1. Introduction
The fall armyworm (FAW),
Spodoptera frugiperda (J.E. Smith), is a voracious insect pest native to the Western Hemisphere, particularly in South America [
1]. It is one of the most rapidly spreading and highly invasive pests of maize across Africa and Asia [
2,
3,
4,
5].
S. frugiperda has become a pest species because of its biological characteristics such as polyphagy, concealed larval feeding habits, high reproductive capacity, adult dispersion, and multiple generations per year [
3,
4,
5]. The polyphagous fall armyworm feeds on more than 350 plants in such families, including Poaceae, Asteraceae, and Fabaceae [
6]. The FAW has two haplotypes that have been recognized for a long time: the “rice strain” (R strain), which prefers to eat rice and grasses, and the “corn strain” (C strain), which prefers to eat maize and sorghum [
7,
8,
9]. It is very important to control
S. frugiperda infestations and spread because it causes severe economic loss to several economically important crop plants and threatens global food security and the livelihoods of many households [
2,
5]. Currently,
S. frugiperda represents a serious problem to maize crops in China and elsewhere in South Asia [
4,
10].
Transgenic plants that express
Bacillus thuringiensis proteins (Bt plants) and synthetic insecticides are the main tactics to control FAW, although widespread usage of synthetic chemicals has resulted in the emergence of resistance [
11,
12,
13,
14]. Unfortunately, the foliar application of chemical insecticides against the
S. frugiperda population in Bt and non-Bt maize crops has low control efficacy [
13]. This may be due to the feeding behavior of
S. frugiperda larvae which stay inside the maize whorl, thus reducing insecticide contact. In addition, Long-term use of chemical pesticides in the field poses serious risks to the environment due to contamination and causes the death of natural enemies, which often leads to pest resurgence, and inevitably has led to insecticide-resistant in different insects population [
11,
15,
16,
17,
18,
19,
20]. Previously, some studies have shown that multiple field populations of
S. frugiperda have developed high-level resistance as well as broad cross-resistance to diverse groups of synthetic insecticides, including pyrethroids, organophosphate, carbamate, chlorantraniliprole, abamectin, emamectin benzoate, lufenuron and spinosad [
11,
12,
13,
14,
18,
19]. In addition, widespread areas of Bt-crops without growing refuge in some mainly tropical countries have increased the evolution of resistance problems to Bt proteins in
S. frugiperda populations [
21,
22,
23]. The effort to control this pest is becoming exceedingly challenging all over the world.
Indoxacarb is a new oxadiazine insecticide with significant toxicity against a variety of lepidopteran, coleopteran, and sucking insect pests in agricultural as well as urban contexts [
24]. Insect esterases or amidases can convert indoxacarb to an N-decarbomethoxylated metabolite (DCJW), a more potent sodium channel blocker than indoxacarb, which causes the target pest species to become paralyzed and die [
25,
26]. Indoxacarb is highly active when ingested, but there have been few reports of contact activity when applied topically [
24,
26,
27,
28]. Indoxacarb is a potent novel insecticide for crop protection because to its safety for humans and non-target organisms, superior environmental and residual qualities, broad spectrum, and quick reduction in insect feeding [
24]. However, numerous studies have indicated that a number of insects have recently evolved resistance to indoxacarb due to its widespread use, including
S. litura [
29], such as
C. rosaceana [
30],
M. domestica [
31],
S. exigua [
32],
P. xylostella [
33] and
H. armigera [
34] have developed a significant level of resistance. Furthermore, resistance to several Bt maize products expressing Cry1F and Cry1Ab proteins has been reported in the field, increasing the use of chemical insecticides against
S. frugiperda in maize [
13,
35]. The detoxification enzymes P450, esterase, and glutathione S-transferase (GST) are involved in the resistance to indoxacarb [
33,
34,
36]. Additionally, novel sodium channel mutations (F1845Y and V1848I) have been reported to be associated with resistance to indoxacarb in
P. xylostella and
T. absoluta [
33,
37]. However, indoxacarb resistance is currently at a relatively low level in
S. frugiperda populations, although the pest has been subjected to indoxacarb selection pressure. Insecticide resistance management in insect pests is a global challenge for entomologists. However, if resistance to a novel insecticide can be monitored and predicted, a proactive resistance management program can be established to reduce the risk of resistance [
23,
38,
39]. Laboratory selection experiments can provide important insect resistance data [
14,
40]. Therefore, it is crucial that we comprehend how insecticides work and how resistance develops in order to develop techniques for managing resistance. Additionally, figuring out the molecular basis behind pesticide resistance may open up new possibilities for the creation of cutting-edge tactics for controlling insect pests. However, indoxacarb resistance mechanism, inheritance, and resistance-associated fitness costs in
S. frugiperda have not been documented to date.
Understanding the resistance mechanism and resistance-associated fitness costs is essential as they directly affect the rate of resistance evolution in the field population and play significant roles in an insect resistance management (IRM) strategy [
41,
42]. Moreover, evaluating the fitness costs associated with resistance can aid in determining if vulnerability can be regained in the context of selection pressure [
43,
44,
45]. In this study, to understand the potential mechanism of the fast-evolved resistance to indoxacarb, the inheritance of resistance and resistance-associated fitness costs in the
S. frugiperda strain from a field population was evaluated. These data are valuable to understand the indoxacarb resistance mechanism and provides vital information for scientific-based guidance of pest management decisions.
4. Discussion
The selection pressure induced by the indiscriminate use of pesticides has resulted in the dramatic evolution of insecticide resistance in insect pests. Indoxacarb belongs to the novel oxadiazine group with wide-spectrum insecticidal activity against several lepidopteran species as well as some other groups of insect pests such as homopteran and coleopteran and has a low toxicity profile for non-target organisms [
24,
60]. Numerous insect species have been used to investigate the mechanisms underlying indoxacarb resistance. Two mutations (F1845Y and V1848I) have been found in indoxacarb-resistant populations of two pest species,
Plutella xylostella [
61] and
Tuta absoluta [
38]. Gao et al. [
32] identified one point mutation (L1014F) in indoxacarb selected strain of
S. exigua, whether L1014F mutation in
S. exigua is associated with indoxacarb resistance or not the functional verification is needed by gene editing or electrophysiology. Similarly, Samantsidis et al. (2019) [
62] and Wang et al. (2022) [
63] found that only F1845Y and V1848I mutations had been proven to confer resistance to indoxacarb in
Plutella xylostella and
Drosophila. In our study, we showed a significant selection response to indoxacarb in a field-collected population of
S. frugiperda. Following continuous laboratory selection for 24 generations, a field-collected population Ind-SEL of
S. frugiperda exhibited a very high level of resistance (472.67-fold) to indoxacarb as compared to the Ind-UNSEL population
It is possible that there was a high frequency of resistance allele in this field-collected population as it was collected from a region where insecticides were used extensively to manage various maize pests. Similarly, Muraro et al. (2021) [
46] evaluated the evolution of resistance to emamectin benzoate in the field-collected population
S. frugiperda, and after 10 generations of continuous selection to pesticide, the resistance ratio increased ∼2283.44-fold. In previous studies, high resistance levels to indoxacarb have been reported in various lepidopteran insects after continuous selection pressure. For example,
P. xylostella (2594-fold after six generations of selection) [
64],
S. exigua (240-fold after 12 generations) [
32],
H. armigera (1239-fold after eight generations) [
65], and
S. litura (95-fold after three generations) [
66]. In contrast, comparatively low level of indoxacarb resistance has been documented in
H. virescens (55-fold after six generations) [
67],
H. armigera (4.43-fold after 11 selected generations) [
68] and
P. xylostella (31.3-fold after ten selected generations) [
69]. These disparities may be due to differences in species’ geographical origin or to the effects of initial sampling.
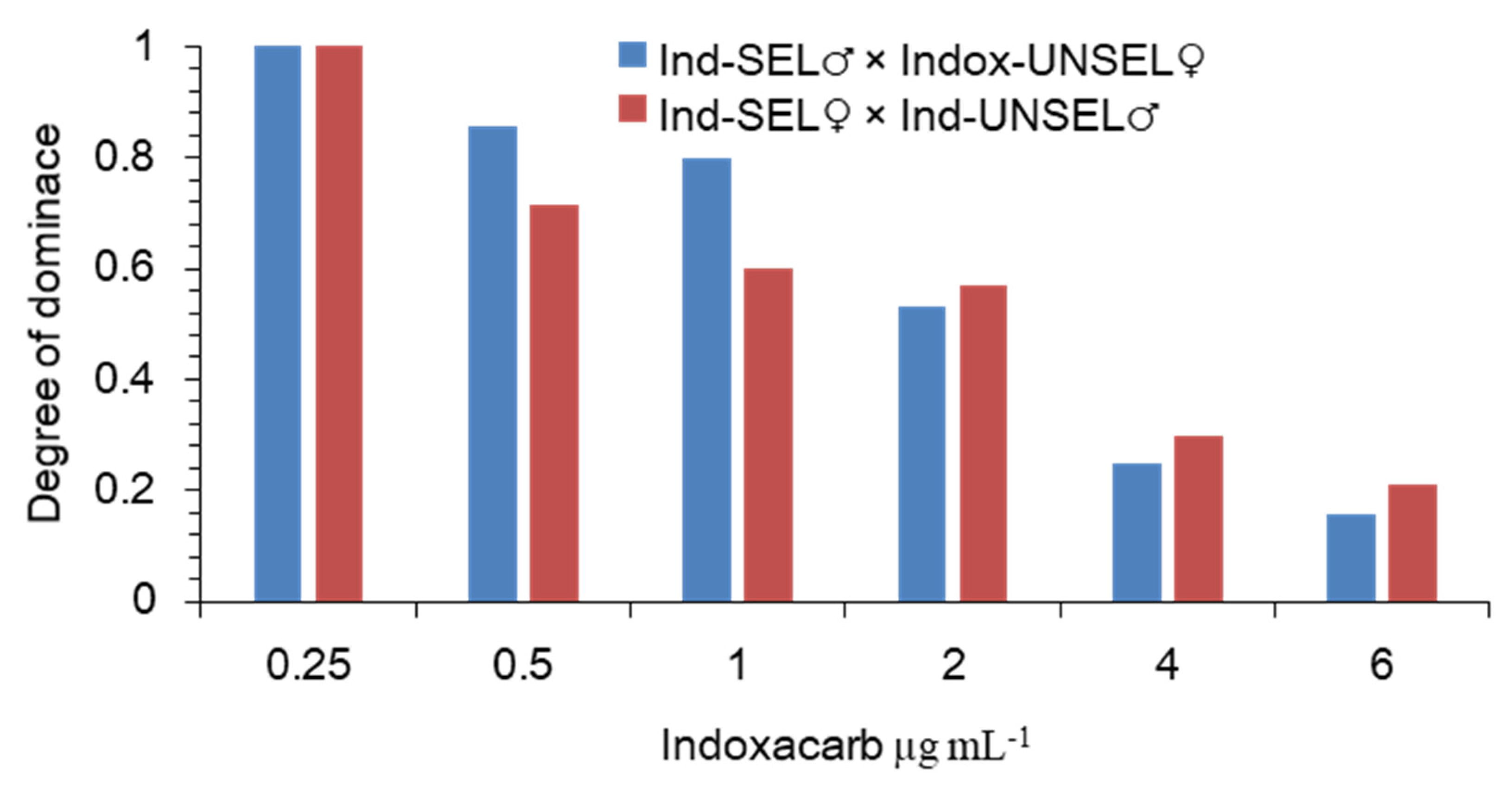
The level of dominance resistance in the field depends on several factors, such as the concentration used, the stage of development of the insect, and the environment[
42,
49]. In the present study, the resistance was characterized as an incompletely recessive trait with polygenic effects when
S. frugiperda was exposed to indoxacarb. A similar pattern of resistance was reported when
S. litura [
66]
, H. armigera [
68],
S. exigua [
32], and
P. solenopsis (Tinsley) were exposed to indoxacarb, respectively [
70]. In contrast, dominant and polygenic resistance to indoxacarb was reported in
S. litura [
66]. Predictions of effective dominance based on laboratory data, however, must be carefully considered because the range of concentrations required to establish dominance may differ between laboratory and field populations, as well as the effect of inducible insecticidal concentration due to chemical degradation [
71]. High levels of cross-resistance between insecticides with the same and different modes of action used in a rotation strategy are one of the key problems for the success of IRM programs. In our study, compared to the Ind-UNSEL strain, the Ind-SEL strain of
S. frugiperda exhibited obvious cross-resistance to deltamethrin (31.23-fold), low and negligible levels of cross-resistance to chlorfenapyr (3.24-fold), spinosad (2.65-fold), respectively. In previous studies, a low and high level of cross-resistance between indoxacarb, spinosad, flubendiamide, fenvalerate, emamectin benzoate and chlorantraniliprole was found in
S. frugiperda,
S. exigua, P. xylostella and
S. litura [
72,
73,
74,
75]. As we know, it has not been reported in other studies for this type of cross-resistance in indoxacarb. It might be that indoxacarb and deltamethrin have the same or cross-molecular targets based on a biochemical mechanism that needs to be investigated.
The oxidative metabolism mediated by cytochrome P450 monooxygenases and the hydrolysis and/or sequestration caused by carboxylesterases is the most frequent mechanisms linked to pesticide resistance in insect pests [
76]. Based on the synergistic effects of metabolic inhibitors on indoxacarb toxicity, the involvement of metabolic mechanisms in indoxacarb resistance has been reported in several insect species [
34,
77]. Present results with synergists showed that the toxicity of indoxacarb against
S. frugiperda was increased by PBO, indicating that mono-oxygenases P450 enzyme may be associated with indoxacarb resistance in the Ind-SEL population. In a Malaysian field-derived strain of
P. xylostella, high-level (813-fold) resistance to indoxacarb was greatly reduced by PBO or a PBO analog specific for esterases, suggesting that indoxacarb resistance was attributable to improved metabolic detoxification by esterases [
78]. Metabolic resistance associated with an increased level of detoxification enzymes, for example, cytochrome P450, carboxy/cholinesterase (CCE), and glutathione S-transferase (GST)) in insecticide-resistant populations have been reported worldwide [
79]. In previous studies, it has been reported that P450, carboxylesterase, and GST were involved in the resistance to indoxacarb in
M. domestica, P. xylostella but carboxylesterase and GST were the main factors in
S. exigua leading to indoxacarb resistance [
27,
78]. Similar to our study, elevated activity of the metabolic enzyme P450 enzyme conferred indoxacarb resistance in
H. armigera and
S. litura [
68,
80]. In a previous study, it was reported that the metabolic inhibitor PBO reduced resistance in the indoxacarb-selected strain, suggesting that metabolic detoxification enzymes were probably involved in indoxacarb resistance in
H. armigera [
34]. These results represent a first step towards understanding the indoxacarb resistance mechanisms in a selected strain of
S. frugiperda.
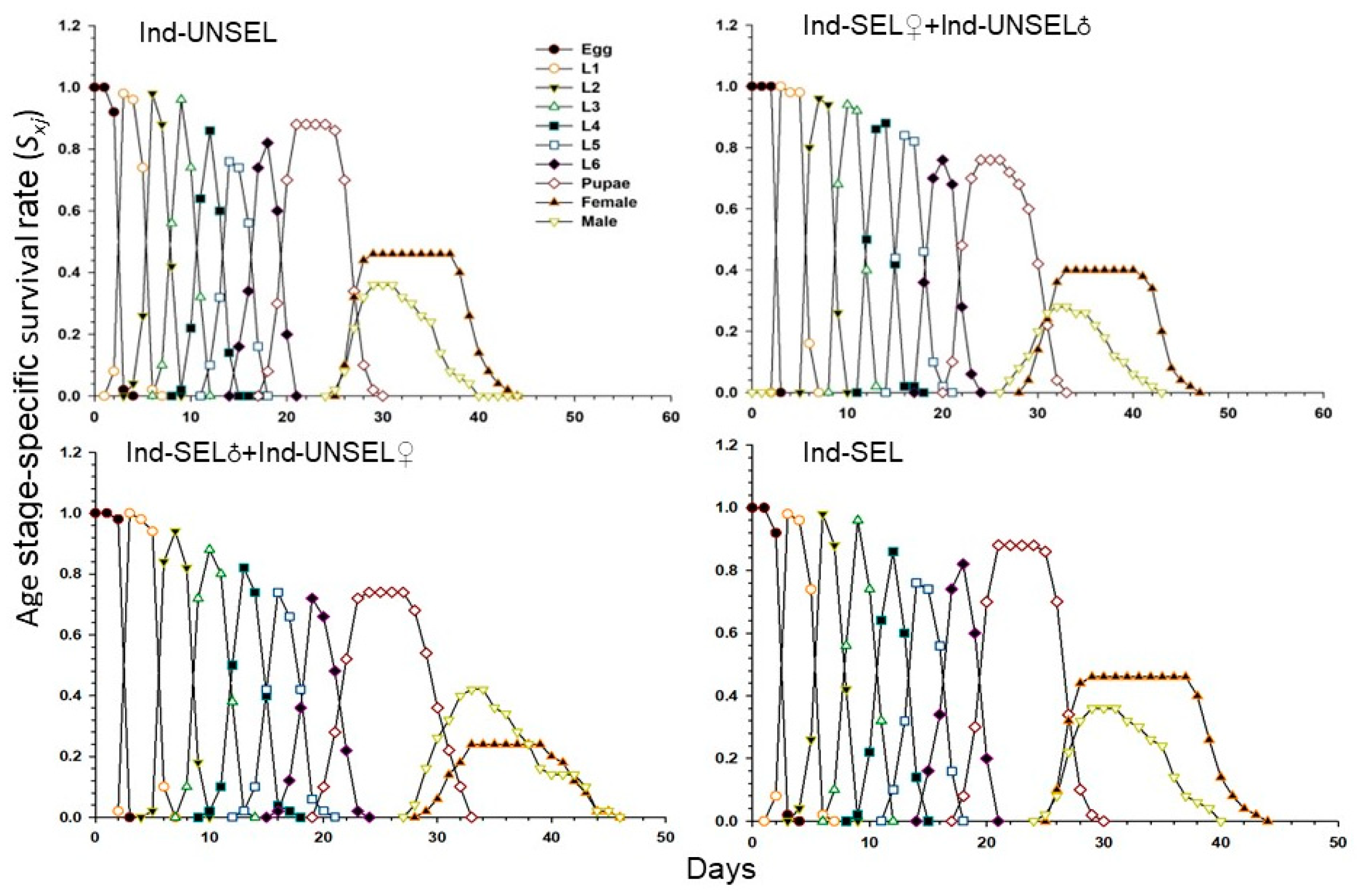
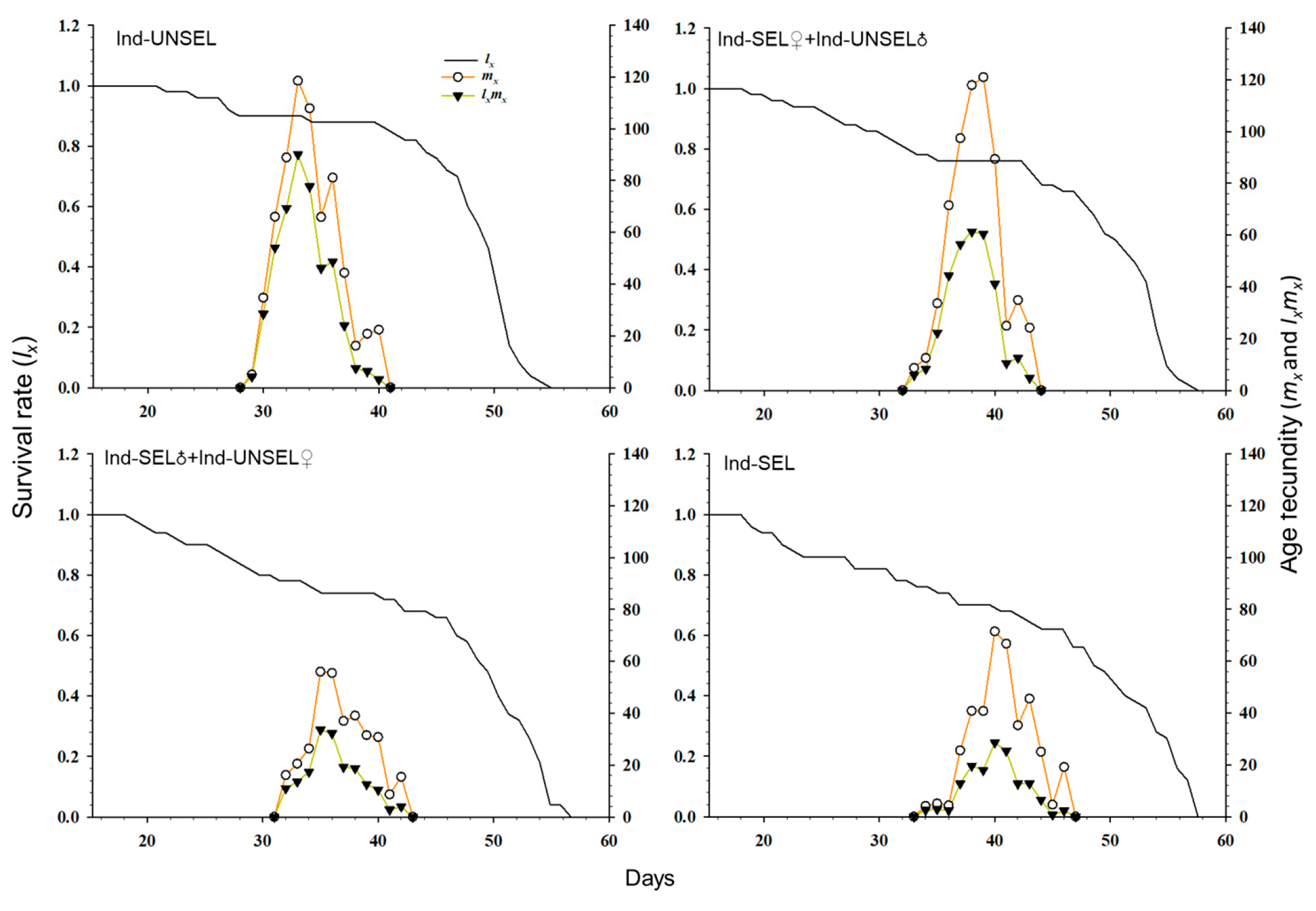
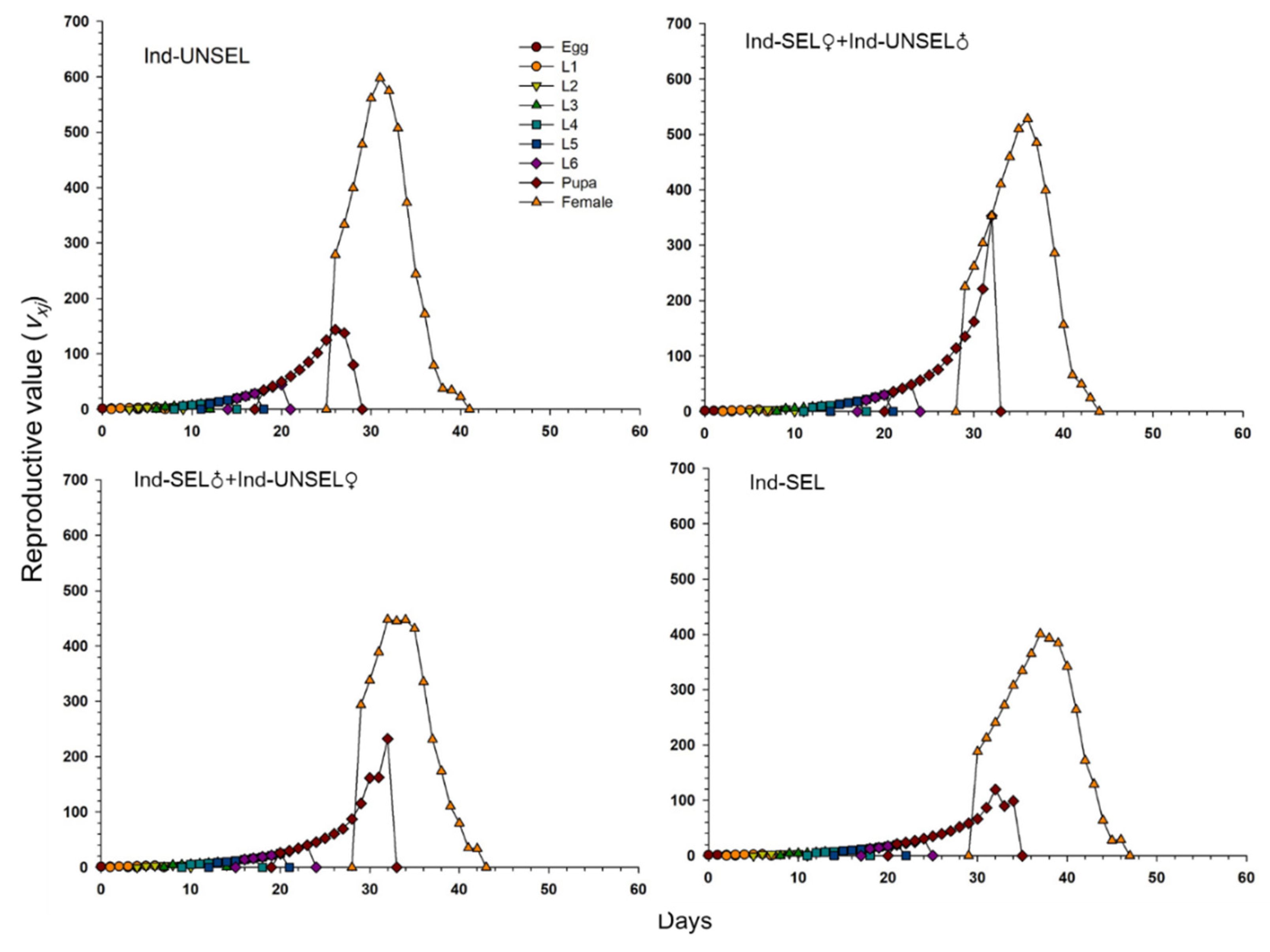
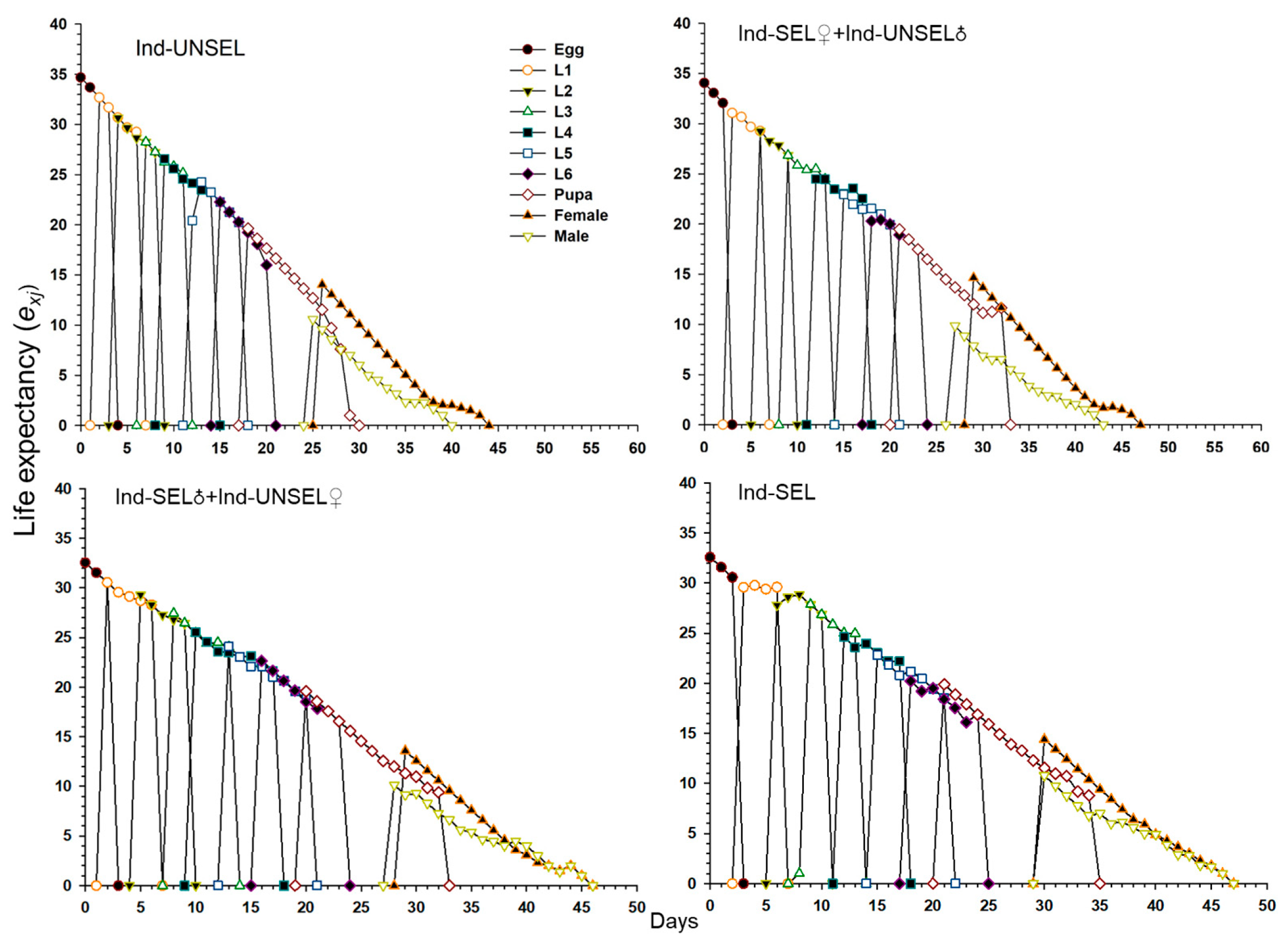
The decline in biological fitness among individuals in different insect populations during the development of resistance can influence their relative abundance and genetic impact on future generations. Traits such as insecticide resistance are advantageous when under selection, and genotypes conferring these phenotypes can rapidly increase in a population [
81]. Resistance-related fitness costs must be assessed in homozygous resistant individuals and heterozygotes that act as carriers of resistant genes in the early stages of resistance [
82]. We evaluated fitness costs in two hybrid populations (Ind-SEL♂ × Ind-UNSEL♀ and Ind-SEL♀ × Ind-UNSEL♂) of the Ind-SEL and the Ind-UNSEL Population. We found significantly longer developmental time of larvae, extended pupal duration, shorter adult longevity, and lower fecundity in the Ind-SEL as compared with the other strains and Ind-UNSEL population. The only parameter that differs between Ind-SEL♂ × Ind-UNSEL♀ (175.68 ± 45.88) and Ind-SEL♀ × Ind-UNSEL♂ (328.03 ± 57.22) was the Net reproductive rate (
R0). On the other hand, all population growth parameters differ between Ind-SEL and Ind-UNSEL strains. Differences in fitness costs associated with insecticide resistance have been reported in many insect populations, including
S. frugiperda,
H. armigera,
H. virescens,
P. xylostella, and
O. hyalinipennis [
14,
66,
67,
83]. Understanding the occurrence of fitness costs associated with insecticide resistance is essential in developing and implementing IRM programs.