Confocal Identification of Immune Molecules in Skin Club Cells of Zebrafish (Danio rerio, Hamilton 1882) and Their Possible Role in Immunity
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Tissue Preparation
2.2. Histology
2.3. Immunoperoxidase
2.4. Immunofluorescence and Laser Confocal Analysis
2.5. Quantitative Analysis
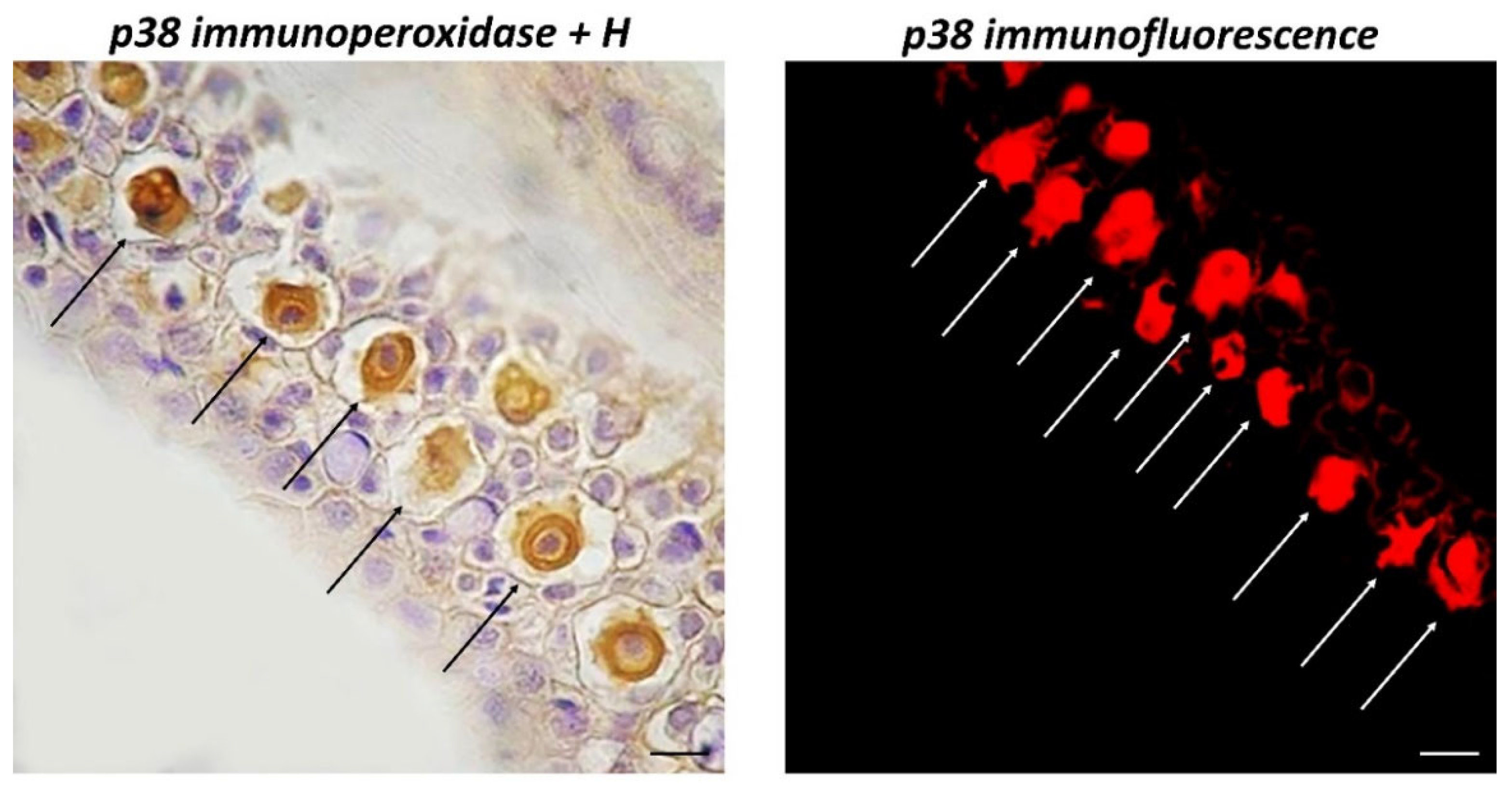
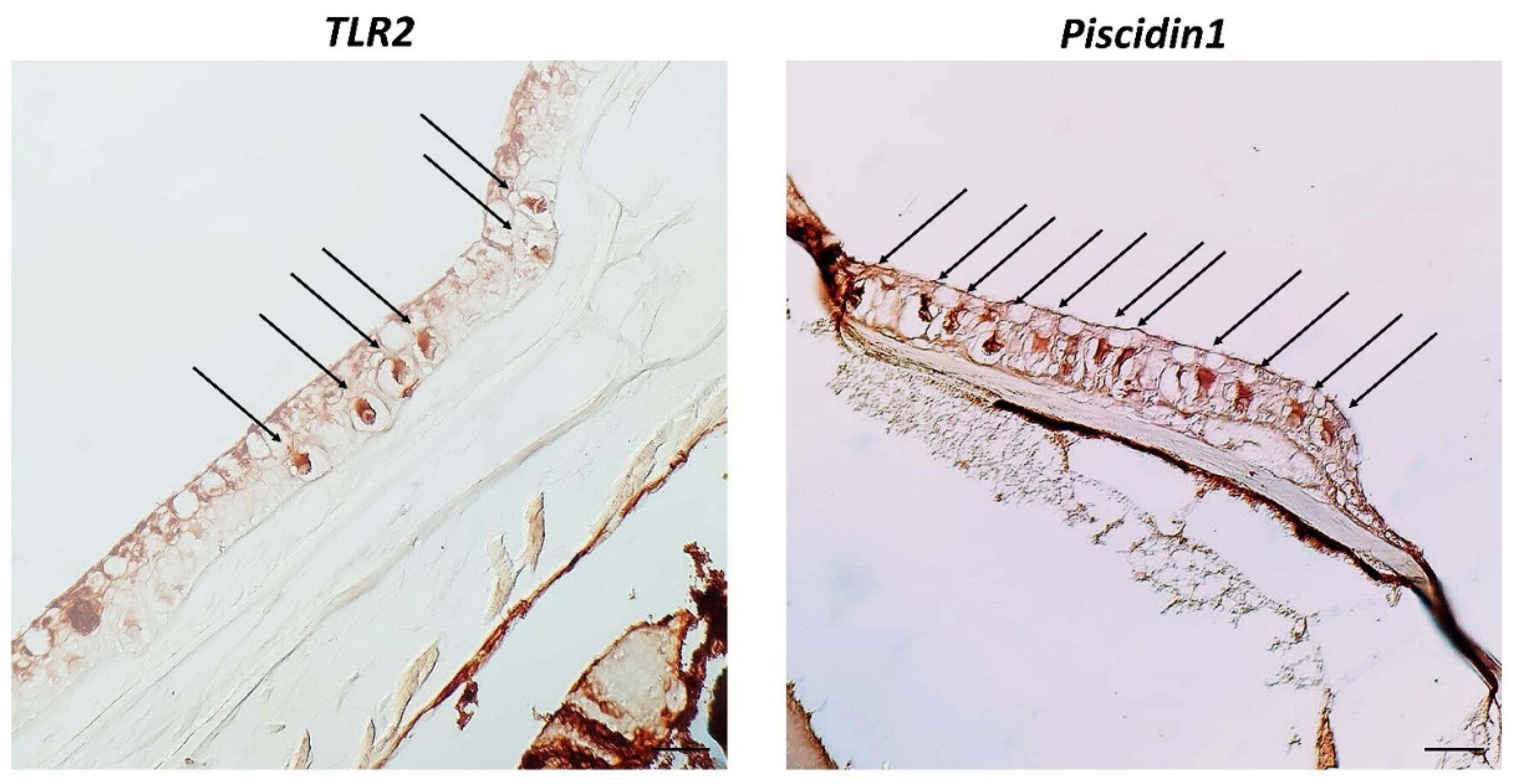
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Biller-Takahashi, J.D.; Urbinati, E.C. Fish Immunology. The Modification and Manipulation of the Innate Immune System: Brazilian Studies. An. Acad. Bras. Ciênc. 2014, 86, 1484–1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayed, R.K.A.; Zaccone, G.; Capillo, G.; Albano, M.; Mokhtar, D.M. Structural and Functional Aspects of the Spleen in Molly Fish Poecilia sphenops (Valenciennes, 1846): Synergistic Interactions of Stem Cells, Neurons, and Immune Cells. Biology 2022, 11, 779. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.C.; Rise, M.L.; Christian, S.L. A Comparison of the Innate and Adaptive Immune Systems in Cartilaginous Fish, Ray-Finned Fish, and Lobe-Finned Fish. Front. Immunol. 2019, 10, 2292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahoo, S.; Banu, H.; Prakash, A.; Tripathi, G. Immune System of Fish: An Evolutionary Perspective. In Antimicrobial Immune Response; del Mar Ortega-Villaizan, M., Chico, V., Eds.; IntechOpen: London, UK, 2021; ISBN 9781839687822. [Google Scholar]
- Riera Romo, M.; Pérez-Martínez, D.; Castillo Ferrer, C. Innate Immunity in Vertebrates: An Overview. Immunology 2016, 148, 125–139. [Google Scholar] [CrossRef]
- Flajnik, M.F.; Kasahara, M. Origin and Evolution of the Adaptive Immune System: Genetic Events and Selective Pressures. Nat. Rev. Genet. 2010, 11, 47–59. [Google Scholar] [CrossRef] [Green Version]
- Brazeau, M.D.; Friedman, M. The Origin and Early Phylogenetic History of Jawed Vertebrates. Nature 2015, 520, 490–497. [Google Scholar] [CrossRef] [Green Version]
- Buchmann, K. Evolution of Innate Immunity: Clues from Invertebrates via Fish to Mammals. Front. Immunol. 2014, 5, 459. [Google Scholar] [CrossRef] [Green Version]
- Ángeles Esteban, M. An Overview of the Immunological Defenses in Fish Skin. ISRN Immunol. 2012, 2012, 853470. [Google Scholar] [CrossRef] [Green Version]
- Proksch, E.; Brandner, J.M.; Jensen, J.-M. The Skin: An Indispensable Barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef]
- Sridhar, A.; Manikandan, D.B.; Palaniyappan, S.; Sekar, R.K.; Ramasamy, T. Correlation Between Three Freshwater Fish Skin Mucus Antiproliferative Effect and Its Elemental Composition Role in Bacterial Growth. Turk. J. Fish. Aquat. Sci. 2021, 21, 233–244. [Google Scholar] [CrossRef]
- Anderson, K.C.; Ghosh, B.; Chetty, T.; Walker, S.P.; Symonds, J.E.; Nowak, B.F. Transcriptomic Characterisation of a Common Skin Lesion in Farmed Chinook Salmon. Fish Shellfish Immunol. 2022, 124, 28–38. [Google Scholar] [CrossRef]
- Whitear, M. Epidermis. In Biology of the Integument; Bereiter-Hahn, J., Matoltsy, A.G., Richards, K.S., Eds.; Springer: Berlin/Heidelberg, Germany, 1986; pp. 8–38. ISBN 9783662009918. [Google Scholar]
- Rakers, S.; Gebert, M.; Uppalapati, S.; Meyer, W.; Maderson, P.; Sell, A.F.; Kruse, C.; Paus, R. ‘Fish Matters’: The Relevance of Fish Skin Biology to Investigative Dermatology. Exp. Dermatol. 2010, 19, 313–324. [Google Scholar] [CrossRef]
- Guerra, R.R.; Santos, N.P.; Cecarelli, P.; Mangetti, A.J.; Silva, J.R.M.C.; Hernandez-Blazquez, F.J. Stratum Adiposum, A Special Structure of the African Catfish Skin (Clarias Gariepinus, Burchell 1822). Anat. Histol. Embryol. J. Vet. Med. Ser. C 2006, 35, 144–146. [Google Scholar] [CrossRef]
- Al-Banaw, A.; Kenngott, R.; Al-Hassan, J.M.; Mehana, N.; Sinowatz, F. Histochemical Analysis of Glycoconjugates in the Skin of a Catfish (Arius Tenuispinis, Day). Anat. Histol. Embryol. 2010, 39, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Chakrabarty, P.; Faircloth, B.C.; Alda, F.; Ludt, W.B.; Mcmahan, C.D.; Near, T.J.; Dornburg, A.; Albert, J.S.; Arroyave, J.; Stiassny, M.L.J.; et al. Phylogenomic Systematics of Ostariophysan Fishes: Ultraconserved Elements Support the Surprising Non-Monophyly of Characiformes. Syst. Biol. 2017, 66, 881–895. [Google Scholar] [CrossRef] [Green Version]
- Chivers, D.P.; Wisenden, B.D.; Hindman, C.J.; Michalak, T.A.; Kusch, R.C.; Kaminskyj, S.G.W.; Jack, K.L.; Ferrari, M.C.O.; Pollock, R.J.; Halbgewachs, C.F.; et al. Epidermal ‘Alarm Substance’ Cells of Fishes Maintained by Non-Alarm Functions: Possible Defence against Pathogens, Parasites and UVB Radiation. Proc. R. Soc. B Biol. Sci. 2007, 274, 2611–2619. [Google Scholar] [CrossRef] [Green Version]
- Helfman, G.; Collette, B.B.; Facey, D.E.; Bowen, B.W. The Diversity of Fishes: Biology, Evolution, and Ecology; John Wiley & Sons: Chichester, UK, 2009. [Google Scholar]
- Park, J.Y.; Oh, M.K.; Kang, E.J.; Kim, C.H.; Beon, M.S. On the Vascularization and Structure of the Skin of a Korean Bullhead Pseudobagrus brevicorpus (Bagridae, Teleostei) Based on Its Entire Body and Appendages. J. Appl. Ichthyol. 2010, 26, 64–70. [Google Scholar] [CrossRef]
- Damasceno, E.M.; Monteiro, J.C.; Duboc, L.F.; Dolder, H.; Mancini, K. Morphology of the Epidermis of the Neotropical Catfish Pimelodella lateristriga (Lichtenstein, 1823) with Emphasis in Club Cells. PLoS ONE 2012, 7, e50255. [Google Scholar] [CrossRef]
- Zaccone, G.; Tagliafierro, G.; Fasulo, S.; Contini, A.; Ainis, L.; Ricca, M.B. Serotonin-like Immunoreactivity in the Epidermal Club Cells of Teleost Fishes. Histochemistry 1990, 93, 355–357. [Google Scholar] [CrossRef]
- Smith, R.J.F. Alarm Signals in Fishes. Rev. Fish Biol. Fish. 1992, 2, 33–63. [Google Scholar] [CrossRef]
- Jung, J.A.; Tonn, W.M. Alarm Substances Elicit Limited Population-Level Responses in Fathead Minnow: Limited Effects of Alarm Substances. Ecol. Freshw. Fish 2011, 20, 220–230. [Google Scholar] [CrossRef]
- Pietrzak, E.; Mazurkiewicz, J.; Slawinska, A. Innate Immune Responses of Skin Mucosa in Common Carp (Cyprinus Carpio) Fed a Diet Supplemented with Galactooligosaccharides. Animals 2020, 10, 438. [Google Scholar] [CrossRef] [Green Version]
- Alesci, A.; Cicero, N.; Fumia, A.; Petrarca, C.; Mangifesta, R.; Nava, V.; Lo Cascio, P.; Gangemi, S.; Di Gioacchino, M.; Lauriano, E.R. Histological and Chemical Analysis of Heavy Metals in Kidney and Gills of Boops Boops: Melanomacrophages Centers and Rodlet Cells as Environmental Biomarkers. Toxics 2022, 10, 218. [Google Scholar] [CrossRef]
- Icardo, J.M.; Colvee, E.; Lauriano, E.R.; Capillo, G.; Guerrera, M.C.; Zaccone, G. The Structure of the Gas Bladder of the Spotted Gar, Lepisosteus Oculatus. J. Morphol. 2015, 276, 90–101. [Google Scholar] [CrossRef]
- Pergolizzi, S.; Rizzo, G.; Favaloro, A.; Alesci, A.; Pallio, S.; Melita, G.; Cutroneo, G.; Lauriano, E.R. Expression of VAChT and 5-HT in Ulcerative Colitis Dendritic Cells. Acta Histochem. 2021, 123, 151715. [Google Scholar] [CrossRef]
- Zaccone, D.; Icardo, J.M.; Kuciel, M.; Alesci, A.; Pergolizzi, S.; Satora, L.; Lauriano, E.R.; Zaccone, G. Polymorphous Granular Cells in the Lung of the Primitive Fish, the Bichir Polypterus senegalus. Acta Zool. 2017, 98, 13–19. [Google Scholar] [CrossRef]
- Lauriano, E.R.; Żuwała, K.; Kuciel, M.; Budzik, K.A.; Capillo, G.; Alesci, A.; Pergolizzi, S.; Dugo, G.; Zaccone, G. Confocal Immunohistochemistry of the Dermal Glands and Evolutionary Considerations in the Caecilian, Typhlonectes natans (Amphibia: Gymnophiona). Acta Zool. 2016, 97, 154–164. [Google Scholar] [CrossRef]
- Manek, A.K.; Ferrari, M.C.O.; Sereda, J.M.; Niyogi, S.; Chivers, D.P. The Effects of Ultraviolet Radiation on a Freshwater Prey Fish: Physiological Stress Response, Club Cell Investment, and Alarm Cue Production. Biol. J. Linn. Soc. 2012, 105, 832–841. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, M.C.O.; Wisenden, B.D.; Chivers, D.P. Chemical Ecology of Predator–Prey Interactions in Aquatic Ecosystems: A Review and Prospectus. Can. J. Zool. 2010, 88, 698–724. [Google Scholar] [CrossRef]
- Frisch, K.V. Die Bedeutung des Geruchsinnes im Leben der Fische. Naturwissenschaften 1941, 29, 321–333. [Google Scholar] [CrossRef]
- Pfeiffer, W. The Distribution of Fright Reaction and Alarm Substance Cells in Fishes. Copeia 1977, 1977, 653. [Google Scholar] [CrossRef]
- Brown, G.E.; Adrian Jr., J.C.; Naderi, N.T.; Harvey, M.C.; Kelly, J.M. Nitrogen Oxides Elicit Antipredator Responses in Juvenile Channel Catfish, But Not in Convict Cichlids or Rainbow Trout: Conservation of the Ostariophysan Alarm Pheromone. J. Chem. Ecol. 2003, 29, 1781–1796. [Google Scholar] [CrossRef]
- Lauriano, E.R.; Guerrera, M.C.; Laurà, R.; Capillo, G.; Pergolizzi, S.; Aragona, M.; Abbate, F.; Germanà, A. Effect of Light on the Calretinin and Calbindin Expression in Skin Club Cells of Adult Zebrafish. Histochem. Cell Biol. 2020, 154, 495–505. [Google Scholar] [CrossRef]
- Meuthen, D.; Meuthen, I.; Bakker, T.C.M.; Thünken, T. Anticipatory Plastic Response of the Cellular Immune System in the Face of Future Injury: Chronic High Perceived Predation Risk Induces Lymphocytosis in a Cichlid Fish. Oecologia 2020, 194, 597–607. [Google Scholar] [CrossRef]
- Barkhymer, A.J.; Garrett, S.G.; Wisenden, B.D. Olfactorily-Mediated Cortisol Response to Chemical Alarm Cues in Zebrafish Danio Rerio. J. Fish Biol. 2019, 95, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Khansari, A.R.; Balasch, J.C.; Vallejos-Vidal, E.; Parra, D.; Reyes-López, F.E.; Tort, L. Comparative Immune- and Stress-Related Transcript Response Induced by Air Exposure and Vibrio anguillarum Bacterin in Rainbow Trout (Oncorhynchus mykiss) and Gilthead Seabream (Sparus aurata) Mucosal Surfaces. Front. Immunol. 2018, 9, 856. [Google Scholar] [CrossRef] [Green Version]
- Pandey, S.; Stockwell, C.A.; Snider, M.R.; Wisenden, B.D. Epidermal Club Cells in Fishes: A Case for Ecoimmunological Analysis. Int. J. Mol. Sci. 2021, 22, 1440. [Google Scholar] [CrossRef]
- Abd-Elhafeez, H.H.; Abdo, W.; Kamal, B.M.; Soliman, S.A. Fish Telocytes and Their Relation to Rodlet Cells in Ruby-Red-Fin Shark (Rainbow Shark) Epalzeorhynchos frenatum (Teleostei: Cyprinidae). Sci. Rep. 2020, 10, 18907. [Google Scholar] [CrossRef]
- Alesci, A.; Pergolizzi, S.; Savoca, S.; Fumia, A.; Mangano, A.; Albano, M.; Messina, E.; Aragona, M.; Lo Cascio, P.; Capillo, G.; et al. Detecting Intestinal Goblet Cells of the Broadgilled Hagfish Eptatretus cirrhatus (Forster, 1801): A Confocal Microscopy Evaluation. Biology 2022, 11, 1366. [Google Scholar] [CrossRef]
- Backström, T.; Winberg, S. Serotonin Coordinates Responses to Social Stress—What We Can Learn from Fish. Front. Neurosci. 2017, 11, 595. [Google Scholar] [CrossRef]
- Gould, S.L.; Winter, M.J.; Norton, W.H.J.; Tyler, C.R. The Potential for Adverse Effects in Fish Exposed to Antidepressants in the Aquatic Environment. Environ. Sci. Technol. 2021, 55, 16299–16312. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Jiang, B.; Zhang, Z.; Huang, Y.; Xu, Z.; Chen, X.; Hou, X.; Cai, J.; Huang, Y.; Jian, J. Serotonin System Is Partially Involved in Immunomodulation of Nile Tilapia (Oreochromis niloticus) Immune Cells. Front. Immunol. 2022, 13, 944388. [Google Scholar] [CrossRef] [PubMed]
- Roumier, A.; Béchade, C.; Maroteaux, L. Serotonin and the Immune System. In Serotonin; Elsevier: Amsterdam, The Netherlands, 2019; pp. 181–196. ISBN 9780128000502. [Google Scholar]
- Tian, Y.; Wen, H.; Qi, X.; Zhang, X.; Li, Y. Identification of Mapk Gene Family in Lateolabrax maculatus and Their Expression Profiles in Response to Hypoxia and Salinity Challenges. Gene 2019, 684, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Neuder, L.E.; Keener, J.M.; Eckert, R.E.; Trujillo, J.C.; Jones, S.L. Role of P38 MAPK in LPS Induced Pro-Inflammatory Cytokine and Chemokine Gene Expression in Equine Leukocytes. Vet. Immunol. Immunopathol. 2009, 129, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Huang, C.; Ma, X.; Wu, R.; Zhu, W.; Li, X.; Liang, Z.; Deng, F.; Wu, J.; Geng, S.; et al. Phthalates Promote Prostate Cancer Cell Proliferation through Activation of ERK5 and P38. Environ. Toxicol. Pharmacol. 2018, 63, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Qian, Y.; Liu, Z.; Wang, C.; Qu, J.; Wang, X.; Wang, S. Perfluorooctane Sulfonate (PFOS) Disrupts Blood-Testis Barrier by down-Regulating Junction Proteins via P38 MAPK/ATF2/MMP9 Signaling Pathway. Toxicology 2016, 373, 1–12. [Google Scholar] [CrossRef]
- Park, K.; Kim, W.-S.; Choi, B.; Kwak, I.-S. Expression Levels of the Immune-Related P38 Mitogen-Activated Protein Kinase Transcript in Response to Environmental Pollutants on Macrophthalmus japonicus Crab. Genes 2020, 11, 958. [Google Scholar] [CrossRef]
- Marshall, W.S.; Cozzi, R.R.F.; Spieker, M. WNK1 and P38-MAPK Distribution in Ionocytes and Accessory Cells of Euryhaline Teleost Fish Implies Ionoregulatory Function. Biol. Open 2017, 6, 956–966. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; Sano, Y.; Todorova, K.; Carlson, B.A.; Arpa, L.; Celada, A.; Lawrence, T.; Otsu, K.; Brissette, J.L.; Arthur, J.S.C.; et al. The Kinase P38α Serves Cell Type–Specific Inflammatory Functions in Skin Injury and Coordinates pro- and Anti-Inflammatory Gene Expression. Nat. Immunol. 2008, 9, 1019–1027. [Google Scholar] [CrossRef] [Green Version]
- Tang, R.; Wang, S.; Han, P.; Zhang, Q.; Zhang, S.; Xing, X.; Shao, R.; Xu, W.; Xu, Q.; Wei, Q.; et al. Toll-like Receptor (TLR) 2 and TLR13 from the Endangered Primitive-Ray Finned Fish Dabry’s Sturgeon (Acipenser dabryanus) and Their Expression Profiling upon Immune Stimulation. Aquac. Rep. 2020, 16, 100247. [Google Scholar] [CrossRef]
- Sahoo, B.R. Structure of Fish Toll-like Receptors (TLR) and NOD-like Receptors (NLR). Int. J. Biol. Macromol. 2020, 161, 1602–1617. [Google Scholar] [CrossRef] [PubMed]
- Alesci, A.; Pergolizzi, S.; Lo Cascio, P.; Fumia, A.; Lauriano, E.R. Neuronal Regeneration: Vertebrates Comparative Overview and New Perspectives for Neurodegenerative Diseases. Acta Zool. 2021, 103, 129–140. [Google Scholar] [CrossRef]
- Alesci, A.; Aragona, M.; Cicero, N.; Lauriano, E.R. Can Nutraceuticals Assist Treatment and Improve Covid-19 Symptoms? Nat. Prod. Res. 2021, 36, 1–20. [Google Scholar] [CrossRef]
- Alesci, A.; Lauriano, E.R.; Fumia, A.; Irrera, N.; Mastrantonio, E.; Vaccaro, M.; Gangemi, S.; Santini, A.; Cicero, N.; Pergolizzi, S. Relationship between Immune Cells, Depression, Stress, and Psoriasis: Could the Use of Natural Products Be Helpful? Molecules 2022, 27, 1953. [Google Scholar] [CrossRef]
- Lauriano, E.R.; Aragona, M.; Alesci, A.; Lo Cascio, P.; Pergolizzi, S. Toll-like Receptor 2 and Alpha-Smooth Muscle Actin Expressed in the Tunica of a Urochordate, Styela plicata. Tissue Cell 2021, 71, 101584. [Google Scholar] [CrossRef]
- Alesci, A.; Pergolizzi, S.; Lo Cascio, P.; Capillo, G.; Lauriano, E.R. Localization of Vasoactive Intestinal Peptide and Toll-like Receptor 2 Immunoreactive Cells in Endostyle of Urochordate Styela plicata (Lesueur, 1823). Microsc. Res. Tech. 2022, 85, 2651–2658. [Google Scholar] [CrossRef]
- Alesci, A.; Capillo, G.; Fumia, A.; Messina, E.; Albano, M.; Aragona, M.; Lo Cascio, P.; Spanò, N.; Pergolizzi, S.; Lauriano, E.R. Confocal Characterization of Intestinal Dendritic Cells from Myxines to Teleosts. Biology 2022, 11, 1045. [Google Scholar] [CrossRef]
- Alesci, A.; Pergolizzi, S.; Fumia, A.; Calabrò, C.; Lo Cascio, P.; Lauriano, E.R. Mast Cells in Goldfish (Carassius Auratus) Gut: Immunohistochemical Characterization. Acta Zool. 2022. [Google Scholar] [CrossRef]
- Alesci, A.; Pergolizzi, S.; Capillo, G.; Lo Cascio, P.; Lauriano, E.R. Rodlet Cells in Kidney of Goldfish (Carassius auratus, Linnaeus 1758): A Light and Confocal Microscopy Study. Acta Histochem. 2022, 124, 151876. [Google Scholar] [CrossRef]
- Marino, A.; Pergolizzi, S.; Lauriano, E.R.; Santoro, G.; Spataro, F.; Cimino, F.; Speciale, A.; Nostro, A.; Bisignano, G. TLR2 Activation in Corneal Stromal Cells by Staphylococcus aureus -Induced Keratitis. APMIS 2015, 123, 163–168. [Google Scholar] [CrossRef]
- Jault, C.; Pichon, L.; Chluba, J. Toll-like Receptor Gene Family and TIR-Domain Adapters in Danio rerio. Mol. Immunol. 2004, 40, 759–771. [Google Scholar] [CrossRef]
- Oshiumi, H.; Matsumoto, M.; Funami, K.; Akazawa, T.; Seya, T. TICAM-1, an Adaptor Molecule That Participates in Toll-like Receptor 3–Mediated Interferon-β Induction. Nat. Immunol. 2003, 4, 161–167. [Google Scholar] [CrossRef]
- Yu, J.; Liu, X.; Yang, N.; Wang, B.; Su, B.; Fu, Q.; Zhang, M.; Tan, F.; Li, C. Characterization of Toll-like Receptor 1 (TLR1) in Turbot (Scophthalmus maximus L.). Fish Shellfish Immunol. 2021, 115, 27–34. [Google Scholar] [CrossRef]
- Pietretti, D.; Spaink, H.P.; Falco, A.; Forlenza, M.; Wiegertjes, G.F. Accessory Molecules for Toll-like Receptors in Teleost Fish. Identification of TLR4 Interactor with Leucine-Rich Repeats (TRIL). Mol. Immunol. 2013, 56, 745–756. [Google Scholar] [CrossRef]
- Chen, Z.; Ceballos-Francisco, D.; Guardiola, F.A.; Huang, D.; Esteban, M.Á. The Alleviation of Skin Wound-Induced Intestinal Barrier Dysfunction via Modulation of TLR Signalling Using Arginine in Gilthead Seabream (Sparus aurata L). Fish Shellfish Immunol. 2020, 107, 519–528. [Google Scholar] [CrossRef]
- Zheng, T.; Song, Z.; Qiang, J.; Tao, Y.; Zhu, H.; Ma, J.; Xu, P. Transport Stress Induces Skin Innate Immunity Response in Hybrid Yellow Catfish (Tachysurus fulvidraco♀ × P. vachellii♂) Through TLR/NLR Signaling Pathways and Regulation of Mucus Secretion. Front. Immunol. 2021, 12, 740359. [Google Scholar] [CrossRef]
- Augustyniak, D.; Nowak, J.; Lundy, F.T. Direct and Indirect Antimicrobial Activities of Neuropeptides and Their Therapeutic Potential. Curr. Protein Pept. Sci. 2012, 13, 723–738. [Google Scholar] [CrossRef] [Green Version]
- Zasloff, M. Antimicrobial Peptides of Multicellular Organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef]
- Corrales, L.; Matson, V.; Flood, B.; Spranger, S.; Gajewski, T.F. Innate Immune Signaling and Regulation in Cancer Immunotherapy. Cell Res. 2017, 27, 96–108. [Google Scholar] [CrossRef] [Green Version]
- Ruangsri, J.; Fernandes, J.M.O.; Rombout, J.H.W.M.; Brinchmann, M.F.; Kiron, V. Ubiquitous Presence of Piscidin-1 in Atlantic Cod as Evidenced by Immunolocalisation. BMC Vet. Res. 2012, 8, 46. [Google Scholar] [CrossRef]
- Rakers, S.; Niklasson, L.; Steinhagen, D.; Kruse, C.; Schauber, J.; Sundell, K.; Paus, R. Antimicrobial Peptides (AMPs) from Fish Epidermis: Perspectives for Investigative Dermatology. J. Investig. Dermatol. 2013, 133, 1140–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calabrese, V.; Mancuso, C.; Calvani, M.; Rizzarelli, E.; Butterfield, D.A.; Giuffrida Stella, A.M. Nitric Oxide in the Central Nervous System: Neuroprotection versus Neurotoxicity. Nat. Rev. Neurosci. 2007, 8, 766–775. [Google Scholar] [CrossRef]
- Xue, Q.; Yan, Y.; Zhang, R.; Xiong, H. Regulation of INOS on Immune Cells and Its Role in Diseases. Int. J. Mol. Sci. 2018, 19, 3805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carey, R.M.; Chen, B.; Adappa, N.D.; Palmer, J.N.; Kennedy, D.W.; Lee, R.J.; Cohen, N.A. Human Upper Airway Epithelium Produces Nitric Oxide in Response to Staphylococcus epidermidis. Int. Forum Allergy Rhinol. 2016, 6, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.E.; Adrian, J.C.; Kaufman, I.H.; Erickson, J.L.; Gershaneck, D. Responses to Nitrogen-Oxides by Characiforme Fishes Suggest Evolutionary Conservation in Ostariophysan Alarm Pheromones. In Chemical Signals in Vertebrates 9; Marchlewska-Koj, A., Lepri, J.J., Müller-Schwarze, D., Eds.; Springer: Boston, MA, USA, 2001; pp. 305–312. ISBN 9781461351870. [Google Scholar]



| Antibody | Supplier | Dilution | Animal Source |
|---|---|---|---|
| MAPK p38 | Sigma-Aldrich, Inc., St. Louis, MO, USA | 1:100 | Rabbit |
| Piscidin1 | GenScript Biotech Corporation, Rijswijk, Netherlands, Europe. Produced on demand | 1:50 | Rabbit |
| TLR2 | Active Motif, La Hulpe, Belgium, Europe | 1:125 | Rabbit |
| 5-HT | Santa Cruz Biotechnology, Inc., Dallas, TX, USA | 1:50 | Mouse |
| 5-HT | Sigma-Aldrich, Inc., St. Louis, MO, USA | 1:300 | Rabbit |
| iNOS | Santa Cruz Biotechnology, Inc., Dallas, TX, USA | 1:200 | Mouse |
| Goat anti-Rabbit IgG Peroxidase conjugated | Sigma Aldrich, Saint Louis, MO, USA | 1:100 | Goat |
| Alexa Fluor 488 Donkey anti-Mouse IgG FITC conjugated | Molecular Probes, Invitrogen | 1:300 | Donkey |
| Alexa Fluor 594 Donkey anti-Rabbit IgG TRITC conjugated | Molecular Probes, Invitrogen | 1:300 | Donkey |
| No. of CCs 1 | ||
|---|---|---|
| Immunoperoxidase | MAPK p38 | 2769.93 ± 345.98 * |
| TLR2 | 2819.05 ± 289.56 ** | |
| Piscidin1 | 2681.94 ± 3 05.67 * | |
| Immunofluorescence | MAPK p38 | 3117.54 ± 319.29 * |
| TLR2 | 3123.55 ± 279.06 * | |
| Piscidin1 | 3114.81 ± 385.90 ** | |
| 5-HT | 3185.46 ± 322.73 ** | |
| iNOS | 3067.84 ± 341.92 * | |
| Colocalization | 5-HT+MAPK p38 | 3067.84 ± 341.92 * |
| 5-HT+TLR2 | 3067.84 ± 341.92 * | |
| 5-HT+Piscidin1 | 3067.84 ± 341.92 * | |
| 5-HT+iNOS | 3067.84 ± 341.92 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alesci, A.; Albano, M.; Savoca, S.; Mokhtar, D.M.; Fumia, A.; Aragona, M.; Lo Cascio, P.; Hussein, M.M.; Capillo, G.; Pergolizzi, S.; et al. Confocal Identification of Immune Molecules in Skin Club Cells of Zebrafish (Danio rerio, Hamilton 1882) and Their Possible Role in Immunity. Biology 2022, 11, 1653. https://doi.org/10.3390/biology11111653
Alesci A, Albano M, Savoca S, Mokhtar DM, Fumia A, Aragona M, Lo Cascio P, Hussein MM, Capillo G, Pergolizzi S, et al. Confocal Identification of Immune Molecules in Skin Club Cells of Zebrafish (Danio rerio, Hamilton 1882) and Their Possible Role in Immunity. Biology. 2022; 11(11):1653. https://doi.org/10.3390/biology11111653
Chicago/Turabian StyleAlesci, Alessio, Marco Albano, Serena Savoca, Doaa M. Mokhtar, Angelo Fumia, Marialuisa Aragona, Patrizia Lo Cascio, Marwa M. Hussein, Gioele Capillo, Simona Pergolizzi, and et al. 2022. "Confocal Identification of Immune Molecules in Skin Club Cells of Zebrafish (Danio rerio, Hamilton 1882) and Their Possible Role in Immunity" Biology 11, no. 11: 1653. https://doi.org/10.3390/biology11111653
APA StyleAlesci, A., Albano, M., Savoca, S., Mokhtar, D. M., Fumia, A., Aragona, M., Lo Cascio, P., Hussein, M. M., Capillo, G., Pergolizzi, S., Spanò, N., & Lauriano, E. R. (2022). Confocal Identification of Immune Molecules in Skin Club Cells of Zebrafish (Danio rerio, Hamilton 1882) and Their Possible Role in Immunity. Biology, 11(11), 1653. https://doi.org/10.3390/biology11111653












