiency of a 2A peptide derived from porcine teschovirus-1 in human cell lines, zebrafish and mice. PLoS ONE 2011, 6, e18556. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Song, T.; Xiao, Y.-L.; Wan, X.; Yang, L.; Li, J.; Zeng, G.; Fang, P.; Wang, Z.-Z.; Gao, R. Enhancement of immune response of piglets to PCV-2 vaccine by porcine IL-2 and fusion IL-4/6 gene entrapped in chitosan nanoparticles. Res. Vet. Sci. 2018, 117, 224–232. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, H.; Chen, J.; Chen, Y.; Li, J.; Song, T.; Zeng, G.; Chen, X.; Lü, X.; Fang, P. Cloning and expression of the tibetan pig interleukin-23 gene and its promotion of immunity of pigs to PCV2 vaccine. Vaccines 2020, 8, 250. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Peng, J.; Xiao, Y.; Liu, Y.; Hao, W.; Yang, X.; Wang, H.; Gao, R. The Construction and Immunoadjuvant Activities of the Oral Interleukin-17B Expressed by Lactobacillus plantarum NC8 Strain in the Infectious Bronchitis Virus Vaccination of Chickens. Vaccines 2020, 8, 282. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, S.; Xiang, Z.; Wang, Y.; He, Z.; Deng, H.; Blaszczyk-Thurin, M.; Ertl, H. Cytokines and costimulatory molecules as genetic adjuvants. Immunol. Cell Biol. 1997, 75, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Paul, W.E. History of interleukin-4. Cytokine 2015, 75, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Batlle, E.; Massagué, J. Transforming growth factor-β signaling in immunity and cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T.R.; Sad, S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol. Today 1996, 17, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Twigg, H.L., III. Humoral immune defense (antibodies) recent advances. Proc. Am. Thorac. Soc. 2005, 2, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, A. The regulation of IgA class switching. Nat. Rev. Immunol. 2008, 8, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Cao, A.T.; Yao, S.; Gong, B.; Elson, C.O.; Cong, Y. Th17 cells upregulate polymeric Ig receptor and intestinal IgA and contribute to intestinal homeostasis. J. Immunol. 2012, 189, 4666–4673. [Google Scholar] [CrossRef]
- Hirota, K.; Turner, J.-E.; Villa, M.; Duarte, J.H.; Demengeot, J.; Steinmetz, O.M.; Stockinger, B. Plasticity of TH17 cells in Peyer’s patches is responsible for the induction of T cell–dependent IgA responses. Nat. Immunol. 2013, 14, 372–379. [Google Scholar] [CrossRef] [PubMed]


| Group | Initial Weight (g) | End Weight (g) | Average Gain (g) |
|---|---|---|---|
| PBS | 18.49 ± 1.07 | 19.08 ± 1.41 | 0.59 ± 0.10 b |
| Po1h-pINA1297 | 18.53 ± 1.55 | 19.50 ± 1.80 | 0.97 ± 0.08 b |
| Po1h-pINA1297-IL-17/22 | 17.93 ± 1.45 | 20.32 ± 1.49 | 2.39 ± 0.19 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, J.; Yang, F.; Chen, J.; Guo, S.; Zhang, L.; Deng, D.; Li, J.; Lv, X.; Gao, R. Porcine Interleukin-17 and 22 Co-Expressed by Yarrowia lipolytica Enhance Immunity and Increase Protection against Bacterial Challenge in Mice and Piglets. Biology 2022, 11, 1747. https://doi.org/10.3390/biology11121747
Peng J, Yang F, Chen J, Guo S, Zhang L, Deng D, Li J, Lv X, Gao R. Porcine Interleukin-17 and 22 Co-Expressed by Yarrowia lipolytica Enhance Immunity and Increase Protection against Bacterial Challenge in Mice and Piglets. Biology. 2022; 11(12):1747. https://doi.org/10.3390/biology11121747
Chicago/Turabian StylePeng, Junjie, Fang Yang, Jianlin Chen, Shaohua Guo, Linhan Zhang, Dinghao Deng, Jiangling Li, Xuebin Lv, and Rong Gao. 2022. "Porcine Interleukin-17 and 22 Co-Expressed by Yarrowia lipolytica Enhance Immunity and Increase Protection against Bacterial Challenge in Mice and Piglets" Biology 11, no. 12: 1747. https://doi.org/10.3390/biology11121747
APA StylePeng, J., Yang, F., Chen, J., Guo, S., Zhang, L., Deng, D., Li, J., Lv, X., & Gao, R. (2022). Porcine Interleukin-17 and 22 Co-Expressed by Yarrowia lipolytica Enhance Immunity and Increase Protection against Bacterial Challenge in Mice and Piglets. Biology, 11(12), 1747. https://doi.org/10.3390/biology11121747









