Cardiolipin Alterations during Obesity: Exploring Therapeutic Opportunities
Abstract
:Simple Summary
Abstract
1. Introduction
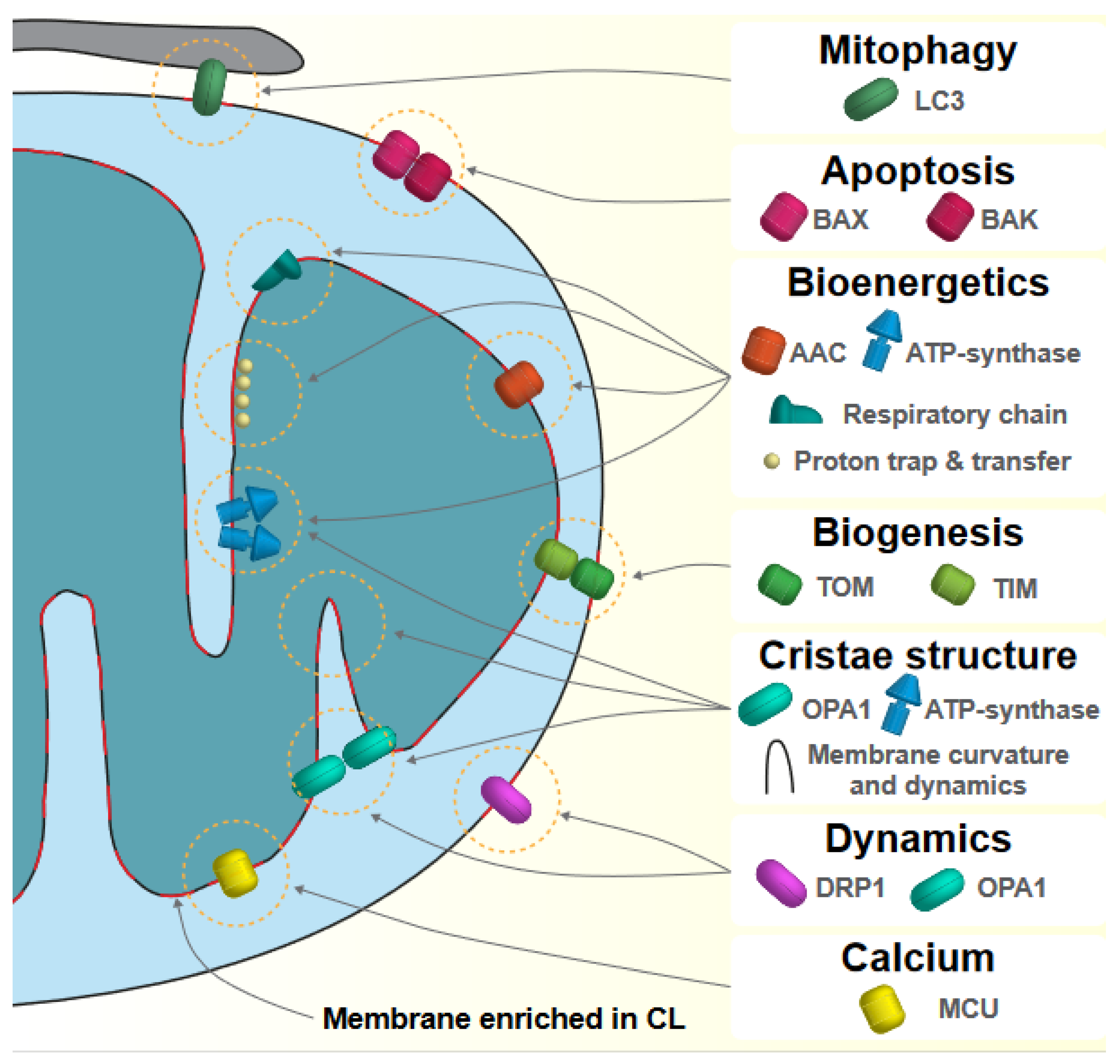
2. Mitochondrial Function and Cardiolipin
2.1. Synthesis of Cardiolipin
2.2. Diversity of Cardiolipin
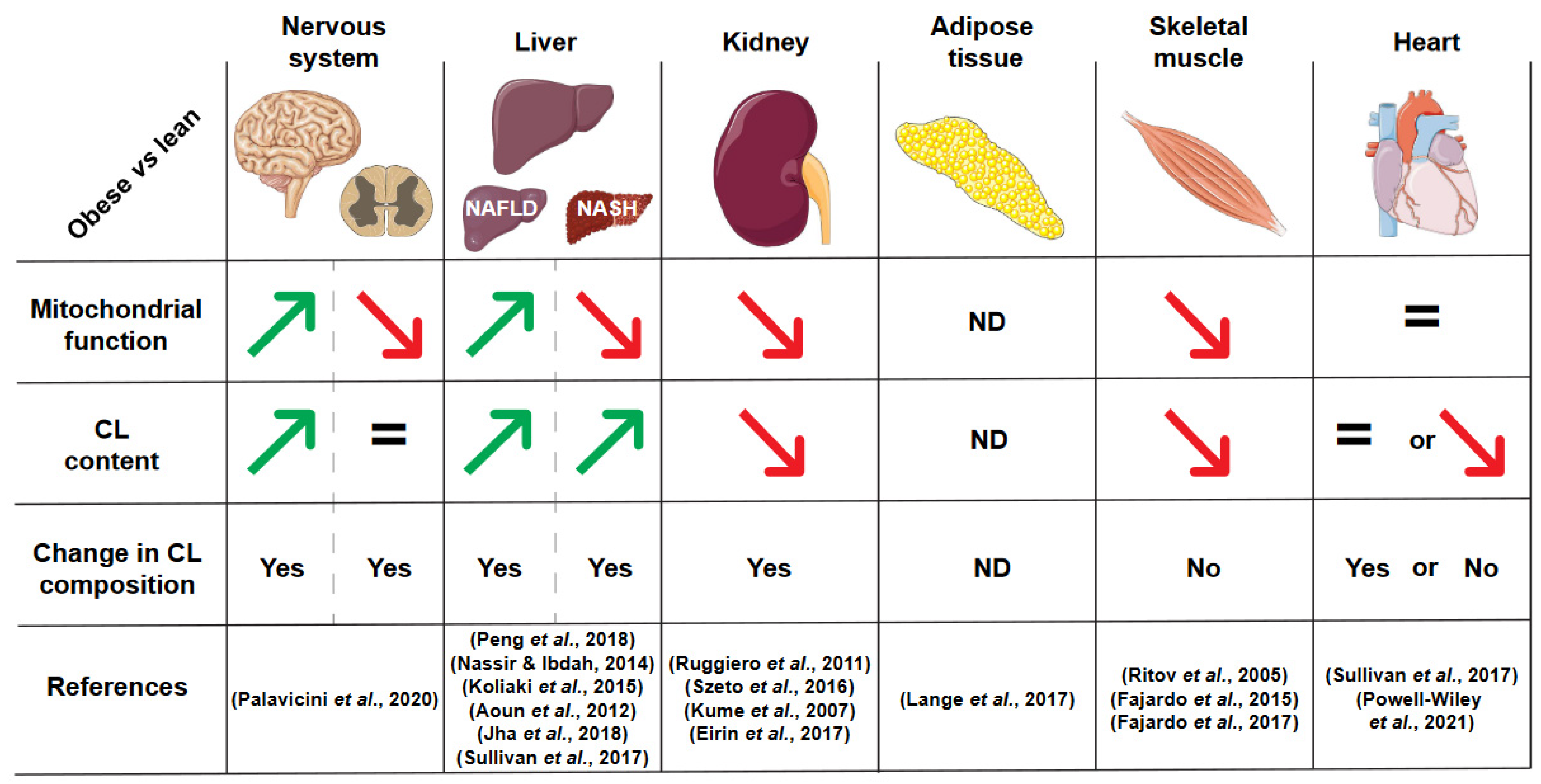
3. Cardiolipin Alterations in Obesity
3.1. Liver
3.2. Kidney
3.3. Heart
3.4. Nervous System
3.5. Adipose Tissue
3.6. Skeletal Muscle
4. Targeting Cardiolipin to Treat Obesity
4.1. Mitigation of Obesity-Associated Comorbidities
4.1.1. Exercise
4.1.2. Modulation of the Cardiolipin Synthetic Pathway
4.1.3. Protection of Cardiolipin against Peroxidation
4.2. Promotion of Weight Loss through Increased Energy Expenditure
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The Metabolic Syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- De Mello, A.H.; Costa, A.B.; Engel, J.D.G.; Rezin, G.T. Mitochondrial Dysfunction in Obesity. Life Sci. 2018, 192, 26–32. [Google Scholar] [CrossRef]
- Heinonen, S.; Buzkova, J.; Muniandy, M.; Kaksonen, R.; Ollikainen, M.; Ismail, K.; Hakkarainen, A.; Lundbom, J.; Lundbom, N.; Vuolteenaho, K.; et al. Impaired Mitochondrial Biogenesis in Adipose Tissue in Acquired Obesity. Diabetes 2015, 64, 3135–3145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Wei, Y.; Sowers, J.R. Role of Mitochondrial Dysfunction in Insulin Resistance. Circ. Res. 2008, 102, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Palavicini, J.P.; Chen, J.; Wang, C.; Wang, J.; Qin, C.; Baeuerle, E.; Wang, X.; Woo, J.A.; Kang, D.E.; Musi, N.; et al. Early Disruption of Nerve Mitochondrial and Myelin Lipid Homeostasis in Obesity-Induced Diabetes. JCI Insight 2020, 5, 137286. [Google Scholar] [CrossRef]
- Peng, K.-Y.; Watt, M.J.; Rensen, S.; Greve, J.W.; Huynh, K.; Jayawardana, K.S.; Meikle, P.J.; Meex, R.C.R. Mitochondrial Dysfunction-Related Lipid Changes Occur in Nonalcoholic Fatty Liver Disease Progression. J. Lipid Res. 2018, 59, 1977–1986. [Google Scholar] [CrossRef] [Green Version]
- Putti, R.; Sica, R.; Migliaccio, V.; Lionetti, L. Diet Impact on Mitochondrial Bioenergetics and Dynamics. Front. Physiol. 2015, 6, 109. [Google Scholar] [CrossRef] [Green Version]
- Ritov, V.B.; Menshikova, E.V.; He, J.; Ferrell, R.E.; Goodpaster, B.H.; Kelley, D.E. Deficiency of Subsarcolemmal Mitochondria in Obesity and Type 2 Diabetes. Diabetes 2005, 54, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Ruggiero, C.; Ehrenshaft, M.; Cleland, E.; Stadler, K. High-Fat Diet Induces an Initial Adaptation of Mitochondrial Bioenergetics in the Kidney despite Evident Oxidative Stress and Mitochondrial ROS Production. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E1047–E1058. [Google Scholar] [CrossRef] [Green Version]
- Szeto, H.H.; Liu, S.; Soong, Y.; Alam, N.; Prusky, G.T.; Seshan, S.V. Protection of Mitochondria Prevents High-Fat Diet–Induced Glomerulopathy and Proximal Tubular Injury. Kidney Int. 2016, 90, 997–1011. [Google Scholar] [CrossRef]
- Yin, X.; Lanza, I.R.; Swain, J.M.; Sarr, M.G.; Nair, K.S.; Jensen, M.D. Adipocyte Mitochondrial Function Is Reduced in Human Obesity Independent of Fat Cell Size. J. Clin. Endocrinol. Metab. 2014, 99, E209–E216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zorzano, A.; Liesa, M.; Palacín, M. Role of Mitochondrial Dynamics Proteins in the Pathophysiology of Obesity and Type 2 Diabetes. Int. J. Biochem. Cell Biol. 2009, 41, 1846–1854. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, C.; Tremblay, A.; Després, J.P.; Nadeau, A.; Lupien, P.J.; Thériault, G.; Dussault, J.; Moorjani, S.; Pinault, S.; Fournier, G. The Response to Long-Term Overfeeding in Identical Twins. N. Engl. J. Med. 1990, 322, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Thrush, A.B.; Antoun, G.; Nikpay, M.; Patten, D.A.; DeVlugt, C.; Mauger, J.-F.; Beauchamp, B.L.; Lau, P.; Reshke, R.; Doucet, É.; et al. Diet-Resistant Obesity Is Characterized by a Distinct Plasma Proteomic Signature and Impaired Muscle Fiber Metabolism. Int. J. Obes. 2018, 42, 353–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerrits, M.F.; Ghosh, S.; Kavaslar, N.; Hill, B.; Tour, A.; Seifert, E.L.; Beauchamp, B.; Gorman, S.; Stuart, J.; Dent, R.; et al. Distinct Skeletal Muscle Fiber Characteristics and Gene Expression in Diet-Sensitive versus Diet-Resistant Obesity. J. Lipid Res. 2010, 51, 2394–2404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mogensen, M.; Sahlin, K.; Fernström, M.; Glintborg, D.; Vind, B.F.; Beck-Nielsen, H.; Højlund, K. Mitochondrial Respiration Is Decreased in Skeletal Muscle of Patients with Type 2 Diabetes. Diabetes 2007, 56, 1592–1599. [Google Scholar] [CrossRef] [Green Version]
- Sun, G.; Ukkola, O.; Rankinen, T.; Joanisse, D.R.; Bouchard, C. Skeletal Muscle Characteristics Predict Body Fat Gain in Response to Overfeeding in Never-Obese Young Men. Metabolism 2002, 51, 451–456. [Google Scholar] [CrossRef]
- Tseng, Y.-H.; Cypess, A.M.; Kahn, C.R. Cellular Bioenergetics as a Target for Obesity Therapy. Nat. Rev. Drug Discov. 2010, 9, 465–482. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, P. Coupling of Phosphorylation to Electron and Hydrogen Transfer by a Chemi-Osmotic Type of Mechanism. Nature 1961, 191, 144–148. [Google Scholar] [CrossRef]
- Mejia, E.M.; Hatch, G.M. Mitochondrial Phospholipids: Role in Mitochondrial Function. J. Bioenerg. Biomembr. 2016, 48, 99–112. [Google Scholar] [CrossRef]
- Ikon, N.; Ryan, R.O. Cardiolipin and Mitochondrial Cristae Organization. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Joubert, F.; Puff, N. Mitochondrial Cristae Architecture and Functions: Lessons from Minimal Model Systems. Membranes 2021, 11, 465. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Gu, J.; Guo, R.; Huang, Y.; Yang, M. Structure of Mammalian Respiratory Supercomplex I1III2IV1. Cell 2016, 167, 1598–1609.e10. [Google Scholar] [CrossRef] [Green Version]
- Letts, J.A.; Fiedorczuk, K.; Sazanov, L.A. The Architecture of Respiratory Supercomplexes. Nature 2016, 537, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, K.; Gohil, V.; Stuart, R.A.; Hunte, C.; Brandt, U.; Greenberg, M.L.; Schägger, H. Cardiolipin Stabilizes Respiratory Chain Supercomplexes. J. Biol. Chem. 2003, 278, 52873–52880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, J.; Wu, M.; Guo, R.; Yan, K.; Lei, J.; Gao, N.; Yang, M. The Architecture of the Mammalian Respirasome. Nature 2016, 537, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Duncan, A.L.; Robinson, A.J.; Walker, J.E. Cardiolipin Binds Selectively but Transiently to Conserved Lysine Residues in the Rotor of Metazoan ATP Synthases. Proc. Natl. Acad. Sci. USA 2016, 113, 8687–8692. [Google Scholar] [CrossRef] [Green Version]
- Eble, K.S.; Coleman, W.B.; Hantgan, R.R.; Cunningham, C.C. Tightly Associated Cardiolipin in the Bovine Heart Mitochondrial ATP Synthase as Analyzed by 31P Nuclear Magnetic Resonance Spectroscopy. J. Biol. Chem. 1990, 265, 19434–19440. [Google Scholar] [CrossRef]
- Mühleip, A.; McComas, S.E.; Amunts, A. Structure of a Mitochondrial ATP Synthase with Bound Native Cardiolipin. eLife 2019, 8, e51179. [Google Scholar] [CrossRef]
- Senoo, N.; Kandasamy, S.; Ogunbona, O.B.; Baile, M.G.; Lu, Y.; Claypool, S.M. Cardiolipin, Conformation, and Respiratory Complex-Dependent Oligomerization of the Major Mitochondrial ADP/ATP Carrier in Yeast. Sci. Adv. 2020, 6, eabb0780. [Google Scholar] [CrossRef]
- Pebay-Peyroula, E.; Dahout-Gonzalez, C.; Kahn, R.; Trézéguet, V.; Lauquin, G.J.-M.; Brandolin, G. Structure of Mitochondrial ADP/ATP Carrier in Complex with Carboxyatractyloside. Nature 2003, 426, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Mileykovskaya, E.; Dowhan, W. Gluing the Respiratory Chain Together. Cardiolipin Is Required for Supercomplex Formation in the Inner Mitochondrial Membrane. J. Biol. Chem. 2002, 277, 43553–43556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheneval, D.; Carafoli, E. Identification and Primary Structure of the Cardiolipin-Binding Domain of Mitochondrial Creatine Kinase. Eur. J. Biochem. 1988, 171, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Schlattner, U.; Wallimann, T. Octamers of Mitochondrial Creatine Kinase Isoenzymes Differ in Stability and Membrane Binding*. J. Biol. Chem. 2000, 275, 17314–17320. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Basu Ball, W.; Madaris, T.R.; Srikantan, S.; Madesh, M.; Mootha, V.K.; Gohil, V.M. An Essential Role for Cardiolipin in the Stability and Function of the Mitochondrial Calcium Uniporter. Proc. Natl. Acad. Sci. USA 2020, 117, 16383–16390. [Google Scholar] [CrossRef]
- Kuwana, T.; Mackey, M.R.; Perkins, G.; Ellisman, M.H.; Latterich, M.; Schneiter, R.; Green, D.R.; Newmeyer, D.D. Bid, Bax, and Lipids Cooperate to Form Supramolecular Openings in the Outer Mitochondrial Membrane. Cell 2002, 111, 331–342. [Google Scholar] [CrossRef] [Green Version]
- Gonzalvez, F.; Pariselli, F.; Dupaigne, P.; Budihardjo, I.; Lutter, M.; Antonsson, B.; Diolez, P.; Manon, S.; Martinou, J.-C.; Goubern, M.; et al. TBid Interaction with Cardiolipin Primarily Orchestrates Mitochondrial Dysfunctions and Subsequently Activates Bax and Bak. Cell Death Differ. 2005, 12, 614–626. [Google Scholar] [CrossRef] [Green Version]
- Rostovtseva, T.K.; Kazemi, N.; Weinrich, M.; Bezrukov, S.M. Voltage Gating of VDAC Is Regulated by Nonlamellar Lipids of Mitochondrial Membranes*. J. Biol. Chem. 2006, 281, 37496–37506. [Google Scholar] [CrossRef] [Green Version]
- Gebert, N.; Joshi, A.S.; Kutik, S.; Becker, T.; McKenzie, M.; Guan, X.L.; Mooga, V.P.; Stroud, D.A.; Kulkarni, G.; Wenk, M.R.; et al. Mitochondrial Cardiolipin Involved in Outer Membrane Protein Biogenesis: Implications for Barth Syndrome. Curr. Biol. 2009, 19, 2133–2139. [Google Scholar] [CrossRef] [Green Version]
- Malhotra, K.; Modak, A.; Nangia, S.; Daman, T.H.; Gunsel, U.; Robinson, V.L.; Mokranjac, D.; May, E.R.; Alder, N.N. Cardiolipin Mediates Membrane and Channel Interactions of the Mitochondrial TIM23 Protein Import Complex Receptor Tim50. Sci. Adv. 2017, 3, e1700532. [Google Scholar] [CrossRef]
- Adachi, Y.; Itoh, K.; Yamada, T.; Cerveny, K.L.; Suzuki, T.L.; Macdonald, P.; Frohman, M.A.; Ramachandran, R.; Iijima, M.; Sesaki, H. Coincident Phosphatidic Acid Interaction Restrains Drp1 in Mitochondrial Division. Mol. Cell 2016, 63, 1034–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ban, T.; Ishihara, T.; Kohno, H.; Saita, S.; Ichimura, A.; Maenaka, K.; Oka, T.; Mihara, K.; Ishihara, N. Molecular Basis of Selective Mitochondrial Fusion by Heterotypic Action between OPA1 and Cardiolipin. Nat. Cell Biol. 2017, 19, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Bustillo-Zabalbeitia, I.; Montessuit, S.; Raemy, E.; Basañez, G.; Terrones, O.; Martinou, J.-C. Specific Interaction with Cardiolipin Triggers Functional Activation of Dynamin-Related Protein 1. PLoS ONE 2014, 9, e102738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montessuit, S.; Somasekharan, S.P.; Terrones, O.; Lucken-Ardjomande, S.; Herzig, S.; Schwarzenbacher, R.; Manstein, D.J.; Bossy-Wetzel, E.; Basañez, G.; Meda, P.; et al. Membrane Remodeling Induced by the Dynamin-Related Protein Drp1 Stimulates Bax Oligomerization. Cell 2010, 142, 889–901. [Google Scholar] [CrossRef] [Green Version]
- Haines, T.H.; Dencher, N.A. Cardiolipin: A Proton Trap for Oxidative Phosphorylation. FEBS Lett. 2002, 528, 35–39. [Google Scholar] [CrossRef] [Green Version]
- Prola, A.; Blondelle, J.; Vandestienne, A.; Piquereau, J.; Denis, R.G.P.; Guyot, S.; Chauvin, H.; Mourier, A.; Maurer, M.; Henry, C.; et al. Cardiolipin Content Controls Mitochondrial Coupling and Energetic Efficiency in Muscle. Sci. Adv. 2021, 7, eabd6322. [Google Scholar] [CrossRef]
- Toth, A.; Meyrat, A.; Stoldt, S.; Santiago, R.; Wenzel, D.; Jakobs, S.; von Ballmoos, C.; Ott, M. Kinetic Coupling of the Respiratory Chain with ATP Synthase, but Not Proton Gradients, Drives ATP Production in Cristae Membranes. Proc. Natl. Acad. Sci. USA 2020, 117, 2412–2421. [Google Scholar] [CrossRef] [Green Version]
- Azzone, G.F.; Pozzan, T.; Viola, E.; Arslan, P. Proton Electrochemical Gradient and Phosphate Potential in Submitochondrial Particles. Biochim. Biophys. Acta BBA Bioenergy 1978, 501, 317–329. [Google Scholar] [CrossRef]
- Rieger, B.; Junge, W.; Busch, K.B. Lateral PH Gradient between OXPHOS Complex IV and F 0 F 1 ATP-Synthase in Folded Mitochondrial Membranes. Nat. Commun. 2014, 5, 3103. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, P. A Commentary on Alternative Hypotheses of Protonic Coupling in the Membrane Systems Catalysing Oxidative and Photosynthetic Phosphorylation. FEBS Lett. 1977, 78, 1–20. [Google Scholar] [CrossRef]
- Kagan, V.E.; Jiang, J.; Huang, Z.; Tyurina, Y.Y.; Desbourdes, C.; Cottet-Rousselle, C.; Dar, H.H.; Verma, M.; Tyurin, V.A.; Kapralov, A.A.; et al. NDPK-D (NM23-H4)-Mediated Externalization of Cardiolipin Enables Elimination of Depolarized Mitochondria by Mitophagy. Cell Death Differ. 2016, 23, 1140–1151. [Google Scholar] [CrossRef] [Green Version]
- Schlattner, U.; Tokarska-Schlattner, M.; Epand, R.M.; Boissan, M.; Lacombe, M.-L.; Kagan, V.E. NME4/Nucleoside Diphosphate Kinase D in Cardiolipin Signaling and Mitophagy. Lab. Investig. 2018, 98, 228–232. [Google Scholar] [CrossRef] [Green Version]
- Kagan, V.E.; Tyurin, V.A.; Jiang, J.; Tyurina, Y.Y.; Ritov, V.B.; Amoscato, A.A.; Osipov, A.N.; Belikova, N.A.; Kapralov, A.A.; Kini, V.; et al. Cytochrome c Acts as a Cardiolipin Oxygenase Required for Release of Proapoptotic Factors. Nat. Chem. Biol. 2005, 1, 223–232. [Google Scholar] [CrossRef]
- Tyurina, Y.Y.; Shrivastava, I.; Tyurin, V.A.; Mao, G.; Dar, H.H.; Watkins, S.; Epperly, M.; Bahar, I.; Shvedova, A.A.; Pitt, B.; et al. “Only a Life Lived for Others Is Worth Living”: Redox Signaling by Oxygenated Phospholipids in Cell Fate Decisions. Antioxid. Redox Signal 2018, 29, 1333–1358. [Google Scholar] [CrossRef] [Green Version]
- Tyurina, Y.Y.; Poloyac, S.M.; Tyurin, V.A.; Kapralov, A.A.; Jiang, J.; Anthonymuthu, T.S.; Kapralova, V.I.; Vikulina, A.S.; Jung, M.-Y.; Epperly, M.W.; et al. A Mitochondrial Pathway for Biosynthesis of Lipid Mediators. Nat. Chem. 2014, 6, 542–552. [Google Scholar] [CrossRef] [Green Version]
- Tamura, Y.; Harada, Y.; Nishikawa, S.; Yamano, K.; Kamiya, M.; Shiota, T.; Kuroda, T.; Kuge, O.; Sesaki, H.; Imai, K.; et al. Tam41 Is a CDP-Diacylglycerol Synthase Required for Cardiolipin Biosynthesis in Mitochondria. Cell Metab. 2013, 17, 709–718. [Google Scholar] [CrossRef] [Green Version]
- Horvath, S.E.; Daum, G. Lipids of Mitochondria. Prog. Lipid Res. 2013, 52, 590–614. [Google Scholar] [CrossRef]
- Chang, S.-C.; Heacock, P.N.; Clancey, C.J.; Dowhan, W. The PEL1 Gene (Renamed PGS1) Encodes the Phosphatidylglycero-Phosphate Synthase OfSaccharomyces Cerevisiae*. J. Biol. Chem. 1998, 273, 9829–9836. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Engel, J.L.; Zhang, J.; Chen, M.J.; Manning, G.; Dixon, J.E. Structural and Functional Analysis of PTPMT1, a Phosphatase Required for Cardiolipin Synthesis. Proc. Natl. Acad. Sci. USA 2011, 108, 11860–11865. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Guan, Z.; Murphy, A.N.; Wiley, S.E.; Perkins, G.A.; Worby, C.A.; Engel, J.L.; Heacock, P.; Nguyen, O.K.; Wang, J.H.; et al. Mitochondrial Phosphatase PTPMT1 Is Essential for Cardiolipin Biosynthesis. Cell Metab. 2011, 13, 690–700. [Google Scholar] [CrossRef]
- Chang, S.-C.; Heacock, P.N.; Mileykovskaya, E.; Voelker, D.R.; Dowhan, W. Isolation and Characterization of the Gene (CLS1) Encoding Cardiolipin Synthase in Saccharomyces Cerevisiae*. J. Biol. Chem. 1998, 273, 14933–14941. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Zhang, X.-Y.; Shi, Y. Identification and Functional Characterization of HCLS1, a Human Cardiolipin Synthase Localized in Mitochondria. Biochem. J. 2006, 398, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Lu, B.; Xu, F.Y.; Jiang, Y.J.; Choy, P.C.; Hatch, G.M.; Grunfeld, C.; Feingold, K.R. Cloning and Characterization of a CDNA Encoding Human Cardiolipin Synthase (HCLS1). J. Lipid Res. 2006, 47, 1140–1145. [Google Scholar] [CrossRef] [Green Version]
- Oemer, G.; Koch, J.; Wohlfarter, Y.; Alam, M.T.; Lackner, K.; Sailer, S.; Neumann, L.; Lindner, H.H.; Watschinger, K.; Haltmeier, M.; et al. Phospholipid Acyl Chain Diversity Controls the Tissue-Specific Assembly of Mitochondrial Cardiolipins. Cell Rep. 2020, 30, 4281–4291.e4. [Google Scholar] [CrossRef]
- Oemer, G.; Lackner, K.; Muigg, K.; Krumschnabel, G.; Watschinger, K.; Sailer, S.; Lindner, H.; Gnaiger, E.; Wortmann, S.B.; Werner, E.R.; et al. Molecular Structural Diversity of Mitochondrial Cardiolipins. Proc. Natl. Acad. Sci. USA 2018, 115, 4158–4163. [Google Scholar] [CrossRef] [Green Version]
- Houtkooper, R.H.; Akbari, H.; van Lenthe, H.; Kulik, W.; Wanders, R.J.A.; Frentzen, M.; Vaz, F.M. Identification and Characterization of Human Cardiolipin Synthase. FEBS Lett. 2006, 580, 3059–3064. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.-W.; Claypool, S.M. Disorders of Phospholipid Metabolism: An Emerging Class of Mitochondrial Disease Due to Defects in Nuclear Genes. Front. Genet. 2015, 6, 3. [Google Scholar] [CrossRef]
- Cao, J.; Liu, Y.; Lockwood, J.; Burn, P.; Shi, Y. A Novel Cardiolipin-Remodeling Pathway Revealed by a Gene Encoding an Endoplasmic Reticulum-Associated Acyl-CoA:Lysocardiolipin Acyltransferase (ALCAT1) in Mouse. J. Biol. Chem. 2004, 279, 31727–31734. [Google Scholar] [CrossRef] [Green Version]
- Taylor, W.A.; Hatch, G.M. Identification of the Human Mitochondrial Linoleoyl-Coenzyme A Monolysocardiolipin Acyltransferase (MLCL AT-1). J. Biol. Chem. 2009, 284, 30360–30371. [Google Scholar] [CrossRef] [Green Version]
- Barth, P.G.; Scholte, H.R.; Berden, J.A.; Moorsel, J.M.V.D.K.-V.; Luyt-Houwen, I.E.M.; Veer-Korthof, E.T.V.; Harten, J.J.V.D.; Sobotka-Plojhar, M.A. An X-Linked Mitochondrial Disease Affecting Cardiac Muscle, Skeletal Muscle and Neutrophil Leucocytes. J. Neurol. Sci. 1983, 62, 327–355. [Google Scholar] [CrossRef]
- Miklas, J.W.; Clark, E.; Levy, S.; Detraux, D.; Leonard, A.; Beussman, K.; Showalter, M.R.; Smith, A.T.; Hofsteen, P.; Yang, X.; et al. TFPa/HADHA Is Required for Fatty Acid Beta-Oxidation and Cardiolipin Re-Modeling in Human Cardiomyocytes. Nat. Commun. 2019, 10, 4671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, W.A.; Mejia, E.M.; Mitchell, R.W.; Choy, P.C.; Sparagna, G.C.; Hatch, G.M. Human Trifunctional Protein Alpha Links Cardiolipin Remodeling to Beta-Oxidation. PLoS ONE 2012, 7, e48628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bione, S.; D’Adamo, P.; Maestrini, E.; Gedeon, A.K.; Bolhuis, P.A.; Toniolo, D. A Novel X-Linked Gene, G4.5. Is Responsible for Barth Syndrome. Nat. Genet. 1996, 12, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Vreken, P.; Valianpour, F.; Nijtmans, L.G.; Grivell, L.A.; Plecko, B.; Wanders, R.J.; Barth, P.G. Defective Remodeling of Cardiolipin and Phosphatidylglycerol in Barth Syndrome. Biochem. Biophys. Res. Commun. 2000, 279, 378–382. [Google Scholar] [CrossRef]
- Valianpour, F.; Mitsakos, V.; Schlemmer, D.; Towbin, J.A.; Taylor, J.M.; Ekert, P.G.; Thorburn, D.R.; Munnich, A.; Wanders, R.J.A.; Barth, P.G.; et al. Monolysocardiolipins Accumulate in Barth Syndrome but Do Not Lead to Enhanced Apoptosis. J. Lipid Res. 2005, 46, 1182–1195. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Erdjument-Bromage, H.; Phoon, C.K.L.; Neubert, T.A.; Ren, M.; Schlame, M. Cardiolipin Remodeling Enables Protein Crowding in the Inner Mitochondrial Membrane. EMBO J. 2021, 40, e108428. [Google Scholar] [CrossRef]
- Oemer, G.; Edenhofer, M.-L.; Wohlfarter, Y.; Lackner, K.; Leman, G.; Koch, J.; Cardoso, L.H.D.; Lindner, H.H.; Gnaiger, E.; Dubrac, S.; et al. Fatty Acyl Availability Modulates Cardiolipin Composition and Alters Mitochondrial Function in HeLa Cells. J. Lipid Res. 2021, 62, 100111. [Google Scholar] [CrossRef]
- Khairallah, R.J.; Kim, J.; O’Shea, K.M.; O’Connell, K.A.; Brown, B.H.; Galvao, T.; Daneault, C.; Rosiers, C.D.; Polster, B.M.; Hoppel, C.L.; et al. Improved Mitochondrial Function with Diet-Induced Increase in Either Docosahexaenoic Acid or Arachidonic Acid in Membrane Phospholipids. PLoS ONE 2012, 7, e34402. [Google Scholar] [CrossRef] [Green Version]
- Bradley, R.M.; Stark, K.D.; Duncan, R.E. Influence of Tissue, Diet, and Enzymatic Remodeling on Cardiolipin Fatty Acyl Profile. Mol. Nutr. Food Res. 2016, 60, 1804–1818. [Google Scholar] [CrossRef]
- Oemer, G.; Koch, J.; Wohlfarter, Y.; Lackner, K.; Gebert, R.E.M.; Geley, S.; Zschocke, J.; Keller, M.A. The Lipid Environment Modulates Cardiolipin and Phospholipid Constitution in Wild Type and Tafazzin-Deficient Cells. J. Inherit. Metab. Dis. 2022, 45, 38–50. [Google Scholar] [CrossRef]
- Sustarsic, E.G.; Ma, T.; Lynes, M.D.; Larsen, M.; Karavaeva, I.; Havelund, J.F.; Nielsen, C.H.; Jedrychowski, M.P.; Moreno-Torres, M.; Lundh, M.; et al. Cardiolipin Synthesis in Brown and Beige Fat Mitochondria Is Essential for Systemic Energy Homeostasis. Cell Metab. 2018, 28, 159–174.e11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berger, A.; Gershwin, M.E.; German, J.B. Effects of Various Dietary Fats on Cardiolipin Acyl Composition during Ontogeny of Mice. Lipids 1992, 27, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.; German, J.B. Phospholipid Fatty Acid Composition of Various Mouse Tissues after Feeding α-Linolenate (18:3n−3) or Eicosatrienoate (20:3n−3). Lipids 1990, 25, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Blomstrand, R.; Svensson, L. The Effects of Partially Hydrogenated Marine Oils on the Mitochondrial Function and Membrane Phospholipid Fatty Acids in Rat Heart. Lipids 1983, 18, 151–170. [Google Scholar] [CrossRef]
- McGree, C.D.; Lieberman, P.; Greenwood, C.E. Dietary Fatty Acid Composition Induces Comparable Changes in Cardiolipin Fatty Acid Profile of Heart and Brain Mitochondria. Lipids 1996, 31, 611–616. [Google Scholar] [CrossRef]
- Stavrovskaya, I.G.; Bird, S.S.; Marur, V.R.; Sniatynski, M.J.; Baranov, S.V.; Greenberg, H.K.; Porter, C.L.; Kristal, B.S. Dietary Macronutrients Modulate the Fatty Acyl Composition of Rat Liver Mitochondrial Cardiolipins. J. Lipid Res. 2013, 54, 2623–2635. [Google Scholar] [CrossRef] [Green Version]
- Coen, P.M.; Menshikova, E.V.; Distefano, G.; Zheng, D.; Tanner, C.J.; Standley, R.A.; Helbling, N.L.; Dubis, G.S.; Ritov, V.B.; Xie, H.; et al. Exercise and Weight Loss Improve Muscle Mitochondrial Respiration, Lipid Partitioning, and Insulin Sensitivity After Gastric Bypass Surgery. Diabetes 2015, 64, 3737–3750. [Google Scholar] [CrossRef] [Green Version]
- Samhan-Arias, A.K.; Ji, J.; Demidova, O.M.; Sparvero, L.J.; Feng, W.; Tyurin, V.; Tyurina, Y.Y.; Epperly, M.W.; Shvedova, A.A.; Greenberger, J.S.; et al. Oxidized Phospholipids as Biomarkers of Tissue and Cell Damage with a Focus on Cardiolipin. Biochim. Biophys. Acta 2012, 1818, 2413–2423. [Google Scholar] [CrossRef] [Green Version]
- Antonenko, Y.N.; Avetisyan, A.V.; Bakeeva, L.E.; Chernyak, B.V.; Chertkov, V.A.; Domnina, L.V.; Ivanova, O.Y.; Izyumov, D.S.; Khailova, L.S.; Klishin, S.S.; et al. Mitochondria-Targeted Plastoquinone Derivatives as Tools to Interrupt Execution of the Aging Program. 1. Cationic Plastoquinone Derivatives: Synthesis and in Vitro Studies. Biochemistry 2008, 73, 1273–1287. [Google Scholar] [CrossRef]
- Marchesini, G.; Bugianesi, E.; Forlani, G.; Cerrelli, F.; Lenzi, M.; Manini, R.; Natale, S.; Vanni, E.; Villanova, N.; Melchionda, N.; et al. Nonalcoholic Fatty Liver, Steatohepatitis, and the Metabolic Syndrome. Hepatology 2003, 37, 917–923. [Google Scholar] [CrossRef]
- Nassir, F.; Ibdah, J.A. Role of Mitochondria in Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2014, 15, 8713–8742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koliaki, C.; Szendroedi, J.; Kaul, K.; Jelenik, T.; Nowotny, P.; Jankowiak, F.; Herder, C.; Carstensen, M.; Krausch, M.; Knoefel, W.T.; et al. Adaptation of Hepatic Mitochondrial Function in Humans with Non-Alcoholic Fatty Liver Is Lost in Steatohepatitis. Cell Metab. 2015, 21, 739–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoun, M.; Fouret, G.; Michel, F.; Bonafos, B.; Ramos, J.; Cristol, J.-P.; Carbonneau, M.-A.; Coudray, C.; Feillet-Coudray, C. Dietary Fatty Acids Modulate Liver Mitochondrial Cardiolipin Content and Its Fatty Acid Composition in Rats with Non Alcoholic Fatty Liver Disease. J. Bioenerg. Biomembr. 2012, 44, 439–452. [Google Scholar] [CrossRef]
- Fouret, G.; Gaillet, S.; Lecomte, J.; Bonafos, B.; Djohan, F.; Barea, B.; Badia, E.; Coudray, C.; Feillet-Coudray, C. 20-Week Follow-up of Hepatic Steatosis Installation and Liver Mitochondrial Structure and Activity and Their Interrelation in Rats Fed a High-Fat–High-Fructose Diet. Br. J. Nutr. 2018, 119, 368–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jha, P.; McDevitt, M.T.; Gupta, R.; Quiros, P.M.; Williams, E.G.; Gariani, K.; Sleiman, M.B.; Diserens, L.; Jochem, A.; Ulbrich, A.; et al. Systems Analyses Reveal Physiological Roles and Genetic Regulators of Liver Lipid Species. Cell Syst. 2018, 6, 722–733.e6. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, E.M.; Fix, A.; Crouch, M.J.; Sparagna, G.C.; Zeczycki, T.N.; Brown, D.A.; Shaikh, S.R. Murine Diet-Induced Obesity Remodels Cardiac and Liver Mitochondrial Phospholipid Acyl Chains with Differential Effects on Respiratory Enzyme Activity. J. Nutr. Biochem. 2017, 45, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Romestaing, C.; Han, X.; Li, Y.; Hao, X.; Wu, Y.; Sun, C.; Liu, X.; Jefferson, L.S.; Xiong, J.; et al. Cardiolipin Remodeling by ALCAT1 Links Oxidative Stress and Mitochondrial Dysfunction to Obesity. Cell Metab. 2010, 12, 154–165. [Google Scholar] [CrossRef] [Green Version]
- Kovesdy, C.P.; Furth, S.L.; Zoccali, C. Obesity and Kidney Disease. Can. J. Kidney Health Dis. 2017, 4, 2054358117698669. [Google Scholar] [CrossRef] [Green Version]
- Herman-Edelstein, M.; Scherzer, P.; Tobar, A.; Levi, M.; Gafter, U. Altered Renal Lipid Metabolism and Renal Lipid Accumulation in Human Diabetic Nephropathy. J. Lipid Res. 2014, 55, 561–572. [Google Scholar] [CrossRef] [Green Version]
- Kume, S.; Uzu, T.; Araki, S.; Sugimoto, T.; Isshiki, K.; Chin-Kanasaki, M.; Sakaguchi, M.; Kubota, N.; Terauchi, Y.; Kadowaki, T.; et al. Role of Altered Renal Lipid Metabolism in the Development of Renal Injury Induced by a High-Fat Diet. J. Am. Soc. Nephrol. 2007, 18, 2715–2723. [Google Scholar] [CrossRef]
- Eirin, A.; Woollard, J.R.; Ferguson, C.M.; Jordan, K.L.; Tang, H.; Textor, S.C.; Lerman, A.; Lerman, L.O. The Metabolic Syndrome Induces Early Changes in the Swine Renal Medullary Mitochondria. Transl. Res. 2017, 184, 45–56.e9. [Google Scholar] [CrossRef] [PubMed]
- Hayasaka, T.; Fuda, H.; Hui, S.-P.; Chiba, H. Imaging Mass Spectrometry Reveals a Decrease of Cardiolipin in the Kidney of NASH Model Mice. Anal Sci. 2016, 32, 473–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Yang, J.; Yang, K.; Zhao, Z.; Abendschein, D.R.; Gross, R.W. Alterations in Myocardial Cardiolipin Content and Composition Occur at the Very Earliest Stages of Diabetes: A Shotgun Lipidomics Study. Biochemistry 2007, 46, 6417–6428. [Google Scholar] [CrossRef] [Green Version]
- Morton, G.J.; Cummings, D.E.; Baskin, D.G.; Barsh, G.S.; Schwartz, M.W. Central Nervous System Control of Food Intake and Body Weight. Nature 2006, 443, 289–295. [Google Scholar] [CrossRef]
- Guarino, D.; Nannipieri, M.; Iervasi, G.; Taddei, S.; Bruno, R.M. The Role of the Autonomic Nervous System in the Pathophysiology of Obesity. Front. Physiol. 2017, 8, 665. [Google Scholar] [CrossRef] [Green Version]
- Leibel, R.L.; Rosenbaum, M.; Hirsch, J. Changes in Energy Expenditure Resulting from Altered Body Weight. N. Engl. J. Med. 1995, 332, 621–628. [Google Scholar] [CrossRef]
- O’Brien, P.D.; Hinder, L.M.; Callaghan, B.C.; Feldman, E.L. Neurological Consequences of Obesity. Lancet Neurol. 2017, 16, 465–477. [Google Scholar] [CrossRef]
- Pointer, C.B.; Klegeris, A. Cardiolipin in Central Nervous System Physiology and Pathology. Cell Mol. Neurobiol. 2017, 37, 1161–1172. [Google Scholar] [CrossRef]
- Ricquier, D. UCP1, the Mitochondrial Uncoupling Protein of Brown Adipocyte: A Personal Contribution and a Historical Perspective. Biochimie 2017, 134, 3–8. [Google Scholar] [CrossRef]
- Lee, Y.; Willers, C.; Kunji, E.R.S.; Crichton, P.G. Uncoupling Protein 1 Binds One Nucleotide per Monomer and Is Stabilized by Tightly Bound Cardiolipin. Proc. Natl. Acad. Sci. USA 2015, 112, 6973–6978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lange, M.; Angelidou, G.; Ni, Z.; Criscuolo, A.; Schiller, J.; Blüher, M.; Fedorova, M. AdipoAtlas: A Reference Lipidome for Human White Adipose Tissue. Cell Rep. Med. 2021, 2, 100407. [Google Scholar] [CrossRef] [PubMed]
- Thyagarajan, B.; Foster, M.T. Beiging of White Adipose Tissue as a Therapeutic Strategy for Weight Loss in Humans. Horm. Mol. Biol. Clin. Investig. 2017, 31. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann, S.; Maurer, S.; Kleigrewe, K.; Klingenspor, M. Spatial Recruitment of Cardiolipins in Inguinal White Adipose Tissue after Cold Stimulation Is Independent of UCP1. Eur. J. Lipid Sci. Technol. 2022, 124, 2100090. [Google Scholar] [CrossRef]
- Rolfe, D.F.; Brown, G.C. Cellular Energy Utilization and Molecular Origin of Standard Metabolic Rate in Mammals. Physiol. Rev. 1997, 77, 731–758. [Google Scholar] [CrossRef] [Green Version]
- Cassidy, S.; Chau, J.Y.; Catt, M.; Bauman, A.; Trenell, M.I. Low Physical Activity, High Television Viewing and Poor Sleep Duration Cluster in Overweight and Obese Adults; a Cross-Sectional Study of 398,984 Participants from the UK Biobank. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 57. [Google Scholar] [CrossRef] [Green Version]
- Kalinkovich, A.; Livshits, G. Sarcopenic Obesity or Obese Sarcopenia: A Cross Talk between Age-Associated Adipose Tissue and Skeletal Muscle Inflammation as a Main Mechanism of the Pathogenesis. Ageing Res. Rev. 2017, 35, 200–221. [Google Scholar] [CrossRef]
- Pileggi, C.A.; Parmar, G.; Harper, M.-E. The Lifecycle of Skeletal Muscle Mitochondria in Obesity. Obes. Rev. 2021, 22, e13164. [Google Scholar] [CrossRef]
- Hannich, J.T.; Loizides-Mangold, U.; Sinturel, F.; Harayama, T.; Vandereycken, B.; Saini, C.; Gosselin, P.; Brulhart-Meynet, M.-C.; Robert, M.; Chanon, S.; et al. Ether Lipids, Sphingolipids and Toxic 1-Deoxyceramides as Hallmarks for Lean and Obese Type 2 Diabetic Patients. Acta Physiol. 2021, 232, e13610. [Google Scholar] [CrossRef]
- Larsen, S.; Nielsen, J.; Hansen, C.N.; Nielsen, L.B.; Wibrand, F.; Stride, N.; Schroder, H.D.; Boushel, R.; Helge, J.W.; Dela, F.; et al. Biomarkers of Mitochondrial Content in Skeletal Muscle of Healthy Young Human Subjects. J. Physiol. 2012, 590, 3349–3360. [Google Scholar] [CrossRef]
- Fajardo, V.A.; McMeekin, L.; Saint, C.; LeBlanc, P.J. Cardiolipin Linoleic Acid Content and Mitochondrial Cytochrome c Oxidase Activity Are Associated in Rat Skeletal Muscle. Chem. Phys. Lipids 2015, 187, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, V.A.; Mikhaeil, J.S.; Leveille, C.F.; Saint, C.; LeBlanc, P.J. Cardiolipin Content, Linoleic Acid Composition, and Tafazzin Expression in Response to Skeletal Muscle Overload and Unload Stimuli. Sci. Rep. 2017, 7, 2060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraschnewski, J.L.; Boan, J.; Esposito, J.; Sherwood, N.E.; Lehman, E.B.; Kephart, D.K.; Sciamanna, C.N. Long-Term Weight Loss Maintenance in the United States. Int. J. Obes. 2010, 34, 1644–1654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westerterp, K.R. Physical Activity, Food Intake, and Body Weight Regulation: Insights from Doubly Labeled Water Studies. Nutr. Rev. 2010, 68, 148–154. [Google Scholar] [CrossRef]
- Wing, R.R.; Phelan, S. Long-Term Weight Loss Maintenance. Am. J. Clin. Nutr. 2005, 82, 222S–225S. [Google Scholar] [CrossRef]
- Gaesser, G.A.; Angadi, S.S. Obesity Treatment: Weight Loss versus Increasing Fitness and Physical Activity for Reducing Health Risks. iScience 2021, 24, 102995. [Google Scholar] [CrossRef]
- Barry, V.W.; Baruth, M.; Beets, M.W.; Durstine, J.L.; Liu, J.; Blair, S.N. Fitness vs. Fatness on All-Cause Mortality: A Meta-Analysis. Prog. Cardiovasc. Dis. 2014, 56, 382–390. [Google Scholar] [CrossRef]
- Carbone, S.; Kirkman, D.L.; Garten, R.S.; Rodriguez-Miguelez, P.; Artero, E.G.; Lee, D.-C.; Lavie, C.J. Muscular Strength and Cardiovascular Disease: An updated state-of-the-art narrative review. J. Cardiopulm. Rehabil. Prev. 2020, 40, 302–309. [Google Scholar] [CrossRef]
- Mendham, A.E.; Goedecke, J.H.; Zeng, Y.; Larsen, S.; George, C.; Hauksson, J.; Fortuin-de Smidt, M.C.; Chibalin, A.V.; Olsson, T.; Chorell, E. Exercise Training Improves Mitochondrial Respiration and Is Associated with an Altered Intramuscular Phospholipid Signature in Women with Obesity. Diabetologia 2021, 64, 1642–1659. [Google Scholar] [CrossRef]
- Toledo, F.G.S.; Menshikova, E.V.; Ritov, V.B.; Azuma, K.; Radikova, Z.; DeLany, J.; Kelley, D.E. Effects of Physical Activity and Weight Loss on Skeletal Muscle Mitochondria and Relationship with Glucose Control in Type 2 Diabetes. Diabetes 2007, 56, 2142–2147. [Google Scholar] [CrossRef]
- Menshikova, E.V.; Ritov, V.B.; Dube, J.J.; Amati, F.; Stefanovic-Racic, M.; Toledo, F.G.S.; Coen, P.M.; Goodpaster, B.H. Calorie Restriction-Induced Weight Loss and Exercise Have Differential Effects on Skeletal Muscle Mitochondria Despite Similar Effects on Insulin Sensitivity. J. Gerontol. Ser. A 2018, 73, 81–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, C.; Xiong, H.; Hu, Y.; Wang, W.; Mei, G.; Wang, H.; Li, Y.; Zhou, Z.; Meng, F.; Zhang, P.; et al. Cardiolipin Synthase 1 Ameliorates NASH Through Activating Transcription Factor 3 Transcriptional Inactivation. Hepatology 2020, 72, 1949–1967. [Google Scholar] [CrossRef] [PubMed]
- Yeung, M.H.Y.; Leung, K.L.; Choi, L.Y.; Yoo, J.S.; Yung, S.; So, P.-K.; Wong, C.-M. Lipidomic Analysis Reveals the Protection Mechanism of GLP-1 Analogue Dulaglutide on High-Fat Diet-Induced Chronic Kidney Disease in Mice. Front. Pharmacol. 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Metreveli, N.S.; Donthi, R.V.; Xia, S.; Xu, M.; Carlson, E.C.; Epstein, P.N. Catalase Protects Cardiomyocyte Function in Models of Type 1 and Type 2 Diabetes. Diabetes 2004, 53, 1336–1343. [Google Scholar] [CrossRef] [Green Version]
- Szeto, H.; Birk, A. Serendipity and the Discovery of Novel Compounds That Restore Mitochondrial Plasticity. Clin. Pharmacol. Ther. 2014, 96, 672–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, J.; Kapralov, A.A.; Yanamala, N.; Tyurina, Y.Y.; Amoscato, A.A.; Pearce, L.; Peterson, J.; Huang, Z.; Jiang, J.; Samhan-Arias, A.K.; et al. A Mitochondria-Targeted Inhibitor of Cytochrome c Peroxidase Mitigates Radiation-Induced Death. Nat. Commun. 2011, 2, 497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epperly, M.W.; Sacher, J.R.; Krainz, T.; Zhang, X.; Wipf, P.; Liang, M.; Fisher, R.; Li, S.; Wang, H.; Greenberger, J.S. Effectiveness of Analogs of the GS-Nitroxide, JP4-039, as Total Body Irradiation Mitigators. In Vivo 2017, 31, 39–43. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Belikova, N.A.; Hoye, A.T.; Zhao, Q.; Epperly, M.W.; Greenberger, J.S.; Wipf, P.; Kagan, V.E. A Mitochondria-Targeted Nitroxide/Hemigramicidin S Conjugate Protects Mouse Embryonic Cells Against Gamma Irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 816–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kagan, V.E.; Bayir, A.; Bayir, H.; Stoyanovsky, D.; Borisenko, G.G.; Tyurina, Y.Y.; Wipf, P.; Atkinson, J.; Greenberger, J.S.; Chapkin, R.S.; et al. Mitochondria-Targeted Disruptors and Inhibitors of Cytochrome c/Cardiolipin Peroxidase Complexes: A New Strategy in Anti-Apoptotic Drug Discovery. Mol. Nutr. Food Res. 2009, 53, 104–114. [Google Scholar] [CrossRef] [Green Version]
- Rajagopalan, M.S.; Gupta, K.; Epperly, M.W.; Franicola, D.; Zhang, X.; Wang, H.; Zhao, H.; Tyurin, V.A.; Pierce, J.G.; Kagan, V.E.; et al. The Mitochondria-Targeted Nitroxide JP4-039 Augments Potentially Lethal Irradiation Damage Repair. In Vivo 2009, 23, 717–726. [Google Scholar]
- Stoyanovsky, D.A.; Huang, Z.; Jiang, J.; Belikova, N.A.; Tyurin, V.; Epperly, M.W.; Greenberger, J.S.; Bayir, H.; Kagan, V.E. A Manganese–Porphyrin Complex Decomposes H2O2, Inhibits Apoptosis, and Acts as a Radiation Mitigator in Vivo. ACS Med. Chem. Lett. 2011, 2, 814–817. [Google Scholar] [CrossRef]
- Skulachev, V.P.; Antonenko, Y.N.; Cherepanov, D.A.; Chernyak, B.V.; Izyumov, D.S.; Khailova, L.S.; Klishin, S.S.; Korshunova, G.A.; Lyamzaev, K.G.; Pletjushkina, O.Y.; et al. Prevention of Cardiolipin Oxidation and Fatty Acid Cycling as Two Antioxidant Mechanisms of Cationic Derivatives of Plastoquinone (SkQs). Biochim. Biophys. Acta BBA Bioenergy 2010, 1797, 878–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colman, E. Dinitrophenol and Obesity: An Early Twentieth-Century Regulatory Dilemma. Regul. Toxicol. Pharmacol. 2007, 48, 115–117. [Google Scholar] [CrossRef]
- Bertholet, A.M.; Kirichok, Y. Mitochondrial H+ Leak and Thermogenesis. Annu. Rev. Physiol. 2022, 84, 381–407. [Google Scholar] [CrossRef] [PubMed]
- Kotova, E.A.; Antonenko, Y.N. Fifty Years of Research on Protonophores: Mitochondrial Uncoupling as a Basis for Therapeutic Action. Acta Nat. 2022, 14, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Thrush, A.B.; Dent, R.; McPherson, R.; Harper, M.-E. Implications of Mitochondrial Uncoupling in Skeletal Muscle in the Development and Treatment of Obesity. FEBS J. 2013, 280, 5015–5029. [Google Scholar] [CrossRef] [PubMed]
- Grundlingh, J.; Dargan, P.I.; El-Zanfaly, M.; Wood, D.M. 2,4-Dinitrophenol (DNP): A Weight Loss Agent with Significant Acute Toxicity and Risk of Death. J. Med. Toxicol. 2011, 7, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Perry, R.J.; Zhang, D.; Zhang, X.-M.; Boyer, J.L.; Shulman, G.I. Controlled-Release Mitochondrial Protonophore Reverses Diabetes and Steatohepatitis in Rats. Science 2015, 347, 1253–1256. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Liu, X.; Nie, J.; Zhang, J.; Kimball, S.R.; Zhang, H.; Zhang, W.J.; Jefferson, L.S.; Cheng, Z.; Ji, Q.; et al. ALCAT1 Controls Mitochondrial Etiology of Fatty Liver Diseases, Linking Defective Mitophagy to Steatosis. Hepatology 2015, 61, 486–496. [Google Scholar] [CrossRef] [Green Version]
- Cole, L.K.; Mejia, E.M.; Vandel, M.; Sparagna, G.C.; Claypool, S.M.; Dyck-Chan, L.; Klein, J.; Hatch, G.M. Impaired Cardiolipin Biosynthesis Prevents Hepatic Steatosis and Diet-Induced Obesity. Diabetes 2016, 65, 3289–3300. [Google Scholar] [CrossRef] [Green Version]
- Acehan, D.; Vaz, F.; Houtkooper, R.H.; James, J.; Moore, V.; Tokunaga, C.; Kulik, W.; Wansapura, J.; Toth, M.J.; Strauss, A.; et al. Cardiac and Skeletal Muscle Defects in a Mouse Model of Human Barth Syndrome*. J. Biol. Chem. 2011, 286, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Cole, L.K.; Dolinsky, V.W.; Hatch, G.M. Tissue Specific Knockout of the Cardiolipin Transacylase Enzyme TAFAZZIN in Both Liver and Pancreatic Beta Cells Protects Mice from Diet-Induced Obesity. bioRxiv 2022. [Google Scholar] [CrossRef]
- Prola, A.; Vandestienne, A.; Baroudi, N.; Joubert, F.; Tiret, L.; Pilot-Storck, F. Isolation and Phospholipid Enrichment of Muscle Mitochondria and Mitoplasts. Bio Protoc. 2021, 11, e4201. [Google Scholar] [CrossRef] [PubMed]
- Gómez Rodríguez, A.; Talamonti, E.; Naudi, A.; Kalinovich, A.V.; Pauter, A.M.; Barja, G.; Bengtsson, T.; Jacobsson, A.; Pamplona, R.; Shabalina, I.G. Elovl2-Ablation Leads to Mitochondrial Membrane Fatty Acid Remodeling and Reduced Efficiency in Mouse Liver Mitochondria. Nutrients 2022, 14, 559. [Google Scholar] [CrossRef]
- Matsuzaka, T.; Kuba, M.; Koyasu, S.; Yamamoto, Y.; Motomura, K.; Arulmozhiraja, S.; Ohno, H.; Sharma, R.; Shimura, T.; Okajima, Y.; et al. Hepatocyte ELOVL Fatty Acid Elongase 6 Determines Ceramide Acyl-Chain Length and Hepatic Insulin Sensitivity in Mice. Hepatology 2020, 71, 1609–1625. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaka, T.; Shimano, H.; Yahagi, N.; Kato, T.; Atsumi, A.; Yamamoto, T.; Inoue, N.; Ishikawa, M.; Okada, S.; Ishigaki, N.; et al. Crucial Role of a Long-Chain Fatty Acid Elongase, Elovl6, in Obesity-Induced Insulin Resistance. Nat. Med. 2007, 13, 1193–1202. [Google Scholar] [CrossRef]
- Pauter, A.M.; Olsson, P.; Asadi, A.; Herslöf, B.; Csikasz, R.I.; Zadravec, D.; Jacobsson, A. Elovl2 Ablation Demonstrates That Systemic DHA Is Endogenously Produced and Is Essential for Lipid Homeostasis in Mice. J. Lipid Res. 2014, 55, 718–728. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.; Weng, S.; Lu, X.; Zhu, S.; Yang, Q.; Chen, Y.Q. 3-Hydroxyacyl-CoA Dehydratase 2 Deficiency Confers Resistance to Diet-Induced Obesity and Glucose Intolerance. Biochem. Biophys. Res. Commun. 2022, 605, 134–140. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prola, A.; Pilot-Storck, F. Cardiolipin Alterations during Obesity: Exploring Therapeutic Opportunities. Biology 2022, 11, 1638. https://doi.org/10.3390/biology11111638
Prola A, Pilot-Storck F. Cardiolipin Alterations during Obesity: Exploring Therapeutic Opportunities. Biology. 2022; 11(11):1638. https://doi.org/10.3390/biology11111638
Chicago/Turabian StyleProla, Alexandre, and Fanny Pilot-Storck. 2022. "Cardiolipin Alterations during Obesity: Exploring Therapeutic Opportunities" Biology 11, no. 11: 1638. https://doi.org/10.3390/biology11111638
APA StyleProla, A., & Pilot-Storck, F. (2022). Cardiolipin Alterations during Obesity: Exploring Therapeutic Opportunities. Biology, 11(11), 1638. https://doi.org/10.3390/biology11111638





