UPLC-QTOF/MS Metabolomics and Biochemical Assays Reveal Changes in Hepatic Nutrition and Energy Metabolism during Sexual Maturation in Female Rainbow Trout (Oncorhynchus mykiss)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Fish and Sampling
2.3. Biochemical Parameters’ Determination
2.4. Metabolomics Analysis
2.4.1. Sample Preparation
2.4.2. UPLC-QTOF/MS Conditions
2.5. Data Processing and Statistical Analysis
3. Results
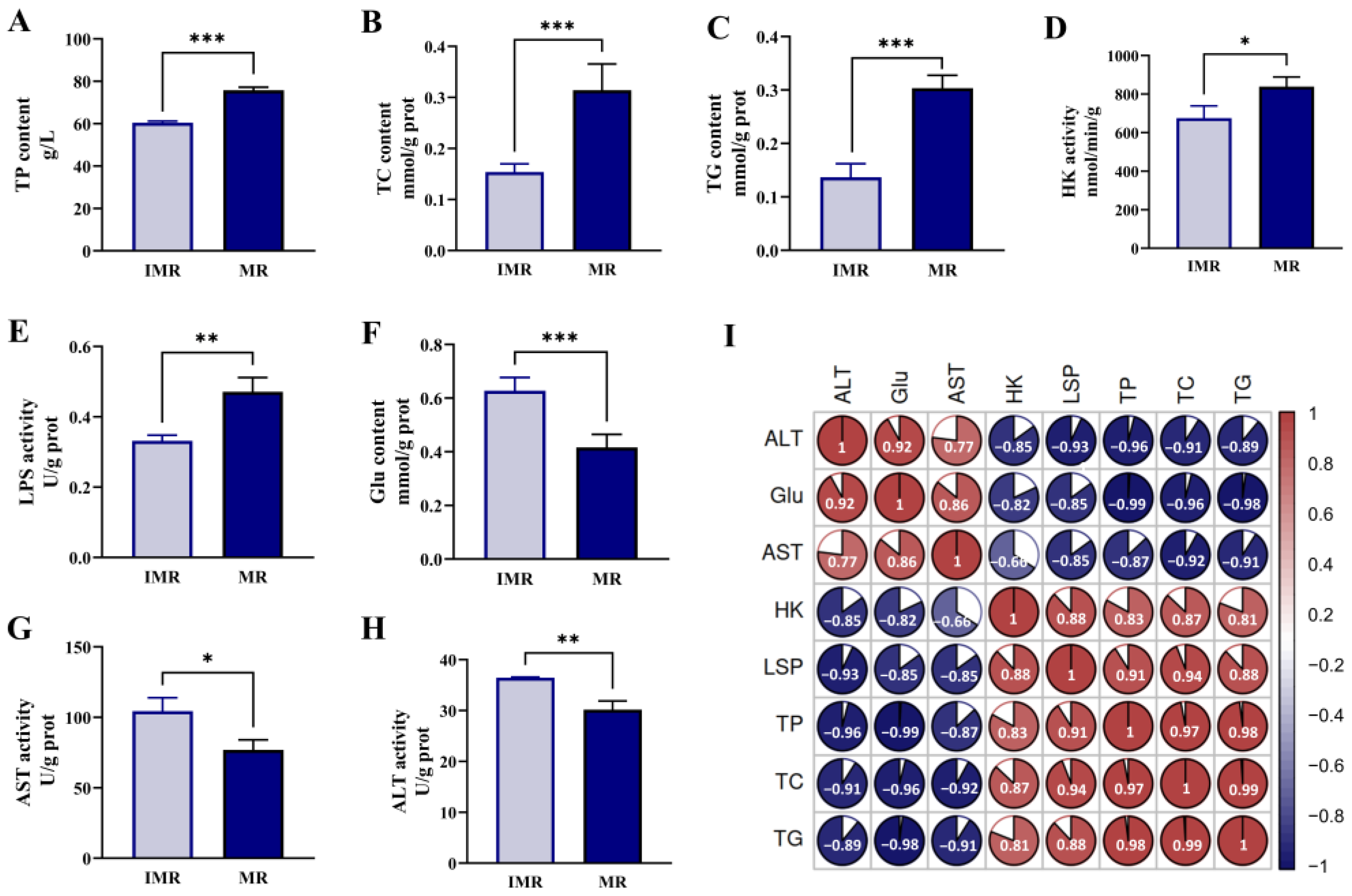
3.1. Biochemical Indicators
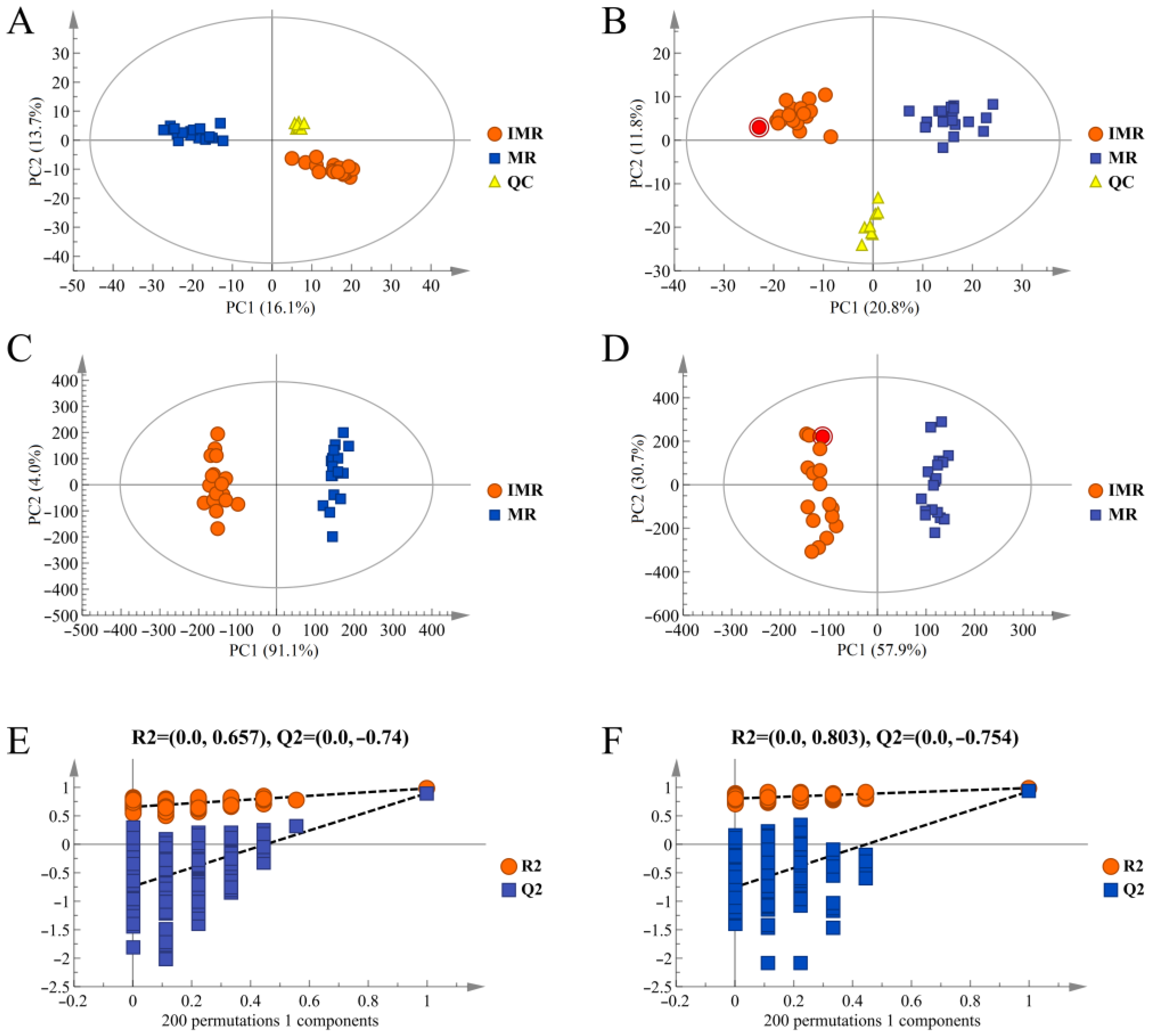
3.2. Metabolic Profiles
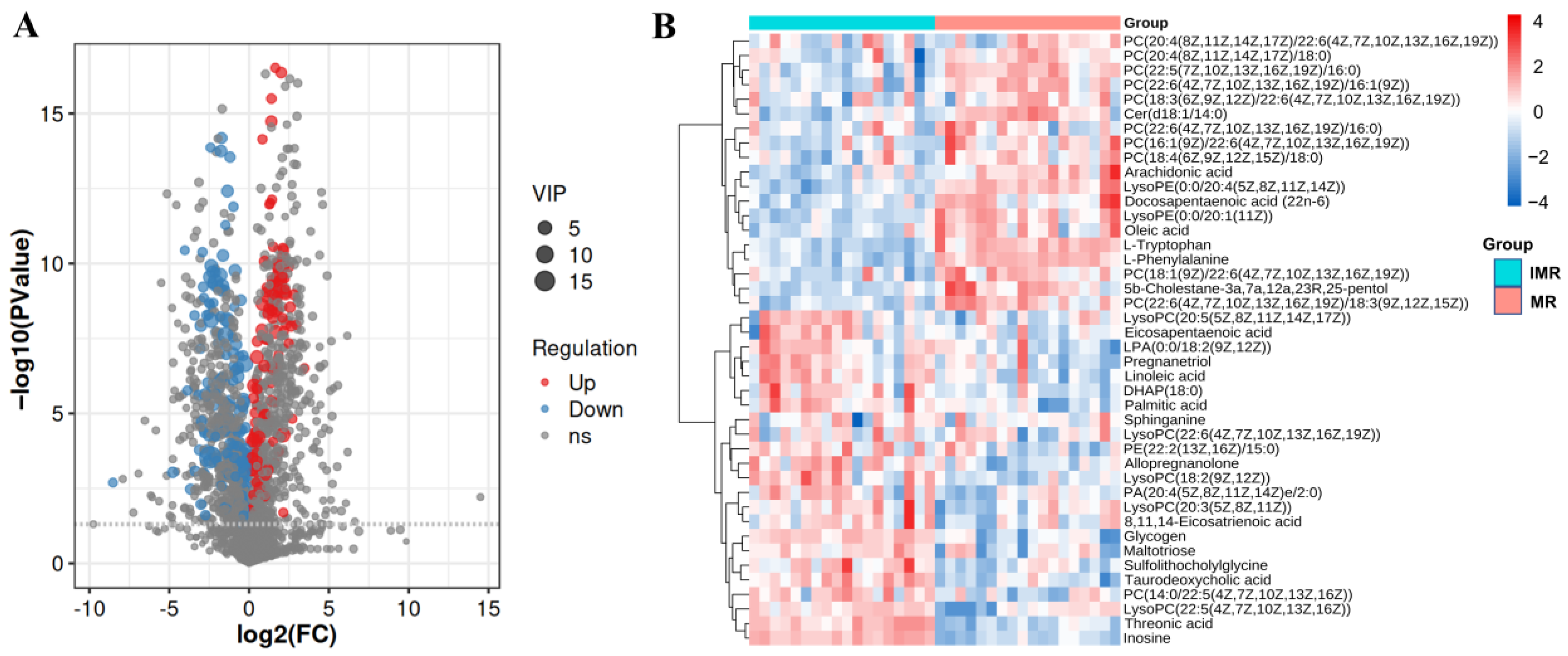
3.3. Identification of Differential Metabolites
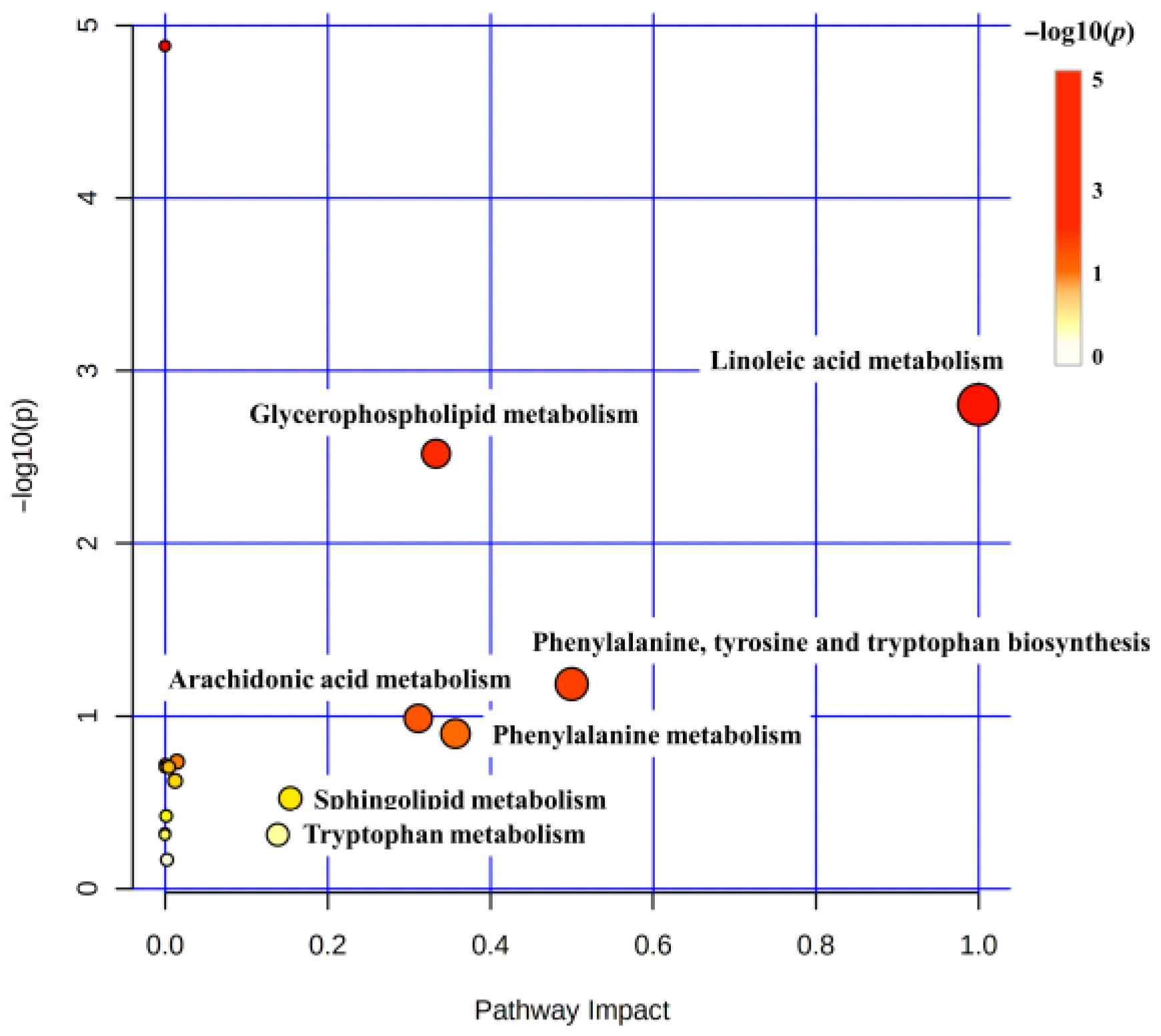
3.4. Metabolic Pathway Analysis
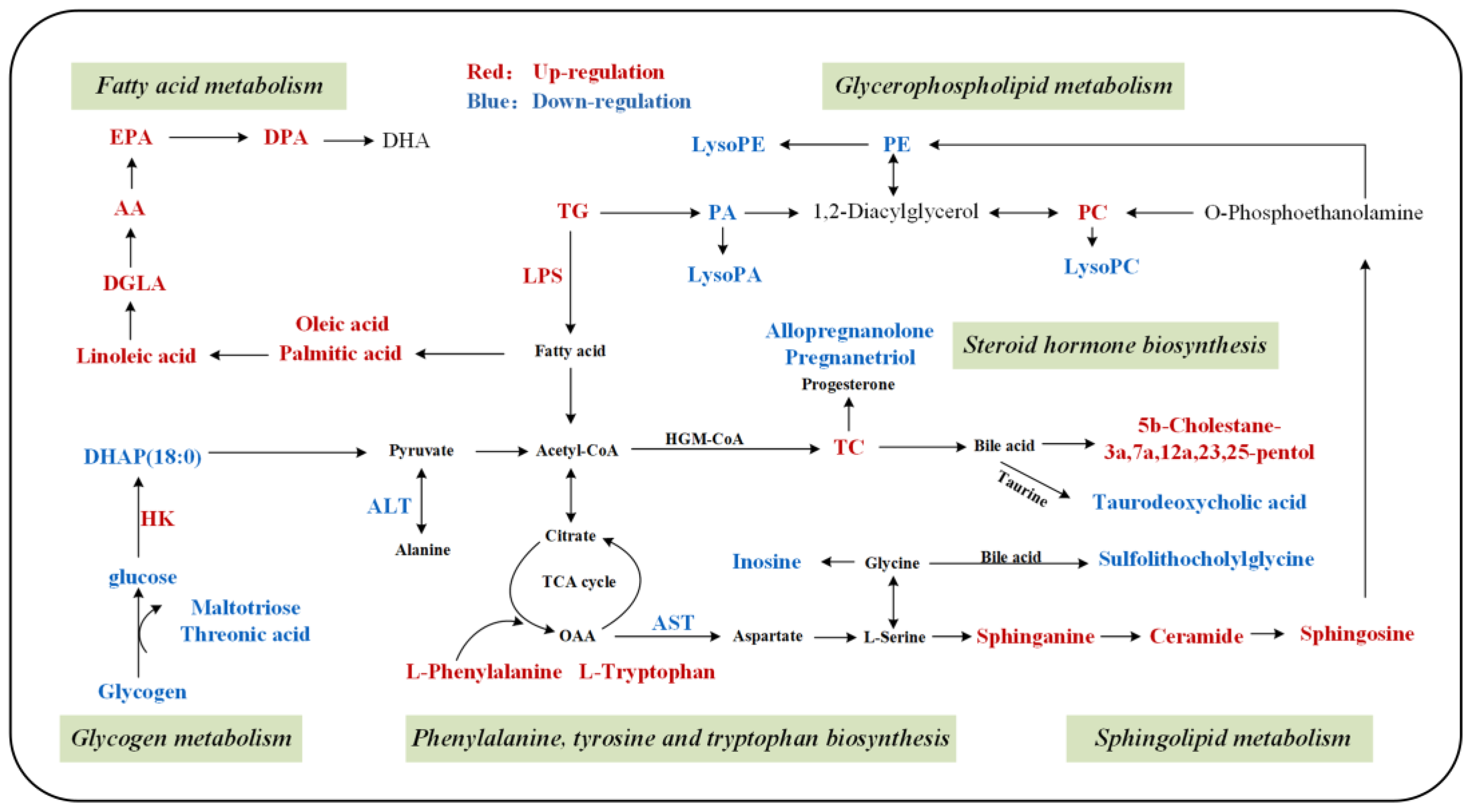
4. Discussion
4.1. Lipid Metabolism
4.1.1. Fatty Acid Metabolism
4.1.2. Phospholipid Metabolism
4.1.3. Steroid Hormone Biosynthesis
4.2. Carbohydrate Metabolism
4.3. Amino Acid Metabolism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yousefi, M.; Ghafarifarsani, H.; Hoseini, S.M.; Hoseinifar, S.H.; Abtahi, B.; Vatnikov, Y.A.; Kulikov, E.V.; Van Doan, H. Effects of dietary thyme essential oil and prebiotic administration on rainbow trout (Oncorhynchus mykiss) welfare and performance. Fish Shellfish. Immunol. 2022, 120, 737–744. [Google Scholar] [CrossRef]
- İnanan, B.E.; Yılmaz, F. Seasonal and age-related changes in semen characteristics and composition of seminal plasma in sex-reverse female rainbow trout (Oncorhynchus mykiss) in comparison with normal males. Anim. Reprod. Sci. 2018, 196, 160–167. [Google Scholar] [CrossRef]
- Harmon, K.J.; Bolinger, M.T.; Rodnick, K.J. Carbohydrate energy reserves and effects of food deprivation in male and female rainbow trout. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2011, 158, 423–431. [Google Scholar] [CrossRef]
- Sun, S.X.; Wu, J.L.; Lv, H.B.; Zhang, H.Y.; Zhang, J.; Limbu, S.M.; Qiao, F.; Chen, L.-Q.; Yang, Y.; Zhang, M.-L. Environmental estrogen exposure converts lipid metabolism in male fish to a female pattern mediated by AMPK and mTOR signaling pathways. J. Hazard. Mater. 2020, 394, 122537. [Google Scholar] [CrossRef]
- Cleveland, B.M.; Weber, G.M.; Raatz, S.K.; Rexroad, C.E.; Picklo, M.J. Fatty acid partitioning varies across fillet regions during sexual maturation in female rainbow trout (Oncorhynchus mykiss). Aquaculture 2017, 475, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Hu, G.; Wang, B. An overview of global rainbow trout breeding industry with insight into reference to China. Chin. J. Fish. 2017, 30, 1–6. [Google Scholar]
- Manor, M.L.; Weber, G.M.; Cleveland, B.M.; Kenney, P.B. Effects of feeding level and sexual maturation on fatty acid composition of energy stores in diploid and triploid rainbow trout (Oncorhynchus mykiss). Aquaculture 2014, 418, 17–25. [Google Scholar] [CrossRef]
- Weber, G.M.; Ma, H.; Birkett, J.; Cleveland, B.M. Effects of feeding level and sexual maturation on expression of genes regulating growth mechanisms in rainbow trout (Oncorhynchus mykiss). Aquaculture 2022, 551, 737917. [Google Scholar] [CrossRef]
- Ye, H.; Xu, M.; Chen, L.; Tan, X.; Chen, S.; Zou, C.; Sun, Z.; Liu, Q.; Ye, C.; Wang, A. Effects of dietary plant protein sources influencing hepatic lipid metabolism and hepatocyte apoptosis in hybrid grouper (Epinephelus lanceolatus♂ × Epinephelus fuscoguttatus♀). Aquaculture 2019, 506, 437–444. [Google Scholar] [CrossRef]
- Kaçar, S.; Başhan, M. Variations in the fatty acid compositions of the liver and gonad tissue of spiny eel (Mastacembelus mastacembelus) from Atatürk Dam Lake. Turk. J. Biochem. 2017, 42, 617–623. [Google Scholar] [CrossRef]
- Bogevik, A.S.; Hayman, E.S.; Bjerke, M.T.; Dessen, J.-E.; Rørvik, K.-A.; Luckenbach, J.A. Phospholipid and LC-PUFA metabolism in Atlantic salmon (Salmo salar) testes during sexual maturation. PLoS ONE 2020, 15, e0233322. [Google Scholar] [CrossRef]
- Pascual, C.; Cruz-Lopez, H.; Mascaró, M.; Gallardo, P.; Sánchez, A.; Domingues, P.; Rosas, C. Changes in biochemical composition and energy reserves associated with sexual maturation of Octopus maya. Front. Physiol. 2020, 11, 22. [Google Scholar] [CrossRef] [Green Version]
- Gu, W.; Xu, G.F.; Hu, G.; Huang, T.Q.; Zhang, Y.Y.; Bai, Q.L.; Wang, B.Q. Comparison of Annual Changes in Serum Steroid Levels Between Male-induced and Normal Diploid Rainbow Trout (Oncorhynchus mykiss). Chin. J. Fish. 2019, 32, 7–11. [Google Scholar]
- Huang, T.; Gu, W.; Liu, E.; Shi, X.; Wang, B.; Wu, W.; Dong, F.; Xu, G. Comprehensive analysis of miRNA-mRNA/lncRNA during gonadal development of triploid female rainbow trout (Oncorhynchus mykiss). Genomics 2021, 113, 3533–3543. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhou, B.; Chen, H.; Tang, X.; Wang, Y. Reactive oxygen species (ROS) and the calcium-(Ca2+) mediated extrinsic and intrinsic pathways underlying BDE-47-induced apoptosis in rainbow trout (Oncorhynchus mykiss) gonadal cells. Sci. Total Environ. 2019, 656, 778–788. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, M.; Chen, P.; Harnly, J.M.; Sun, J. Mass Spectrometry-Based Nontargeted and Targeted Analytical Approaches in Fingerprinting and Metabolomics of Food and Agricultural Research. J. Agric. Food Chem. 2022, 70, 11138–11153. [Google Scholar] [CrossRef]
- Gong, B.; Bao, F.; Wang, Y.; Liu, H.; Xiao, M.; He, J. Metabonomics study on the effect of traditional Chinese medicines feed addition on growth performance and serum metabolic profile of juvenile Chinese softshell turtle (Pelodiscus sinensis Wiegmann). Aquac. Rep. 2021, 20, 100632. [Google Scholar] [CrossRef]
- Sun, Y.C.; Han, S.C.; Yao, M.Z.; Liu, H.B.; Wang, Y.M. Exploring the metabolic biomarkers and pathway changes in crucian under carbonate alkalinity exposure using high-throughput metabolomics analysis based on UPLC-ESI-QTOF-MS. RSC Adv. 2020, 10, 1552–1571. [Google Scholar] [CrossRef]
- Ladisa, C.; Ma, Y.; Habibi, H.R. Seasonally related metabolic changes and energy allocation associated with growth and reproductive phases in the liver of male goldfish (Carassius auratus). J. Proteom. 2021, 241, 104237. [Google Scholar] [CrossRef]
- Yi, S.; Liu, L.F.; Zhou, L.F.; Zhao, B.W.; Wang, W.M.; Gao, Z.X. Screening of biomarkers related to ovarian maturation and spawning in blunt snout bream (Megalobrama amblycephala) based on metabolomics and transcriptomics. Mar. Biotechnol. 2020, 22, 180–193. [Google Scholar] [CrossRef]
- Du, H.; Lv, H.; Xu, Z.; Zhao, S.; Huang, T.; Manyande, A.; Xiong, S. The mechanism for improving the flesh quality of grass carp (Ctenopharyngodon idella) following the micro-flowing water treatment using a UPLC-QTOF/MS based metabolomics method. Food Chem. 2020, 327, 126777. [Google Scholar] [CrossRef]
- Meng, Q.W.; Su, J.X.; Li, W.D. Comparative Anatomy of Fishes; Science Press: Beijing, China, 1987; pp. 1–367. [Google Scholar]
- Pang, Z.; Zhou, G.; Ewald, J.; Chang, L.; Hacariz, O.; Basu, N.; Xia, J. Using MetaboAnalyst 5.0 for LC–HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat. Protoc. 2022, 17, 1735–1761. [Google Scholar] [CrossRef]
- Fontana, R.; Della Torre, S. The deep correlation between energy metabolism and reproduction: A view on the effects of nutrition for women fertility. Nutrients 2016, 8, 87. [Google Scholar] [CrossRef]
- Lam, S.M.; Shui, G. Lipidomics as a principal tool for advancing biomedical research. J. Genet. Genom. 2013, 40, 375–390. [Google Scholar] [CrossRef]
- Singh, R.; Kaushik, S.; Wang, Y.; Xiang, Y.; Novak, I.; Komatsu, M.; Tanaka, K.; Cuervo, A.M.; Czaja, M.J. Autophagy regulates lipid metabolism. Nature 2009, 458, 1131–1135. [Google Scholar] [CrossRef] [Green Version]
- Gu, L.; Liu, H.; Gu, X.; Boots, C.; Moley, K.H.; Wang, Q. Metabolic control of oocyte development: Linking maternal nutrition and reproductive outcomes. Cell. Mol. Life Sci. 2015, 72, 251–271. [Google Scholar] [CrossRef]
- Meesapyodsuk, D.; Qiu, X. The front-end desaturase: Structure, function, evolution and biotechnological use. Lipids 2012, 47, 227–237. [Google Scholar] [CrossRef]
- Suloma, A.; Ogata, H. Arachidonic acid is a major component in gonadal fatty acids of tropical coral reef fish in the Philippines and Japan. J. Aquac. Res. Dev. 2011, 2, 111. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.F.; Tong, W.F.; Ruan, Y.; Andrew, J.S.; Li, D. Different metabolism of EPA, DPA and DHA in humans, A double-blind cross-over study. Prostaglandins Leukot. Essent. Fat. Acids 2020, 158, 102033. [Google Scholar] [CrossRef]
- Røjbek, M.; Støttrup, J.; Jacobsen, C.; Tomkiewicz, J.; Nielsen, A.; Trippel, E. Effects of dietary fatty acids on the production and quality of eggs and larvae of Atlantic cod (Gadus morhua L.). Aquac. Nutr. 2014, 20, 654–666. [Google Scholar] [CrossRef]
- Zhu, Y.; Tan, Q.; Zhang, L.; Yao, J.; Zhou, H.; Hu, P.; Liang, X.; Liu, H. The migration of docosahexenoic acid (DHA) to the developing ovary of female zebrafish (Danio rerio). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2019, 233, 97–105. [Google Scholar] [CrossRef]
- Li, S.; Wen, W.; Gong, X.; Huang, X.; Chen, N. Variation of lipids and fatty acids composition in the tissues of wild devil stinger (Inimicus japonicas) during sexual maturation. Aquac. Fish. 2018, 3, 115–121. [Google Scholar] [CrossRef]
- Long, S.; Dong, X.; Liu, H.; Yan, X.; Tan, B.; Zhang, S.; Chi, S.; Yang, Q.; Liu, H.; Yang, Y. Effect of dietary oxidized fish oil on liver function in hybrid grouper (♀ Epinephelus fuscoguttatus × ♂ Epinephelus lanceolatus). Aquac. Rep. 2022, 22, 101000. [Google Scholar] [CrossRef]
- Lutkewitte, A.J.; Finck, B.N. Regulation of signaling and metabolism by lipin-mediated phosphatidic acid phosphohydrolase activity. Biomolecules 2020, 10, 1386. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef]
- Ledeen, R.W.; Wu, G. Sphingolipids of the nucleus and their role in nuclear signaling. Biochim. Et Biophys. Acta Mol. Cell Biol. Lipids 2006, 1761, 588–598. [Google Scholar] [CrossRef]
- Shirai, N.; Suzuki, H.; Toukairin, S.; Wada, S. Spawning and season affect lipid content and fatty acid composition of ovary and liver in Japanese catfish (Silurus asotus). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2001, 129, 185–195. [Google Scholar] [CrossRef]
- Ladisa, C.; Ma, Y.; Habibi, H.R. Metabolic Changes During Growth and Reproductive Phases in the Liver of Female Goldfish (Carassius auratus). Front. Cell Dev. Biol. 2022, 10, 834688. [Google Scholar] [CrossRef]
- Batetta, B.; Sanna, F. Cholesterol metabolism during cell growth, Which role for the plasma membrane? Eur. J. Lipid Sci. Technol. 2006, 108, 687–699. [Google Scholar] [CrossRef]
- Li, T.; Matozel, M.; Boehme, S.; Kong, B.; Nilsson, L.M.; Guo, G.; Ellis, E.; Chiang, J.Y. Overexpression of cholesterol 7α-hydroxylase promotes hepatic bile acid synthesis and secretion and maintains cholesterol homeostasis. Hepatology 2011, 53, 996–1006. [Google Scholar] [CrossRef] [Green Version]
- Jinn, S.; Brandis, K.A.; Ren, A.; Chacko, A.; Dudley-Rucker, N.; Gale, S.E.; Sidhu, R.; Fujiwara, H.; Jiang, H.; Olsen, B.N. snoRNA U17 regulates cellular cholesterol trafficking. Cell Metab. 2015, 21, 855–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Hong, X.; Mesiano, S.; Muglia, L.J.; Wang, X.; Snyder, M.; Stevenson, D.K.; Shaw, G.M. Natural selection has differentiated the progesterone receptor among human populations. Am. J. Hum. Genet. 2018, 103, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Adel, M.; Safari, R.; Yeganeh, S.; Satheesh Kumar, P.; Safaie, P. Hematological and biochemical profile of pike breeders (Esox lucius Linneaus, 1758) from the Anzali Wetland, Caspian Sea. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2017, 87, 1271–1276. [Google Scholar] [CrossRef]
- Munakata, A. Migratory Behaviors in Masu Salmon (Oncorhynchus masou) and the Influence of Endocrinological Factors; Terrapub: Tokyo, Japan, 2012; pp. 29–65. [Google Scholar]
- Strauss, J.F., III; Williams, C.J. Ovarian Life Cycle. In Yen and Jaffe’s Reproductive Endocrinology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 167–205.e169. [Google Scholar]
- Harrison, K.E. The role of nutrition in maturation, reproduction and embryonic development of decapod crustacean: A review. J. Shellfish. Res. 1990, 9, 1–28. [Google Scholar]
- Kamalam, B.S.; Medale, F.; Panserat, S. Utilisation of dietary carbohydrates in farmed fishes: New insights on influencing factors, biological limitations and future strategies. Aquaculture 2017, 467, 3–27. [Google Scholar] [CrossRef]
- Leung, L.; Woo, N. Influence of dietary carbohydrate level on endocrine status and hepatic carbohydrate metabolism in the marine fish Sparus sarba. Fish Physiol. Biochem. 2012, 38, 543–554. [Google Scholar] [CrossRef]
- Zhao, H.; Ke, H.; Zhang, L.; Zhao, Z.; Lai, J.; Zhou, J.; Huang, Z.; Li, H.; Du, J.; Li, Q. Integrated analysis about the effects of heat stress on physiological responses and energy metabolism in Gymnocypris chilianensis. Sci. Total Environ. 2022, 806, 151252. [Google Scholar] [CrossRef]
- Soengas, J.; Barciela, P.; Aldegunde, M. Variations in carbohydrate metabolism during gonad maturation in female turbot (Scophthalmus maximus). Mar. Biol. 1995, 123, 11–18. [Google Scholar] [CrossRef]
- Bo, Q.K.; Lu, Y.Z.; Ma, C.; Mi, H.J.; Geng, X.Y. Reproductive biology and biochemical changes in female mantis shrimp Oratosquilla oratoria (Stomatopoda) with ovary development from the Tianjin coastal zone of Bohai Bay. Aquaculture 2021, 534, 736239. [Google Scholar] [CrossRef]
- Cho, S.Y.; Kwon, Y.K.; Nam, M.; Vaidya, B.; Kim, S.R.; Lee, S.; Kwon, J.; Kim, D.; Hwang, G.S. Integrated profiling of global metabolomic and transcriptomic responses to viral hemorrhagic septicemia virus infection in olive flounder. Fish Shellfish. Immunol. 2017, 71, 220–229. [Google Scholar] [CrossRef]
- Zhao, M.; Zhu, X.; Shan, D.; Huang, X.; Xu, Q. Metabolomics in liver injury induced by dietary cadmium exposure and protective effect of calcium supplementation. Anal. Biochem. 2022, 641, 114556. [Google Scholar] [CrossRef] [PubMed]
- Anesi, A.; Rubert, J.; Oluwagbemigun, K.; Orozco-Ruiz, X.; Nöthlings, U.; Breteler, M.M.; Mattivi, F. Metabolic profiling of human plasma and urine, targeting tryptophan, tyrosine and branched chain amino acid pathways. Metabolites 2019, 9, 261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Wu, J.; Leng, X.; Du, H.; Wu, J.; He, S.; Luo, J.; Liang, X.; Liu, H.; Wei, Q. Metabolomics and gene expressions revealed the metabolic changes of lipid and amino acids and the related energetic mechanism in response to ovary development of Chinese sturgeon (Acipenser sinensis). PLoS ONE 2020, 15, e0235043. [Google Scholar] [CrossRef]
- Jiang, J.; Zhao, Z.; Gao, S.; Chen, Z.; Dong, Y.; He, P.; Wang, B.; Pan, Y.; Wang, X.; Guan, X. Divergent metabolic responses to sex and reproduction in the sea cucumber Apostichopus japonicus. Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 39, 100845. [Google Scholar] [CrossRef] [PubMed]
- Suljević, D.; Alijagić, A.; Islamagić, E. Temporal influence of spawning on serum biochemical parameters in brown trout Salmo trutta (teleostei, Salmonidae). Bulg. J. Agric. Sci. 2017, 23, 485–490. [Google Scholar]
- Akhavan, S.R.; Salati, A.P.; Falahatkar, B.; Jalali, S.A.H. Changes of vitellogenin and Lipase in captive Sterlet sturgeon Acipenser ruthenus females during previtellogenesis to early atresia. Fish Physiol. Biochem. 2016, 42, 967–978. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, L.; Liu, Y.; Kang, M.; Wei, X.; Geng, C.; Liu, W.; Han, L.; Yuan, F.; Wang, P.; Wang, B.; et al. UPLC-QTOF/MS Metabolomics and Biochemical Assays Reveal Changes in Hepatic Nutrition and Energy Metabolism during Sexual Maturation in Female Rainbow Trout (Oncorhynchus mykiss). Biology 2022, 11, 1679. https://doi.org/10.3390/biology11111679
Ding L, Liu Y, Kang M, Wei X, Geng C, Liu W, Han L, Yuan F, Wang P, Wang B, et al. UPLC-QTOF/MS Metabolomics and Biochemical Assays Reveal Changes in Hepatic Nutrition and Energy Metabolism during Sexual Maturation in Female Rainbow Trout (Oncorhynchus mykiss). Biology. 2022; 11(11):1679. https://doi.org/10.3390/biology11111679
Chicago/Turabian StyleDing, Lu, Yingjie Liu, Meng Kang, Xiaofeng Wei, Chuanye Geng, Wenzhi Liu, Lin Han, Fangying Yuan, Peng Wang, Bingqian Wang, and et al. 2022. "UPLC-QTOF/MS Metabolomics and Biochemical Assays Reveal Changes in Hepatic Nutrition and Energy Metabolism during Sexual Maturation in Female Rainbow Trout (Oncorhynchus mykiss)" Biology 11, no. 11: 1679. https://doi.org/10.3390/biology11111679




