Mitochondrial Impairments in Peripheral Blood Mononuclear Cells of Multiple Sclerosis Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. LS-OCMB Detection
2.3. Metabolic Seahorse Assays
2.4. Flow Cytometry
2.5. Statistical Analysis
3. Results
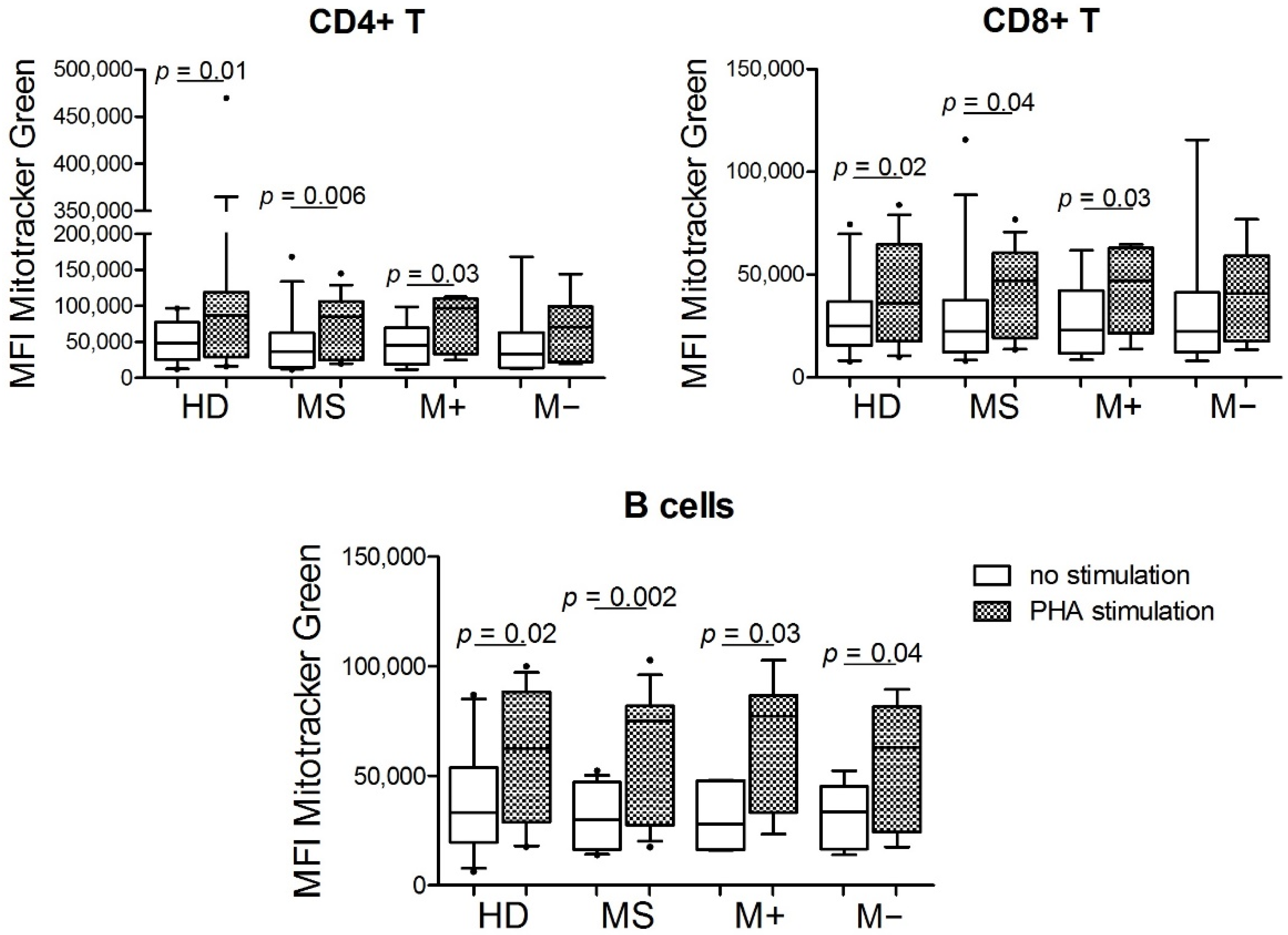
3.1. Mitochondrial Mass from CD4+ T Cells, CD8+ T Cells, and B Cells under Stimulus Was Higher than without Stimulus
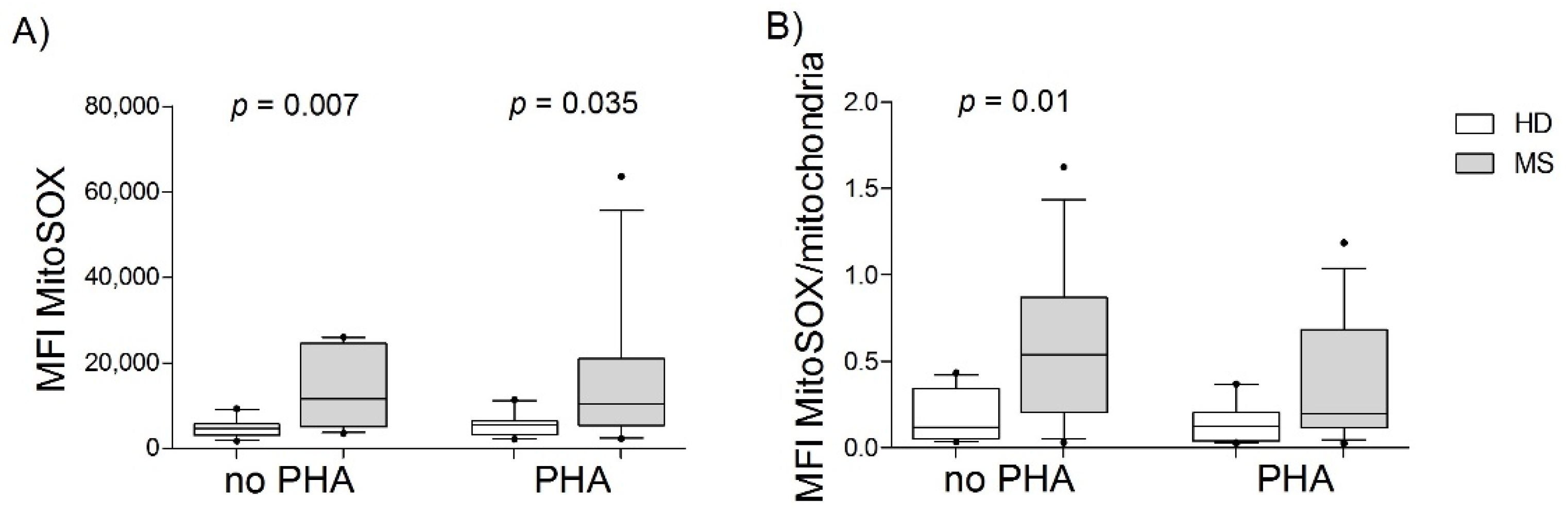
3.2. PBMCs from MS Patients Had Greater Superoxide Production than Those from HDs
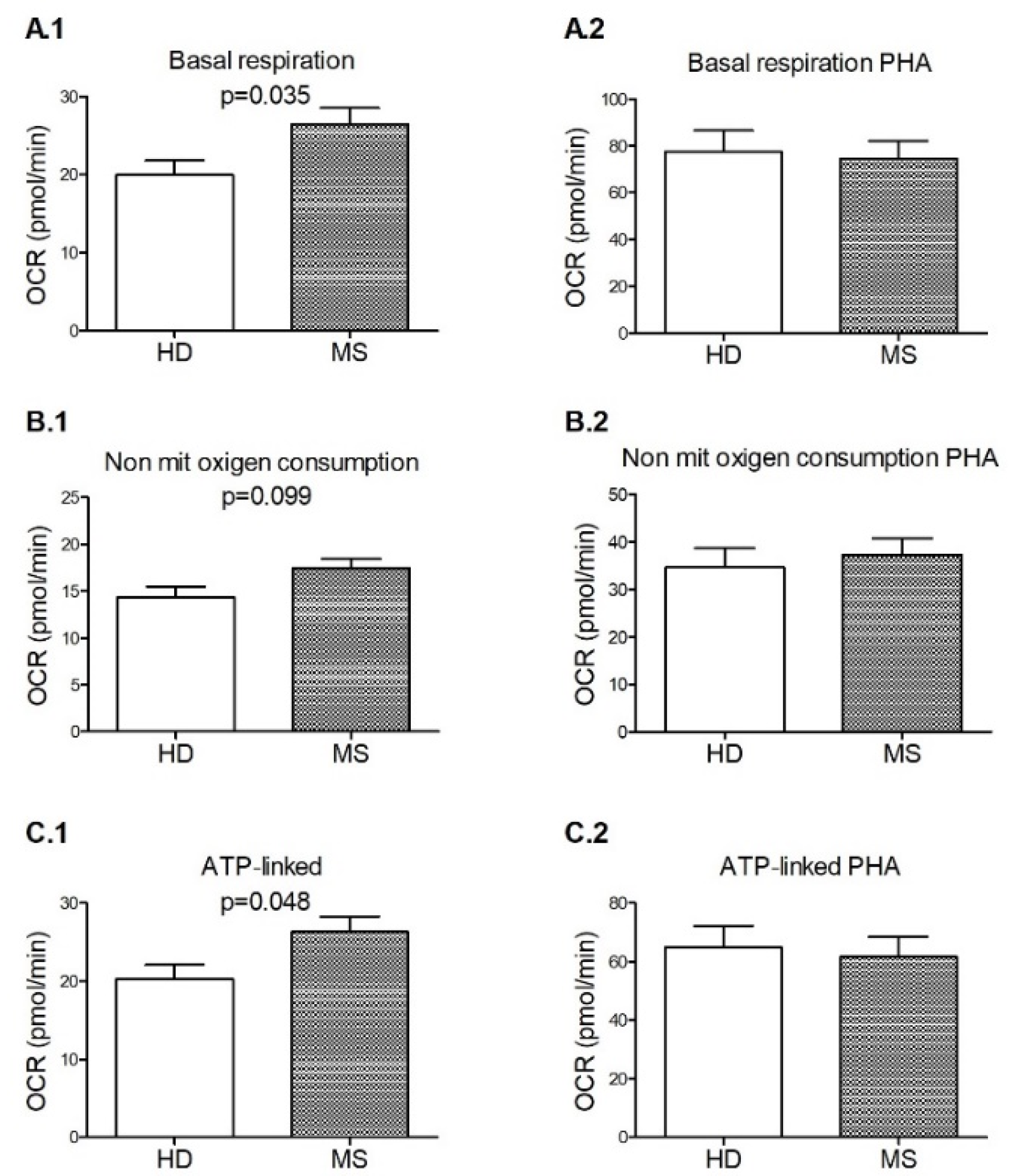
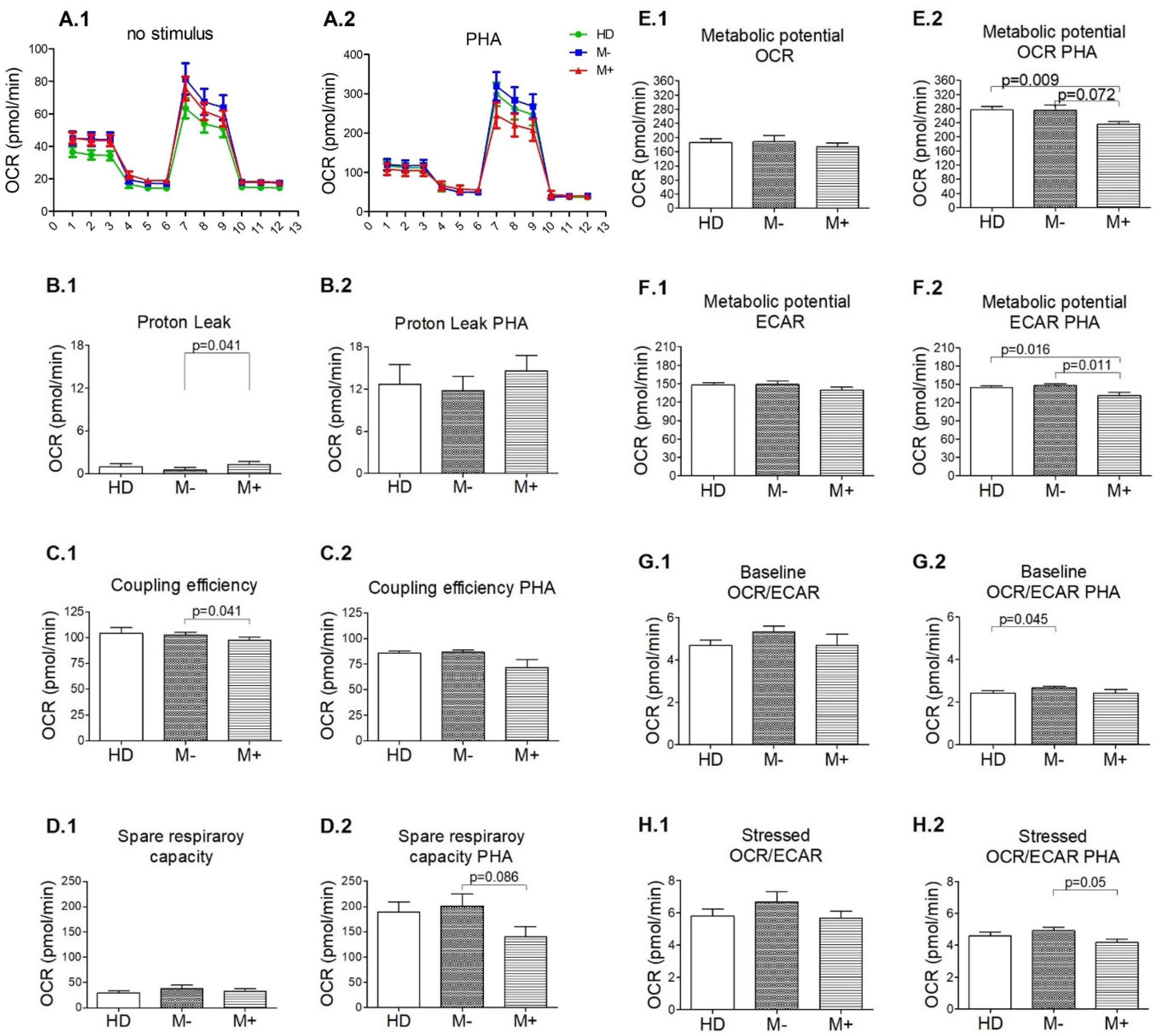
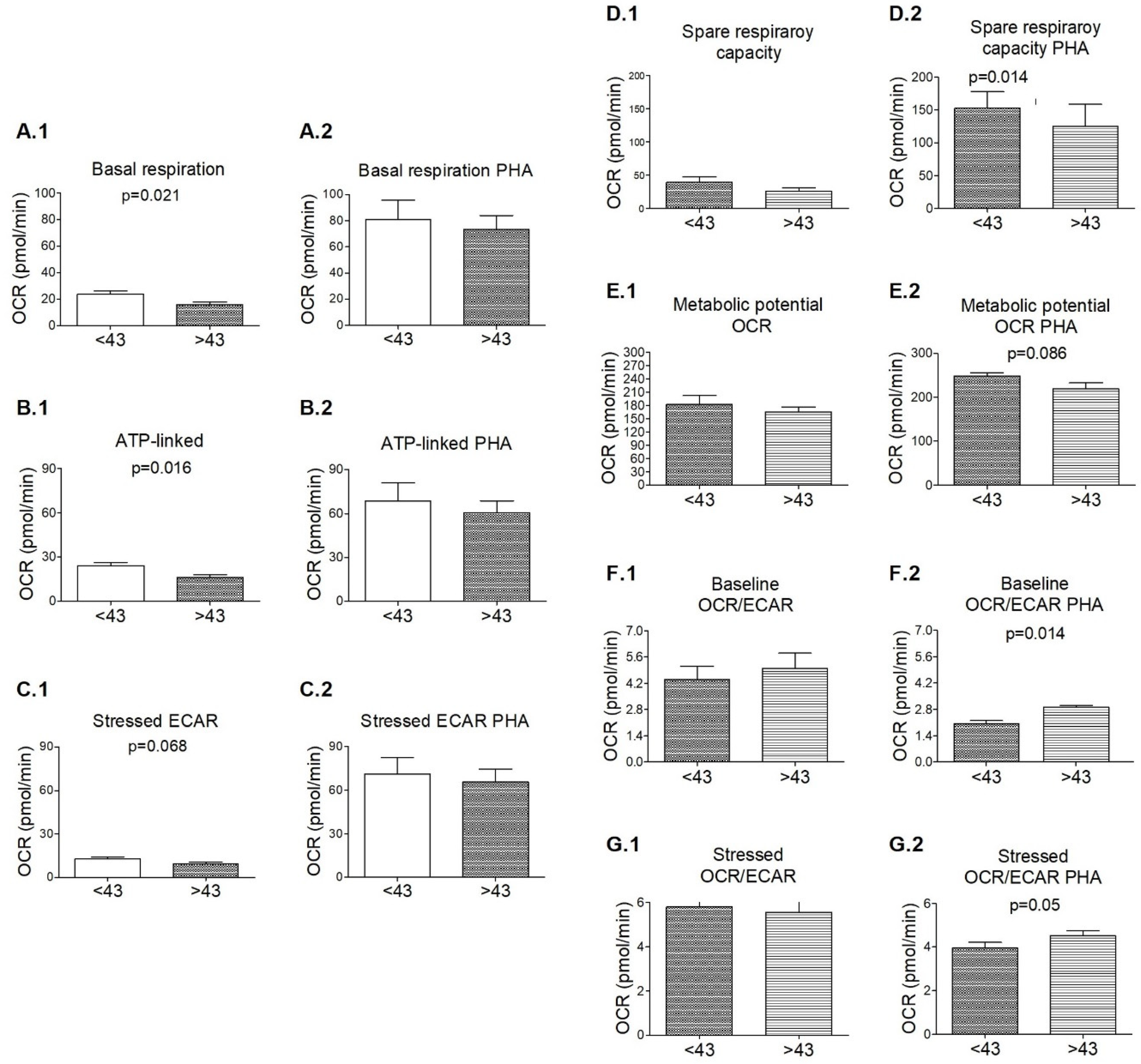
3.3. Signs of Functional Mitochondrial Impairments among M+ Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lublin, F.D.; Reingold, S.C.; Cohen, J.A.; Cutter, G.R.; Sørensen, P.S.; Thompson, A.J.; Wolinsky, J.S.; Balcer, L.J.; Banwell, B.; Barkhof, F.; et al. Defining the Clinical Course of Multiple Sclerosis: The 2013 Revisions. Neurology 2014, 83, 278–286. [Google Scholar] [CrossRef] [Green Version]
- Mahad, D.; Ziabreva, I.; Lassmann, H.; Turnbull, D. Mitochondrial Defects in Acute Multiple Sclerosis Lesions. Brain 2009, 132 Pt 5, 1161–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, G.R.; Worrall, J.T.; Mahad, D.J. The Central Role of Mitochondria in Axonal Degeneration in Multiple Sclerosis. Mult. Scler. 2014, 20, 1806–1813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Biasi, S.; Simone, A.M.; Bianchini, E.; Lo Tartaro, D.; Pecorini, S.; Nasi, M.; Patergnani, S.; Carnevale, G.; Gibellini, L.; Ferraro, D.; et al. Mitochondrial Functionality and Metabolism in T Cells from Progressive Multiple Sclerosis Patients. Eur. J. Immunol. 2019, 49, 2204–2221. [Google Scholar] [CrossRef] [PubMed]
- La Rocca, C.; Carbone, F.; De Rosa, V.; Colamatteo, A.; Galgani, M.; Perna, F.; Lanzillo, R.; Brescia Morra, V.; Orefice, G.; Cerillo, I.; et al. Immunometabolic Profiling of T Cells from Patients with Relapsing-Remitting Multiple Sclerosis Reveals an Impairment in Glycolysis and Mitochondrial Respiration. Metab. Metab. Clin. Exp. 2017, 77, 39–46. [Google Scholar] [CrossRef] [Green Version]
- López-Cotarelo, P.; González-Jiménez, A.; Agudo-Jiménez, T.; Abarca-Zabalía, J.; Aladro, Y.; Pilo, B.; Comabella, M.; Espino-Paisán, L.; Urcelay, E. Genetic Variation in NDFIP1 Modifies the Metabolic Patterns in Immune Cells of Multiple Sclerosis Patients. Sci. Rep. 2021, 11, 21371. [Google Scholar] [CrossRef]
- Gonzalo, H.; Nogueras, L.; Gil-Sánchez, A.; Hervás, J.V.; Valcheva, P.; González-Mingot, C.; Martin-Gari, M.; Canudes, M.; Peralta, S.; Solana, M.J.; et al. Impairment of Mitochondrial Redox Status in Peripheral Lymphocytes of Multiple Sclerosis Patients. Front. Neurosci. 2019, 13, 938. [Google Scholar] [CrossRef]
- Callender, L.A.; Carroll, E.C.; Bober, E.A.; Akbar, A.N.; Solito, E.; Henson, S.M. Mitochondrial Mass Governs the Extent of Human T Cell Senescence. Aging Cell 2020, 19, e13067. [Google Scholar] [CrossRef] [Green Version]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of Multiple Sclerosis: 2017 Revisions of the McDonald Criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Villar, L.M.; Masjuan, J.; González-Porqué, P.; Plaza, J.; Sádaba, M.C.; Roldán, E.; Bootello, A.; Alvarez-Cermeño, J.C. Intrathecal IgM Synthesis Is a Prognostic Factor in Multiple Sclerosis. Ann. Neurol. 2003, 53, 222–226. [Google Scholar] [CrossRef]
- Pfuhl, C.; Grittner, U.; Gieß, R.M.; Scheel, M.; Behrens, J.R.; Rasche, L.; Pache, F.C.; Wenzel, R.; Brandt, A.U.; Bellmann-Strobl, J.; et al. Intrathecal IgM Production Is a Strong Risk Factor for Early Conversion to Multiple Sclerosis. Neurology 2019, 93, e1439–e1451. [Google Scholar] [CrossRef]
- Perini, P.; Ranzato, F.; Calabrese, M.; Battistin, L.; Gallo, P. Intrathecal IgM Production at Clinical Onset Correlates with a More Severe Disease Course in Multiple Sclerosis. J. Neurol. Neurosurg. Psychiatry. 2006, 77, 953–955. [Google Scholar] [CrossRef] [Green Version]
- Durante, L.; Zaaraoui, W.; Rico, A.; Crespy, L.; Wybrecht, D.; Faivre, A.; Reuter, F.; Malikova, I.; Pommier, G.; Confort-Gouny, S.; et al. Intrathecal Synthesis of IgM Measured after a First Demyelinating Event Suggestive of Multiple Sclerosis Is Associated with Subsequent MRI Brain Lesion Accrual. Mult. Scler. 2012, 18, 587–591. [Google Scholar] [CrossRef]
- Huss, A.; Abdelhak, A.; Halbgebauer, S.; Mayer, B.; Senel, M.; Otto, M.; Tumani, H. Intrathecal Immunoglobulin M Production: A Promising High-Risk Marker in Clinically Isolated Syndrome Patients. Ann. Neurol. 2018, 83, 1032–1036. [Google Scholar] [CrossRef]
- Fonderico, M.; Biagioli, T.; Lanzilao, L.; Bellinvia, A.; Fratangelo, R.; Pastò, L.; Prestipino, E.; Razzolini, L.; Tudisco, L.; Ginestroni, A.; et al. Prognostic Role of Intrathecal IgM Synthesis in Multiple Sclerosis: Results from a Clinical Series. Mult. Scler. 2021, 27, 198–207. [Google Scholar] [CrossRef]
- Villar, L.M.; Sádaba, M.C.; Roldán, E.; Masjuan, J.; González-Porqué, P.; Villarrubia, N.; Espiño, M.; García-Trujillo, J.A.; Bootello, A.; Alvarez-Cermeño, J.C. Intrathecal Synthesis of Oligoclonal IgM against Myelin Lipids Predicts an Aggressive Disease Course in MS. J. Clin. Investg. 2005, 115, 187–194. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.; Huang, Y.; Jiang, H.; Zhang, D.; Yang, Y.; Geng, X.; Li, B. Functional Differences and Similarities in Activated Peripheral Blood Mononuclear Cells by Lipopolysaccharide or Phytohemagglutinin Stimulation between Human and Cynomolgus Monkeys. Ann. Transl. Med. 2021, 9, 257. [Google Scholar] [CrossRef]
- Nagy, G.; Barcza, M.; Gonchoroff, N.; Phillips, P.E.; Perl, A. Nitric Oxide-Dependent Mitochondrial Biogenesis Generates Ca2+ Signaling Profile of Lupus T Cells. J. Immunol. 2004, 173, 3676–3683. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Gao, C.; Luo, J. Metabolism Characteristics of Th17 and Regulatory T Cells in Autoimmune Diseases. Front. Immunol. 2022, 13, 828191. [Google Scholar] [CrossRef]
- de Bilbao, F.; Arsenijevic, D.; Vallet, P.; Hjelle, O.P.; Ottersen, O.P.; Bouras, C.; Raffin, Y.; Abou, K.; Langhans, W.; Collins, S.; et al. Resistance to Cerebral Ischemic Injury in UCP2 Knockout Mice: Evidence for a Role of UCP2 as a Regulator of Mitochondrial Glutathione Levels. J. Neurochem. 2004, 89, 1283–1292. [Google Scholar] [CrossRef]
- Beckman, J.S.; Estévez, A.G.; Crow, J.P.; Barbeito, L. Superoxide Dismutase and the Death of Motoneurons in ALS. Trends. Neurosci. 2001, 24, S15–S20. [Google Scholar] [CrossRef]
- Rego, A.C.; Oliveira, C.R. Mitochondrial Dysfunction and Reactive Oxygen Species in Excitotoxicity and Apoptosis: Implications for the Pathogenesis of Neurodegenerative Diseases. Neurochem. Res. 2003, 28, 1563–1574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tieu, K.; Ischiropoulos, H.; Przedborski, S. Nitric Oxide and Reactive Oxygen Species in Parkinson’s Disease. IUBMB Life 2003, 55, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Sena, L.A.; Li, S.; Jairaman, A.; Prakriya, M.; Ezponda, T.; Hildeman, D.A.; Wang, C.-R.; Schumacker, P.T.; Licht, J.D.; Perlman, H.; et al. Mitochondria Are Required for Antigen-Specific T Cell Activation through Reactive Oxygen Species Signaling. Immunity 2013, 38, 225–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chacko, B.K.; Kramer, P.A.; Ravi, S.; Benavides, G.A.; Mitchell, T.; Dranka, B.P.; Ferrick, D.; Singal, A.K.; Ballinger, S.W.; Bailey, S.M.; et al. The Bioenergetic Health Index: A New Concept in Mitochondrial Translational Research. Clin. Sci. 2014, 127, 367–373. [Google Scholar] [CrossRef] [Green Version]
- Michalek, R.D.; Gerriets, V.A.; Jacobs, S.R.; Macintyre, A.N.; MacIver, N.J.; Mason, E.F.; Sullivan, S.A.; Nichols, A.G.; Rathmell, J.C. Cutting Edge: Distinct Glycolytic and Lipid Oxidative Metabolic Programs Are Essential for Effector and Regulatory CD4+ T Cell Subsets. J. Immunol. 2011, 186, 3299–3303. [Google Scholar] [CrossRef] [Green Version]
- Boothby, M.; Rickert, R.C. Metabolic Regulation of the Immune Humoral Response. Immunity 2017, 46, 743–755. [Google Scholar] [CrossRef] [Green Version]
- De Riccardis, L.; Rizzello, A.; Ferramosca, A.; Urso, E.; De Robertis, F.; Danieli, A.; Giudetti, A.M.; Trianni, G.; Zara, V.; Maffia, M. Bioenergetics Profile of CD4(+) T Cells in Relapsing Remitting Multiple Sclerosis Subjects. J. Biotechnol. 2015, 202, 31–39. [Google Scholar] [CrossRef]
- Wimmer, I.; Scharler, C.; Zrzavy, T.; Kadowaki, T.; Mödlagl, V.; Rojc, K.; Tröscher, A.R.; Kitic, M.; Ueda, S.; Bradl, M.; et al. Microglia Pre-Activation and Neurodegeneration Precipitate Neuroinflammation without Exacerbating Tissue Injury in Experimental Autoimmune Encephalomyelitis. Acta. Neuropathol. Commun. 2019, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Fastenackels, S.; Sauce, D.; Vigouroux, C.; Avettand-Fènoël, V.; Bastard, J.-P.; Fellahi, S.; Nailler, L.; Arezes, E.; Rouzioux, C.; Warszawski, J.; et al. HIV-Mediated Immune Aging in Young Adults Infected Perinatally or during Childhood. AIDS 2019, 33, 1705–1710. [Google Scholar] [CrossRef]
- Gubser, P.M.; Bantug, G.R.; Razik, L.; Fischer, M.; Dimeloe, S.; Hoenger, G.; Durovic, B.; Jauch, A.; Hess, C. Rapid Effector Function of Memory CD8+ T Cells Requires an Immediate-Early Glycolytic Switch. Nat. Immunol. 2013, 14, 1064–1072. [Google Scholar] [CrossRef]
- Gerriets, V.A.; Rathmell, J.C. Metabolic Pathways in T Cell Fate and Function. Trends Immunol. 2012, 33, 168–173. [Google Scholar] [CrossRef] [Green Version]
- Picón, C.; Tejeda-Velarde, A.; Fernández-Velasco, J.I.; Comabella, M.; Álvarez-Lafuente, R.; Quintana, E.; Sainz de la Maza, S.; Monreal, E.; Villarrubia, N.; Álvarez-Cermeño, J.C.; et al. Identification of the Immunological Changes Appearing in the CSF During the Early Immunosenescence Process Occurring in Multiple Sclerosis. Front. Immunol. 2021, 12, 685139. [Google Scholar] [CrossRef]
- Wherry, E.J. T Cell Exhaustion. Nat. Immunol. 2011, 12, 492–499. [Google Scholar] [CrossRef]
- Wolf, Y.; Anderson, A.C.; Kuchroo, V.K. TIM3 Comes of Age as an Inhibitory Receptor. Nat. Rev. Immunol. 2020, 20, 173–185. [Google Scholar] [CrossRef]
- Klotz, L.; Eschborn, M.; Lindner, M.; Liebmann, M.; Herold, M.; Janoschka, C.; Torres Garrido, B.; Schulte-Mecklenbeck, A.; Gross, C.C.; Breuer, J.; et al. Teriflunomide Treatment for Multiple Sclerosis Modulates T Cell Mitochondrial Respiration with Affinity-Dependent Effects. Sci. Transl. Med. 2019, 11, eaao5563. [Google Scholar] [CrossRef] [Green Version]
- Hui, C.K.; Dedkova, E.N.; Montgomery, C.; Cortopassi, G. Dimethyl Fumarate Dose-Dependently Increases Mitochondrial Gene Expression and Function in Muscle and Brain of Friedreich’s Ataxia Model Mice. Hum. Mol. Genet. 2021, 29, 3954–3965. [Google Scholar] [CrossRef]
- Li, R.; Tang, H.; Burns, J.C.; Hopkins, B.T.; Le Coz, C.; Zhang, B.; de Barcelos, I.P.; Romberg, N.; Goldstein, A.C.; Banwell, B.L.; et al. BTK Inhibition Limits B-Cell-T-Cell Interaction through Modulation of B-Cell Metabolism: Implications for Multiple Sclerosis Therapy. Acta Neuropathol. 2022, 143, 505–521. [Google Scholar] [CrossRef]
| Non-Treated Multiple Sclerosis Patients | Healthy Donors (n = 17) | ||
|---|---|---|---|
| M− (n = 10) | M+ (n = 9) | ||
| Age (years, median (P25, P75) | 43 (37.8, 49.5) | 39 (34.5, 46) | 42 (37, 49.5) |
| Sex (M/F) (%) | 30/70 | 11/89 | 24/76 |
| Disease duration (years, median (P25, P75) | 9.8 (1.1, 19.3) | 5.8 (2.2, 15.9) | |
| EDSS | 1.5 (1.5, 6.5) | 1.5 (1, 5.8) | |
| Relapses 6 months before | 0 (0, 1) | 1 (0, 1.5) | |
| Relapses 6 months after | 0 (0, 0) | 0 (0, 0) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domínguez-Mozo, M.I.; García-Frontini Nieto, M.C.; Gómez-Calcerrada, M.I.; Pérez-Pérez, S.; García-Martínez, M.Á.; Villar, L.M.; Villarrubia, N.; Costa-Frossard, L.; Arroyo, R.; Alvarez-Lafuente, R. Mitochondrial Impairments in Peripheral Blood Mononuclear Cells of Multiple Sclerosis Patients. Biology 2022, 11, 1633. https://doi.org/10.3390/biology11111633
Domínguez-Mozo MI, García-Frontini Nieto MC, Gómez-Calcerrada MI, Pérez-Pérez S, García-Martínez MÁ, Villar LM, Villarrubia N, Costa-Frossard L, Arroyo R, Alvarez-Lafuente R. Mitochondrial Impairments in Peripheral Blood Mononuclear Cells of Multiple Sclerosis Patients. Biology. 2022; 11(11):1633. https://doi.org/10.3390/biology11111633
Chicago/Turabian StyleDomínguez-Mozo, María Inmaculada, María Celeste García-Frontini Nieto, María Isabel Gómez-Calcerrada, Silvia Pérez-Pérez, María Ángel García-Martínez, Luisa María Villar, Noelia Villarrubia, Lucienne Costa-Frossard, Rafael Arroyo, and Roberto Alvarez-Lafuente. 2022. "Mitochondrial Impairments in Peripheral Blood Mononuclear Cells of Multiple Sclerosis Patients" Biology 11, no. 11: 1633. https://doi.org/10.3390/biology11111633
APA StyleDomínguez-Mozo, M. I., García-Frontini Nieto, M. C., Gómez-Calcerrada, M. I., Pérez-Pérez, S., García-Martínez, M. Á., Villar, L. M., Villarrubia, N., Costa-Frossard, L., Arroyo, R., & Alvarez-Lafuente, R. (2022). Mitochondrial Impairments in Peripheral Blood Mononuclear Cells of Multiple Sclerosis Patients. Biology, 11(11), 1633. https://doi.org/10.3390/biology11111633






