Simple Summary
Flickering light is used in research in many different fields. Despite growing interest in the subject, there is still little known about its effects on the brain. The researchers used flickering light in different variations, so it is important to analyse how these modifications may affect the obtained results. This review relates to both neurophysiological and anatomical aspects of this topic, including processing visual stimuli in the brain, especially colour and motion. Since the results of flickering light-based tests (e.g., flicker test) have been linked to arousal levels in the literature, this review also describes this topic, along with attentional processes and detection of signals in the visual field.
Abstract
The aim of this review is to summarise current knowledge about flickering light and the underlying processes that occur during its processing in the brain. Despite the growing interest in the topic of flickering light, its clinical applications are still not well understood. Studies using EEG indicate an appearing synchronisation of brain wave frequencies with the frequency of flickering light, and hopefully, it could be used in memory therapy, among other applications. Some researchers have focused on using the flicker test as an indicator of arousal, which may be useful in clinical studies if the background for such a relationship is described. Since flicker testing has a risk of inducing epileptic seizures, however, every effort must be made to avoid high-risk combinations, which include, for example, red-blue light flashing at 15 Hz. Future research should focus on the usage of neuroimaging methods to describe the specific neuropsychological and neurophysiological processes occurring in the brain during the processing of flickering light so that its clinical utility can be preliminarily determined and randomised clinical trials can be initiated to test existing reports.
1. Introduction
In the last hundred years, flickering light has attracted increasingly more attention from researchers around the world. From 1933 to 2021, more than 6000 related articles appeared in the PubMed database alone. Critical flicker fusion frequency (CFF or CFFF), as a frequency at which flickering light stops being visible and starts being perceived as a steady light, has been used in various fields of research and various groups or species. Research has focused on dementia [1,2,3], visual impairment [4], cognitive functioning [5,6,7,8] and divers [9,10,11,12,13,14], among other topics.
Despite growing interest in the topic, the neurophysiological and neuropsychological mechanisms behind the processing of flickering light stimuli are still not fully understood. This review aims to integrate existing knowledge and determine a valid basis for the mentioned processes that occur during observing light and detecting its blinking or stability. This knowledge could contribute to the development of new clinical and research interventions and diagnostic procedures.
2. Flickering Light
The CFF threshold in humans is estimated to be 50–90 Hz [15,16], although some researchers have reported that distinguishing between flickering and steady light can also occur at higher frequencies, i.e., 500 Hz [17], which they explain by the emergence of the unconscious, rapid, saccadic eye movements that are made along the edges of the projected image. The results of previous studies are not consistent with each other, as some researchers have reported that eye movements can positively increase the frequency needed to perceive a stable image [18,19], while others reported no such regularity [15,20].
Distinguishing between flickering and steady light is dependent on the factors mentioned in the review article by Mankowska et al. [21]: (1) the frequency of modulation, (2) the amplitude of modulation, (3) the average illumination intensity, (4) the position on the retina at which the stimulus occurs, (5) the wavelength or colour of the light-emitting diode, (6) the intensity of ambient light, or (7) the viewing distance and (8) size of the stimulus. According to Bullough and colleagues [22], the combination of light flickering at 100 Hz or more with a stable, stationary background is associated with: (1) the perception of that light as a more constant and (2) less stroboscopic effect. However, there have been reports that a stroboscopic effect can occur even with the light flickering at more than 1000 Hz if objects in the rest of the visual field are moving [23,24].
Rider et al. [25] summarised results from research conducted over the last twenty years and concluded that CFF has limited value as a clinical measurement because while it contributes to a better understanding of the visual system’s overall gain, it does not provide much information on processing speed. The CFF is dependent on both gain and processing speed, so measurements need to take several intensity levels into account to acquire information about this dependence. It would be beneficial to follow damage in different parts of the visual pathway since it causes diverse reactions.
3. Visual System and Visual Information Processing
Flickering light is a visual stimulus of relatively high complexity, as part of its processing by the visual system, not only the size is analysed, but also the motion (flickering), colour, the size of the stimulus and spatial relations (the arrangement of the stimulus and whether a single element or a more complex arrangement, such as a checkerboard, is presented). Thus, it is necessary to understand how the visual system processes information of different types.
Light comes into the eye through the pupil, before being focused by the lens and cornea, through which it is projected onto the retina (Figure 1). The retina contains photoreceptors that convert photon energy into neuronal activity [26]. The left half of the retina processes information from the right visual field and the right from the left visual field. The image on the retina is upside down, just like in a camera. The retinas of vertebrates have two types of receptors, with about 5 million cones and 120 million rods [27]. Rods are responsible for scotopic (night) vision, while cones relate to photopic vision (when there is plenty of light) and play a significant role in colour perception. Both have visual pigments that emit energy when exposed to light. Rhodopsin is a photosensitive pigment of rods and absorbs wavelengths of about 400–600 nm to create action potential and transmit information from the retina to the brain. Cones have four types of pigments, absorbing short, medium and long wavelengths [27,28].
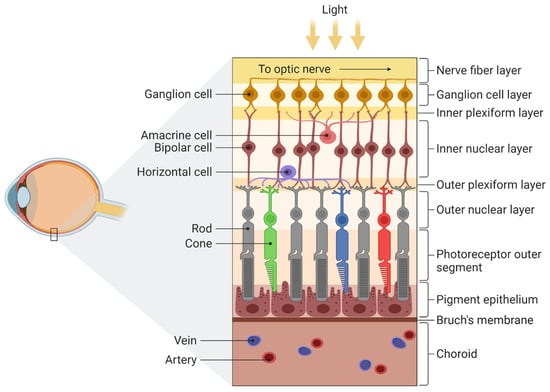
Figure 1.
Structure of the retina. There are four main layers: (1) pigment epithelium, (2) the light-sensitive cells such as rods and cones that transmit information to a layer of neurons called (3) bipolar cells which are connected to (4) ganglion cells, located directly under the optic nerve. For more detailed information see Mahabadi & Khalili [29]. Adapted from “Structure of the Retina”, by BioRender.com (2022). Retrieved from https://app.biorender.com/biorender-templates, accessed on 9 September 2022.
Information from the retina is transmitted to the visual cortex through the lateral geniculate nucleus, after receiving it from ganglion cells, which corresponds to a receptive field where the transmitted light causes a change during the electrical activity. There are three types of responses from ganglion cells: on, off and on-off. They show, respectively: (1) increased activity occurring only during light stimulation, (2) inhibited activity when the light occurs and acceleration after turning the light off and (3) increased activity immediately after turning the light on and off [30,31].
The most common types of ganglion cells are magno and parvo. Magno cells process information about luminance, motion, stereopsis and depth of objects since they receive information from all cones and can quickly conduct this information (about 40 m/s). Parvo cells are slower (20 m/s), more numerous than magno (80% of total compared to 10% of magno cells) and more sensitive to colours and stationary patterns such as the material and form of objects. This differentiation is reflected in the connection with visual information processing pathways: while information from magno cells is processed by the “where” pathway (dorsal stream), the “what” pathway (ventral stream) processes information from parvo cells [32]. Figure 2 shows a course of both pathways [33,34]. The V1–V6 indicates brain regions, as described in Table 1 and shown in Figure 3.
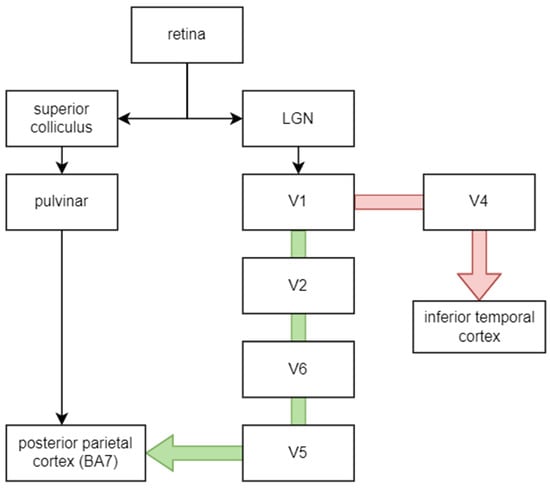
Figure 2.
The visual information pathway. The green arrow indicates the dorsal stream, while the red one indicates the ventral stream. LGN—lateral geniculate nucleus.

Table 1.
Brain regions associated with visual pathways, their location, function and the most common effects of damage [35,36,37,38].
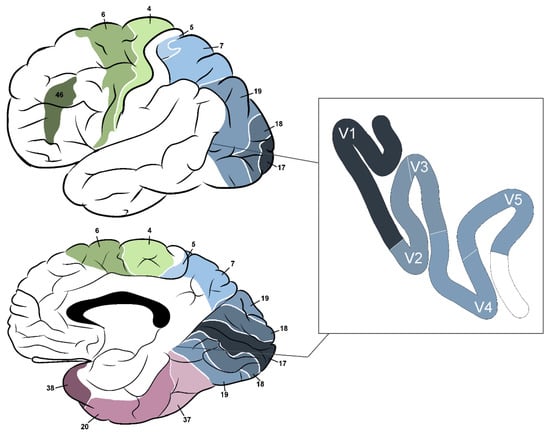
Figure 3.
Brodmann’s areas relevant to the visual pathway; 4—primary motor cortex; 5—superior parietal lobule; 6—premotor cortex and supplementary motor area; 7—medial part: precuneus, lateral part: superior parietal lobule; 17—primary visual cortex; 18, 19—secondary visual cortex; 20—inferior temporal gyrus; 37—fusiform gyrus; 38—temporopolar area; 46—dorsolateral prefrontal cortex.
Some research showed that reduced magnocellular sensitivity is associated with problems in rapid visual processing and thus with poor reading and dyslexia, while there is no such a correlation in parvocellular ganglion cells, in neither better nor poor readers [39,40]. This mechanism is probably associated with visual attention and eye fixation on words. As reported by Stein [40], reading in these individuals may be improved by boosting M-performance by using yellow filters or training eye fixation. Graves et al. [41] indicated that adults with reading disabilities had problems with localising small, briefly presented visual stimuli. Moreover, Peters and colleagues [42] found that dyslexic individuals compared to neurotypicals have a lower ability to detect flickering at high temporal frequencies, but they also emphasise that this difference is not sufficient to discriminate between groups.
Foxe et al. [43], in their experiment with the use of electroencephalography (EEG) and visual evoked potentials (VEPs), showed that the C1 component occurred in the central parieto-occipital area, which is only seen when parvo cells are excited (with chromatic isoluminant stimuli). It has been possible to extract other meaningful information from event-related potentials as well. Some researchers showed that the timing of display of a target stimulus among some non-target stimuli could be identified by a single trial [43,44]. MRI studies would be useful to explore this topic further.
Experiments with positron emission tomography (PET) scans revealed that the lateral part of Brodmann’s area (BA) 19 is responsible for movement perception in the contralateral visual hemifield. Area 7, more widely known as the posterior parietal cortex, receives main projections from area 19 and is involved in stereopsis (three-dimensional vision). This area is connected to the pulvinar and projects to the ipsilateral frontal eye field and premotor cortex (PMC) via the superior longitudinal fasciculus [36]. The electrical stimulation of BA 17, 18 and 19 may lead to the production of visual sensations (usually in the contralateral field), often described as flickering lights, stars, lines and spots, among others [45].
Berryhill and Olson [46] performed case-study experiments with two patients with bilateral parietal lobe damage (revealed in MRI) and showed that this part of the brain may be relevant to performing tasks using visual working memory, especially in the retrieval of this kind of material. These findings were replicated in a different group of seven patients with right parietal lobe damage, also confirmed by MRI [47]. They conducted a few experiments based on remembering items to compare performance in tasks with cued recall and old/new recognition. This choice was made after consideration of the research and review of Marshuetz and colleagues [48,49]; they found order working memory-related activity in functional MRI in bilateral parietal regions (BA 7 and 40) which was deemed important for mediation storage operations, e.g., tracking the temporal spacing between items.
Working memory, like some aspects of attention, is considered to belong to executive functions, which may be relevant in analysing the mechanisms of flickering light. The parietal cortex is thought to be activated in tasks requiring sequencing, especially motor sequencing. Gonzalez and Burke [50] examined 12 young participants with functional MRI and an eye tracker to observe brain activity during the visual learning of simple and more complex trajectories. They found the following: (1) the activation of memory and attention-related areas (BA10 and dorsolateral prefrontal cortex) during the shorter sequences and (2) activation within areas that corresponded to the pre-motor (BA6) and motor (BA4) cortex during longer sequences as an effect of movement planning and the preparation of movement. In the comparison of both short and long tasks, there were no differences within the parietal cortex; however, the higher activation was observed in BA40 for predictive versus random conditions.
4. Colour Vision
In the literature, flickering stimuli are presented in different colours. As is known, particular rods and cones process slightly different wavelengths of visible light. Interactions between different colours are important, among other things, by their ability to induce epileptic seizures, as described in Section 10.
Humans can perceive light with a wavelength of approximately 350–700 nm [51]. The shortest wavelengths are responsible for the impression of the violet colour and the longest of red (see Figure 4). Perception relies on the activity of multiple neurons because any one of them can simultaneously transmit information about brightness and colour [34].
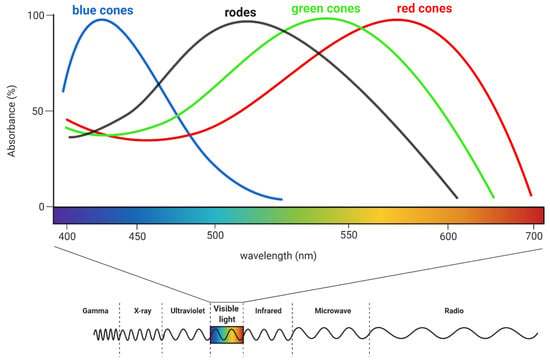
Figure 4.
The sensitivity of rods and cones on different light wavelengths. Humans can perceive electromagnetic waves with a length of about 350–700 nm. For comparison, gamma-ray waves have a length less than 10−12 m, while radio waves are longer than 1 mm. Created with BioRender.com.
5. Motion Processing
As mentioned above, the brain structures responsible for analysing motion are also involved in processing flickering stimuli. The most important area associated with the perception of motion stimuli, as described by researchers so far, is area V5 (medial temporal, MT) [52]. Researchers used various neuroimaging and neurostimulation techniques such as PET [53], MRI [54], functional MRI [55,56], transcranial magnetic stimulation (TMS) [57] and magnetoencephalography (MEG). The latter two were used to describe its localisation more specifically: “human V5 is located near the occipito-temporal border in a minor sulcus immediately below the superior temporal sulcus” [54]. Damage to this area results in akinetopsia, firstly described by Zihl et al. [58] and confirmed by Shipp et al. [59] using MRI.
Area V5 is divided into two subdivisions which allow primates to (1) perceive the motion of objects and (2) analyse object images after making a head movement [60]. Ashida and Osaka [61] described motion aftereffect (MAE) which occurs after gazing at a stimulus moving in some direction for a long period of time; a later viewed stationary scene seems to move then in the opposite direction. There are two types of MAE: (1) static, where the maximum aftereffect is reached when the spatial frequency of both adapting and test stimuli are equals because of reflection of Fourier power and (2) flicker, which follows the pattern direction. Researchers concluded that flicker MAE is probably more dependent on velocity than on temporal frequency, unlike static MAE, which shows spatial frequency selectivity. It is highly possible that these types of MAE are processed in different stages of the visual pathway; while static is analysed in V1, flicker MAE needs the integration of more information entering V5.
6. Flickering Light in Neuroimaging Studies
EEG is a non-invasive method of studying the bioelectrical activity of the brain that has been used successfully in clinical studies of patients with epilepsy, sleep disorders and other neurological problems, diseases and psychiatric disorders, among others [62]. It is also useful in studies of healthy subjects, for example, to observe brain activity at rest or when focusing attention on cognitively demanding tasks. Each state of brain activity is reflected in different frequencies of brain waves. The electrodes used in research vary in number, depending on how accurate the information needed is.
Human visual perception tends to change in synchronisation with neural oscillations, especially in the delta, theta and alpha frequencies in the EEG. Some researchers focus on alpha oscillations, as variations in individual alpha frequency are the most prominent and correlate with discrimination between stimuli presented in the same location [63]. For example, Spaak et al. [64] showed that 10 Hz flicker caused changes in entraining that persisted for approximately 300 ms after ceasing stimulus observation. Gray and Emmanouil [63] tested whether rhythmic visual stimulation at lower and higher alpha frequencies (8.3 and 12.5 Hz) may have an impact on differentiating one from two flashes. They found that individual alpha frequency only reflects neural processes which are independent of environmental factors and regulate perception internally, but they emphasised that further research is needed to unambiguously explain this phenomenon.
According to Herrmann [65], external stimulation can drive the visual cortex up to 50 Hz. Moreover, in the steady state VEP (SSVEP), the resonance phenomena are visible, which indicates a stronger response on some frequencies (10, 20, 40 and 80 Hz), probably because of the preferred oscillation frequency, resulting from axonal connections between neurons in a specific system. In research with patients with multiple sclerosis, Salmi [66] found that the flicker test may have additional diagnostic value in optic neuritis compared to VEP from O1 and O2 electrodes. Whether there were differences in the CFF threshold between both patients’ eyes was examined. Interocular discrepancies were taken as an indicator of abnormal functioning of the visual pathway in some optic neuritis cases. The abnormal CFF threshold was found in 46% of the group of sixty-three patients and it was significantly lower than in the controls, which is consistent with previous research in larger groups of patients (48–78% of abnormal results, depending on the method used) [67]. For comparison, VEP values were pathological in 69% of cases, compared to previous results—in 81–95% of definite and 26–50% of possible cases of multiple sclerosis [68,69,70].
Another helpful method for studying brain activity is functional magnetic resonance imaging (fMRI), which uses analysis of the level of oxygen consumption by brain tissue. Since its inception in the 1990s, several hundred thousand scientific papers have been written, primarily in neuroscience and psychiatry. Its applications are wide-ranging and include, among others, presurgical planning, monitoring the effectiveness of therapy, or comparing patterns of brain activity in different clinical groups [71]. In addition, it allows the mapping of brain activity and interactions between activated areas, which in research on flickering light makes it possible to unveil structures involved in its processing.
In 2006, Carmel and colleagues analysed brain activity when observing a flickering point of light, as examined by fMRI [72]. They noted increased activity in the cortex of both frontal lobes and the left parietal cortex. These areas have previously been reported to be associated with awareness during non-temporal tasks (with the perception of intervals), but the results of the mentioned study suggest that they may nevertheless play a role in these types of tasks as well. These results are consistent with theories of visual awareness that arise from the interplay of multiple networks within the brain. However, whether frontoparietal areas play a role in the detection of flickering or whether they are activated by the performance of the flicker detection task itself cannot be determined from fMRI studies.
In a functional MRI study, Zafiris and colleagues [73] analysed neuronal mechanisms of processing flickering light in patients with liver cirrhosis. They used part of the computerised neuropsychological test battery (Vienna Test System) and the range of flicker frequency used in their paradigm was 25–50 Hz, slowly decreasing from higher values by 0.5 Hz. They tested 9 patients and 10 control subjects and found activation differences in the right inferior parietal cortex, the parietooccipital cortex (cuneus), the anterior cingulate cortex, the intraparietal sulcus, the medial temporal lobe, the thalamus, prefrontal polar cortex and striate and extrastriate visual cortices. Moreover, the functional MRI signal in patients was reduced in the inferior parietal cortex compared to healthy controls, which may indicate the importance of this area for visual attention. Interestingly, this finding is correlated with enhanced signals in the temporal pole (see Table 1 for its functions in visual processing), probably as the compensatory mechanism. As researchers emphasised, according to Butterworth [74], chronic liver failure may result in the production of brain cells of Alzheimer’s type II; thus, a better understanding of these matters would benefit researchers, clinicians and, most importantly, patients.
In their research with a comparison of EEG and functional MRI responses, Mullinger et al. [75] recorded data from 17 subjects (mean age: 26 years) and used a black-white checkerboard in two conditions: static and flickering at 3 Hz (in functional MRI). The aim of their study was to differentiate whether weakening of the functional MRI signal (in response to 8 Hz flicker) after the cessation of stimulus observation is due to neuronal or vascular changes. Their results provide evidence that the origin of this phase response is neuronal as the amplitudes of functional MRI signal and cerebral blood flow signals were dependent on the post-stimulus power of the occipital alpha EEG neuronal activity. When the EEG powers were the highest, the lowest activity in functional MRI was observed in the contralateral visual cortex. Mullinger and colleagues pointed out that a more accurate analysis could contribute to a better understanding of cognitive dysfunctions and neurological diseases. There is limited evidence that the visual cortex responds to a flickering stimulus, not only when the flickering is perceivable, but also when the stimulus seem to be steady. Few healthy subjects participated in the study by Jiang et al. [76] and observed a 5 and 30 Hz full contrast chromatic flicker. When they perceived light flickering at lower frequencies, they showed a large functional MRI signal in the visual cortex which also persisted at higher stimulus frequencies.
To the best of our knowledge, there is a lack of studies comprehensively describing the neuropsychological and neurophysiological mechanisms of flickering light processing in the brain. Mentioned studies showed that processing this type of stimuli is complex and involves many brain regions—not only the visual cortex is involved in processing flickering light, but also frontal areas associated with attention and decision-making processes. Examples of conducted studies show that flickering light can have diagnostic and therapeutic potential, but at this point, there is no robust explanation of the principles of these interactions.
7. Visual Attention
When two stimuli are presented concurrently, competition between them appears. It is a mechanism of selective attention, described by Desimone and Duncan [77] as a “biased competition model” which assumes that these stimuli would suppress the response from neurons; this was confirmed by Fuchs et al. [78] when they showed that SSVEP amplitude decreased in these conditions. The amplitude of SSVEPs may be increased by paying attention to flickering stimuli [79,80,81]. Any flicker frequency is responsible for activating different cortical networks that are responding to specific frequencies, for example, flickering at lower frequencies (to 10 Hz) activates a global cortical network that is distinguishable from other regions [80,81].
To investigate these competitive interactions and attention, the frequency-tagging method might be used. Its main concept is that different frequencies are assigned to each stimulus to elicit SSVEP in EEG. As the SSVEP amplitude changes in response to the perceived frequency, it is possible to separate them in analysis [82].
De Lissa et al. [83] used the frequency tagging method to assess visual attention in the smooth-pursuit paradigm, where 17 participants had to follow a moving target and react when it reached the goal area (1st paradigm) or follow this target and switch their attention when the second one, flickering at 30 Hz, occurs and follow it until it reaches the goal area (2nd paradigm). Researchers found that SSVEP power decreased early and rapidly when the individuals had to divide their attention. These findings are consistent with Kahnemann’s and Lavie’s theories about shared attentional resources for covert and overt attention [84,85], which shows that not only the current task is relevant but also surroundings to which, as needed, attention resources are shifting. In a laboratory environment, where task performance is strictly controlled by researchers, this naturally has implications for the ability to compare patterns of neuronal activity. In everyday life, when a variety of stimuli arrive, affecting different modalities, these relationships become less obvious. It is therefore important to consider how performance is affected without this control.
8. Psychophysics of Stimuli Detection
This section attempts to describe how various stimuli (especially flickering light), more or less relevant to the individual’s current expectations, are processed by this individual. Basically, the perception of flickering light can be considered in the context of bottom-up and top-down processing theories. In bottom-up processing, incoming stimulus information comes from sensory data and is transferred to the higher levels of the brain for further analysis. Top-down processing is reliant on an individual’s knowledge and prior experiences to analyse current stimuli. Currently, research shows that both processes support orienting to stimuli and the human dorsal frontoparietal network is activated in its searching and detecting. It interacts with the extrastriate cortex and the brain activity occurs as soon as the search field is seen and persists until the target is detected, which was confirmed with neuroimaging methods. The ventral parietal network might be activated in tasks with the detection of low-frequency stimuli at an expected location or when the stimuli were fixed at the centre of gaze (see review by Corbetta & Shulman [86]). These authors summarised the areas of the brain that are involved in performing tasks of the first kind, which are the right temporoparietal junction and ventral frontal cortex (including the inferior frontal gyrus, middle frontal gyrus and frontal operculum). It is worth noting that lateralisation to the right hemisphere was observed. Referring to the mentioned review, it could therefore be hypothesized that similar brain regions will show their activity when observing a flickering light and when making decisions about its properties.
According to signal detection theory (or sensory decision theory), making statements about perception is based on two parameters: the level of observer’s sensitivity and the process of decision making [87]. Any sensory message can be described in two parts: signal and noise. The signal is a stimulus that one attempts to detect and the noise is also known as a distractor, which may be a physiological activity, thought, requirement of the experimenter, noise, etc. When an observer must decide whether the stimulus is perceived or not, any response can be assigned to one of four categories: hit, miss, false alarm or correct rejection (Table 2). The number of responses depends on the observer’s decision-making style—conservative ones would commit fewer false alarms and hits compared to lax observers [27].

Table 2.
The four typical situations of the signal detection theory.
When observers must decide whether stimuli are different from each other, another sensory ability may be seen. The minimum intensity difference required for differentiating two stimuli is called the difference threshold [27]. The value of the threshold can be estimated by many methods, but the most reasonable and common [66,88,89,90] in the flicker test seems to be the method of limits. This method is based on the presentation of two series of stimuli: ascending and descending (in flicker test it would be manipulating of frequency value). This strategy allows problems associated with anticipation and habituation errors to be excluded. As Grondin [27] indicates, responses in each sample may vary considerably, so it is necessary to calculate average response values over all samples to estimate the absolute threshold. Thus, based on theoretical models, it seems that the usage of the limit method is reasonable and could allow for a smaller margin of error in flickering light judgement.
Interpretation of tasks using flickering light depends on the protocol used. Flickering stimuli appear in scientific research in different variants—from simpler ones (e.g., a flickering dot on a uniform background or accompanied by a stable reference dot) to more complex ones (e.g., flickering checkerboards). In signal detection theory terms, this complexity (i.e., usually more noise) can translate into difficulties in the decision-making process about signal properties. Therefore, it is essential to remember that comparing brain activity in response to the processing of these different stimuli should be done with extreme caution in concluding.
9. Does the Flicker Test Examine Arousal?
There are a number of studies using the flicker test and neuropsychological tests that seem to indicate a connection between the flicker test and arousal [21]. However, comparing CFF and psychological test results does not provide insight into the underlying processes behind these associations.
In previous studies, the flicker test has been used to assess arousal status and visual processing. These aspects have been studied most often using EEG [63,91,92,93,94,95] and less frequently, functional fMRI [73,76,96] or no method other than the flicker test [11,97].
The validation for using the flicker test to assess alertness and arousal is provided, among others, in a study of 10 patients with narcolepsy and 10 age- and sex-matched volunteers conducted by German researchers [97]. The mean age of subjects was 42 years and they performed a test every 15 min for 10 h; narcoleptic patients had higher variability in the level of task performance throughout the day compared to the control group, suggesting that their alertness fluctuated over time, although the CFF threshold differences were not significant between the two groups.
A study by Ronzhina and colleagues [98] assessed whether simply performing a flicker test affects an individual’s arousal state. The EEG electrodes were arranged according to the international 10–20 system, but the analysis used recordings from three electrodes (Pz, O1, O2) because they did not show blinking artifacts and because they clearly depict alpha wave activity (8–12 Hz), which is characteristic of a relaxed state. The authors reported that performing the flicker test did not affect participant arousal or alertness levels, but it is important to note that the study included only seven participants after sleep deprivation (they slept for 4 h).
Lecca et al. [99] conducted a study in which they assessed the mental fatigue of 30 professional drivers caused by driving a bus. The study collected information from the flicker test and heart rate variability (HRV), among other things. Their results showed a slight decrease in CFF at the end of a 6 h drive only in the condition of assessing the increasing frequency of flickering light (p = 0.041). They also found that heart rate decreases over a few hours of driving (in comparison of initial, central and final values).
Thus, the results of the presented research so far are inconclusive and it is hard to answer the question presented in the title of subsection. Although the flicker test is used in studies as a method of assessing arousal, for now it does not appear to be a sensitive and robust method. It would be beneficial to conduct further research to evaluate the use of this test in research on this topic. It is necessary to find out what relationships connect the CFF with other psychometric tests and physiological factors so it can be considered a reliable tool. The purpose of this work was to summarize the current knowledge of flickering light and its effects on the brain, so given the question posed in this section, it is necessary to consider what brain and cognitive processes could reflect the possibilities in the perception of the changing properties of flickering light.
10. Flickering Light, Headaches and Epilepsy
Occasionally, light from lamps flickering at a high frequency, i.e., 100–120 Hz, can induce headaches or migraines [100]. Discomfort from visual strain can also result from observing specific stimuli, such as stripes [101,102] or blurred images [103]. It is worth noting that these stimuli elicit a discomfort response often related to the subject’s state (stress level, cognitive functioning, fatigue, light sensitivity, etc.). According to a study by Yoshimoto et al. [104], strong subjective discomfort is caused by light that has excessively strong contrast and flashes at a medium frequency (around 15 Hz), which confirms a previous study published by Lin et al. [105].
Watching flickering lights can induce epileptic seizures, particularly in individuals who have photogenic epilepsy. However, photoparoxysmal response (PPR) can also be induced in people who have never experienced them before. In the literature, cases of epileptic seizures have been reported in children while watching an episode of the television show Pokémon in which an intense blue-red light flashed at a frequency of 15 Hz [106,107]. Later studies confirmed this was due to stimulation of the most numerous, red cones in the retina [108], so the red light, with a wavelength of about 700 nm, might be the most harmful in these conditions [109]. This relationship is not clear, because Binnie and collaborators [110] showed that some individuals might respond similarly to green light if they had greater sensitivity to green cones than to red cones. Furthermore, patients who had seizures during the colour stimulation also had them during stimulation with a black and white pattern and the number of PPRs was greater than in patients who did not respond to any of these, which was probably a sign of increased photosensitivity [111].
The onset of PPRs is derived from the orientation of stimulus patterns, which is due to the activity of neurons of the visual cortex [102]. Therefore, some people might be more or less sensitive to particular directions [111] although this sensitivity may change over time [112]. Based on their research, Parra and colleagues [111] concluded that the photosensitivity is larger during one-colour stimulation compared to alternating colours stimulation; this is because of the different response mechanisms in visual system pathways: (1) magnocellular responses rely on luminance and high frequencies, (2) parvocellular are more sensitive to longer wavelengths (red-green) and (3) koniocellular to blue-yellow as they have shorter wavelengths. Consequently, the Pokémon incident and its confirmation in research suggest that a red-blue alternating pattern at 15 Hz involves more neurons and cortical space compared to a white/single-color stimulus [111].
To minimise the risk of inducing seizures in people who have never had them before, experiments using potential risk factors should consider the findings of Parra and colleagues [111]. They noted that white lights flashing at higher frequencies (above 20 Hz) can induce epileptic seizures, but that for coloured stimuli (e.g., blue and red) the risk is greatest at frequencies around 15 Hz (see Table 3 for summary). Among the coloured stimuli, it appears that the combination of blue and green is the safest (they elicited the fewest epileptiform responses). Moving forward, Takano and his team [113,114] presented results indicating that participants observing blue-green light experienced less discomfort and performed better than those observing traditional white-grey matrices.

Table 3.
The effect of flickering light of different colours on the evoking of epileptic seizures. Adapted from: Parra et al. [111].
The phenomena and relationships described above should guide future research. First, it is worth monitoring the level of the mentioned discomfort and, on this basis, consider planning possible breaks—so its high level does not significantly affect the obtained results. Second, for ethical reasons, one should design experiments to minimize the risk of inducing epileptic seizures. Third, the mentioned studies indicate that the colours of the stimuli used in flickering light studies affect elements of the visual system differently. Therefore, comparing different experimental designs may have limited relevance.
11. Future Directions
To the best of our knowledge, no review has yet been written that summarises the neurophysiological and neuropsychological mechanisms underlying the visual processing of flickering light. Therefore, we have brought together the available information and research findings in this review, which may contribute to the implementation of new interventions among patients from different clinical groups.
Current, but rather limited, knowledge suggests that the processing of flickering light is complex and involves numerous brain regions. However, none of the studies we found explicitly describes the mentioned mechanisms with the step-by-step tracing of the activity of different brain regions. There are not many studies that have combined the analysis of flickering light in EEG and functional MRI and such analysis from different neuroimaging methods could provide more reliable, accurate data.
Future research could also explore the use of flickering light in new technologies. One of them is the brain–computer interface (BCI), which allows neurophysiological signals to be used to control external devices or computers. In past studies, BCI has been tested among patients with amyotrophic lateral sclerosis and cervical spinal cord injury [115,116,117,118,119]. Developing this knowledge could allow the development of forms of examination and support for people with disabilities, particularly those with mobility and communication problems for whom limited options are using standard methods.
In 2021, Norton et al. [120] described BCI-based method for assessing colour vision which is independent from subject’s active participation so it could be used among people who may have difficulty responding, e.g., children or people with cognitive and motor impairments. This method uses SSVEP to the identification of metamers by two flickering light sources. Moreover, they suggest the application potential of this method in the industry. Another promising result of the experiments carried out is that the mentioned method may allow differentiating people with and without colour vision deficits, which, however, needs to be confirmed by further studies using other methods, i.e., anomaloscope. The usage of this method can also contribute to understanding the neural basis of colour perception and to designing therapeutic interventions for people with colour perception disorders.
Combining flickering light with virtual reality would also be an interesting research direction. Recently, a study by Moncada et al. [121] was published in which VR with flickering light (1–50 Hz) was used to identify PPR and photic-driving responses in healthy subjects and light-sensitive subjects. Extending this research into clinical trials among a larger group of patients would be beneficial from the perspective of detecting and treating neurological diseases.
12. Conclusions
This review brings together information on flickering light, its features processed by the visual system, and its possible applications in neuroimaging and clinical studies. Information on visual stimulus processing in general, as well as its colour and motion aspects are also included. All information has been analysed from neurophysiological and neuropsychological perspectives.
To summarize the existing knowledge on the described topic: (1) it is known that flickering light is a complex stimulus, processed by many brain structures that work closely together, (2) there are attempts to use flickering light in the assessment of arousal, (3) flickering stimuli can induce epileptic seizures, depending on their colour and flickering frequency, (4) flickering light can cause discomfort and headaches, (5) processing of information about flickering light is dependent on attentional resources and (6) comparative studies using neuroimaging methods are needed to further verify the described information.
Author Contributions
Conceptualization, N.D.M. and P.J.W.; Methodology, N.D.M.; Validation, M.G. and A.B.M.; Formal Analysis, N.D.M., M.G. and A.B.M.; Investigation, N.D.M.; Resources, M.G. and P.J.W.; Writing—Original Draft Preparation, N.D.M.; Writing—Review and Editing, N.D.M., M.G., P.J.W. and A.B.M.; Visualization, N.D.M.; Supervision, M.G., P.J.W. and A.B.M.; Project Administration, N.D.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abiyev, A.; Yakaryılmaz, F.D.; Öztürk, Z.A. A New Diagnostic Approach in Alzheimer’s Disease: The Critical Flicker Fusion Threshold. Dement. Neuropsychol. 2022, 16, 89–96. [Google Scholar] [CrossRef]
- Curran, S.; Wilson, S.; Musa, S.; Wattis, J. Critical Flicker Fusion Threshold in Patients with Alzheimer’s Disease and Vascular Dementia. Int. J. Geriatr. Psychiatry 2004, 19, 575–581. [Google Scholar] [CrossRef]
- Park, S.-S.; Park, H.-S.; Kim, C.-J.; Baek, S.-S.; Park, S.-Y.; Anderson, C.; Kim, M.-K.; Park, I.-R.; Kim, T.-W. Combined Effects of Aerobic Exercise and 40-Hz Light Flicker Exposure on Early Cognitive Impairments in Alzheimer’s Disease of 3×Tg Mice. J. Appl. Physiol. 2022, 132, 1054–1068. [Google Scholar] [CrossRef]
- Shankar, H.; Pesudovs, K. Critical Flicker Fusion Test of Potential Vision. J. Cataract Refract. Surg. 2007, 33, 232–239. [Google Scholar] [CrossRef]
- Casey, B.J.; Tottenham, N.; Liston, C.; Durston, S. Imaging the Developing Brain: What Have We Learned about Cognitive Development? Trends Cogn. Sci. 2005, 9, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Mewborn, C.; Renzi, L.M.; Hammond, B.R.; Stephen Miller, L. Critical Flicker Fusion Predicts Executive Function in Younger and Older Adults. Arch. Clin. Neuropsychol. 2015, 30, 605–610. [Google Scholar] [CrossRef][Green Version]
- Prabu Kumar, A.; Omprakash, A.; Kuppusamy, M.; Maruthy, K.N.; Sathiyasekaran, B.W.C.; Vijayaraghavan, P.V.; Padmavathi, R. How Does Cognitive Function Measured by the Reaction Time and Critical Flicker Fusion Frequency Correlate with the Academic Performance of Students? BMC Med. Educ. 2020, 20, 507. [Google Scholar] [CrossRef]
- Saint, S.E.; Hammond, B.R.; O’Brien, K.J.; Frick, J.E. Developmental Trends in Infant Temporal Processing Speed. Vis. Res. 2017, 138, 71–77. [Google Scholar] [CrossRef]
- Ardestani, B.S.; Balestra, C.; Bouzinova, E.V.; Loennechen, Ø.; Pedersen, M.; Berenji Ardestani, S.; Balestra, C.; Bouzinova, E.V.; Loennechen, Ø.; Pedersen, M.; et al. Evaluation of Divers’ Neuropsychometric Effectiveness and High-Pressure Neurological Syndrome via Computerized Test Battery Package and Questionnaires in Operational Setting. Front. Physiol. 2019, 10, 1386. [Google Scholar] [CrossRef]
- Balestra, C.; Lafere, P.; Germonpre, P. Persistence of Critical Flicker Fusion Frequency Impairment after a 33 Mfw SCUBA Dive: Evidence of Prolonged Nitrogen Narcosis? Eur. J. Appl. Physiol. 2012, 112, 4063–4068. [Google Scholar] [CrossRef][Green Version]
- Balestra, C.; Machado, M.-L.L.; Theunissen, S.; Balestra, A.; Cialoni, D.; Clot, C.; Besnard, S.; Kammacher, L.; Delzenne, J.; Germonpré, P.; et al. Critical Flicker Fusion Frequency: A Marker of Cerebral Arousal during Modified Gravitational Conditions Related to Parabolic Flights. Front. Physiol. 2018, 9, 1403. [Google Scholar] [CrossRef] [PubMed]
- Lafère, P.; Balestra, C.; Hemelryck, W.; Donda, N.; Sakr, A.; Taher, A.; Marroni, S.; Germonpré, P. Evaluation of Critical Flicker Fusion Frequency and Perceived Fatigue in Divers after Air and Enriched Air Nitrox Diving. Diving Hyperb. Med. 2010, 40, 114–118. [Google Scholar]
- Piispanen, W.W.; Lundell, R.V.; Tuominen, L.J.; Räisänen-Sokolowski, A.K. Assessment of Alertness and Cognitive Performance of Closed Circuit Rebreather Divers With the Critical Flicker Fusion Frequency Test in Arctic Diving Conditions. Front. Physiol. 2021, 12, 722915. [Google Scholar] [CrossRef] [PubMed]
- Vrijdag, X.C.E.; van Waart, H.; Sleigh, J.W.; Balestra, C.; Mitchell, S.J. Investigating Critical Flicker Fusion Frequency for Monitoring Gas Narcosis in Divers. Diving Hyperb. Med. 2020, 50, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.M.; Karasev, V.I.; Banks, M.S. Temporal Presentation Protocols in Stereoscopic Displays: Flicker Visibility, Perceived Motion, and Perceived Depth. J. Soc. Inf. Disp. 2011, 19, 271–297. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.L. Display Interfaces: Fundamentals and Standards; John Wiley & Sons: Chichester, UK, 2002; ISBN 0-471-49946-3. [Google Scholar]
- Davis, J.; Hsieh, Y.-H.H.; Lee, H.-C.C. Humans Perceive Flicker Artifacts at 500 Hz. Sci. Rep. 2015, 5, 7861. [Google Scholar] [CrossRef] [PubMed]
- Deubel, H.; Elsner, T. Threshold Perception and Saccadic Eye Movements. Biol. Cybern. 1986, 54, 351–358. [Google Scholar] [CrossRef]
- Watson, A.B. High Frame Rates and Human Vision: A View through the Window of Visibility. SMPTE Motion Imaging J. 2013, 122, 18–32. [Google Scholar] [CrossRef]
- Baccino, T.; Jaschinski, W.; Bussolon, J. The Influence of Bright Background Flicker during Different Saccade Periods on Saccadic Performance. Vis. Res. 2001, 41, 3909–3916. [Google Scholar] [CrossRef]
- Mankowska, N.D.; Marcinkowska, A.B.; Waskow, M.; Sharma, R.I.; Kot, J.; Winklewski, P.J. Critical Flicker Fusion Frequency: A Narrative Review. Medicina 2021, 57, 1096. [Google Scholar] [CrossRef]
- Bullough, J.D.; Sweater Hickcox, K.; Klein, T.R.; Narendran, N. Effects of Flicker Characteristics from Solid-State Lighting on Detection, Acceptability and Comfort. Light. Res. Technol. 2011, 43, 337–348. [Google Scholar] [CrossRef]
- Bullough, J.D.; Hickcox, K.S.; Klein, T.R.; Lok, A.; Narendran, N. Detection and Acceptability of Stroboscopic Effects from Flicker. Light. Res. Technol. 2012, 44, 477–483. [Google Scholar] [CrossRef]
- Roberts, J.E.; Wilkins, A.J. Flicker Can Be Perceived during Saccades at Frequencies in Excess of 1 KHz. Light. Res. Technol. 2013, 45, 124–132. [Google Scholar] [CrossRef]
- Rider, A.T.; Henning, G.B.; Stockman, A. A Reinterpretation of Critical Flicker-Frequency (CFF) Data Reveals Key Details about Light Adaptation and Normal and Abnormal Visual Processing. Prog. Retin. Eye Res. 2022, 87, 101001. [Google Scholar] [CrossRef]
- Donner, K. Temporal Vision: Measures, Mechanisms and Meaning. J. Exp. Biol. 2021, 224, jeb222679. [Google Scholar] [CrossRef] [PubMed]
- Grondin, S. Psychology of Perception; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 9783319317915. [Google Scholar]
- Imamoto, Y.; Shichida, Y. Cone Visual Pigments. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2014, 1837, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Mahabadi, N.; Al Khalili, Y. Neuroanatomy, Retina; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Hartline, H.K. The Receptive Fields of Optic Nerve Fibers. Am. J. Physiol. 1940, 130, 690–699. [Google Scholar] [CrossRef]
- Kuffler, S.W. Discharge Patterns and Functional Organization of Mammalian Retina. J. Neurophysiol. 1953, 16, 37–68. [Google Scholar] [CrossRef]
- Callaway, E.M. Structure and Function of Parallel Pathways in the Primate Early Visual System. J. Physiol. 2005, 566, 13–19. [Google Scholar] [CrossRef]
- Goodale, M.A.; Milner, A.D. Separate Visual Pathways for Perception and Action. Trends Neurosci. 1992, 15, 20–25. [Google Scholar] [CrossRef]
- Snowden, R.; Snowden, R.J.; Thompson, P.; Troscianko, T. Basic Vision: An Introduction to Visual Perception; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Bernal, B.; Perdomo, J. Brodmann’s Interactive Atlas 1. Available online: https://www.fmriconsulting.com/brodmann/Introduction.html (accessed on 12 April 2022).
- Mtui, E.; Gruener, G.; Dockery, P. Fitzgerald’s Clinical Neuroanatomy and Neuroscience, 7th ed.; Elsevier Health Sciences: Philadelphia, PA, USA, 2015. [Google Scholar]
- Felten, D.L.; O’Banion, M.K.; Maida, M.S. Netter’s Atlas of Neuroscience, 4th ed.; Elsevier Health Sciences: Philadelphia, PA, USA, 2021; ISBN 978-0-323-75654-9. [Google Scholar]
- Khan, A.Z.; Pisella, L.; Vighetto, A.; Cotton, F.; Luauté, J.; Boisson, D.; Salemme, R.; Crawford, J.D.; Rossetti, Y. Optic Ataxia Errors Depend on Remapped, Not Viewed, Target Location. Nat. Neurosci. 2005, 8, 418–420. [Google Scholar] [CrossRef] [PubMed]
- Brannan, J.R.; Williams, M.C. The Effects of Age and Reading Ability on Flicker Threshold. Clin. Vis. Sci. 1988, 3, 137–142. [Google Scholar]
- Stein, J. Visual Motion Sensitivity and Reading. Neuropsychologia 2003, 41, 1785–1793. [Google Scholar] [CrossRef]
- Graves, R.E.; Frerichs, R.J.; Cook, J.A. Visual Localization in Dyslexia. Neuropsychology 1999, 13, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.L.; Bavin, E.L.; Brown, A.; Crewther, D.P.; Crewther, S.G. Flicker Fusion Thresholds as a Clinical Identifier of a Magnocellular-Deficit Dyslexic Subgroup. Sci. Rep. 2020, 10, 21638. [Google Scholar] [CrossRef]
- Foxe, J.J.; Strugstad, E.C.; Sehatpour, P.; Molholm, S.; Pasieka, W.; Schroeder, C.E.; McCourt, M.E. Parvocellular and Magnocellular Contributions to the Initial Generators of the Visual Evoked Potential: High-Density Electrical Mapping of the “C1” Component. Brain Topogr. 2008, 21, 11–21. [Google Scholar] [CrossRef]
- Luo, A.; Sajda, P. Using Single-Trial EEG to Estimate the Timing of Target Onset during Rapid Serial Visual Presentation. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006. [Google Scholar]
- Pollen, D.A. Some Perceptual Effects of Electrical Stimulation of the Visual Cortex in Man. Nerv. Syst. 1975, 2, 519–528. [Google Scholar]
- Berryhill, M.E.; Olson, I.R. Is the Posterior Parietal Lobe Involved in Working Memory Retrieval? Evidence from Patients with Bilateral Parietal Lobe Damage. Neuropsychologia 2008, 46, 1775–1786. [Google Scholar] [CrossRef]
- Berryhill, M.E.; Olson, I.R. The Right Parietal Lobe Is Critical for Visual Working Memory. Neuropsychologia 2008, 46, 1767–1774. [Google Scholar] [CrossRef]
- Marshuetz, C. Order Information in Working Memory: An Integrative Review of Evidence from Brain and Behavior. Psychol. Bull. 2005, 131, 323–339. [Google Scholar] [CrossRef]
- Marshuetz, C.; Smith, E.E.; Jonides, J.; DeGutis, J.; Chenevert, T.L. Order Information in Working Memory: FMRI Evidence for Parietal and Prefrontal Mechanisms. J. Cogn. Neurosci. 2000, 12, 130–144. [Google Scholar] [CrossRef]
- Gonzalez, C.C.; Burke, M.R. Motor Sequence Learning in the Brain: The Long and Short of It. Neuroscience 2018, 389, 85–98. [Google Scholar] [CrossRef]
- Sliney, D.H. What Is Light? The Visible Spectrum and Beyond. Eye 2016, 30, 222–229. [Google Scholar] [CrossRef]
- Eysenck, M.; Keane, M. Cognitive Psychology: A Student’s Handbook, 8th ed.; Psychology Press: London, UK, 2020; ISBN 9781138482234. [Google Scholar]
- Zeki, S.; Watson, J.D.G.; Lueck, C.J.; Friston, K.J.; Kennard, C.; Frackowiak, R.S.J. A Direct Demonstration of Functional Specialization in Human Visual Cortex. J. Neurosci. 1991, 11, 641–649. [Google Scholar] [CrossRef]
- Anderson, S.J.; Holliday, I.E.; Singh, K.D.; Harding, G.F.A. Localization and Functional Analysis of Human Cortical Area V5 Using Magneto-Encephalography. Proc. R. Soc. B Biol. Sci. 1996, 263, 423–431. [Google Scholar] [CrossRef]
- Tootell, R.B.H.; Reppas, J.B.; Kwong, K.K.; Malach, R.; Born, R.T.; Brady, T.J.; Rosen, B.R.; Belliveau, J.W. Functional Analysis of Human MT and Related Visual Cortical Areas Using Magnetic Resonance Imaging. J. Neurosci. 1995, 15, 3215–3230. [Google Scholar] [CrossRef]
- Tootell, R.B.H.; Reppas, J.B.; Dale, A.M.; Look, R.B.; Sereno, M.I.; Malach, R.; Brady, T.J.; Rosen, B.R. Visual Motion Aftereffect in Human Cortical Area MT Revealed by Functional Magnetic Resonance Imaging. Nature 1995, 375, 139–141. [Google Scholar] [CrossRef]
- Beckers, G.; Zeki, S. The Consequences of Inactivating Areas V1 and V5 on Visual Motion Perception. Brain 1995, 118, 49–60. [Google Scholar] [CrossRef]
- Zihl, J.; von Cramon, D.; Mai, N. Selective Disturbance of Movement Vision after Bilateral Brain Damage. Brain 1983, 106, 313–340. [Google Scholar] [CrossRef]
- Shipp, S.; Jong, B.M.D.; Zihl, J.; Frackowiak, R.S.J.; Zeki, S. The Brain Activity Related to Residual Motion Vision in a Patient with Bilateral Lesions of V5. Brain 1994, 117, 1023–1038. [Google Scholar] [CrossRef]
- van Essen, D.C.; Gallant, J.L. Neural Mechanisms of Form and Motion Processing in the Primate Visual System. Neuron 1994, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ashida, H.; Osaka, N. Motion Aftereffect with Flickering Test Stimuli Depends on Adapting Velocity. Vis. Res. 1995, 35, 1825–1833. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mecarelli, O. (Ed.) Clinical Electroencephalography; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Gray, M.J.; Emmanouil, T.A. Individual Alpha Frequency Increases during a Task but Is Unchanged by Alpha-Band Flicker. Psychophysiology 2020, 57, e13480. [Google Scholar] [CrossRef] [PubMed]
- Spaak, E.; de Lange, F.P.; Jensen, O. Local Entrainment of Alpha Oscillations by Visual Stimuli Causes Cyclic Modulation of Perception. J. Neurosci. 2014, 34, 3536–3544. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, C.S. Human EEG Responses to 1–100 Hz Flicker: Resonance Phenomena in Visual Cortex and Their Potential Correlation to Cognitive Phenomena. Exp. Brain Res. 2001, 137, 346–353. [Google Scholar] [CrossRef]
- Salmi, T. Critical Flicker Frequencies in MS Patients with Normal or Abnormal Pattern VEP. Acta Neurol. Scand. 1985, 71, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Daley, M.L.; Swank, R.L.; Ellison, C.M. Flicker Fusion Thresholds in Multiple Sclerosis: A Functional Measure of Neurological Damage. Arch. Neurol. 1979, 36, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Chiappa, K.H. Pattern Shift Visual, Brainstem Auditory, and Short-latency Somatosensory Evoked Potentials in Multiple Sclerosis. Neurology 1980, 30, 110–123. [Google Scholar] [CrossRef]
- Halliday, A.M.; Mcdonald, W.I.; Mushin, J. Delayed Visual Evoked Response in Optic Neuritis. Lancet 1972, 299, 982–985. [Google Scholar] [CrossRef]
- Trojaborg, W.; Böttcher, J.; Saxtrup, O. Evoked Potentials and Immunoglobulin Abnormalities in Multiple Sclerosis. Neurology 1981, 31, 866. [Google Scholar] [CrossRef]
- Glover, G.H. Overview of Functional Magnetic Resonance Imaging. Neurosurg. Clin. N. Am. 2011, 22, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Carmel, D.; Lavie, N.; Rees, G. Conscious Awareness of Flicker in Humans Involves Frontal and Parietal Cortex. Curr. Biol. 2006, 16, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Zafiris, O.; Kircheis, G.; Rood, H.A.; Boers, F.; Häussinger, D.; Zilles, K. Neural Mechanism Underlying Impaired Visual Judgement in the Dysmetabolic Brain: An FMRI Study. Neuroimage 2004, 22, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, R.F. Metabolic Encephalopathies. In Basic Neurochemistry: Molecular, Cellular and Medical Aspects; Siegiel, G.J., Ed.; Lippincott-Raven: Philadelphia, PA, USA, 1999; pp. 316–333. [Google Scholar]
- Mullinger, K.J.; Cherukara, M.T.; Buxton, R.B.; Francis, S.T.; Mayhew, S.D. Post-Stimulus FMRI and EEG Responses: Evidence for a Neuronal Origin Hypothesised to Be Inhibitory. Neuroimage 2017, 157, 388–399. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, K.; He, S. Human Visual Cortex Responds to Invisible Chromatic Flicker. Nat. Neurosci. 2007, 10, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Desimone, R.; Duncan, J. Neural Mechanisms of Selective Visual Attention. Annu. Rev. Neurosci. 1995, 18, 193–222. [Google Scholar] [CrossRef]
- Fuchs, S.; Andersen, S.K.; Gruber, T.; Müller, M.M. Attentional Bias of Competitive Interactions in Neuronal Networks of Early Visual Processing in the Human Brain. Neuroimage 2008, 41, 1086–1101. [Google Scholar] [CrossRef]
- Morgan, S.T.; Hansen, J.C.; Hillyard, S.A. Selective Attention to Stimulus Location Modulates the Steady-State Visual Evoked Potential. Proc. Natl. Acad. Sci. USA 1996, 93, 4770–4774. [Google Scholar] [CrossRef]
- Müller, M.M.; Malinowski, P.; Gruber, T.; Hillyard, S.A. Sustained Division of the Attentional Spotlight. Nature 2003, 424, 309–312. [Google Scholar] [CrossRef]
- Müller, M.M.; Picton, T.W.; Valdes-Sosa, P.; Riera, J.; Teder-Sälejärvi, W.A.; Hillyard, S.A. Effects of Spatial Selective Attention on the Steady-State Visual Evoked Potential in the 20–28 Hz Range. Cogn. Brain Res. 1998, 6, 249–261. [Google Scholar] [CrossRef]
- Keitel, C.; Andersen, S.K.; Müller, M.M. Competitive Effects on Steady-State Visual Evoked Potentials with Frequencies in- and Outside the Alpha Band. Exp. Brain Res. 2010, 205, 489–495. [Google Scholar] [CrossRef]
- de Lissa, P.; Caldara, R.; Nicholls, V.; Miellet, S. In Pursuit of Visual Attention: SSVEP Frequency-Tagging Moving Targets. PLoS ONE 2020, 15, e0236967. [Google Scholar] [CrossRef]
- Egeth, H.; Kahneman, D. Attention and Effort. Am. J. Psychol. 1975, 88, 339. [Google Scholar] [CrossRef]
- Lavie, N.; Hirst, A.; de Fockert, J.W.; Viding, E. Load Theory of Selective Attention and Cognitive Control. J. Exp. Psychol. Gen. 2004, 133, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Corbetta, M.; Shulman, G.L. Control of Goal-Directed and Stimulus-Driven Attention in the Brain. Nat. Rev. Neurosci. 2002, 3, 201–215. [Google Scholar] [CrossRef]
- Hautus, M.J.; Macmillan, N.A.; Creelman, C.D. (Eds.) Detection Theory, 3rd ed.; Routledge: New York, NY, USA, 2021; ISBN 9781003203636. [Google Scholar]
- Chang, T.T.L.; Ciuffreda, K.J.; Kapoor, N. Critical Flicker Frequency and Related Symptoms in Mild Traumatic Brain Injury. Brain Inj. 2007, 21, 1055–1062. [Google Scholar] [CrossRef]
- Clark, W.C.; Rutschmann, J.; Link, R.; Brown, J.C. Comparison of Flicker-Fusion Thresholds Obtained by the Methods of Forced-Choice and Limits on Psychiatric Patients. Percept. Mot. Ski. 1963, 16, 19–30. [Google Scholar] [CrossRef]
- Eisen-Enosh, A.; Farah, N.; Burgansky-Eliash, Z.; Polat, U.; Mandel, Y. Evaluation of Critical Flicker-Fusion Frequency Measurement Methods for the Investigation of Visual Temporal Resolution. Sci. Rep. 2017, 7, 15621. [Google Scholar] [CrossRef]
- Becker, D.; Creutzfeldt, O.D.; Schwibbe, M.; Wuttke, W. Changes in Physiological, EEG and Psychological Parameters in Women during the Spontaneous Menstrual Cycle and Following Oral Contraceptives. Psychoneuroendocrinology 1982, 7, 75–90. [Google Scholar] [CrossRef]
- Berka, C.; Levendowski, D.; Lumicao, M.; Yau, A.; Davis, G.; Zivkovic, V.; Olmstead, R.; Tremoulet, P.; Craven, P. EEG Correlates of Task Engagement and Mental Workload in Vigilance, Learning, and Memory Tasks. Aviat. Space Environ. Med. 2007, 78, B231–B244. [Google Scholar]
- Brown, B.B. Specificity of EEG Photic Flicker Responses To Color As Related To Visual Imagery Ability. Psychophysiology 1966, 2, 197–207. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Yotsumoto, Y. The Amount of Time Dilation for Visual Flickers Corresponds to the Amount of Neural Entrainments Measured by EEG. Front. Comput. Neurosci. 2018, 12, 30. [Google Scholar] [CrossRef]
- Küller, R.; Laike, T. The Impact of Flicker from Fluorescent Lighting on Well-Being, Performance and Physiological Arousal. Ergonomics 1998, 41, 433–447. [Google Scholar] [CrossRef]
- Ikegami, S.; Takano, K.; Wada, M.; Saeki, N.; Kansaku, K. Effect of the Green/Blue Flicker Matrix for P300-Based Brain-Computer Interface: An EEG-FMRI Study. Front. Neurol. 2012, 3, 113. [Google Scholar] [CrossRef]
- Schulz, H.; Wilde-Frenz, J. The Disturbance of Cognitive Processes in Narcolepsy. J. Sleep Res. 1995, 4, 10–14. [Google Scholar] [CrossRef]
- Ronzhina, M.; Bubnik, K.; Gajdos, M.; Kolarova, J.; Honzik, P.; Provaznik, I. Use of EEG for Validation of Flicker-Fusion Test. In Proceedings of the ISABEL ‘11: International Symposium on Applied Sciences in Biomedical and Communication Technologies, Barcelona, Spain, 26–29 October 2011. [Google Scholar] [CrossRef]
- Lecca, L.I.; Fadda, P.; Fancello, G.; Medda, A.; Meloni, M. Cardiac Autonomic Control and Neural Arousal as Indexes of Fatigue in Professional Bus Drivers. Saf. Health Work 2022, 13, 148–154. [Google Scholar] [CrossRef]
- Wilkins, A.J.; Nimmo-Smith, I.; Slater, A.I.; Bedocs, L. Fluorescent Lighting, Headaches and Eyestrain. Light. Res. Technol. 1989, 21, 11–18. [Google Scholar] [CrossRef]
- Chatrian, G.E.; Lettich, E.; Miller, L.H.; Green, J.R. Pattern-Sensitive Epilepsy. Part 1. An Electrographic Study of Its Mechanisms. Epilepsia 1970, 11, 125–149. [Google Scholar] [CrossRef]
- Wilkins, A.J. Visual Stress; Oxford University Press: New York, NY, USA, 1995; ISBN 0-19-852174-X. [Google Scholar]
- O’Hare, L.; Hibbard, P.B. Visual Discomfort and Blur. J. Vis. 2013, 13, 7. [Google Scholar] [CrossRef]
- Yoshimoto, S.; Garcia, J.; Jiang, F.; Wilkins, A.J.; Takeuchi, T.; Webster, M.A. Visual Discomfort and Flicker. Vis. Res. 2017, 138, 18–28. [Google Scholar] [CrossRef]
- Lin, M.-W.; Hsieh, P.-H.; Chang, E.C.; Chen, Y.-C. Flicker-Glare and Visual-Comfort Assessments of Light Emitting Diode Billboards. Appl. Opt. 2014, 53, E61–E68. [Google Scholar] [CrossRef]
- Ishida, S.; Yamashita, Y.; Matsuishi, T.; Ohshima, M.; Ohshima, H.; Kato, H.; Maeda, H. Photosensitive Seizures Provoked While Viewing “Pocket Monsters,” a Made-for-Televison Animation Program in Japan. Epilepsia 1998, 39, 1340–1344. [Google Scholar] [CrossRef]
- Takahashi, T.; Tsukahara, Y. Pocket Monster Incident and Low Luminance Visual Stimuli: Special Reference to Deep Red Flicker Stimulation. Pediatr. Int. 1998, 40, 631–637. [Google Scholar] [CrossRef]
- Harding, G.F.A. TV Can Be Bad for Your Health. Nat. Med. 1998, 4, 265–267. [Google Scholar] [CrossRef]
- Takahashi, T.; Tsukahara, Y. Influence of Color on the Photoconvulsive Response. Electroencephalogr. Clin. Neurophysiol. 1976, 41, 124–136. [Google Scholar] [CrossRef]
- Binnie, C.D.; Estevez, O.; Kasteleijn-Nolst Trenité, D.G.A.; Peters, A. Colour and Photosensitive Epilepsy. Electroencephalogr. Clin. Neurophysiol. 1984, 58, 387–391. [Google Scholar] [CrossRef]
- Parra, J.; Lopes Da Silva, F.H.; Stroink, H.; Kalitzin, S. Is Colour Modulation an Independent Factor in Human Visual Photosensitivity? Brain 2007, 130, 1679–1689. [Google Scholar] [CrossRef]
- Klass, D.W. Pattern Activation of Seizures. In Epileptic Seizures: Pathophysiology and Clinical Semiology; Luders, H.O., Noachtar, S., Eds.; Churchill Livingstone: Philadelphia, PA, USA, 2000; pp. 598–608. [Google Scholar]
- Takano, K.; Ikegami, S.; Komatsu, T.; Kansaku, K. Green/Blue Flicker Matrices for the P300 BCI Improve the Subjective Feeling of Comfort. Neurosci. Res. 2009, 65, S182. [Google Scholar] [CrossRef]
- Takano, K.; Komatsu, T.; Hata, N.; Nakajima, Y.; Kansaku, K. Visual Stimuli for the P300 Brain-Computer Interface: A Comparison of White/Gray and Green/Blue Flicker Matrices. Clin. Neurophysiol. 2009, 120, 1562–1566. [Google Scholar] [CrossRef]
- Hoffmann, U.; Vesin, J.M.; Ebrahimi, T.; Diserens, K. An Efficient P300-Based Brain–Computer Interface for Disabled Subjects. J. Neurosci. Methods 2008, 167, 115–125. [Google Scholar] [CrossRef]
- Ikegami, S.; Takano, K.; Saeki, N.; Kansaku, K. Operation of a P300-Based Brain–Computer Interface by Individuals with Cervical Spinal Cord Injury. Clin. Neurophysiol. 2011, 122, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Nijboer, F.; Sellers, E.W.; Mellinger, J.; Jordan, M.A.; Matuz, T.; Furdea, A.; Halder, S.; Mochty, U.; Krusienski, D.J.; Vaughan, T.M.; et al. A P300-Based Brain–Computer Interface for People with Amyotrophic Lateral Sclerosis. Clin. Neurophysiol. 2008, 119, 1909–1916. [Google Scholar] [CrossRef] [PubMed]
- Piccione, F.; Giorgi, F.; Tonin, P.; Priftis, K.; Giove, S.; Silvoni, S.; Palmas, G.; Beverina, F. P300-Based Brain Computer Interface: Reliability and Performance in Healthy and Paralysed Participants. Clin. Neurophysiol. 2006, 117, 531–537. [Google Scholar] [CrossRef]
- Sellers, E.W.; Donchin, E. A P300-Based Brain–Computer Interface: Initial Tests by ALS Patients. Clin. Neurophysiol. 2006, 117, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Norton, J.J.S.; DiRisio, G.F.; Carp, J.S.; Norton, A.E.; Kochan, N.S.; Wolpaw, J.R. Brain-Computer Interface-Based Assessment of Color Vision. J. Neural Eng. 2021, 18, 066024. [Google Scholar] [CrossRef]
- Moncada, F.; Martín, S.; González, V.M.; Álvarez, V.M.; García-López, B.; Gómez-Menéndez, A.I.; Villar, J.R. Virtual Reality and Machine Learning in the Automatic Photoparoxysmal Response Detection. Neural Comput. Appl. 2022, 1–17. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).