Transcriptomic Analysis Revealed Candidate Genes Involved in Pseudomale Sperm Abnormalities in Chinese Tongue Sole (Cynoglossus semilaevis)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Genetic Sex Identification
2.3. Total RNA Extraction and Library Preparation
2.4. RNA Sequencing and Differentially Expressed Gene (DEG) Analysis
2.5. qPCR Validation
3. Results
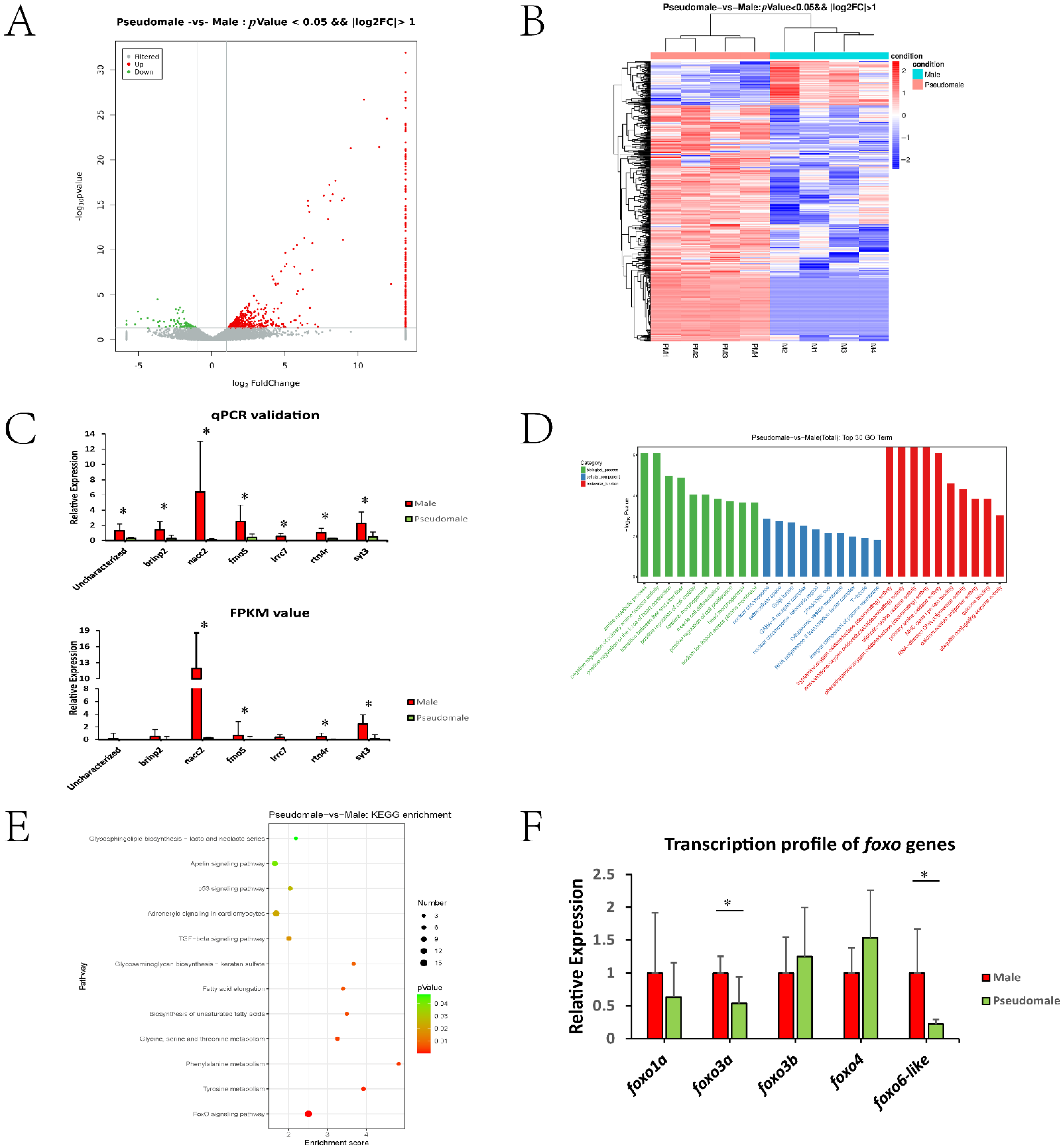
3.1. Transcriptomic Overview and Identification of DEGs
3.2. GO and KEGG Enrichment
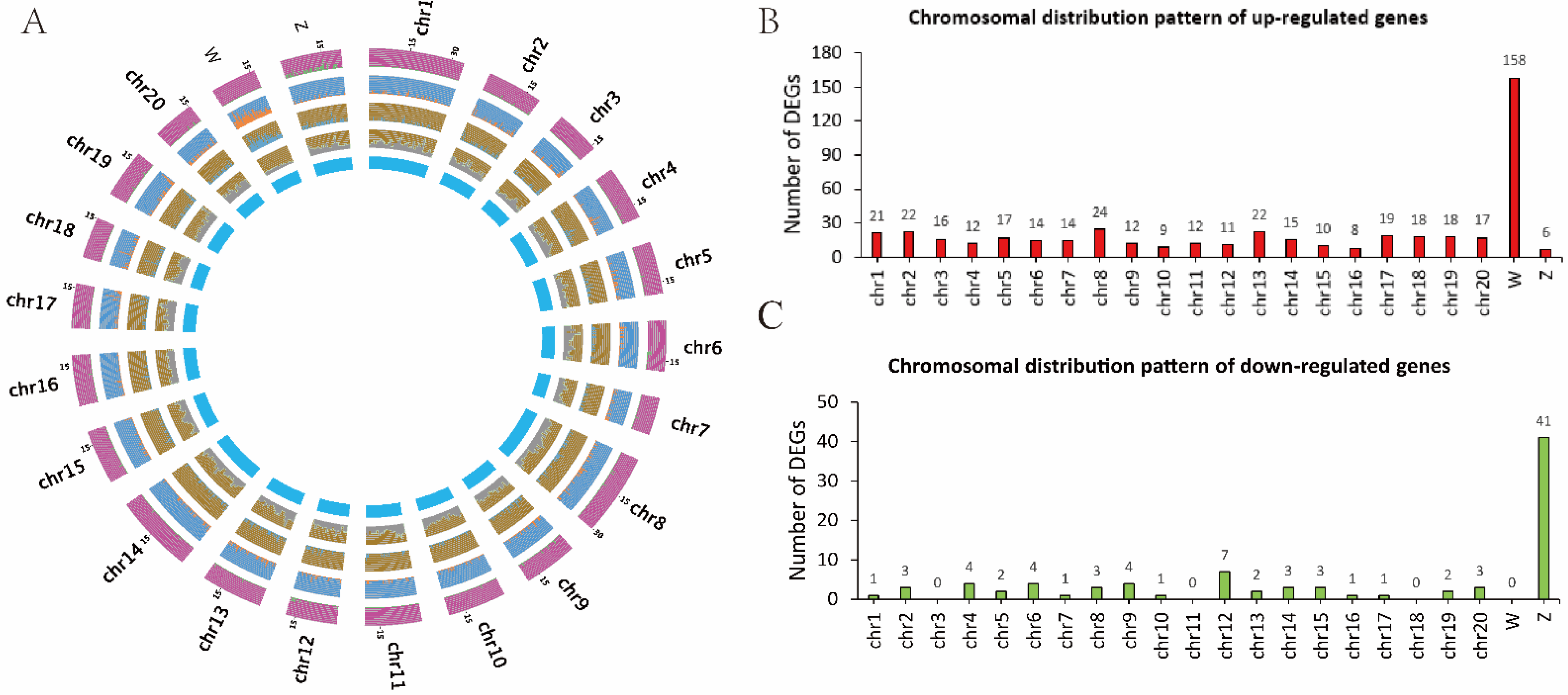
3.3. Chromosomal Distribution Pattern of DEGs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Chen, S.-L.; Tian, Y.-S.; Yang, J.-F.; Shao, C.-W.; Ji, X.-S.; Zhai, J.-M.; Liao, X.-L.; Zhuang, Z.-M.; Su, P.-Z.; Xu, J.-Y.; et al. Artificial Gynogenesis and Sex Determination in Half-Smooth Tongue Sole (Cynoglossus semilaevis). Mar. Biotechnol. 2009, 11, 243–251. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, G.; Shao, C.; Huang, Q.; Liu, G.; Zhang, P.; Song, W.; An, N.; Chalopin, D.; Volff, J.-N.; et al. Whole-genome sequence of a flatfish provides insights into ZW sex chromosome evolution and adaptation to a benthic lifestyle. Nat. Genet. 2014, 46, 253–260. [Google Scholar] [CrossRef]
- Meng, L.; Zhu, Y.; Zhang, N.; Liu, W.; Liu, Y.; Shao, C.; Wang, N.; Chen, S. Cloning and characterization of tesk1, a novel spermatogenesis-related gene, in the tongue sole (Cynoglossus semilaevis). PloS ONE 2014, 9, e107922. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Li, Q.; Chen, S.; Zhang, P.; Lian, J.; Hu, Q.; Sun, B.; Jin, L.; Liu, S.; Wang, Z.; et al. Epigenetic modification and inheritance in sexual reversal of fish. Genome Res. 2014, 24, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Krausz, C.; Riera-Escamilla, A. Genetics of male infertility. Nat. Rev. Urol. 2018, 15, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Li, H.; Dong, Z.; Cui, Z.; Zhang, N.; Meng, L.; Zhu, Y.; Liu, Y.; Li, Y.; Guo, H.; et al. Ubiquitin ligase gene neurl3 plays a role in spermatogenesis of half-smooth tongue sole (Cynoglossus semilaevis) by regulating testis protein ubiquitination. Gene 2016, 592, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Liu, Y.; Wang, W.; Wang, Q.; Zhang, N.; Lin, F.; Wang, N.; Shao, C.; Dong, Z.; Li, Y.; et al. Genome editing reveals dmrt1 as an essential male sex-determining gene in Chinese tongue sole (Cynoglossus semilaevis). Sci. Rep. 2017, 7, 42213. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Gao, D.; Lu, J.; Sun, X. Transcriptome Profiling Reveals the Sexual Dimorphism of Gene Expression Patterns during Gonad Differentiation in the Half-Smooth Tongue Sole (Cynoglossus semilaevis). Mar. Biotechnol. 2021, 23, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, X.; Jin, C.; Du, X.; He, Y.; Zhang, Q. Transcriptome Profiling Insights the Feature of Sex Reversal Induced by High Temperature in Tongue Sole Cynoglossus semilaevis. Front. Genet. 2019, 10, 522. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, K.; Feng, B.; Zhang, Z.; Wang, R.; Tang, L.; Li, W.; Li, Q.; Piferrer, F.; Shao, C. Transcriptome of Gonads From High Temperature Induced Sex Reversal During Sex Determination and Differentiation in Chinese Tongue Sole, Cynoglossus semilaevis. Front. Genet. 2019, 10, 1128. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, S.; Gao, F.; Meng, L.; Hu, Q.; Song, W.; Shao, C.; Lv, W. SCAR transformation of sex-specific SSR marker and its application in half-smooth tongue sole(Cynoglossus semiliaevis). J. Agric. Biotechnol. 2014, 22, 787–792. [Google Scholar]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; Van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef]
- Wang, N.; Yang, Q.; Wang, J.; Shi, R.; Li, M.; Gao, J.; Xu, W.; Yang, Y.; Chen, Y.; Chen, S. Integration of Transcriptome and Methylome Highlights the Roles of Cell Cycle and Hippo Signaling Pathway in Flatfish Sexual Size Dimorphism. Front. Cell Dev. Biol. 2021, 9, 743722. [Google Scholar] [CrossRef]
- Zhang, X.; Hao, X.; Ma, W.; Zhu, T.; Zhang, Z.; Wang, Q.; Liu, K.; Shao, C.; Wang, H.-Y. Transcriptome Analysis Indicates Immune Responses against Vibrio harveyi in Chinese Tongue Sole (Cynoglossus semilaevis). Animals 2022, 12, 1144. [Google Scholar] [CrossRef]
- Guo, L.; Wang, Y.; Liang, S.; Lin, G.; Chen, S.; Yang, G. Tissue-overlapping response of half-smooth tongue sole (Cynoglossus semilaevis) to thermostressing based on transcriptome profiles. Gene 2016, 586, 97–104. [Google Scholar] [CrossRef]
- Xu, W.; Cui, Z.; Wang, N.; Zhang, M.; Wang, J.; Xu, X.; Liu, Y.; Chen, S. Transcriptomic analysis revealed gene expression profiles during the sex differentiation of Chinese tongue sole (Cynoglossus semilaevis). Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 40, 100919. [Google Scholar] [CrossRef]
- Goertz, M.J.; Wu, Z.; Gallardo, T.D.; Hamra, F.K.; Castrillon, D.H. Foxo1 is required in mouse spermatogonial stem cells for their maintenance and the initiation of spermatogenesis. J. Clin. Investig. 2011, 121, 3456–3466. [Google Scholar] [CrossRef]
- Huang, P.; Zhou, Z.; Shi, F.; Shao, G.; Wang, R.; Wang, J.; Wang, K.; Ding, W. Effects of the IGF-1/PTEN/Akt/FoxO signaling pathway on male reproduction in rats subjected to water immersion and restraint stress. Mol. Med. Rep. 2016, 14, 5116–5124. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Wang, Q.; Zhang, G.; Li, Z.; Wang, Q. Differentially expressed miRNAs and potential therapeutic targets for asthenospermia. Andrologia 2021, 54, e14265. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Luo, S.; Xi, Y.; Tu, X.; Yang, X.; Zhang, H.; Feng, J.; Wang, C.; Zhang, Y. Integrative bioinformatics approaches for identifying potential biomarkers and pathways involved in non-obstructive azoospermia. Transl. Androl. Urol. 2021, 10, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Guo, L.; Wang, J.; Li, M.; Appiah, M.O.; Liu, H.; Zhao, J.; Yang, L.; Lu, W. Photoperiodic effect on the testicular transcriptome in broiler roosters. J. Anim. Physiol. Anim. Nutr. 2020, 104, 918–927. [Google Scholar] [CrossRef]
- Ghaffari, R.; Di Bona, K.R.; Riley, C.L.; Richburg, J.H. Copper transporter 1 (CTR1) expression by mouse testicular germ cells, but not Sertoli cells, is essential for functional spermatogenesis. PLoS ONE 2019, 14, e0215522. [Google Scholar] [CrossRef] [PubMed]

| Gene ID | Localization | Annotation | Gene ID | Localization | Annotation |
|---|---|---|---|---|---|
| LOC103397162 | W | ankyrin repeat domain-containing protein 13A-like | LOC103397320 | Z | ankyrin repeat domain 13A |
| LOC103397197 | W | SET-binding protein-like | LOC103398277 | Z | SET binding protein 1 |
| LOC103397030 | W | ras-related protein Rab-35-like | LOC103397428 | Z | ras-related protein Rab-35-like |
| LOC103396891 | W | transcriptional activator protein Pur-beta-like | LOC103397871 | Z | transcriptional activator protein Pur-beta |
| LOC103396909 | W | G protein-coupled receptor kinase 5-like | LOC103398033 | Z | G protein-coupled receptor kinase 5-like |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Li, M.; Cui, Z.; Zhang, M.; Zhang, T.; Li, L.; Wang, N.; Xu, X.; Wei, M.; Xu, W. Transcriptomic Analysis Revealed Candidate Genes Involved in Pseudomale Sperm Abnormalities in Chinese Tongue Sole (Cynoglossus semilaevis). Biology 2022, 11, 1716. https://doi.org/10.3390/biology11121716
Sun Y, Li M, Cui Z, Zhang M, Zhang T, Li L, Wang N, Xu X, Wei M, Xu W. Transcriptomic Analysis Revealed Candidate Genes Involved in Pseudomale Sperm Abnormalities in Chinese Tongue Sole (Cynoglossus semilaevis). Biology. 2022; 11(12):1716. https://doi.org/10.3390/biology11121716
Chicago/Turabian StyleSun, Yuxuan, Ming Li, Zhongkai Cui, Mengqian Zhang, Tingting Zhang, Lu Li, Na Wang, Xiwen Xu, Min Wei, and Wenteng Xu. 2022. "Transcriptomic Analysis Revealed Candidate Genes Involved in Pseudomale Sperm Abnormalities in Chinese Tongue Sole (Cynoglossus semilaevis)" Biology 11, no. 12: 1716. https://doi.org/10.3390/biology11121716
APA StyleSun, Y., Li, M., Cui, Z., Zhang, M., Zhang, T., Li, L., Wang, N., Xu, X., Wei, M., & Xu, W. (2022). Transcriptomic Analysis Revealed Candidate Genes Involved in Pseudomale Sperm Abnormalities in Chinese Tongue Sole (Cynoglossus semilaevis). Biology, 11(12), 1716. https://doi.org/10.3390/biology11121716







