Aging and Mesenchymal Stem Cells: Basic Concepts, Challenges and Strategies
Abstract
Simple Summary
Abstract
1. Introduction
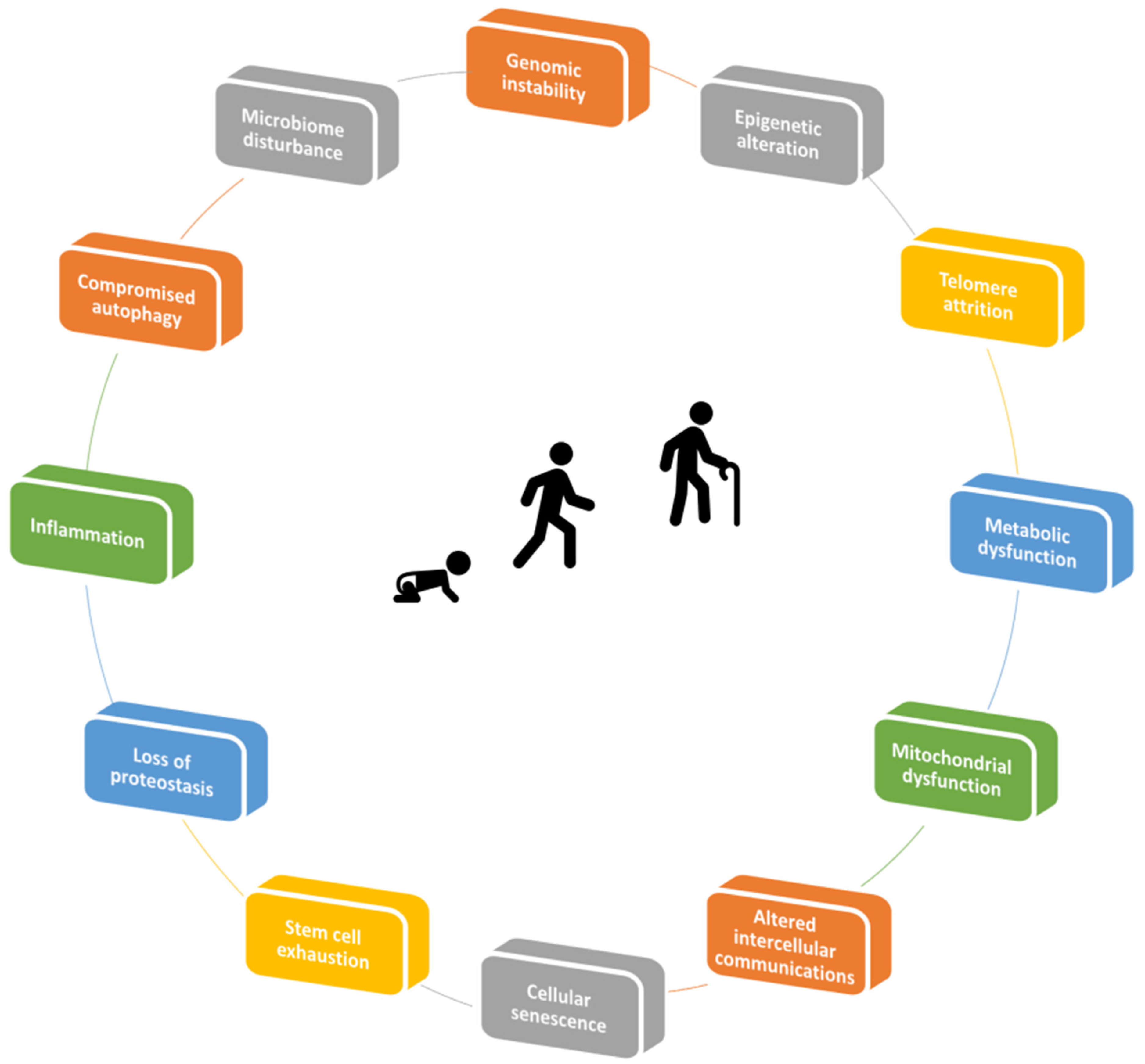
2. Mechanisms of Aging
2.1. The Importance of the Relationship between Aging and Immune System
3. Aging and Disease
4. MSC and Tissue Homeostasis
4.1. Anti-Inflammatory Effects
4.2. Regenerative Effects
4.3. Antioxidative Stress Effects
4.4. Antitumor Effects
4.5. Antifibrotic Effects
4.6. Antimicrobial Effects
5. MSC and Aging
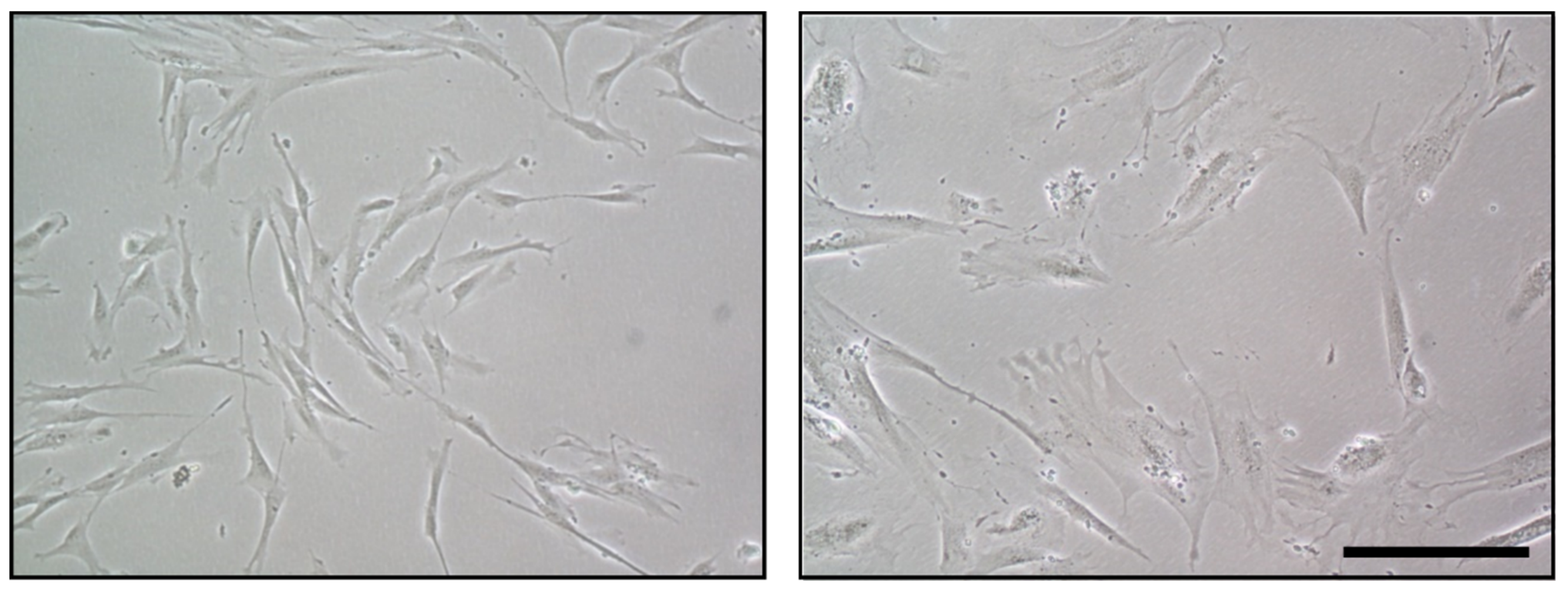
5.1. Functional Alterations of MSC in Aging and Related Diseases
6. MSC as Basis of Anti-Aging Strategies
6.1. Senolytics: Elimination of Senescent Cells
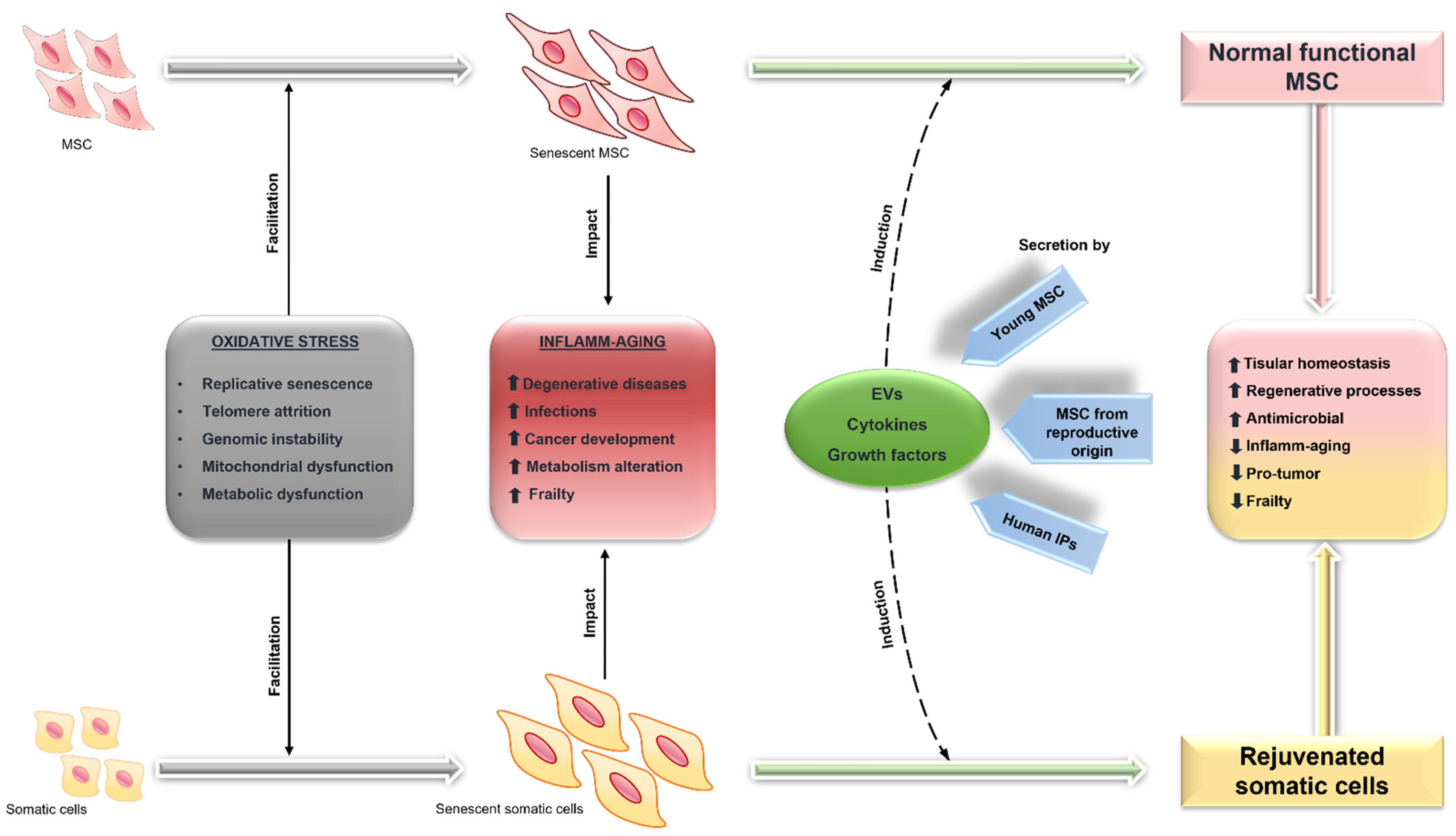
6.2. Rejuvenation of MSC
6.3. Replacing MSC
6.4. Therapy Based on MSC Secretome-Derived Products: A New Proposal for Aging Frailty Treatment
7. The Challenge to Implement a Therapy Based on ERRISC for Aging Frailty Treatment
7.1. More Precise Diagnosis of the Aging Frailty
7.2. The More Suitable Source of MSC
7.3. The Need for Mass Production of MSC
7.4. Standardization, Functional Test for Anti-Aging Effects, and Mechanisms
7.5. The Selection of Administration Route
8. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Population Ageing, 2019 Highlights; United Nations Department of Economic and Social Affairs: New York, NY, USA, 2019; Volume 430.
- Gautam, N.; Das, S.; Kar Mahapatra, S.; Chakraborty, S.P.; Kundu, P.K.; Roy, S. Age associated oxidative damage in lymphocytes. Oxid. Med. Cell Longev. 2010, 3, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Suzman, R.; Beard, J.R.; Boerma, T.; Chatterji, S. Health in an ageing world--what do we know? Lancet 2015, 385, 484–486. [Google Scholar] [CrossRef]
- Wimmer, B.C.; Cross, A.J.; Jokanovic, N.; Wiese, M.D.; George, J.; Johnell, K.; Diug, B.; Bell, J.S. Clinical Outcomes Associated with Medication Regimen Complexity in Older People: A Systematic Review. J. Am. Geriatr. Soc. 2017, 65, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Crimmins, E.M.; Beltrán-Sánchez, H. Mortality and morbidity trends: Is there compression of morbidity? J. Gerontol. B. Psychol. Sci. Soc. Sci. 2011, 66, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Dziechciaż, M.; Filip, R. Biological psychological and social determinants of old age: Bio-psycho-social aspects of human aging. Ann. Agric. Env. Med. 2014, 21, 835–838. [Google Scholar] [CrossRef]
- Cohen, A.A. Complex systems dynamics in aging: New evidence, continuing questions. Biogerontology 2016, 17, 205–220. [Google Scholar] [CrossRef]
- da Costa, J.P.; Vitorino, R.; Silva, G.M.; Vogel, C.; Duarte, A.C.; Rocha-Santos, T. A synopsis on aging-Theories, mechanisms and future prospects. Ageing Res. Rev. 2016, 29, 90–112. [Google Scholar] [CrossRef]
- Cohen, A.A.; Kennedy, B.K.; Anglas, U.; Bronikowski, A.M.; Deelen, J.; Dufour, F.; Ferbeyre, G.; Ferrucci, L.; Franceschi, C.; Frasca, D.; et al. Lack of consensus on an aging biology paradigm? A global survey reveals an agreement to disagree, and the need for an interdisciplinary framework. Mech. Ageing Dev. 2020, 191, 111316. [Google Scholar] [CrossRef]
- Schmauck-Medina, T.; Moliere, A.; Lautrup, S.; Zhang, J.; Chlopicki, S.; Madsen, H.B.; Cao, S.; Soendenbroe, C.; Mansell, E.; Vestergaard, M.B.; et al. New hallmarks of ageing: A 2022 Copenhagen ageing meeting summary. Aging 2022, 14, 6829–6839. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Lidzbarsky, G.; Gutman, D.; Shekhidem, H.A.; Sharvit, L.; Atzmon, G. Genomic Instabilities, Cellular Senescence, and Aging: In Vitro, In Vivo and Aging-Like Human Syndromes. Front. Med. 2018, 5, 104. [Google Scholar] [CrossRef] [PubMed]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef]
- Toussaint, O.; Medrano, E.E.; von Zglinicki, T. Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes. Exp. Gerontol. 2000, 35, 927–945. [Google Scholar] [CrossRef]
- Stolzing, A.; Coleman, N.; Scutt, A. Glucose-induced replicative senescence in mesenchymal stem cells. Rejuvenation Res. 2006, 9, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, Q.; Wang, Y.; Li, L.; Bu, H.; Bao, J. Senescence of mesenchymal stem cells (Review). Int. J. Mol. Med. 2017, 39, 775–782. [Google Scholar] [CrossRef]
- Wirawan, E.; Vanden Berghe, T.; Lippens, S.; Agostinis, P.; Vandenabeele, P. Autophagy: For better or for worse. Cell Res. 2012, 22, 43–61. [Google Scholar] [CrossRef]
- Rubinsztein, D.C.; Mariño, G.; Kroemer, G. Autophagy and aging. Cell 2011, 146, 682–695. [Google Scholar] [CrossRef]
- Xie, W.; Baylin, S.B.; Easwaran, H. DNA methylation in senescence, aging and cancer. Oncoscience 2019, 6, 291–293. [Google Scholar] [CrossRef]
- Cakouros, D.; Gronthos, S. Epigenetic Regulation of Bone Marrow Stem Cell Aging: Revealing Epigenetic Signatures associated with Hematopoietic and Mesenchymal Stem Cell Aging. Aging Dis. 2019, 10, 174–189. [Google Scholar] [CrossRef]
- Wagner, W. The Link Between Epigenetic Clocks for Aging and Senescence. Front. Genet. 2019, 10, 303. [Google Scholar] [CrossRef]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef] [PubMed]
- Yeo, G.E.C.; Ng, M.H.; Nordin, F.B.; Law, J.X. Potential of Mesenchymal Stem Cells in the Rejuvenation of the Aging Immune System. Int. J. Mol. Sci. 2021, 22, 5749. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Larbi, A.; Dupuis, G.; Le Page, A.; Frost, E.H.; Cohen, A.A.; Witkowski, J.M.; Franceschi, C. Immunosenescence and Inflamm-Aging As Two Sides of the Same Coin: Friends or Foes? Front. Immunol. 2017, 8, 1960. [Google Scholar] [CrossRef]
- Yao, X.; Li, H.; Leng, S.X. Inflammation and immune system alterations in frailty. Clin. Geriatr. Med. 2011, 27, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Wenisch, C.; Patruta, S.; Daxböck, F.; Krause, R.; Hörl, W. Effect of age on human neutrophil function. J. Leukoc. Biol. 2000, 67, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Gounder, S.S.; Abdullah, B.J.J.; Radzuanb, N.; Zain, F.; Sait, N.B.M.; Chua, C.; Subramani, B. Effect of Aging on NK Cell Population and Their Proliferation at Ex Vivo Culture Condition. Anal. Cell Pathol. 2018, 2018, 7871814. [Google Scholar] [CrossRef]
- Van den Biggelaar, A.H.; Huizinga, T.W.; de Craen, A.J.; Gussekloo, J.; Heijmans, B.T.; Frölich, M.; Westendorp, R.G. Impaired innate immunity predicts frailty in old age. The Leiden 85-plus study. Exp. Gerontol. 2004, 39, 1407–1414. [Google Scholar] [CrossRef]
- Panda, A.; Qian, F.; Mohanty, S.; van Duin, D.; Newman, F.K.; Zhang, L.; Chen, S.; Towle, V.; Belshe, R.B.; Fikrig, E.; et al. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J. Immunol. 2010, 184, 2518–2527. [Google Scholar] [CrossRef]
- Lee, G.H.; Hong, K.T.; Choi, J.Y.; Shin, H.Y.; Lee, W.W.; Kang, H.J. Immunosenescent characteristics of T cells in young patients following haploidentical haematopoietic stem cell transplantation from parental donors. Clin. Transl. Immunol. 2020, 9, e1124. [Google Scholar] [CrossRef]
- Ma, S.; Wang, C.; Mao, X.; Hao, Y. B Cell Dysfunction Associated With Aging and Autoimmune Diseases. Front. Immunol. 2019, 10, 318. [Google Scholar] [CrossRef]
- Holodick, N.E.; Rothstein, T.L. B cells in the aging immune system: Time to consider B-1 cells. Ann. N. Y. Acad. Sci. 2015, 1362, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Lelic, A.; Verschoor, C.P.; Ventresca, M.; Parsons, R.; Evelegh, C.; Bowdish, D.; Betts, M.R.; Loeb, M.B.; Bramson, J.L. The polyfunctionality of human memory CD8+ T cells elicited by acute and chronic virus infections is not influenced by age. PLoS Pathog 2012, 8, e1003076. [Google Scholar] [CrossRef] [PubMed]
- Frasca, D.; Diaz, A.; Romero, M.; Blomberg, B.B. Human peripheral late/exhausted memory B cells express a senescent-associated secretory phenotype and preferentially utilize metabolic signaling pathways. Exp. Gerontol. 2017, 87, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; de Luca, M.; Ottaviani, E.; de Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Fedarko, N.S. The biology of aging and frailty. Clin. Geriatr. Med. 2011, 27, 27–37. [Google Scholar] [CrossRef]
- Schulman, I.H.; Balkan, W.; Hare, J.M. Mesenchymal Stem Cell Therapy for Aging Frailty. Front. Nutr. 2018, 5, 108. [Google Scholar] [CrossRef]
- Thomas, R.; Wang, W.; Su, D.M. Contributions of Age-Related Thymic Involution to Immunosenescence and Inflammaging. Immun. Ageing 2020, 17, 2. [Google Scholar] [CrossRef]
- Hsieh, T.H.; Tsai, T.T.; Chen, C.L.; Shen, T.J.; Jhan, M.K.; Tseng, P.C.; Lin, C.F. Senescence in Monocytes Facilitates Dengue Virus Infection by Increasing Infectivity. Front. Cell Infect. Microbiol. 2020, 10, 375. [Google Scholar] [CrossRef]
- Bewick, T.; Sheppard, C.; Greenwood, S.; Slack, M.; Trotter, C.; George, R.; Lim, W.S. Serotype prevalence in adults hospitalised with pneumococcal non-invasive community-acquired pneumonia. Thorax 2012, 67, 540–545. [Google Scholar] [CrossRef]
- Martin-Loeches, I.; Guia, M.C.; Vallecoccia, M.S.; Suarez, D.; Ibarz, M.; Irazabal, M.; Ferrer, R.; Artigas, A. Risk factors for mortality in elderly and very elderly critically ill patients with sepsis: A prospective, observational, multicenter cohort study. Ann. Intensive Care 2019, 9, 26. [Google Scholar] [CrossRef]
- Borst, J.; Ahrends, T.; Bąbała, N.; Melief, C.J.M.; Kastenmüller, W. CD4(+) T cell help in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2018, 18, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Blasi, F.; Akova, M.; Bonanni, P.; Dartois, N.; Sauty, E.; Webber, C.; Torres, A. Community-acquired pneumonia in adults: Highlighting missed opportunities for vaccination. Eur. J. Intern. Med. 2017, 37, 13–18. [Google Scholar] [CrossRef]
- Weinberger, B. Vaccines for the elderly: Current use and future challenges. Immun. Ageing 2018, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Aspinall, R.; Del Giudice, G.; Effros, R.B.; Grubeck-Loebenstein, B.; Sambhara, S. Challenges for vaccination in the elderly. Immun. Ageing 2007, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Lang, P.O.; Mendes, A.; Socquet, J.; Assir, N.; Govind, S.; Aspinall, R. Effectiveness of influenza vaccine in aging and older adults: Comprehensive analysis of the evidence. Clin. Interv. Aging 2012, 7, 55–64. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen Med. 2019, 4, 22. [Google Scholar] [CrossRef]
- Sanders, K.M.; Ward, S.M.; Koh, S.D. Interstitial cells: Regulators of smooth muscle function. Physiol. Rev. 2014, 94, 859–907. [Google Scholar] [CrossRef]
- Firestein, G.S.; Gabriel, S.E.; McInnes, I.B.; O′Dell, J.R. Kelley & Firestein′s Textbook of Rheumatology, 10th ed.; Elsevier: Philadelphia, PA, USA, 2017. [Google Scholar]
- Rinn, J.L.; Bondre, C.; Gladstone, H.B.; Brown, P.O.; Chang, H.Y. Anatomic demarcation by positional variation in fibroblast gene expression programs. PLoS Genet. 2006, 2, e119. [Google Scholar] [CrossRef]
- Driscoll, J.; Patel, T. The mesenchymal stem cell secretome as an acellular regenerative therapy for liver disease. J. Gastroenterol. 2019, 54, 763–773. [Google Scholar] [CrossRef]
- Vizoso, F.J.; Eiro, N.; Costa, L.; Esparza, P.; Landin, M.; Diaz-Rodriguez, P.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cells in Homeostasis and Systemic Diseases: Hypothesis, Evidences, and Therapeutic Opportunities. Int. J. Mol. Sci. 2019, 20, 3738. [Google Scholar] [CrossRef]
- Squillaro, T.; Peluso, G.; Galderisi, U. Clinical Trials With Mesenchymal Stem Cells: An Update. Cell Transpl. 2016, 25, 829–848. [Google Scholar] [CrossRef] [PubMed]
- Landgraf, K.; Brunauer, R.; Lepperdinger, G.; Grubeck-Loebenstein, B. The suppressive effect of mesenchymal stromal cells on T cell proliferation is conserved in old age. Transpl. Immunol. 2011, 25, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, M.E.; Avanzini, M.A.; Ciccocioppo, R.; Perotti, C.; Cometa, A.M.; Moretta, A.; Marconi, M.; Valli, M.; Novara, F.; Bonetti, F.; et al. Phenotypical/functional characterization of in vitro-expanded mesenchymal stromal cells from patients with Crohn′s disease. Cytotherapy 2009, 11, 825–836. [Google Scholar] [CrossRef]
- Al Demour, S.; Jafar, H.; Adwan, S.; AlSharif, A.; Alhawari, H.; Alrabadi, A.; Zayed, A.; Jaradat, A.; Awidi, A. Safety and Potential Therapeutic Effect of Two Intracavernous Autologous Bone Marrow Derived Mesenchymal Stem Cells injections in Diabetic Patients with Erectile Dysfunction: An Open Label Phase I Clinical Trial. Urol. Int. 2018, 101, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Iacobaeus, E.; Kadri, N.; Lefsihane, K.; Boberg, E.; Gavin, C.; Törnqvist Andrén, A.; Lilja, A.; Brundin, L.; Blanc, K.L. Short and Long Term Clinical and Immunologic Follow up after Bone Marrow Mesenchymal Stromal Cell Therapy in Progressive Multiple Sclerosis-A Phase I Study. J. Clin. Med. 2019, 8, 2102. [Google Scholar] [CrossRef]
- Gyöngyösi, M.; Wojakowski, W.; Lemarchand, P.; Lunde, K.; Tendera, M.; Bartunek, J.; Marban, E.; Assmus, B.; Henry, T.D.; Traverse, J.H.; et al. Meta-Analysis of Cell-based CaRdiac stUdiEs (ACCRUE) in patients with acute myocardial infarction based on individual patient data. Circ. Res. 2015, 116, 1346–1360. [Google Scholar] [CrossRef]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Larrick, J.W.; Mendelsohn, A.R. Mesenchymal Stem Cells for Frailty? Rejuvenation Res. 2017, 20, 525–529. [Google Scholar] [CrossRef]
- Mushahary, D.; Spittler, A.; Kasper, C.; Weber, V.; Charwat, V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytom. A 2018, 93, 19–31. [Google Scholar] [CrossRef]
- Rasmusson, I. Immune modulation by mesenchymal stem cells. Exp. Cell Res. 2006, 312, 2169–2179. [Google Scholar] [CrossRef] [PubMed]
- Nauta, A.J.; Fibbe, W.E. Immunomodulatory properties of mesenchymal stromal cells. Blood 2007, 110, 3499–3506. [Google Scholar] [CrossRef] [PubMed]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef]
- Weiss, A.R.R.; Dahlke, M.H. Immunomodulation by Mesenchymal Stem Cells (MSCs): Mechanisms of Action of Living, Apoptotic, and Dead MSCs. Front. Immunol. 2019, 10, 1191. [Google Scholar] [CrossRef] [PubMed]
- Eggenhofer, E.; Hoogduijn, M.J. Mesenchymal stem cell-educated macrophages. Transpl. Res. 2012, 1, 12. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hematti, P. Mesenchymal stem cell-educated macrophages: A novel type of alternatively activated macrophages. Exp. Hematol 2009, 37, 1445–1453. [Google Scholar] [CrossRef]
- Jiang, X.X.; Zhang, Y.; Liu, B.; Zhang, S.X.; Wu, Y.; Yu, X.D.; Mao, N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 2005, 105, 4120–4126. [Google Scholar] [CrossRef]
- Park, S.R.; Kim, J.W.; Jun, H.S.; Roh, J.Y.; Lee, H.Y.; Hong, I.S. Stem Cell Secretome and Its Effect on Cellular Mechanisms Relevant to Wound Healing. Mol. Ther. 2018, 26, 606–617. [Google Scholar] [CrossRef]
- Ahangar, P.; Mills, S.J.; Cowin, A.J. Mesenchymal Stem Cell Secretome as an Emerging Cell-Free Alternative for Improving Wound Repair. Int. J. Mol. Sci. 2020, 21, 7038. [Google Scholar] [CrossRef]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef]
- Efimenko, A.; Starostina, E.; Kalinina, N.; Stolzing, A. Angiogenic properties of aged adipose derived mesenchymal stem cells after hypoxic conditioning. J. Transl. Med. 2011, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Mohsin, S.; Khan, S.N.; Riazuddin, S. Repair of senescent myocardium by mesenchymal stem cells is dependent on the age of donor mice. J. Cell Mol. Med. 2011, 15, 1515–1527. [Google Scholar] [CrossRef]
- Lo Furno, D.; Mannino, G.; Giuffrida, R. Functional role of mesenchymal stem cells in the treatment of chronic neurodegenerative diseases. J. Cell Physiol. 2018, 233, 3982–3999. [Google Scholar] [CrossRef] [PubMed]
- Duncan, T.; Valenzuela, M. Alzheimer′s disease, dementia, and stem cell therapy. Stem Cell Res. 2017, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Valle-Prieto, A.; Conget, P.A. Human mesenchymal stem cells efficiently manage oxidative stress. Stem Cells Dev. 2010, 19, 1885–1893. [Google Scholar] [CrossRef]
- Gorbunov, N.V.; Garrison, B.R.; McDaniel, D.P.; Zhai, M.; Liao, P.J.; Nurmemet, D.; Kiang, J.G. Adaptive redox response of mesenchymal stromal cells to stimulation with lipopolysaccharide inflammagen: Mechanisms of remodeling of tissue barriers in sepsis. Oxid. Med. Cell Longev. 2013, 2013, 186795. [Google Scholar] [CrossRef]
- Stavely, R.; Nurgali, K. The emerging antioxidant paradigm of mesenchymal stem cell therapy. Stem Cells Transl. Med. 2020, 9, 985–1006. [Google Scholar] [CrossRef]
- Eiro, N.; Sendon-Lago, J.; Cid, S.; Saa, J.; de Pablo, N.; Vega, B.; Bermudez, M.A.; Perez-Fernandez, R.; Vizoso, F.J. Conditioned medium from human uterine cervical stem cells regulates oxidative stress and angiogenesis of retinal pigment epithelial cells. Ophthalmic Res. 2022. [Google Scholar] [CrossRef]
- Pallis, A.G.; Fortpied, C.; Wedding, U.; van Nes, M.C.; Penninckx, B.; Ring, A.; Lacombe, D.; Monfardini, S.; Scalliet, P.; Wildiers, H. EORTC elderly task force position paper: Approach to the older cancer patient. Eur. J. Cancer 2010, 46, 1502–1513. [Google Scholar] [CrossRef]
- Pilleron, S.; Sarfati, D.; Janssen-Heijnen, M.; Vignat, J.; Ferlay, J.; Bray, F.; Soerjomataram, I. Global cancer incidence in older adults, 2012 and 2035: A population-based study. Int. J. Cancer 2019, 144, 49–58. [Google Scholar] [CrossRef]
- Stout, N.L.; Wagner, S.S. Antineoplastic therapy side effects and polypharmacy in older adults with cancer. Top. Geriatr. Rehabil. 2019, 35, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Rhee, K.J.; Lee, J.I.; Eom, Y.W. Mesenchymal Stem Cell-Mediated Effects of Tumor Support or Suppression. Int. J. Mol. Sci. 2015, 16, 30015–30033. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Huang, L.; Li, Y.; Fang, B.; Li, G.; Chen, L.; Xu, L. Mesenchymal Stem Cells and Cancer: Clinical Challenges and Opportunities. Biomed. Res. Int. 2019, 2019, 2820853. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.A.; Eiro, N.; Fraile, M.; Gonzalez, L.O.; Saá, J.; Garcia-Portabella, P.; Vega, B.; Schneider, J.; Vizoso, F.J. Functional heterogeneity of mesenchymal stem cells from natural niches to culture conditions: Implications for further clinical uses. Cell Mol. Life Sci. 2021, 78, 447–467. [Google Scholar] [CrossRef]
- Leng, L.; Wang, Y.; He, N.; Wang, D.; Zhao, Q.; Feng, G.; Su, W.; Xu, Y.; Han, Z.; Kong, D.; et al. Molecular imaging for assessment of mesenchymal stem cells mediated breast cancer therapy. Biomaterials 2014, 35, 5162–5170. [Google Scholar] [CrossRef]
- Lozito, T.P.; Tuan, R.S. Mesenchymal stem cells inhibit both endogenous and exogenous MMPs via secreted TIMPs. J. Cell Physiol. 2011, 226, 385–396. [Google Scholar] [CrossRef]
- Eiró, N.; Sendon-Lago, J.; Seoane, S.; Bermúdez, M.A.; Lamelas, M.L.; Garcia-Caballero, T.; Schneider, J.; Perez-Fernandez, R.; Vizoso, F.J. Potential therapeutic effect of the secretome from human uterine cervical stem cells against both cancer and stromal cells compared with adipose tissue stem cells. Oncotarget 2014, 5, 10692–10708. [Google Scholar] [CrossRef]
- Ueda, N.; Atsuta, I.; Ayukawa, Y.; Yamaza, T.; Furuhashi, A.; Narimatsu, I.; Matsuura, Y.; Kondo, R.; Watanabe, Y.; Zhang, X.; et al. Novel Application Method for Mesenchymal Stem Cell Therapy Utilizing Its Attractant-Responsive Accumulation Property. Appl. Sci. 2019, 9, 4908. [Google Scholar] [CrossRef]
- Wu, S.; Ju, G.Q.; Du, T.; Zhu, Y.J.; Liu, G.H. Microvesicles derived from human umbilical cord Wharton′s jelly mesenchymal stem cells attenuate bladder tumor cell growth in vitro and in vivo. PLoS ONE 2013, 8, e61366. [Google Scholar] [CrossRef]
- Reza, A.; Choi, Y.J.; Yasuda, H.; Kim, J.H. Human adipose mesenchymal stem cell-derived exosomal-miRNAs are critical factors for inducing anti-proliferation signalling to A2780 and SKOV-3 ovarian cancer cells. Sci. Rep. 2016, 6, 38498. [Google Scholar] [CrossRef]
- Greco, K.A.; Franzen, C.A.; Foreman, K.E.; Flanigan, R.C.; Kuo, P.C.; Gupta, G.N. PLK-1 Silencing in Bladder Cancer by siRNA Delivered With Exosomes. Urology 2016, 91, e241–e247. [Google Scholar] [CrossRef] [PubMed]
- Smyth, T.J.; Redzic, J.S.; Graner, M.W.; Anchordoquy, T.J. Examination of the specificity of tumor cell derived exosomes with tumor cells in vitro. Biochim. Biophys. Acta 2014, 1838, 2954–2965. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.; Park, S.E.; Jeong, J.B.; Choi, S.J.; Oh, S.Y.; Ryu, G.H.; Lee, J.; Jeon, H.B.; Chang, J.W. Anti-Fibrotic Effect of Human Wharton′s Jelly-Derived Mesenchymal Stem Cells on Skeletal Muscle Cells, Mediated by Secretion of MMP-1. Int. J. Mol. Sci. 2020, 21, 6269. [Google Scholar] [CrossRef]
- Ishiuchi, N.; Nakashima, A.; Doi, S.; Yoshida, K.; Maeda, S.; Kanai, R.; Yamada, Y.; Ike, T.; Doi, T.; Kato, Y.; et al. Hypoxia-preconditioned mesenchymal stem cells prevent renal fibrosis and inflammation in ischemia-reperfusion rats. Stem Cell Res. 2020, 11, 130. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.T.; Sharma, V.; Elmén, L.; Peterson, S.N. Immune homeostasis, dysbiosis and therapeutic modulation of the gut microbiota. Clin. Exp. Immunol. 2015, 179, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Zhao, J.; Wu, L.; Carru, C.; Biagi, E.; Franceschi, C. Microbiomes other than the gut: Inflammaging and age-related diseases. Semin Immunopathol. 2020, 42, 589–605. [Google Scholar] [CrossRef] [PubMed]
- DeJong, E.N.; Surette, M.G.; Bowdish, D.M.E. The Gut Microbiota and Unhealthy Aging: Disentangling Cause from Consequence. Cell Host Microbe 2020, 28, 180–189. [Google Scholar] [CrossRef]
- Ragonnaud, E.; Biragyn, A. Gut microbiota as the key controllers of "healthy" aging of elderly people. Immun. Ageing 2021, 18, 2. [Google Scholar] [CrossRef]
- Bosco, N.; Noti, M. The aging gut microbiome and its impact on host immunity. Genes Immun. 2021, 22, 289–303. [Google Scholar] [CrossRef]
- Wu, X.; Dao Thi, V.L.; Huang, Y.; Billerbeck, E.; Saha, D.; Hoffmann, H.H.; Wang, Y.; Silva, L.A.V.; Sarbanes, S.; Sun, T.; et al. Intrinsic Immunity Shapes Viral Resistance of Stem Cells. Cell 2018, 172, 423–438. [Google Scholar] [CrossRef]
- Bailey, C.C.; Zhong, G.; Huang, I.C.; Farzan, M. IFITM-Family Proteins: The Cell′s First Line of Antiviral Defense. Annu. Rev. Virol. 2014, 1, 261–283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Gallo, R.L. Antimicrobial peptides. Curr. Biol. 2016, 26, R14–R19. [Google Scholar] [CrossRef] [PubMed]
- Yagi, H.; Chen, A.F.; Hirsch, D.; Rothenberg, A.C.; Tan, J.; Alexander, P.G.; Tuan, R.S. Antimicrobial activity of mesenchymal stem cells against Staphylococcus aureus. Stem Cell Res. 2020, 11, 293. [Google Scholar] [CrossRef]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef]
- Agerberth, B.; Charo, J.; Werr, J.; Olsson, B.; Idali, F.; Lindbom, L.; Kiessling, R.; Jörnvall, H.; Wigzell, H.; Gudmundsson, G.H. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood 2000, 96, 3086–3093. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Narayana, J.L.; Mishra, B.; Zhang, Y.; Wang, F.; Wang, C.; Zarena, D.; Lushnikova, T.; Wang, X. Design of Antimicrobial Peptides: Progress Made with Human Cathelicidin LL-37. Adv. Exp. Med. Biol. 2019, 1117, 215–240. [Google Scholar] [CrossRef] [PubMed]
- De Smet, K.; Contreras, R. Human antimicrobial peptides: Defensins, cathelicidins and histatins. Biotechnol. Lett. 2005, 27, 1337–1347. [Google Scholar] [CrossRef] [PubMed]
- López-García, B.; Lee, P.H.; Yamasaki, K.; Gallo, R.L. Anti-fungal activity of cathelicidins and their potential role in Candida albicans skin infection. J. Invest. Derm. 2005, 125, 108–115. [Google Scholar] [CrossRef]
- Bergman, P.; Walter-Jallow, L.; Broliden, K.; Agerberth, B.; Söderlund, J. The antimicrobial peptide LL-37 inhibits HIV-1 replication. Curr. HIV Res. 2007, 5, 410–415. [Google Scholar] [CrossRef]
- Meisel, R.; Brockers, S.; Heseler, K.; Degistirici, O.; Bülle, H.; Woite, C.; Stuhlsatz, S.; Schwippert, W.; Jäger, M.; Sorg, R.; et al. Human but not murine multipotent mesenchymal stromal cells exhibit broad-spectrum antimicrobial effector function mediated by indoleamine 2,3-dioxygenase. Leukemia 2011, 25, 648–654. [Google Scholar] [CrossRef]
- Yang, R.; Liu, Y.; Kelk, P.; Qu, C.; Akiyama, K.; Chen, C.; Atsuta, I.; Chen, W.; Zhou, Y.; Shi, S. A subset of IL-17(+) mesenchymal stem cells possesses anti-Candida albicans effect. Cell Res. 2013, 23, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.P.; Wright, W.E.; Shay, J.W. Comparison of telomere length measurement methods. Philos Trans. R. Soc. Lond B Biol. Sci. 2018, 373, 20160451. [Google Scholar] [CrossRef] [PubMed]
- Chikenji, T.S.; Saito, Y.; Konari, N.; Nakano, M.; Mizue, Y.; Otani, M.; Fujimiya, M. p16(INK4A)-expressing mesenchymal stromal cells restore the senescence-clearance-regeneration sequence that is impaired in chronic muscle inflammation. EBioMedicine 2019, 44, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Mas-Bargues, C.; Viña-Almunia, J.; Inglés, M.; Sanz-Ros, J.; Gambini, J.; Ibáñez-Cabellos, J.S.; García-Giménez, J.L.; Viña, J.; Borrás, C. Role of p16(INK4a) and BMI-1 in oxidative stress-induced premature senescence in human dental pulp stem cells. Redox Biol. 2017, 12, 690–698. [Google Scholar] [CrossRef]
- Park, I.K.; Morrison, S.J.; Clarke, M.F. Bmi1, stem cells, and senescence regulation. J. Clin. Invest. 2004, 113, 175–179. [Google Scholar] [CrossRef]
- Liu, H.; Xia, X.; Li, B. Mesenchymal stem cell aging: Mechanisms and influences on skeletal and non-skeletal tissues. Exp. Biol. Med. 2015, 240, 1099–1106. [Google Scholar] [CrossRef]
- Chen, X.; Wang, L.; Hou, J.; Li, J.; Chen, L.; Xia, J.; Wang, Z.; Xiao, M.; Wang, Y. Study on the Dynamic Biological Characteristics of Human Bone Marrow Mesenchymal Stem Cell Senescence. Stem Cells Int. 2019, 2019, 9271595. [Google Scholar] [CrossRef]
- Jeong, S.G.; Cho, G.W. Endogenous ROS levels are increased in replicative senescence in human bone marrow mesenchymal stromal cells. Biochem. Biophys. Res. Commun. 2015, 460, 971–976. [Google Scholar] [CrossRef]
- Franzen, J.; Zirkel, A.; Blake, J.; Rath, B.; Benes, V.; Papantonis, A.; Wagner, W. Senescence-associated DNA methylation is stochastically acquired in subpopulations of mesenchymal stem cells. Aging Cell 2017, 16, 183–191. [Google Scholar] [CrossRef]
- Mortensen, M.; Watson, A.S.; Simon, A.K. Lack of autophagy in the hematopoietic system leads to loss of hematopoietic stem cell function and dysregulated myeloid proliferation. Autophagy 2011, 7, 1069–1070. [Google Scholar] [CrossRef]
- Revuelta, M.; Matheu, A. Autophagy in stem cell aging. Aging Cell 2017, 16, 912–915. [Google Scholar] [CrossRef] [PubMed]
- Herbig, U.; Ferreira, M.; Condel, L.; Carey, D.; Sedivy, J.M. Cellular senescence in aging primates. Science 2006, 311, 1257. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Hong, Y.; Zhang, H.; Li, X. Mesenchymal Stem Cell Senescence and Rejuvenation: Current Status and Challenges. Front. Cell Dev. Biol. 2020, 8, 364. [Google Scholar] [CrossRef] [PubMed]
- Gaur, M.; Dobke, M.; Lunyak, V.V. Methods and Strategies for Procurement, Isolation, Characterization, and Assessment of Senescence of Human Mesenchymal Stem Cells from Adipose Tissue. Methods Mol. Biol. 2019, 2045, 37–92. [Google Scholar] [CrossRef]
- Abuna, R.P.; Stringhetta-Garcia, C.T.; Fiori, L.P.; Dornelles, R.C.; Rosa, A.L.; Beloti, M.M. Aging impairs osteoblast differentiation of mesenchymal stem cells grown on titanium by favoring adipogenesis. J. Appl. Oral. Sci. 2016, 24, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Marędziak, M.; Marycz, K.; Tomaszewski, K.A.; Kornicka, K.; Henry, B.M. The Influence of Aging on the Regenerative Potential of Human Adipose Derived Mesenchymal Stem Cells. Stem Cells Int. 2016, 2016, 2152435. [Google Scholar] [CrossRef]
- Infante, A.; Rodríguez, C.I. Osteogenesis and aging: Lessons from mesenchymal stem cells. Stem Cell Res. 2018, 9, 244. [Google Scholar] [CrossRef]
- Yin, Y.; Wu, R.X.; He, X.T.; Xu, X.Y.; Wang, J.; Chen, F.M. Influences of age-related changes in mesenchymal stem cells on macrophages during in-vitro culture. Stem Cell Res. 2017, 8, 153. [Google Scholar] [CrossRef]
- Rodier, F.; Campisi, J. Four faces of cellular senescence. J. Cell Biol. 2011, 192, 547–556. [Google Scholar] [CrossRef]
- Reitinger, S.; Schimke, M.; Klepsch, S.; de Sneeuw, S.; Yani, S.L.; Gaßner, R.; Ertl, P.; Lepperdinger, G. Systemic impact molds mesenchymal stromal/stem cell aging. Transfus. Apher. Sci. 2015, 52, 285–289. [Google Scholar] [CrossRef]
- Urbanelli, L.; Buratta, S.; Sagini, K.; Tancini, B.; Emiliani, C. Extracellular Vesicles as New Players in Cellular Senescence. Int. J. Mol. Sci. 2016, 17, 1408. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; Liu, T.; Gao, F.; Xie, H.; Sun, L.; Zhao, A.; Ren, W.; Guo, H.; Zhang, L.; Wang, H.; et al. Microvesicles as Potential Biomarkers for the Identification of Senescence in Human Mesenchymal Stem Cells. Theranostics 2017, 7, 2673–2689. [Google Scholar] [CrossRef] [PubMed]
- Weilner, S.; Schraml, E.; Wieser, M.; Messner, P.; Schneider, K.; Wassermann, K.; Micutkova, L.; Fortschegger, K.; Maier, A.B.; Westendorp, R.; et al. Secreted microvesicular miR-31 inhibits osteogenic differentiation of mesenchymal stem cells. Aging Cell 2016, 15, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.D.; Paine, M.S.; Brooks, A.M.; McCubrey, J.A.; Renegar, R.H.; Wang, R.; Terrian, D.M. Senescence-associated exosome release from human prostate cancer cells. Cancer Res. 2008, 68, 7864–7871. [Google Scholar] [CrossRef] [PubMed]
- Takasugi, M.; Okada, R.; Takahashi, A.; Virya Chen, D.; Watanabe, S.; Hara, E. Small extracellular vesicles secreted from senescent cells promote cancer cell proliferation through EphA2. Nat. Commun. 2017, 8, 15729. [Google Scholar] [CrossRef] [PubMed]
- Fafián-Labora, J.A.; Morente-López, M.; Arufe, M.C. Effect of aging on behaviour of mesenchymal stem cells. World J. Stem Cells 2019, 11, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Fujii, M.; Kawai, Y.; Endoh, M.; Hossain, M.N.; Nakabayashi, K.; Ayusawa, D. Expression of RAB27B is up-regulated in senescent human cells. Mech. Ageing Dev. 2006, 127, 639–642. [Google Scholar] [CrossRef]
- Takahashi, A.; Okada, R.; Nagao, K.; Kawamata, Y.; Hanyu, A.; Yoshimoto, S.; Takasugi, M.; Watanabe, S.; Kanemaki, M.T.; Obuse, C.; et al. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat. Commun. 2017, 8, 15287. [Google Scholar] [CrossRef]
- Soysal, P.; Stubbs, B.; Lucato, P.; Luchini, C.; Solmi, M.; Peluso, R.; Sergi, G.; Isik, A.T.; Manzato, E.; Maggi, S.; et al. Inflammation and frailty in the elderly: A systematic review and meta-analysis. Ageing Res. Rev. 2016, 31, 1–8. [Google Scholar] [CrossRef]
- Fickert, S.; Fiedler, J.; Brenner, R.E. Identification of subpopulations with characteristics of mesenchymal progenitor cells from human osteoarthritic cartilage using triple staining for cell surface markers. Arthritis Res. 2004, 6, R422–R432. [Google Scholar] [CrossRef]
- Su, X.; Zuo, W.; Wu, Z.; Chen, J.; Wu, N.; Ma, P.; Xia, Z.; Jiang, C.; Ye, Z.; Liu, S.; et al. CD146 as a new marker for an increased chondroprogenitor cell sub-population in the later stages of osteoarthritis. J. Orthop. Res. 2015, 33, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Malaise, O.; Tachikart, Y.; Constantinides, M.; Mumme, M.; Ferreira-Lopez, R.; Noack, S.; Krettek, C.; Noël, D.; Wang, J.; Jorgensen, C.; et al. Mesenchymal stem cell senescence alleviates their intrinsic and seno-suppressive paracrine properties contributing to osteoarthritis development. Aging 2019, 11, 9128–9146. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Gao, Y.; Jayasuriya, C.T.; Liu, W.; Du, H.; Ding, J.; Feng, M.; Chen, Q. Chondrogenic induction of human osteoarthritic cartilage-derived mesenchymal stem cells activates mineralization and hypertrophic and osteogenic gene expression through a mechanomiR. Arthritis Res. 2019, 21, 167. [Google Scholar] [CrossRef]
- Martinez, F.J.; Collard, H.R.; Pardo, A.; Raghu, G.; Richeldi, L.; Selman, M.; Swigris, J.J.; Taniguchi, H.; Wells, A.U. Idiopathic pulmonary fibrosis. Nat. Rev. Dis. Primers 2017, 3, 17074. [Google Scholar] [CrossRef] [PubMed]
- Cárdenes, N.; Álvarez, D.; Sellarés, J.; Peng, Y.; Corey, C.; Wecht, S.; Nouraie, S.M.; Shanker, S.; Sembrat, J.; Bueno, M.; et al. Senescence of bone marrow-derived mesenchymal stem cells from patients with idiopathic pulmonary fibrosis. Stem Cell Res. 2018, 9, 257. [Google Scholar] [CrossRef]
- Vicinanza, C.; Aquila, I.; Scalise, M.; Cristiano, F.; Marino, F.; Cianflone, E.; Mancuso, T.; Marotta, P.; Sacco, W.; Lewis, F.C.; et al. Adult cardiac stem cells are multipotent and robustly myogenic: C-kit expression is necessary but not sufficient for their identification. Cell Death Differ. 2017, 24, 2101–2116. [Google Scholar] [CrossRef]
- Castaldi, A.; Dodia, R.M.; Orogo, A.M.; Zambrano, C.M.; Najor, R.H.; Gustafsson, Å.B.; Heller Brown, J.; Purcell, N.H. Decline in cellular function of aged mouse c-kit(+) cardiac progenitor cells. J. Physiol. 2017, 595, 6249–6262. [Google Scholar] [CrossRef]
- Lewis-McDougall, F.C.; Ruchaya, P.J.; Domenjo-Vila, E.; Shin Teoh, T.; Prata, L.; Cottle, B.J.; Clark, J.E.; Punjabi, P.P.; Awad, W.; Torella, D.; et al. Aged-senescent cells contribute to impaired heart regeneration. Aging Cell 2019, 18, e12931. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Boisvert, M.M.; Erikson, G.A.; Shokhirev, M.N.; Allen, N.J. The Aging Astrocyte Transcriptome from Multiple Regions of the Mouse Brain. Cell Rep. 2018, 22, 269–285. [Google Scholar] [CrossRef]
- Newman, A.B.; Gottdiener, J.S.; McBurnie, M.A.; Hirsch, C.H.; Kop, W.J.; Tracy, R.; Walston, J.D.; Fried, L.P. Associations of subclinical cardiovascular disease with frailty. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M158–M166. [Google Scholar] [CrossRef] [PubMed]
- Polidoro, A.; Stefanelli, F.; Ciacciarelli, M.; Pacelli, A.; Di Sanzo, D.; Alessandri, C. Frailty in patients affected by atrial fibrillation. Arch. Gerontol. Geriatr. 2013, 57, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997, 127, 990s–991s. [Google Scholar] [CrossRef]
- Brown, M. Skeletal muscle and bone: Effect of sex steroids and aging. Adv. Physiol Educ 2008, 32, 120–126. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Dattilo, M.; Macut, D.; Duntas, L.; Gonos, E.S.; Goulis, D.G.; Gantenbein, C.K.; Kapetanou, M.; Koukkou, E.; Lambrinoudaki, I.; et al. MECHANISMS IN ENDOCRINOLOGY: Aging and anti-aging: A Combo-Endocrinology overview. Eur. J. Endocrinol. 2017, 176, R283–R308. [Google Scholar] [CrossRef]
- Clegg, A.; Hassan-Smith, Z. Frailty and the endocrine system. Lancet Diabetes Endocrinol. 2018, 6, 743–752. [Google Scholar] [CrossRef]
- Mahindran, E.; Law, J.X.; Ng, M.H.; Nordin, F. Mesenchymal Stem Cell Transplantation for the Treatment of Age-Related Musculoskeletal Frailty. Int. J. Mol. Sci. 2021, 22, 10542. [Google Scholar] [CrossRef]
- Zhu, Y.; Ge, J.; Huang, C.; Liu, H.; Jiang, H. Application of mesenchymal stem cell therapy for aging frailty: From mechanisms to therapeutics. Theranostics 2021, 11, 5675–5685. [Google Scholar] [CrossRef]
- Abd El-Kader, S.M.; Al-Shreef, F.M. Inflammatory cytokines and immune system modulation by aerobic versus resisted exercise training for elderly. Afr. Health Sci. 2018, 18, 120–131. [Google Scholar] [CrossRef]
- De Vita, F.; Lauretani, F.; Bauer, J.; Bautmans, I.; Shardell, M.; Cherubini, A.; Bondi, G.; Zuliani, G.; Bandinelli, S.; Pedrazzoni, M.; et al. Relationship between vitamin D and inflammatory markers in older individuals. Age 2014, 36, 9694. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Liu, K.; Daviglus, M.L.; Jenny, N.S.; Mayer-Davis, E.; Jiang, R.; Steffen, L.; Siscovick, D.; Tsai, M.; Herrington, D. Associations of dietary long-chain n-3 polyunsaturated fatty acids and fish with biomarkers of inflammation and endothelial activation (from the Multi-Ethnic Study of Atherosclerosis [MESA]). Am. J. Cardiol. 2009, 103, 1238–1243. [Google Scholar] [CrossRef] [PubMed]
- Apóstolo, J.; Cooke, R.; Bobrowicz-Campos, E.; Santana, S.; Marcucci, M.; Cano, A.; Vollenbroek-Hutten, M.; Germini, F.; D′Avanzo, B.; Gwyther, H.; et al. Effectiveness of interventions to prevent pre-frailty and frailty progression in older adults: A systematic review. JBI Database Syst. Rev. Implement. Rep. 2018, 16, 140–232. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Q. Senescent Mesenchymal Stem Cells: Disease Mechanism and Treatment Strategy. Curr. Mol. Biol. Rep. 2020, 6, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, J.L.; Tchkonia, T.; Zhu, Y.; Niedernhofer, L.J.; Robbins, P.D. The Clinical Potential of Senolytic Drugs. J. Am. Geriatr. Soc. 2017, 65, 2297–2301. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, A.; Fairbrother, W.J.; Leverson, J.D.; Souers, A.J. From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors. Nat. Rev. Drug Discov. 2017, 16, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.; Giorgadze, N.; Palmer, A.K.; Ikeno, Y.; Hubbard, G.B.; Lenburg, M.; et al. The Achilles′ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell 2015, 14, 644–658. [Google Scholar] [CrossRef]
- Zhu, Y.; Tchkonia, T.; Fuhrmann-Stroissnigg, H.; Dai, H.M.; Ling, Y.Y.; Stout, M.B.; Pirtskhalava, T.; Giorgadze, N.; Johnson, K.O.; Giles, C.B.; et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 2016, 15, 428–435. [Google Scholar] [CrossRef]
- Justice, J.N.; Nambiar, A.M.; Tchkonia, T.; LeBrasseur, N.K.; Pascual, R.; Hashmi, S.K.; Prata, L.; Masternak, M.M.; Kritchevsky, S.B.; Musi, N.; et al. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine 2019, 40, 554–563. [Google Scholar] [CrossRef]
- Zhu, Y.; Doornebal, E.J.; Pirtskhalava, T.; Giorgadze, N.; Wentworth, M.; Fuhrmann-Stroissnigg, H.; Niedernhofer, L.J.; Robbins, P.D.; Tchkonia, T.; Kirkland, J.L. New agents that target senescent cells: The flavone, fisetin, and the BCL-X(L) inhibitors, A1331852 and A1155463. Aging 2017, 9, 955–963. [Google Scholar] [CrossRef]
- Ocansey, D.K.W.; Pei, B.; Yan, Y.; Qian, H.; Zhang, X.; Xu, W.; Mao, F. Improved therapeutics of modified mesenchymal stem cells: An update. J. Transl. Med. 2020, 18, 42. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Teng, S.; Ma, C.; Yu, Y.; Wang, P.; Yi, C. Ascorbic acid inhibits senescence in mesenchymal stem cells through ROS and AKT/mTOR signaling. Cytotechnology 2018, 70, 1301–1313. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Jeong, A.J.; Kim, G.Y.; Jo, A.; Lee, J.E.; Leem, S.H.; Yoon, J.H.; Ye, S.K.; Chung, J.W. Lactoferrin Protects Human Mesenchymal Stem Cells from Oxidative Stress-Induced Senescence and Apoptosis. J. Microbiol. Biotechnol. 2017, 27, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.M.; Tsai, J.L.; Lin, S.D.; Lai, C.S.; Chang, C.C. Accelerated growth and prolonged lifespan of adipose tissue-derived human mesenchymal stem cells in a medium using reduced calcium and antioxidants. Stem Cells Dev. 2005, 14, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jung, H.K.; Han, Y.S.; Yoon, Y.M.; Yun, C.W.; Sun, H.Y.; Cho, H.W.; Lee, S.H. Antioxidant effects of Cirsium setidens extract on oxidative stress in human mesenchymal stem cells. Mol. Med. Rep. 2016, 14, 3777–3784. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, H.; Liang, X.; Hong, Y.; Mao, M.; Han, Q.; He, H.; Tao, W.; Jiang, G.; Zhang, Y.; et al. Adipose-Derived Mesenchymal Stem Cells Isolated from Patients with Abdominal Aortic Aneurysm Exhibit Senescence Phenomena. Oxid. Med. Cell Longev. 2019, 2019, 1305049. [Google Scholar] [CrossRef]
- Khorraminejad-Shirazi, M.; Sani, M.; Talaei-Khozani, T.; Dorvash, M.; Mirzaei, M.; Faghihi, M.A.; Monabati, A.; Attar, A. AICAR and nicotinamide treatment synergistically augment the proliferation and attenuate senescence-associated changes in mesenchymal stromal cells. Stem Cell Res. 2020, 11, 45. [Google Scholar] [CrossRef]
- Lee, J.H.; Yoon, Y.M.; Song, K.H.; Noh, H.; Lee, S.H. Melatonin suppresses senescence-derived mitochondrial dysfunction in mesenchymal stem cells via the HSPA1L-mitophagy pathway. Aging Cell 2020, 19, e13111. [Google Scholar] [CrossRef]
- Liu, J.; Ding, Y.; Liu, Z.; Liang, X. Senescence in Mesenchymal Stem Cells: Functional Alterations, Molecular Mechanisms, and Rejuvenation Strategies. Front. Cell Dev. Biol. 2020, 8, 258. [Google Scholar] [CrossRef]
- Simonsen, J.L.; Rosada, C.; Serakinci, N.; Justesen, J.; Stenderup, K.; Rattan, S.I.; Jensen, T.G.; Kassem, M. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat. Biotechnol. 2002, 20, 592–596. [Google Scholar] [CrossRef]
- Liang, X.; Ding, Y.; Lin, F.; Zhang, Y.; Zhou, X.; Meng, Q.; Lu, X.; Jiang, G.; Zhu, H.; Chen, Y.; et al. Overexpression of ERBB4 rejuvenates aged mesenchymal stem cells and enhances angiogenesis via PI3K/AKT and MAPK/ERK pathways. FASEB J. 2019, 33, 4559–4570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, W.; He, H.; Fan, B.; Deng, R.; Hong, Y.; Liang, X.; Zhao, H.; Li, X.; Zhang, F. Macrophage migration inhibitory factor rejuvenates aged human mesenchymal stem cells and improves myocardial repair. Aging 2019, 11, 12641–12660. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; An, X.; Yang, Y.; Xu, H.; Fan, L.; Deng, L.; Li, T.; Weng, X.; Zhang, J.; Chunhua Zhao, R. MiR-1292 Targets FZD4 to Regulate Senescence and Osteogenic Differentiation of Stem Cells in TE/SJ/Mesenchymal Tissue System via the Wnt/β-catenin Pathway. Aging Dis. 2018, 9, 1103–1121. [Google Scholar] [CrossRef] [PubMed]
- Sotiropoulou, P.A.; Perez, S.A.; Salagianni, M.; Baxevanis, C.N.; Papamichail, M. Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells 2006, 24, 462–471. [Google Scholar] [CrossRef]
- Duggal, S.; Brinchmann, J.E. Importance of serum source for the in vitro replicative senescence of human bone marrow derived mesenchymal stem cells. J. Cell Physiol. 2011, 226, 2908–2915. [Google Scholar] [CrossRef]
- Baker, N.; Boyette, L.B.; Tuan, R.S. Characterization of bone marrow-derived mesenchymal stem cells in aging. Bone 2015, 70, 37–47. [Google Scholar] [CrossRef]
- Golpanian, S.; DiFede, D.L.; Pujol, M.V.; Lowery, M.H.; Levis-Dusseau, S.; Goldstein, B.J.; Schulman, I.H.; Longsomboon, B.; Wolf, A.; Khan, A.; et al. Rationale and design of the allogeneiC human mesenchymal stem cells (hMSC) in patients with aging fRAilTy via intravenoUS delivery (CRATUS) study: A phase I/II, randomized, blinded and placebo controlled trial to evaluate the safety and potential efficacy of allogeneic human mesenchymal stem cell infusion in patients with aging frailty. Oncotarget 2016, 7, 11899–11912. [Google Scholar] [CrossRef]
- Premer, C.; Wanschel, A.; Porras, V.; Balkan, W.; Legendre-Hyldig, T.; Saltzman, R.G.; Dong, C.; Schulman, I.H.; Hare, J.M. Mesenchymal Stem Cell Secretion of SDF-1α Modulates Endothelial Function in Dilated Cardiomyopathy. Front. Physiol. 2019, 10, 1182. [Google Scholar] [CrossRef]
- Babenko, V.A.; Silachev, D.N.; Danilina, T.I.; Goryunov, K.V.; Pevzner, I.B.; Zorova, L.D.; Popkov, V.A.; Chernikov, V.P.; Plotnikov, E.Y.; Sukhikh, G.T.; et al. Age-Related Changes in Bone-Marrow Mesenchymal Stem Cells. Cells 2021, 10, 1273. [Google Scholar] [CrossRef]
- Das, M.; Mayilsamy, K.; Mohapatra, S.S.; Mohapatra, S. Mesenchymal stem cell therapy for the treatment of traumatic brain injury: Progress and prospects. Rev. Neurosci. 2019, 30, 839–855. [Google Scholar] [CrossRef]
- Mukhamedshina, Y.; Shulman, I.; Ogurcov, S.; Kostennikov, A.; Zakirova, E.; Akhmetzyanova, E.; Rogozhin, A.; Masgutova, G.; James, V.; Masgutov, R.; et al. Mesenchymal Stem Cell Therapy for Spinal Cord Contusion: A Comparative Study on Small and Large Animal Models. Biomolecules 2019, 9, 811. [Google Scholar] [CrossRef] [PubMed]
- Goradel, N.H.; Hour, F.G.; Negahdari, B.; Malekshahi, Z.V.; Hashemzehi, M.; Masoudifar, A.; Mirzaei, H. Stem Cell Therapy: A New Therapeutic Option for Cardiovascular Diseases. J. Cell Biochem. 2018, 119, 95–104. [Google Scholar] [CrossRef]
- Doeppner, T.R.; Hermann, D.M. Mesenchymal stem cells in the treatment of ischemic stroke: Progress and possibilities. Stem Cells Cloning 2010, 3, 157–163. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mahmood, A.; Seetharaman, R.; Kshatriya, P.; Patel, D.; Srivastava, A.S. Stem Cell Transplant for Advanced Stage Liver Disorders: Current Scenario and Future Prospects. Curr. Med. Chem. 2020, 27, 6276–6293. [Google Scholar] [CrossRef] [PubMed]
- Raggi, C.; Berardi, A.C. Mesenchymal stem cells, aging and regenerative medicine. Muscles Ligaments Tendons J. 2012, 2, 239–242. [Google Scholar]
- Golpanian, S.; DiFede, D.L.; Khan, A.; Schulman, I.H.; Landin, A.M.; Tompkins, B.A.; Heldman, A.W.; Miki, R.; Goldstein, B.J.; Mushtaq, M.; et al. Allogeneic Human Mesenchymal Stem Cell Infusions for Aging Frailty. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1505–1512. [Google Scholar] [CrossRef]
- Tompkins, B.A.; DiFede, D.L.; Khan, A.; Landin, A.M.; Schulman, I.H.; Pujol, M.V.; Heldman, A.W.; Miki, R.; Goldschmidt-Clermont, P.J.; Goldstein, B.J.; et al. Allogeneic Mesenchymal Stem Cells Ameliorate Aging Frailty: A Phase II Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1513–1522. [Google Scholar] [CrossRef]
- Fernández-Francos, S.; Eiro, N.; Costa, L.A.; Escudero-Cernuda, S.; Fernández-Sánchez, M.L.; Vizoso, F.J. Mesenchymal Stem Cells as a Cornerstone in a Galaxy of Intercellular Signals: Basis for a New Era of Medicine. Int. J. Mol. Sci. 2021, 22, 3576. [Google Scholar] [CrossRef]
- Chimenti, I.; Smith, R.R.; Li, T.S.; Gerstenblith, G.; Messina, E.; Giacomello, A.; Marbán, E. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ. Res. 2010, 106, 971–980. [Google Scholar] [CrossRef]
- Timmers, L.; Lim, S.K.; Hoefer, I.E.; Arslan, F.; Lai, R.C.; van Oorschot, A.A.; Goumans, M.J.; Strijder, C.; Sze, S.K.; Choo, A.; et al. Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem Cell Res. 2011, 6, 206–214. [Google Scholar] [CrossRef]
- Parekkadan, B.; Milwid, J.M. Mesenchymal stem cells as therapeutics. Annu. Rev. Biomed. Eng. 2010, 12, 87–117. [Google Scholar] [CrossRef] [PubMed]
- Toma, C.; Pittenger, M.F.; Cahill, K.S.; Byrne, B.J.; Kessler, P.D. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 2002, 105, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Péault, B.; Huard, J. Regenerative Translation of Human Blood-Vessel-Derived MSC Precursors. Stem Cells Int. 2015, 2015, 375187. [Google Scholar] [CrossRef] [PubMed]
- Bagno, L.; Hatzistergos, K.E.; Balkan, W.; Hare, J.M. Mesenchymal Stem Cell-Based Therapy for Cardiovascular Disease: Progress and Challenges. Mol. Ther. 2018, 26, 1610–1623. [Google Scholar] [CrossRef]
- Fontaine, M.J.; Shih, H.; Schäfer, R.; Pittenger, M.F. Unraveling the Mesenchymal Stromal Cells′ Paracrine Immunomodulatory Effects. Transfus. Med. Rev. 2016, 30, 37–43. [Google Scholar] [CrossRef]
- Teixeira, F.G.; Salgado, A.J. Mesenchymal stem cells secretome: Current trends and future challenges. Neural Regen Res. 2020, 15, 75–77. [Google Scholar] [CrossRef]
- Wang, B.; Lee, W.Y.; Huang, B.; Zhang, J.F.; Wu, T.; Jiang, X.; Wang, C.C.; Li, G. Secretome of Human Fetal Mesenchymal Stem Cell Ameliorates Replicative Senescen. Stem Cells Dev. 2016, 25, 1755–1766. [Google Scholar] [CrossRef]
- Platas, J.; Guillén, M.I.; Pérez Del Caz, M.D.; Gomar, F.; Castejón, M.A.; Mirabet, V.; Alcaraz, M.J. Paracrine effects of human adipose-derived mesenchymal stem cells in inflammatory stress-induced senescence features of osteoarthritic chondrocytes. Aging 2016, 8, 1703–1717. [Google Scholar] [CrossRef]
- Pan, S.; Gong, S.; Zhang, J.; Jia, S.; Wang, M.; Pan, Y.; Wang, X.; Jiang, D. Anti-aging effects of fetal dermal mesenchymal stem cells in a D-galactose-induced aging model of adult dermal fibroblasts. Vitr. Cell Dev. Biol. Anim. 2021, 57, 795–807. [Google Scholar] [CrossRef]
- Tofiño-Vian, M.; Guillén, M.I.; Pérez Del Caz, M.D.; Castejón, M.A.; Alcaraz, M.J. Extracellular Vesicles from Adipose-Derived Mesenchymal Stem Cells Downregulate Senescence Features in Osteoarthritic Osteoblasts. Oxid. Med. Cell Longev. 2017, 2017, 7197598. [Google Scholar] [CrossRef]
- Liu, S.; Mahairaki, V.; Bai, H.; Ding, Z.; Li, J.; Witwer, K.W.; Cheng, L. Highly Purified Human Extracellular Vesicles Produced by Stem Cells Alleviate Aging Cellular Phenotypes of Senescent Human Cells. Stem Cells 2019, 37, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Dorronsoro, A.; Santiago, F.E.; Grassi, D.; Zhang, T.; Lai, R.C.; McGowan, S.J.; Angelini, L.; Lavasani, M.; Corbo, L.; Lu, A.; et al. Mesenchymal stem cell-derived extracellular vesicles reduce senescence and extend health span in mouse models of aging. Aging Cell 2021, 20, e13337. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wei, J.; Da Fonseca Ferreira, A.; Wang, H.; Zhang, L.; Zhang, Q.; Bellio, M.A.; Chu, X.M.; Khan, A.; Jayaweera, D.; et al. Rejuvenation of Senescent Endothelial Progenitor Cells by Extracellular Vesicles Derived From Mesenchymal Stromal Cells. JACC Basic Transl. Sci. 2020, 5, 1127–1141. [Google Scholar] [CrossRef] [PubMed]
- Jujo, K.; Ii, M.; Losordo, D.W. Endothelial progenitor cells in neovascularization of infarcted myocardium. J. Mol. Cell Cardiol. 2008, 45, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Imanishi, T.; Tsujioka, H.; Akasaka, T. Endothelial progenitor cells dysfunction and senescence: Contribution to oxidative stress. Curr. Cardiol. Rev. 2008, 4, 275–286. [Google Scholar] [CrossRef][Green Version]
- Kulkarni, R.; Bajaj, M.; Ghode, S.; Jalnapurkar, S.; Limaye, L.; Kale, V.P. Intercellular Transfer of Microvesicles from Young Mesenchymal Stromal Cells Rejuvenates Aged Murine Hematopoietic Stem Cells. Stem Cells 2018, 36, 420–433. [Google Scholar] [CrossRef]
- Huang, R.; Qin, C.; Wang, J.; Hu, Y.; Zheng, G.; Qiu, G.; Ge, M.; Tao, H.; Shu, Q.; Xu, J. Differential effects of extracellular vesicles from aging and young mesenchymal stem cells in acute lung injury. Aging 2019, 11, 7996–8014. [Google Scholar] [CrossRef]
- Mishra, P.; Martin, D.C.; Androulakis, I.P.; Moghe, P.V. Fluorescence Imaging of Actin Turnover Parses Early Stem Cell Lineage Divergence and Senescence. Sci. Rep. 2019, 9, 10377. [Google Scholar] [CrossRef]
- Ang, J.; Lee, Y.A.; Raghothaman, D.; Jayaraman, P.; Teo, K.L.; Khan, F.J.; Reuveny, S.; Chang, Y.T.; Kang, N.Y.; Oh, S. Rapid Detection of Senescent Mesenchymal Stromal Cells by a Fluorescent Probe. Biotechnol. J. 2019, 14, e1800691. [Google Scholar] [CrossRef]
- Berebichez-Fridman, R.; Montero-Olvera, P.R. Sources and Clinical Applications of Mesenchymal Stem Cells: State-of-the-art review. Sultan Qaboos Univ. Med. J. 2018, 18, e264–e277. [Google Scholar] [CrossRef]
- Spath, L.; Rotilio, V.; Alessandrini, M.; Gambara, G.; de Angelis, L.; Mancini, M.; Mitsiadis, T.A.; Vivarelli, E.; Naro, F.; Filippini, A.; et al. Explant-derived human dental pulp stem cells enhance differentiation and proliferation potentials. J. Cell Mol. Med. 2010, 14, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Kichenbrand, C.; Velot, E.; Menu, P.; Moby, V. Dental Pulp Stem Cell-Derived Conditioned Medium: An Attractive Alternative for Regenerative Therapy. Tissue Eng. Part B Rev. 2019, 25, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Tobias, R.S.; Cassidy, N.; Bégue-Kirn, C.; Ruch, J.V.; Lesot, H. Influence of Substrate Nature and Immobilization of Implanted Dentin Matrix Components During Induction of Reparative Dentinogenesis. Connect. Tissue Res. 1995, 32, 291–296. [Google Scholar] [CrossRef]
- Morad, G.; Kheiri, L.; Khojasteh, A. Dental pulp stem cells for in vivo bone regeneration: A systematic review of literature. Arch. Oral. Biol. 2013, 58, 1818–1827. [Google Scholar] [CrossRef] [PubMed]
- Achilleos, A.; Trainor, P.A. Neural crest stem cells: Discovery, properties and potential for therapy. Cell Res. 2012, 22, 288–304. [Google Scholar] [CrossRef]
- Cecoro, G.; Guida, L.; Iuorio, A.; Nucci, L.; Pesce, P.; Annunziata, M. Cell-based therapies for the surgical treatment of periodontal intrabony defects: A systematic review. Eur. Rev. Med. Pharm. Sci. 2021, 25, 6592–6602. [Google Scholar] [CrossRef]
- Suda, S.; Nito, C.; Ihara, M.; Iguchi, Y.; Urabe, T.; Matsumaru, Y.; Sakai, N.; Kimura, K.; J-REPAIR trial group. Randomised placebo-controlled multicentre trial to evaluate the efficacy and safety of JTR-161, allogeneic human dental pulp stem cells, in patients with Acute Ischaemic stRoke (J-REPAIR). BMJ Open 2022, 12, e054269. [Google Scholar] [CrossRef]
- Mason, J.B.; Habermehl, T.L.; Underwood, K.B.; Schneider, A.; Brieño-Enriquez, M.A.; Masternak, M.M.; Parkinson, K.C. The interrelationship between female reproductive aging and survival. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 77, 75–83. [Google Scholar] [CrossRef]
- Eisenberg, M.L.; Li, S.; Behr, B.; Cullen, M.R.; Galusha, D.; Lamb, D.J.; Lipshultz, L.I. Semen quality, infertility and mortality in the USA. Hum. Reprod. 2014, 29, 1567–1574. [Google Scholar] [CrossRef]
- Thom, T.; Haase, N.; Rosamond, W.; Howard, V.J.; Rumsfeld, J.; Manolio, T.; Zheng, Z.J.; Flegal, K.; O′Donnell, C.; Kittner, S.; et al. Heart disease and stroke statistics--2006 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2006, 113, e85–e151. [Google Scholar] [CrossRef]
- Shuster, L.T.; Rhodes, D.J.; Gostout, B.S.; Grossardt, B.R.; Rocca, W.A. Premature menopause or early menopause: Long-term health consequences. Maturitas 2010, 65, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.; Doblhammer, G.; Rau, R.; Vaupel, J.W. Ageing populations: The challenges ahead. Lancet 2009, 374, 1196–1208. [Google Scholar] [CrossRef]
- Macintyre, S.; Ford, G.; Hunt, K. Do women ′over-report′ morbidity? Men′s and women′s responses to structured prompting on a standard question on long standing illness. Soc. Sci. Med. 1999, 48, 89–98. [Google Scholar] [CrossRef]
- Eiro, N.; Fraile, M.; Schneider, J.; Vizoso, F.J. Non Pregnant Human Uterus as Source of Mesenchymal Stem Cells. Curr. Stem Cell Res. 2018, 13, 423–431. [Google Scholar] [CrossRef]
- Bermudez, M.A.; Sendon-Lago, J.; Seoane, S.; Eiro, N.; Gonzalez, F.; Saa, J.; Vizoso, F.; Perez-Fernandez, R. Anti-inflammatory effect of conditioned medium from human uterine cervical stem cells in uveitis. Exp. Eye Res. 2016, 149, 84–92. [Google Scholar] [CrossRef]
- Sendon-Lago, J.; Rio, L.G.; Eiro, N.; Diaz-Rodriguez, P.; Avila, L.; Gonzalez, L.O.; Vizoso, F.J.; Perez-Fernandez, R.; Landin, M. Tailored Hydrogels as Delivery Platforms for Conditioned Medium from Mesenchymal Stem Cells in a Model of Acute Colitis in Mice. Pharmaceutics 2021, 13, 1127. [Google Scholar] [CrossRef]
- Bermudez, M.A.; Sendon-Lago, J.; Eiro, N.; Treviño, M.; Gonzalez, F.; Yebra-Pimentel, E.; Giraldez, M.J.; Macia, M.; Lamelas, M.L.; Saa, J.; et al. Corneal epithelial wound healing and bactericidal effect of conditioned medium from human uterine cervical stem cells. Invest. Ophthalmol. Vis. Sci. 2015, 56, 983–992. [Google Scholar] [CrossRef]
- Sendon-Lago, J.; Seoane, S.; Martinez-Ordoñez, A.; Eiro, N.; Saa, J.; Vizoso, F.J.; Gonzalez, F.; Perez-Fernandez, R.; Bermudez, M.A. Corneal regeneration by conditioned medium of human uterine cervical stem cells is mediated by TIMP-1 and TIMP-2. Exp. Eye Res. 2019, 180, 110–121. [Google Scholar] [CrossRef]
- Schneider, J.; Mateo, E.; Marcos-Arias, C.; Eiró, N.; Vizoso, F.; Pérez-Fernández, R.; Eraso, E.; Quindós, G. Antifungal Activity of the Human Uterine Cervical Stem Cells Conditioned Medium (hUCESC-CM) Against Candida albicans and Other Medically Relevant Species of Candida. Front. Microbiol. 2018, 9, 2818. [Google Scholar] [CrossRef]
- Eiro, N.; Fraile, M.; Fernández-Francos, S.; Sánchez, R.; Costa, L.A.; Vizoso, F.J. Importance of the origin of mesenchymal (stem) stromal cells in cancer biology: "alliance" or "war" in intercellular signals. Cell Biosci. 2021, 11, 109. [Google Scholar] [CrossRef]
- Schimke, M.M.; Marozin, S.; Lepperdinger, G. Patient-Specific Age: The Other Side of the Coin in Advanced Mesenchymal Stem Cell Therapy. Front. Physiol. 2015, 6, 362. [Google Scholar] [CrossRef] [PubMed]
- Banfi, A.; Bianchi, G.; Notaro, R.; Luzzatto, L.; Cancedda, R.; Quarto, R. Replicative aging and gene expression in long-term cultures of human bone marrow stromal cells. Tissue Eng. 2002, 8, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Baxter, M.A.; Wynn, R.F.; Jowitt, S.N.; Wraith, J.E.; Fairbairn, L.J.; Bellantuono, I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells 2004, 22, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Lechanteur, C.; Briquet, A.; Giet, O.; Delloye, O.; Baudoux, E.; Beguin, Y. Clinical-scale expansion of mesenchymal stromal cells: A large banking experience. J. Transl. Med. 2016, 14, 145. [Google Scholar] [CrossRef] [PubMed]
- Sabapathy, V.; Kumar, S. hiPSC-derived iMSCs: NextGen MSCs as an advanced therapeutically active cell resource for regenerative medicine. J. Cell Mol. Med. 2016, 20, 1571–1588. [Google Scholar] [CrossRef]
- Hynes, K.; Menicanin, D.; Han, J.; Marino, V.; Mrozik, K.; Gronthos, S.; Bartold, P.M. Mesenchymal stem cells from iPS cells facilitate periodontal regeneration. J. Dent. Res. 2013, 92, 833–839. [Google Scholar] [CrossRef]
- Kabat, M.; Bobkov, I.; Kumar, S.; Grumet, M. Trends in mesenchymal stem cell clinical trials 2004-2018: Is efficacy optimal in a narrow dose range? Stem Cells Transl. Med. 2020, 9, 17–27. [Google Scholar] [CrossRef]
- Olsen, T.R.; Ng, K.S.; Lock, L.T.; Ahsan, T.; Rowley, J.A. Peak MSC-Are We There Yet? Front. Med. 2018, 5, 178. [Google Scholar] [CrossRef]
- Moutsatsou, P.; Ochs, J.; Schmitt, R.H.; Hewitt, C.J.; Hanga, M.P. Automation in cell and gene therapy manufacturing: From past to future. Biotechnol Lett 2019, 41, 1245–1253. [Google Scholar] [CrossRef]
- Simón, M. Bioreactor design for adherent cell culture: The bolt-on bioreactor project, part 2-process automation. BioProcess Int. 2015, 13, 28–33. [Google Scholar]
- Chen, A.K.; Reuveny, S.; Oh, S.K. Application of human mesenchymal and pluripotent stem cell microcarrier cultures in cellular therapy: Achievements and future direction. Biotechnol. Adv. 2013, 31, 1032–1046. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Kornmuller, A.; Brown, C.; Hoare, T.; Flynn, L.E. Decellularized adipose tissue microcarriers as a dynamic culture platform for human adipose-derived stem/stromal cell expansion. Biomaterials 2017, 120, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Leber, J.; Barekzai, J.; Blumenstock, M.; Pospisil, B.; Salzig, D.; Czermak, P. Microcarrier choice and bead-to-bead transfer for human mesenchymal stem cells in serum-containing and chemically defined media. Process. Biochem. 2017, 59, 255–265. [Google Scholar] [CrossRef]
- Czermak, P.; Pörtner, R.; Brix, A. Special engineering aspects. In Cell and Tissue Reaction Engineering: With a Contribution by Martin Fussenegger and Wilfried Weber; Eibl, R., Eibl, D., Pörtner, R., Catapano, G., Czermak, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 83–172. [Google Scholar]
- Ban, J.J.; Lee, M.; Im, W.; Kim, M. Low pH increases the yield of exosome isolation. Biochem. Biophys. Res. Commun. 2015, 461, 76–79. [Google Scholar] [CrossRef]
- Holzwarth, C.; Vaegler, M.; Gieseke, F.; Pfister, S.M.; Handgretinger, R.; Kerst, G.; Müller, I. Low physiologic oxygen tensions reduce proliferation and differentiation of human multipotent mesenchymal stromal cells. BMC Cell Biol. 2010, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Huang, W.; Wani, M.; Yu, X.; Ashraf, M. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS ONE 2014, 9, e88685. [Google Scholar] [CrossRef]
- Han, K.H.; Kim, A.K.; Jeong, G.J.; Jeon, H.R.; Bhang, S.H.; Kim, D.I. Enhanced Anti-Cancer Effects of Conditioned Medium from Hypoxic Human Umbilical Cord-Derived Mesenchymal Stem Cells. Int. J. Stem Cells 2019, 12, 291–303. [Google Scholar] [CrossRef]
- Rosová, I.; Dao, M.; Capoccia, B.; Link, D.; Nolta, J.A. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells 2008, 26, 2173–2182. [Google Scholar] [CrossRef]
- Stroncek, D.F.; Jin, P.; McKenna, D.H.; Takanashi, M.; Fontaine, M.J.; Pati, S.; Schäfer, R.; Peterson, E.; Benedetti, E.; Reems, J.-A. Human Mesenchymal Stromal Cell (MSC) Characteristics Vary Among Laboratories When Manufactured From the Same Source Material: A Report by the Cellular Therapy Team of the Biomedical Excellence for Safer Transfusion (BEST) Collaborative. Front. Cell Dev. Biol. 2020, 8, 458. [Google Scholar] [CrossRef]
- Billing, A.M.; Ben Hamidane, H.; Dib, S.S.; Cotton, R.J.; Bhagwat, A.M.; Kumar, P.; Hayat, S.; Yousri, N.A.; Goswami, N.; Suhre, K.; et al. Comprehensive transcriptomic and proteomic characterization of human mesenchymal stem cells reveals source specific cellular markers. Sci. Rep. 2016, 6, 21507. [Google Scholar] [CrossRef]
- Menard, C.; Pacelli, L.; Bassi, G.; Dulong, J.; Bifari, F.; Bezier, I.; Zanoncello, J.; Ricciardi, M.; Latour, M.; Bourin, P.; et al. Clinical-grade mesenchymal stromal cells produced under various good manufacturing practice processes differ in their immunomodulatory properties: Standardization of immune quality controls. Stem Cells Dev. 2013, 22, 1789–1801. [Google Scholar] [CrossRef]
- Isobe, Y.; Koyama, N.; Nakao, K.; Osawa, K.; Ikeno, M.; Yamanaka, S.; Okubo, Y.; Fujimura, K.; Bessho, K. Comparison of human mesenchymal stem cells derived from bone marrow, synovial fluid, adult dental pulp, and exfoliated deciduous tooth pulp. Int. J. Oral. Maxillofac. Surg. 2016, 45, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewska, A.; Catar, R.; Schoon, J.; Qazi, T.H.; Sass, F.A.; Jacobi, D.; Blankenstein, A.; Reinke, S.; Krüger, D.; Streitz, M.; et al. Multi-Parameter Analysis of Biobanked Human Bone Marrow Stromal Cells Shows Little Influence for Donor Age and Mild Comorbidities on Phenotypic and Functional Properties. Front. Immunol. 2019, 10, 2474. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, R.; Spohn, G.; Baer, P.C. Mesenchymal Stem/Stromal Cells in Regenerative Medicine: Can Preconditioning Strategies Improve Therapeutic Efficacy? Transfus. Med. Hemother. 2016, 43, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Cunha, B.; Aguiar, T.; Carvalho, S.B.; Silva, M.M.; Gomes, R.A.; Carrondo, M.J.T.; Gomes-Alves, P.; Peixoto, C.; Serra, M.; Alves, P.M. Bioprocess integration for human mesenchymal stem cells: From up to downstream processing scale-up to cell proteome characterization. J. Biotechnol. 2017, 248, 87–98. [Google Scholar] [CrossRef]
- Martin, C.; Olmos, É.; Collignon, M.-L.; de Isla, N.; Blanchard, F.; Chevalot, I.; Marc, A.; Guedon, E. Revisiting MSC expansion from critical quality attributes to critical culture process parameters. Process. Biochem. 2017, 59, 231–243. [Google Scholar] [CrossRef]
- Bouleftour, W.; Magne, N. Aging preclinical models in oncology field: From cells to aging. Aging Clin. Exp. Res. 2021, 1–5. [Google Scholar] [CrossRef]
- Braid, L.R.; Wood, C.A.; Wiese, D.M.; Ford, B.N. Intramuscular administration potentiates extended dwell time of mesenchymal stromal cells compared to other routes. Cytotherapy 2018, 20, 232–244. [Google Scholar] [CrossRef]
- Roux, C.; Saviane, G.; Pini, J.; Belaïd, N.; Dhib, G.; Voha, C.; Ibáñez, L.; Boutin, A.; Mazure, N.M.; Wakkach, A.; et al. Immunosuppressive Mesenchymal Stromal Cells Derived from Human-Induced Pluripotent Stem Cells Induce Human Regulatory T Cells In Vitro and In Vivo. Front. Immunol. 2017, 8, 1991. [Google Scholar] [CrossRef]
- Castelo-Branco, M.T.; Soares, I.D.; Lopes, D.V.; Buongusto, F.; Martinusso, C.A.; do Rosario, A., Jr.; Souza, S.A.; Gutfilen, B.; Fonseca, L.M.; Elia, C.; et al. Intraperitoneal but not intravenous cryopreserved mesenchymal stromal cells home to the inflamed colon and ameliorate experimental colitis. PLoS ONE 2012, 7, e33360. [Google Scholar] [CrossRef]
- Gonçalves Fda, C.; Schneider, N.; Pinto, F.O.; Meyer, F.S.; Visioli, F.; Pfaffenseller, B.; Lopez, P.L.; Passos, E.P.; Cirne-Lima, E.O.; Meurer, L.; et al. Intravenous vs intraperitoneal mesenchymal stem cells administration: What is the best route for treating experimental colitis? World J. Gastroenterol. 2014, 20, 18228–18239. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Guingab-Cagmat, J.; Kobeissy, F.; Zoltewicz, S.; Mondello, S.; Gao, M.; Hafeez, A.; Li, N.; Geng, X.; Larner, S.F.; et al. A neuroproteomic and systems biology analysis of rat brain post intracerebral hemorrhagic stroke. Brain Res. Bull. 2014, 102, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, T.; Kibayashi, T.; Katayama, T.; Yamashita, Y.; Suzuki, S.; Kawamata, J.; Honmou, O.; Minami, M.; Shimohama, S. Mesenchymal stem cells transmigrate across brain microvascular endothelial cell monolayers through transiently formed inter-endothelial gaps. Neurosci. Lett. 2011, 502, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Eckert, M.A.; Riazifar, H.; Kang, D.K.; Agalliu, D.; Zhao, W. From blood to the brain: Can systemically transplanted mesenchymal stem cells cross the blood-brain barrier? Stem Cells Int. 2013, 2013, 435093. [Google Scholar] [CrossRef]
- Bao, X.; Wei, J.; Feng, M.; Lu, S.; Li, G.; Dou, W.; Ma, W.; Ma, S.; An, Y.; Qin, C.; et al. Transplantation of human bone marrow-derived mesenchymal stem cells promotes behavioral recovery and endogenous neurogenesis after cerebral ischemia in rats. Brain Res. 2011, 1367, 103–113. [Google Scholar] [CrossRef]
- Ruan, G.P.; Han, Y.B.; Wang, T.H.; Xing, Z.G.; Zhu, X.B.; Yao, X.; Ruan, G.H.; Wang, J.X.; Pang, R.Q.; Cai, X.M.; et al. Comparative study among three different methods of bone marrow mesenchymal stem cell transplantation following cerebral infarction in rats. Neurol. Res. 2013, 35, 212–220. [Google Scholar] [CrossRef]
- Lou, G.; Chen, Z.; Zheng, M.; Liu, Y. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp. Mol. Med. 2017, 49, e346. [Google Scholar] [CrossRef]
- Kooijmans, S.A.; Vader, P.; van Dommelen, S.M.; van Solinge, W.W.; Schiffelers, R.M. Exosome mimetics: A novel class of drug delivery systems. Int. J. Nanomed. 2012, 7, 1525–1541. [Google Scholar] [CrossRef]
- Harrell, C.R.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases. Cells 2019, 8, 1605. [Google Scholar] [CrossRef]
- Zhuang, X.; Xiang, X.; Grizzle, W.; Sun, D.; Zhang, S.; Axtell, R.C.; Ju, S.; Mu, J.; Zhang, L.; Steinman, L.; et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol. Ther. 2011, 19, 1769–1779. [Google Scholar] [CrossRef]
- Santamaria, G.; Brandi, E.; Vitola, P.; Grandi, F.; Ferrara, G.; Pischiutta, F.; Vegliante, G.; Zanier, E.R.; Re, F.; Uccelli, A.; et al. Intranasal delivery of mesenchymal stem cell secretome repairs the brain of Alzheimer′s mice. Cell Death Differ. 2021, 28, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Demaria, M.; O′Leary, M.N.; Chang, J.; Shao, L.; Liu, S.; Alimirah, F.; Koenig, K.; Le, C.; Mitin, N.; Deal, A.M.; et al. Cellular Senescence Promotes Adverse Effects of Chemotherapy and Cancer Relapse. Cancer Discov. 2017, 7, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Mosteiro, L.; Pantoja, C.; Alcazar, N.; Marión, R.M.; Chondronasiou, D.; Rovira, M.; Fernandez-Marcos, P.J.; Muñoz-Martin, M.; Blanco-Aparicio, C.; Pastor, J.; et al. Tissue damage and senescence provide critical signals for cellular reprogramming In Vivo. Science 2016, 354, aaf4445. [Google Scholar] [CrossRef] [PubMed]
- Ritschka, B.; Storer, M.; Mas, A.; Heinzmann, F.; Ortells, M.C.; Morton, J.P.; Sansom, O.J.; Zender, L.; Keyes, W.M. The senescence-associated secretory phenotype induces cellular plasticity and tissue regeneration. Genes Dev. 2017, 31, 172–183. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2018, 217, 65–77. [Google Scholar] [CrossRef]
- Wilson, W.H.; O′Connor, O.A.; Czuczman, M.S.; LaCasce, A.S.; Gerecitano, J.F.; Leonard, J.P.; Tulpule, A.; Dunleavy, K.; Xiong, H.; Chiu, Y.L.; et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: A phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 2010, 11, 1149–1159. [Google Scholar] [CrossRef]
- Kornienko, J.S.; Smirnova, I.S.; Pugovkina, N.A.; Ivanova, J.S.; Shilina, M.A.; Grinchuk, T.M.; Shatrova, A.N.; Aksenov, N.D.; Zenin, V.V.; Nikolsky, N.N.; et al. High doses of synthetic antioxidants induce premature senescence in cultivated mesenchymal stem cells. Sci. Rep. 2019, 9, 1296. [Google Scholar] [CrossRef]
- Osugi, M.; Katagiri, W.; Yoshimi, R.; Inukai, T.; Hibi, H.; Ueda, M. Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects. Tissue Eng. Part A 2012, 18, 1479–1489. [Google Scholar] [CrossRef]
- Hu, J.L.; Todhunter, M.E.; LaBarge, M.A.; Gartner, Z.J. Opportunities for organoids as new models of aging. J. Cell Biol. 2018, 217, 39–50. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fraile, M.; Eiro, N.; Costa, L.A.; Martín, A.; Vizoso, F.J. Aging and Mesenchymal Stem Cells: Basic Concepts, Challenges and Strategies. Biology 2022, 11, 1678. https://doi.org/10.3390/biology11111678
Fraile M, Eiro N, Costa LA, Martín A, Vizoso FJ. Aging and Mesenchymal Stem Cells: Basic Concepts, Challenges and Strategies. Biology. 2022; 11(11):1678. https://doi.org/10.3390/biology11111678
Chicago/Turabian StyleFraile, Maria, Noemi Eiro, Luis A. Costa, Arancha Martín, and Francisco J. Vizoso. 2022. "Aging and Mesenchymal Stem Cells: Basic Concepts, Challenges and Strategies" Biology 11, no. 11: 1678. https://doi.org/10.3390/biology11111678
APA StyleFraile, M., Eiro, N., Costa, L. A., Martín, A., & Vizoso, F. J. (2022). Aging and Mesenchymal Stem Cells: Basic Concepts, Challenges and Strategies. Biology, 11(11), 1678. https://doi.org/10.3390/biology11111678





