The Activity of Polyhomoarginine against Acanthamoeba castellanii
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Amoeba Culturing
2.2. Polyhomoarginines
2.3. Amoebicidal Assay
2.4. Amoebistatic Assay
2.5. Encystation Assay
2.6. Excystment Assay
2.7. Lysis of Horse Red Blood Cells (HRBCs)
/ (absorbance of positive control) − (absorbance of diluent) × 100
2.8. Statistical Analyses
3. Results
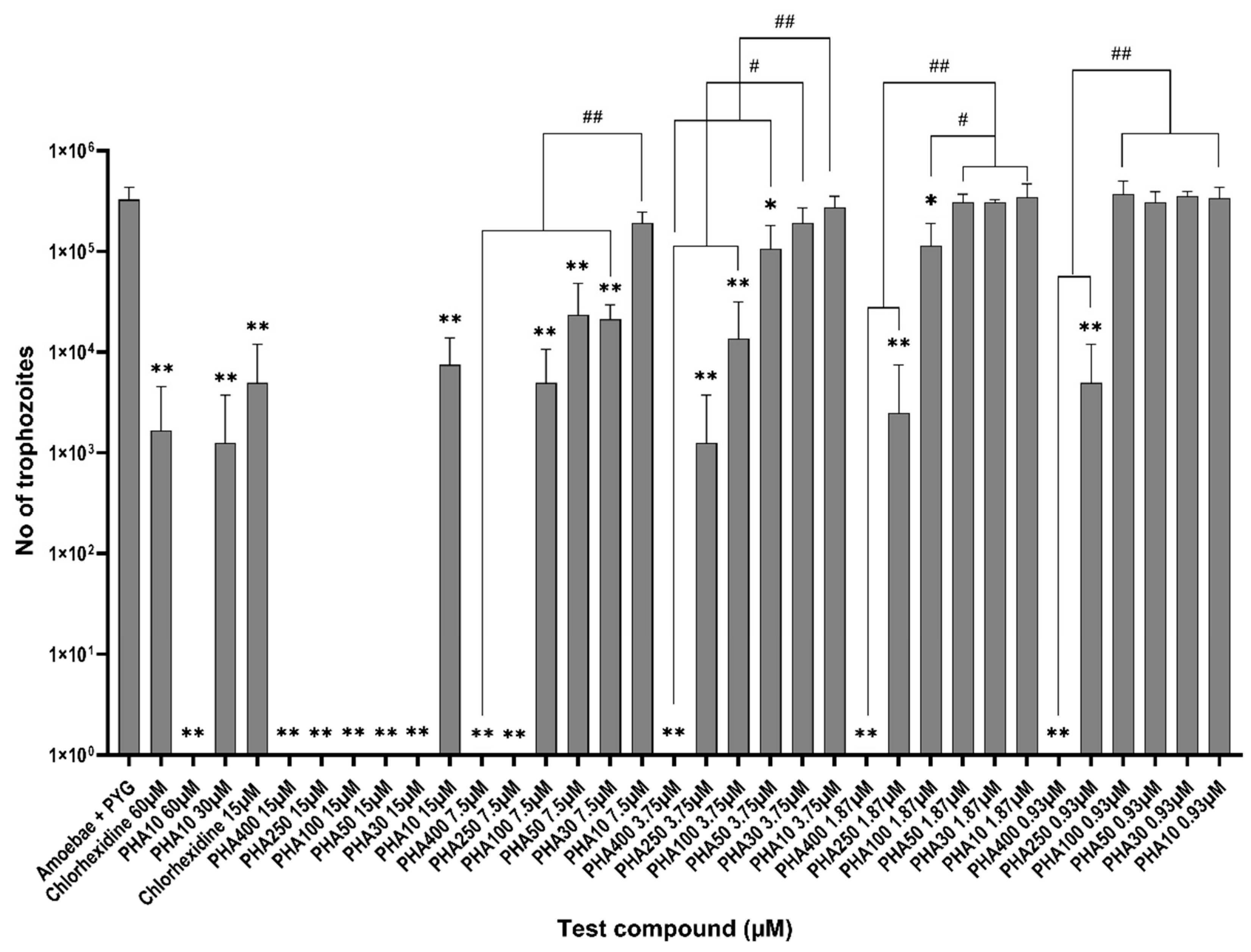
3.1. Amoebicidal Assay
3.2. Amoebistatic Assay
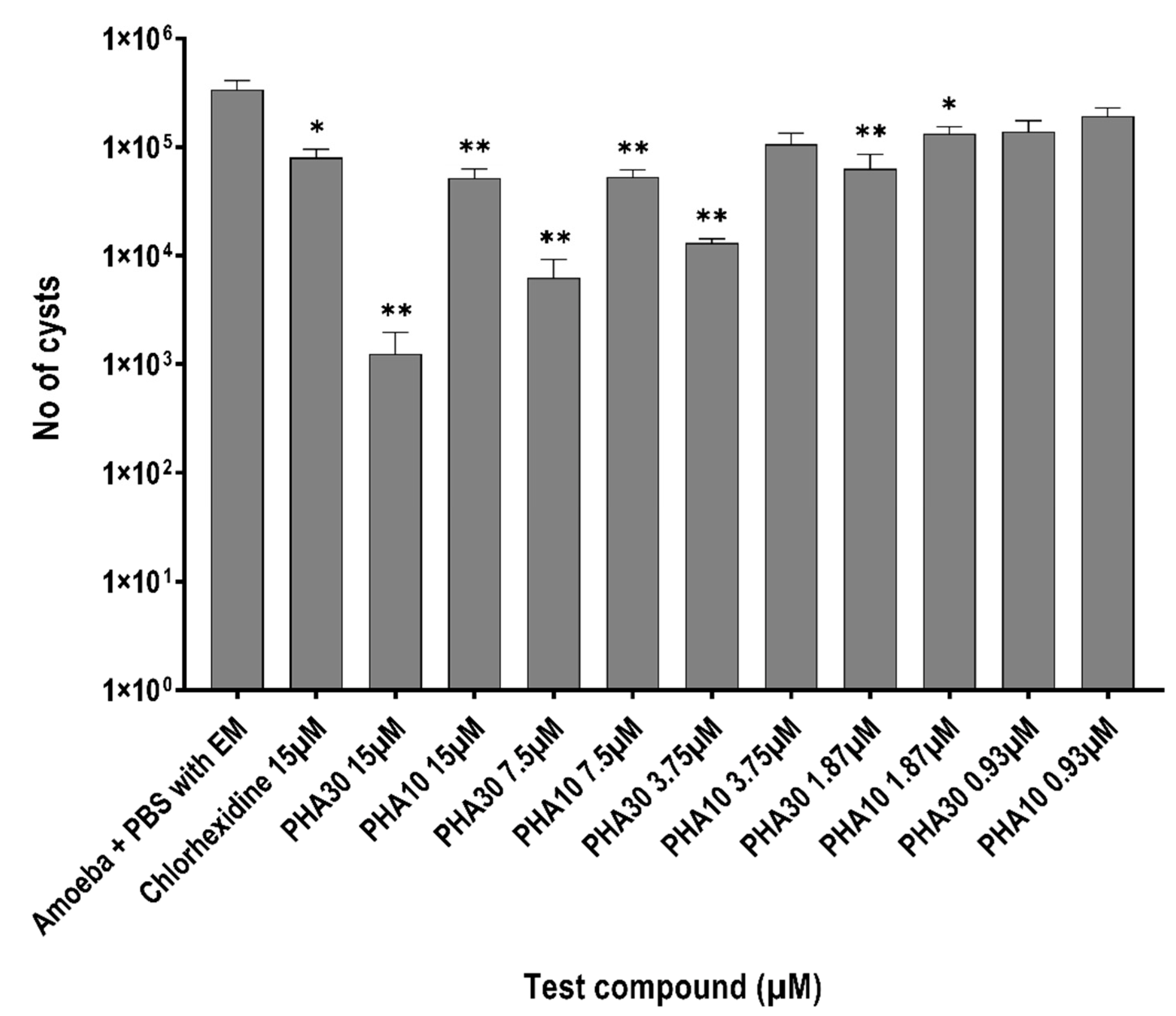
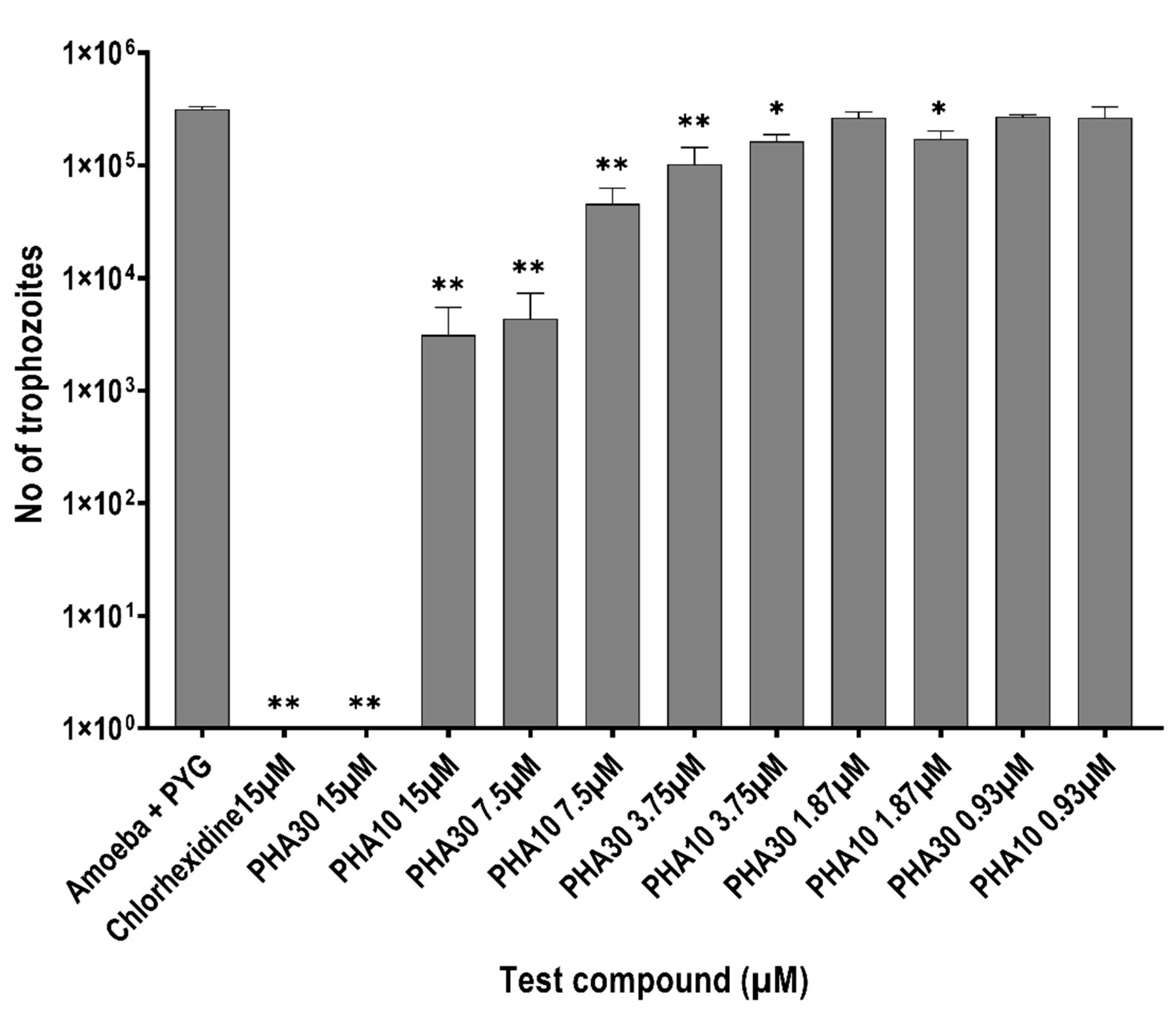
3.3. Encystment and Excystment Assays
3.4. Lysis of HRBCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bechinger, B.; Gorr, S.U. Antimicrobial Peptides: Mechanisms of Action and Resistance. J. Dent. Res. 2017, 96, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef] [PubMed]
- Mollica, A.; Macedonio, G.; Stefanucci, A.; Costante, R.; Carradori, S.; Cataldi, V.; Di Giulio, M.; Cellini, L.; Silvestri, R.; Giordano, C.; et al. Arginine- and Lysine-rich Peptides: Synthesis, Characterization and Antimicrobial Activity. Lett. Drug Des. Discov. 2018, 15, 220–226. [Google Scholar] [CrossRef]
- Schmidt, N.; Mishra, A.; Lai, G.H.; Wong, G.C. Arginine-rich cell-penetrating peptides. FEBS Lett. 2010, 584, 1806–1813. [Google Scholar] [CrossRef]
- Futaki, S.; Suzuki, T.; Ohashi, W.; Yagami, T.; Tanaka, S.; Ueda, K.; Sugiura, Y. Arginine-rich peptides. An abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. J. Biol. Chem. 2001, 276, 5836–5840. [Google Scholar] [CrossRef] [PubMed]
- Chafai, A.; Fromm, M.F.; Konig, J.; Maas, R. The prognostic biomarker L-homoarginine is a substrate of the cationic amino acid transporters CAT1, CAT2A and CAT2B. Sci. Rep. 2017, 7, 4767. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Meinitzer, A.; Gaksch, M.; Grubler, M.; Verheyen, N.; Drechsler, C.; Hartaigh, B.O.; Lang, F.; Alesutan, I.; Voelkl, J.; et al. Homoarginine in the renal and cardiovascular systems. Amino Acids 2015, 47, 1703–1713. [Google Scholar] [CrossRef]
- Lin, C.W.; Fishman, W.H. L-Homoarginine. An organ-specific, uncompetitive inhibitor of human liver and bone alkaline phosphohydrolases. J. Biol. Chem. 1972, 247, 3082–3087. [Google Scholar] [CrossRef]
- Mitchell, D.J.; Kim, D.T.; Steinman, L.; Fathman, C.G.; Rothbard, J.B. Polyarginine enters cells more efficiently than other polycationic homopolymers. J. Pept. Res. 2000, 56, 318–325. [Google Scholar] [CrossRef]
- Praveen, K.; Das, S.; Dhaware, V.; Pandey, B.; Mondal, B.; Gupta, S.S. pH-Responsive "Supra-Amphiphilic" Nanoparticles Based on Homoarginine Polypeptides. ACS Appl. Bio. Mater. 2019, 2, 4162–4172. [Google Scholar] [CrossRef]
- Sun, V.Z.; Li, Z.; Deming, T.J.; Kamei, D.T. Intracellular fates of cell-penetrating block copolypeptide vesicles. Biomacromolecules 2011, 12, 10–13. [Google Scholar] [CrossRef][Green Version]
- Duncan, V.; Smith, D.; Simpson, L.; Lovie, E.; Katvars, L.; Berge, L.; Robertson, J.; Smith, S.; Munro, C.; Mercer, D.; et al. Preliminary Characterization of NP339, a Novel Polyarginine Peptide with Broad Antifungal Activity. Antimicrob. Agents Chemother. 2021, 65, e0234520. [Google Scholar] [CrossRef]
- Sepahi, M.; Jalal, R.; Mashreghi, M. Antibacterial activity of poly-l-arginine under different conditions. Iran. J. Microbiol. 2017, 9, 103–111. [Google Scholar]
- Marz, W.; Meinitzer, A.; Drechsler, C.; Pilz, S.; Krane, V.; Kleber, M.E.; Fischer, J.; Winkelmann, B.R.; Bohm, B.O.; Ritz, E.; et al. Homoarginine, cardiovascular risk, and mortality. Circulation 2010, 122, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Atzler, D.; Schwedhelm, E.; Choe, C.U. L-homoarginine and cardiovascular disease. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 83–88. [Google Scholar] [CrossRef]
- Miyazaki, M.; Yoneyama, M.; Sugai, S. Anion-induced conformational transition of poly(l-arginine) and its two homologues. Polymer 1978, 19, 995–1000. [Google Scholar] [CrossRef]
- Guzman, F.; Marshall, S.; Ojeda, C.; Albericio, F.; Carvajal-Rondanelli, P. Inhibitory effect of short cationic homopeptides against gram-positive bacteria. J. Pept. Sci. 2013, 19, 792–800. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, W.; Cong, Z.; Chen, K.; She, Y.; Zhong, C.; Zhang, W.; Chen, M.; Zhou, M.; Shao, N.; et al. An Effective Strategy to Develop Potent and Selective Antifungal Agents from Cell Penetrating Peptides in Tackling Drug-Resistant Invasive Fungal Infections. J. Med. Chem. 2022, 65, 7296–7311. [Google Scholar] [CrossRef]
- Randag, A.C.; van Rooij, J.; van Goor, A.T.; Verkerk, S.; Wisse, R.P.L.; Saelens, I.E.Y.; Stoutenbeek, R.; van Dooren, B.T.H.; Cheng, Y.Y.Y.; Eggink, C.A. The rising incidence of Acanthamoeba keratitis: A 7-year nationwide survey and clinical assessment of risk factors and functional outcomes. PLoS ONE 2019, 14, e0222092. [Google Scholar] [CrossRef]
- Carnt, N.; Hoffman, J.M.; Verma, S.; Hau, S.; Radford, C.F.; Minassian, D.C.; Dart, J.K.G. Acanthamoeba keratitis: Confirmation of the UK outbreak and a prospective case-control study identifying contributing risk factors. Br. J. Ophthalmol. 2018, 102, 1621–1628. [Google Scholar] [CrossRef]
- Siddiqui, R.; Khan, N.A. Biology and pathogenesis of Acanthamoeba. Parasit. Vectors 2012, 5, 6. [Google Scholar] [CrossRef]
- List, W.; Glatz, W.; Riedl, R.; Mossboeck, G.; Steinwender, G.; Wedrich, A. Evaluation of Acanthamoeba keratitis cases in a tertiary medical care centre over 21 years. Sci. Rep. 2021, 11, 1036. [Google Scholar] [CrossRef]
- Lim, N.; Goh, D.; Bunce, C.; Xing, W.; Fraenkel, G.; Poole, T.R.; Ficker, L. Comparison of polyhexamethylene biguanide and chlorhexidine as monotherapy agents in the treatment of Acanthamoeba keratitis. Am. J. Ophthalmol. 2008, 145, 130–135. [Google Scholar] [CrossRef]
- Szentmary, N.; Daas, L.; Shi, L.; Laurik, K.L.; Lepper, S.; Milioti, G.; Seitz, B. Acanthamoeba keratitis—Clinical signs, differential diagnosis and treatment. J. Curr. Ophthalmol. 2019, 31, 16–23. [Google Scholar] [CrossRef]
- Carnt, N.; Robaei, D.; Minassian, D.C.; Dart, J.K.G. Acanthamoeba keratitis in 194 patients: Risk factors for bad outcomes and severe inflammatory complications. Br. J. Ophthalmol. 2018, 102, 1431–1435. [Google Scholar] [CrossRef]
- Carnt, N.; Stapleton, F. Strategies for the prevention of contact lens-related Acanthamoeba keratitis: A review. Ophthalmic Physiol. Opt. J. Br. Coll. Ophthalmic Opt. 2016, 36, 77–92. [Google Scholar] [CrossRef]
- Schuster, F.L.; Visvesvara, G.S. Opportunistic amoebae: Challenges in prophylaxis and treatment. Drug Resist. Updates 2004, 7, 41–51. [Google Scholar] [CrossRef]
- Vijay, A.K.; Bandara, M.; Zhu, H.; Willcox, M.D. Protamine as a potential amoebicidal agent for contact lens disinfection. Optom. Vis. Sci. 2013, 90, 119–124. [Google Scholar] [CrossRef]
- Bandara, M.K.; Masoudi, S.; Zhu, H.; Bandara, R.; Willcox, M.D. Evaluation of Protamine as a Disinfectant for Contact Lenses. Optom. Vis. Sci. 2016, 93, 1349–1355. [Google Scholar] [CrossRef]
- Dutta, D.; Cole, N.; Kumar, N.; Willcox, M.D. Broad spectrum antimicrobial activity of melimine covalently bound to contact lenses. Investig. Ophthalmol. Vis. Sci. 2013, 54, 175–182. [Google Scholar] [CrossRef]
- Willcox, M.D.; Hume, E.B.; Aliwarga, Y.; Kumar, N.; Cole, N. A novel cationic-peptide coating for the prevention of microbial colonization on contact lenses. J. Appl. Microbiol. 2008, 105, 1817–1825. [Google Scholar] [CrossRef]
- Elder, M.; Kilvington, S.; Dart, J. A clinicopathologic study of in vivo sensitivity testing and Acanthamoeba keratitis. Investig. Ophthalmol. Vis. Sci. 1994, 35, 1059–1064. [Google Scholar]
- Narasimhan, S.; Madhavan, H.N.; Therese, K.L. Development and application of an in vitro susceptibility test for Acanthamoeba species isolated from keratitis to polyhexamethylene biguanide and chlorhexidine. Cornea 2002, 21, 203–205. [Google Scholar] [CrossRef]
- Baig, A.M.; Iqbal, J.; Khan, N.A. In vitro efficacies of clinically available drugs against growth and viability of an Acanthamoeba castellanii keratitis isolate belonging to the T4 genotype. Antimicrob. Agents Chemother. 2013, 57, 3561–3567. [Google Scholar] [CrossRef]
- Anwar, A.; Chi Fung, L.; Anwar, A.; Jagadish, P.; Numan, A.; Khalid, M.; Shahabuddin, S.; Siddiqui, R.; Khan, N.A. Effects of Shape and Size of Cobalt Phosphate Nanoparticles against Acanthamoeba castellanii. Pathogens 2019, 8, 260. [Google Scholar] [CrossRef]
- Gomart, G.; Denis, J.; Bourcier, T.; Dory, A.; Abou-Bacar, A.; Candolfi, E.; Sauer, A. In Vitro Amoebicidal Activity of Titanium Dioxide/UV-A Combination Against Acanthamoeba. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4567–4571. [Google Scholar] [CrossRef]
- Aqeel, Y.; Siddiqui, R.; Iftikhar, H.; Khan, N.A. The effect of different environmental conditions on the encystation of Acanthamoeba castellanii belonging to the T4 genotype. Exp. Parasitol. 2013, 135, 30–35. [Google Scholar] [CrossRef]
- Kilvington, S.; Heaselgrave, W.; Lally, J.M.; Ambrus, K.; Powell, H. Encystment of Acanthamoeba during incubation in multipurpose contact lens disinfectant solutions and experimental formulations. Eye Contact Lens 2008, 34, 133–139. [Google Scholar] [CrossRef]
- Sabir, S.; Yu, T.T.; Kuppusamy, R.; Almohaywi, B.; Iskander, G.; Das, T.; Willcox, M.D.P.; Black, D.S.; Kumar, N. Novel Seleno- and Thio-Urea Containing Dihydropyrrol-2-One Analogues as Antibacterial Agents. Antibiotics 2021, 10, 321. [Google Scholar] [CrossRef]
- Yasir, M.; Dutta, D.; Willcox, M.D.P. Mode of action of the antimicrobial peptide Mel4 is independent of Staphylococcus aureus cell membrane permeability. PLoS ONE 2019, 14, e0215703. [Google Scholar] [CrossRef]
- Carvajal-Rondanelli, P.; Aróstica, M.; Marshall, S.H.; Albericio, F.; Álvarez, C.A.; Ojeda, C.; Aguilar, L.F.; Guzmán, F. Inhibitory effect of short cationic homopeptides against Gram-negative bacteria. Amino Acids 2016, 48, 1445–1456. [Google Scholar] [CrossRef]
- Redd, T.K.; Talbott, M.; Cevallos, V.; Lalitha, P.; Seitzman, G.D.; Lietman, T.M.; Keenan, J.D. In Vitro Comparison of the Acanthamoeba Cysticidal Activity of Povidone Iodine, Natamycin, and Chlorhexidine. Ophthalmol. Sci. 2021, 1, 100025. [Google Scholar] [CrossRef]
- Li, Q.; Xu, M.; Cui, Y.; Huang, C.; Sun, M. Arginine-rich membrane-permeable peptides are seriously toxic. Pharmacol. Res. Perspect. 2017, 5, e00334. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peguda, H.K.; Lakshminarayanan, R.; Carnt, N.A.; Gu, Z.; Willcox, M.D.P. The Activity of Polyhomoarginine against Acanthamoeba castellanii. Biology 2022, 11, 1726. https://doi.org/10.3390/biology11121726
Peguda HK, Lakshminarayanan R, Carnt NA, Gu Z, Willcox MDP. The Activity of Polyhomoarginine against Acanthamoeba castellanii. Biology. 2022; 11(12):1726. https://doi.org/10.3390/biology11121726
Chicago/Turabian StylePeguda, Hari Kumar, Rajamani Lakshminarayanan, Nicole A. Carnt, Zi Gu, and Mark D. P. Willcox. 2022. "The Activity of Polyhomoarginine against Acanthamoeba castellanii" Biology 11, no. 12: 1726. https://doi.org/10.3390/biology11121726
APA StylePeguda, H. K., Lakshminarayanan, R., Carnt, N. A., Gu, Z., & Willcox, M. D. P. (2022). The Activity of Polyhomoarginine against Acanthamoeba castellanii. Biology, 11(12), 1726. https://doi.org/10.3390/biology11121726








