Cellular Immuno-Profile in Septic Human Host: A Scoping Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- Characteristic changes in neutrophil and monocyte function in sepsis.
- Characteristic functional and phenotypic changes in adaptive immune system cells during sepsis.
- Techniques that are useful for the study of circulating cells in sepsis and to understand if immune cells act as a “biopsy sample”.
- Can extracorporeal and non-blood purification therapies alter cell phenotypes and/or change the function of leukocytes?
- COVID-19 and a “cytokine storm”: the role of blood purification.
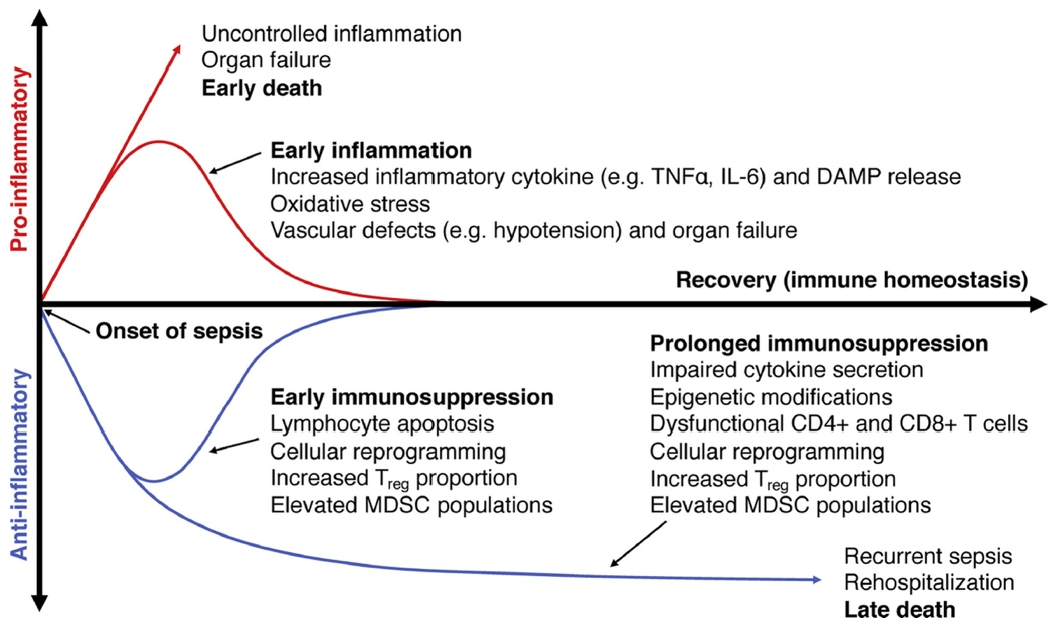
3. Discussion
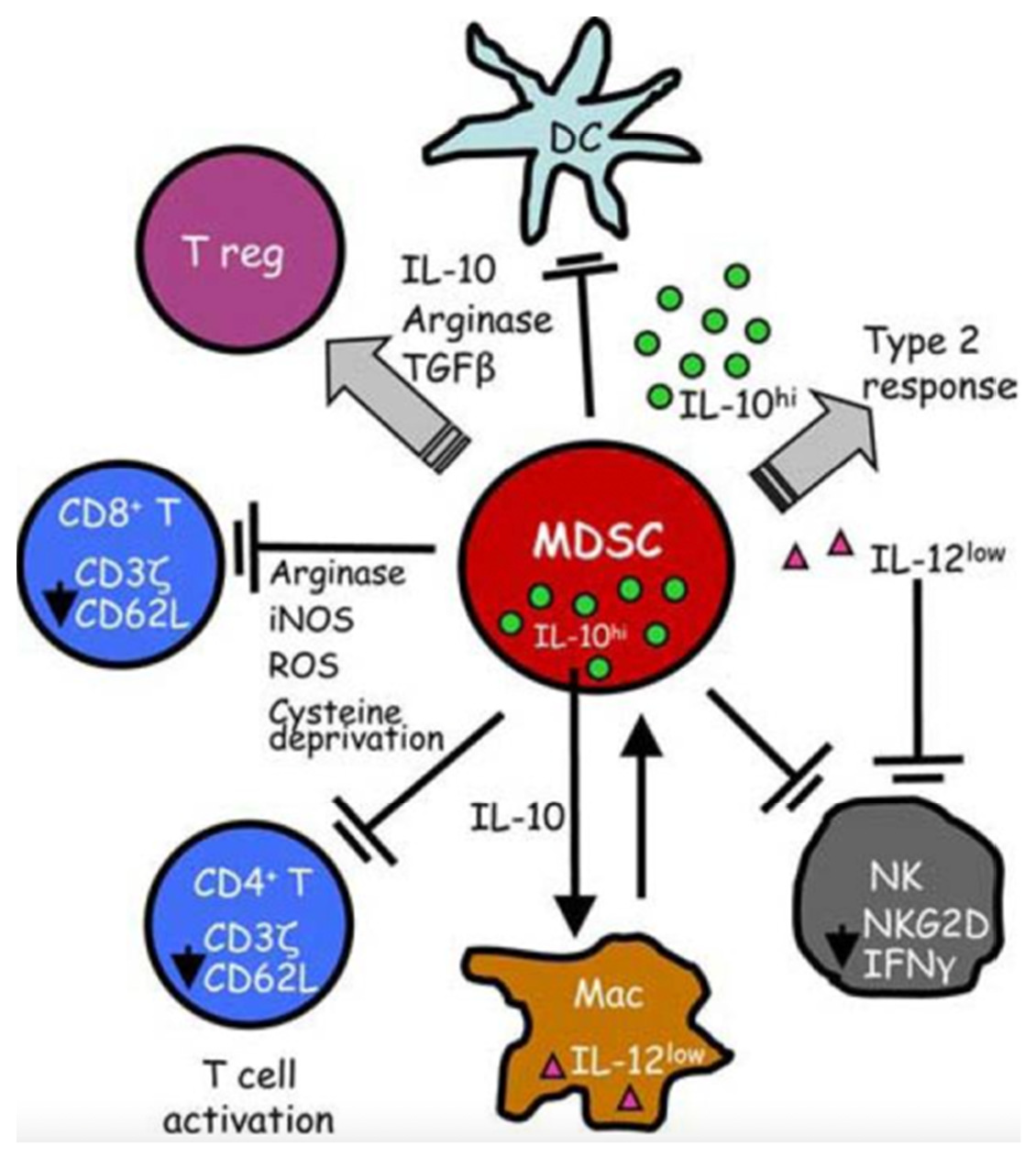
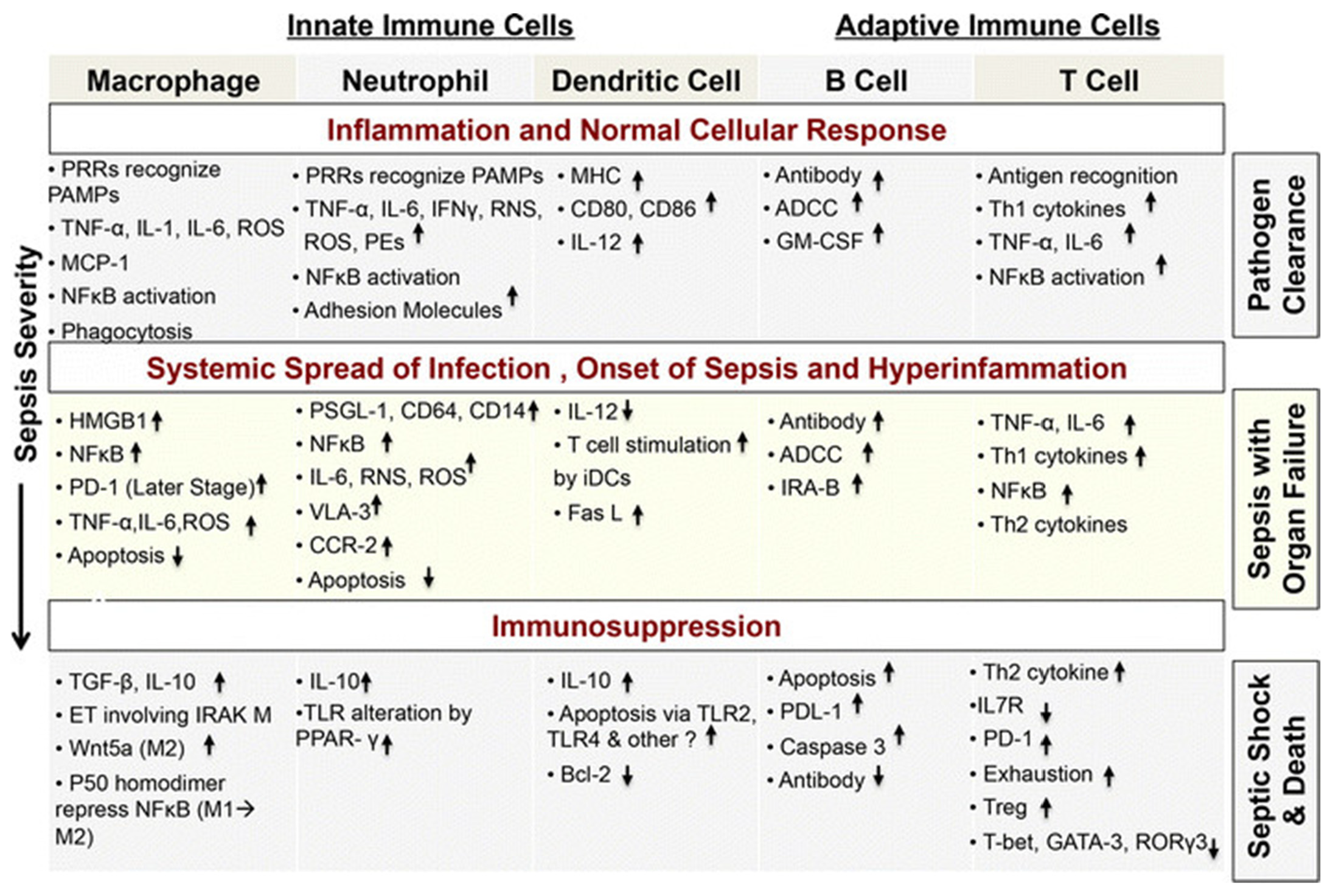
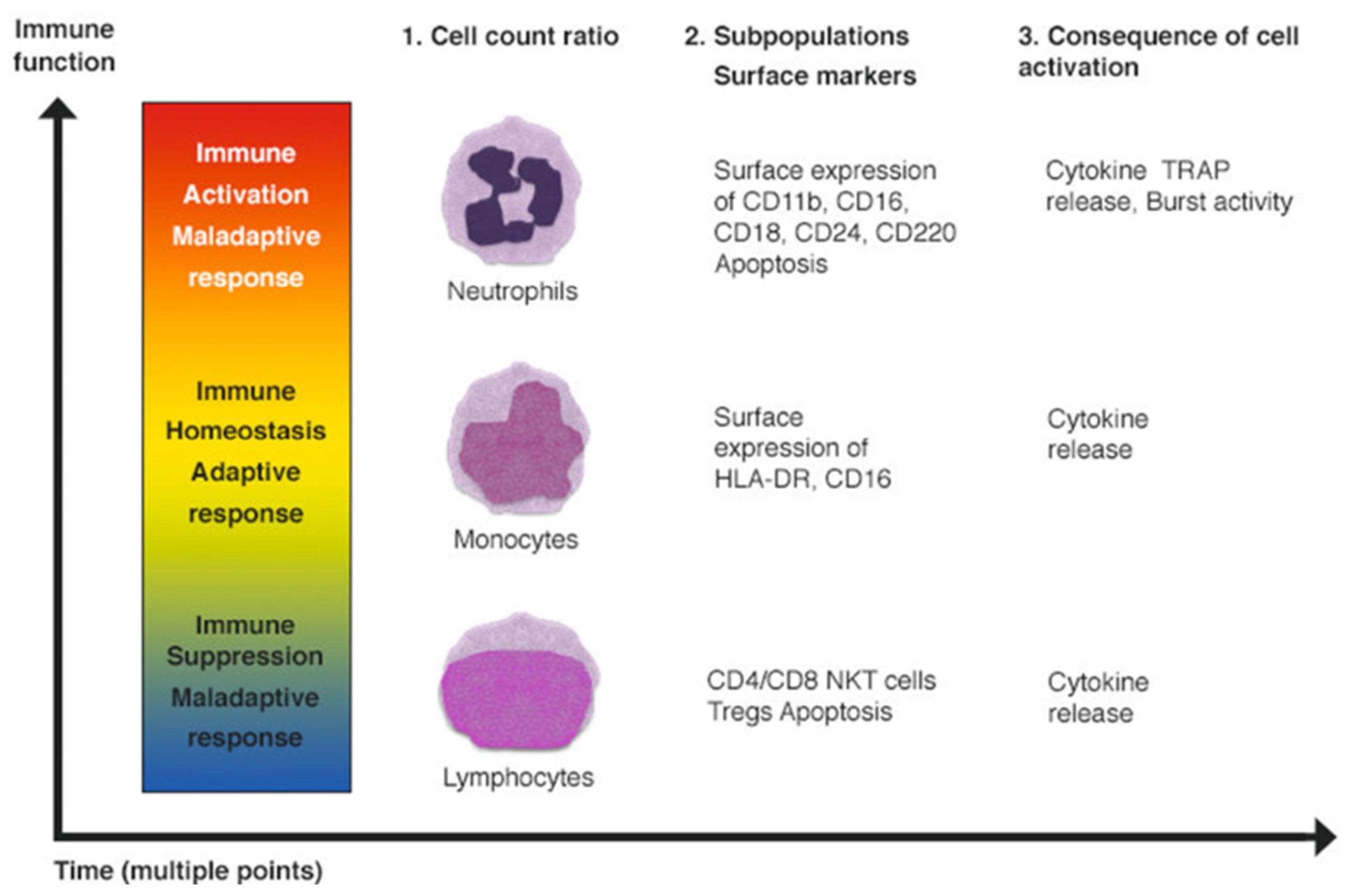
3.1. Characteristic Changes in Neutrophil and Monocyte Function in Sepsis
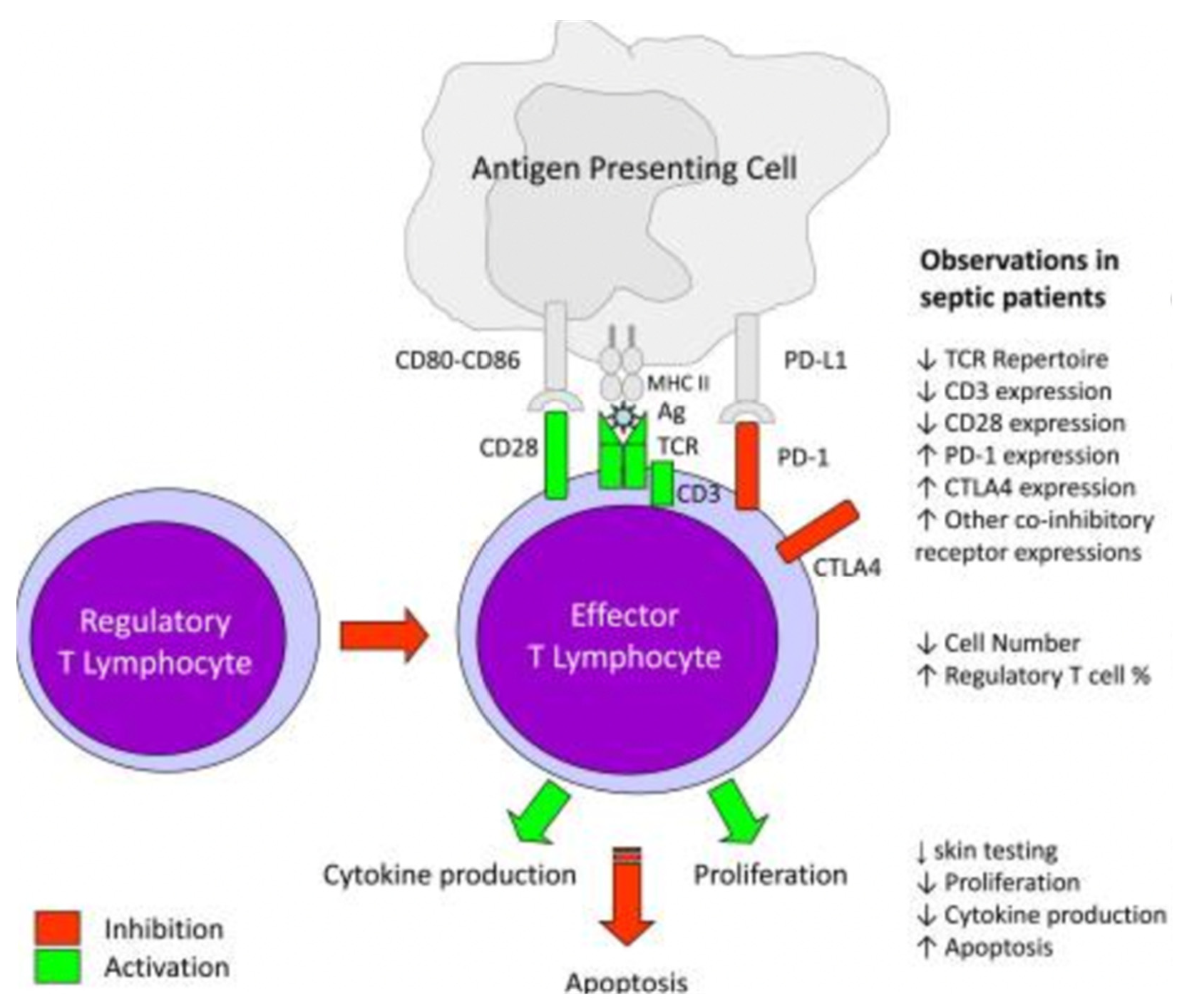
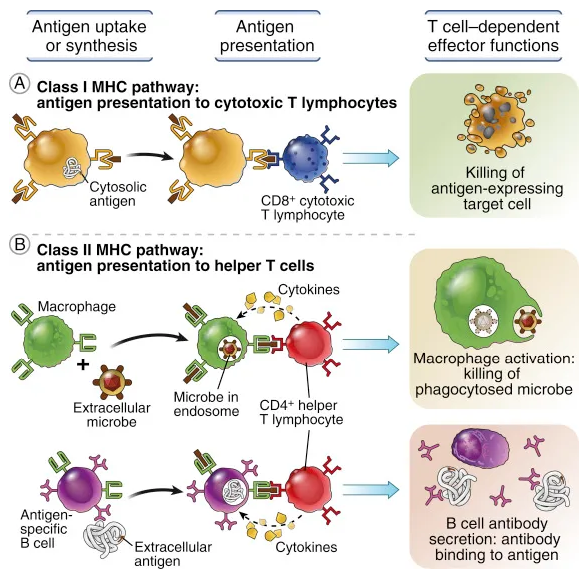
3.2. Characteristic Functional and Phenotypic Changes in Adaptive Immune System Cells during Sepsis
3.3. Techniques That Are Useful for the Study of Circulating Cells in Sepsis and to Understand If Immune Cells Act as a “Biopsy Sample”
3.4. Can Extracorporeal and Non-Blood Purification Therapies Alter Cell Phenotypes and/or Change the Function of Leukocytes?
3.5. COVID-19 and “Cytokine Storm”: The Role of Blood Purification
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mitchell, M.; Levy, A.R. The Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup*Surviving Sepsis Campaign: International Guidelines for Management of Severe Sepsis and Septic Shock, 2012. Intensive Care Med. 2013, 39, 165–228. [Google Scholar]
- Singer, M.; Deutschman, C.S.; Seymour, C.W. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Marshall, J.C. Assessment of the worldwide burden of critical illness: The intensive care over nations (ICON) audit. Lancet Respir. Med. 2014, 2, 380–386. [Google Scholar] [CrossRef]
- Marshall, J.C. Why have clinical trials in sepsis failed? Trends Mol. Med. 2014, 20, 195–203. [Google Scholar] [CrossRef]
- Piccioni, A.; Saviano, A.; Cicchinelli, S.; Valletta, F.; Santoro, M.C.; de Cunzo, T.; Zanza, C.; Longhitano, Y.; Tullo, G.; Tilli, P.; et al. Proadrenomedullin in Sepsis and Septic Shock: A Role in the Emergency Department. Medicina 2021, 57, 920. [Google Scholar] [CrossRef]
- Faix, J.D. Biomarker of sepsis. Crit. Rev. Clin. Lab. Sci. 2013, 50, 23–26. [Google Scholar] [CrossRef] [Green Version]
- Kellum, J.A.; Murray, P.; Ronco, C. Acute dialysis quality initiative (ADQI) XIV sepsis phenotypes and targets for blood purification in sepsis: The Bogota Consensus. Shock 2016, 45, 242–248. [Google Scholar] [CrossRef]
- Savill, J.S.; Wyllie, A.H.; Henson, J.E.; Henson, P.M.; Haslett, C. Macrophage phagocytosis of aging neutrophils in inflammation. J. Clin. Investig. 1989, 83, 865–875. [Google Scholar] [CrossRef] [Green Version]
- Pillay, J.; Den Braber, I.; Vrisekoop, N.; Kwast, L.M.; de Boer, R.J. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood 2010, 116, 625–627. [Google Scholar] [CrossRef] [Green Version]
- Chung, H.; Lee, J.H.; Jo, Y.H.; Hwang, J.E.; Kim, J. Circulating Monocyte Counts and its Impact on Outcomes in Patients with Severe Sepsis Including Septic Shock. Shock 2019, 51, 423–429. [Google Scholar] [CrossRef]
- Yipp, B.G.; Kubes, P. NETosis: How vital is it? Blood 2013, 122, 2784–2794. [Google Scholar] [CrossRef] [PubMed]
- Ahm, N.A.; McGill, S.; Christou, N.V. Mechanisms for the diminished neutrophil exudation to secondary inflammatory sites in infected patients with a systemic inflammatory response (sepsis). Crit. Care Med. 1999, 27, 2459–2468. [Google Scholar]
- Romaschin, A.D.; Foster, D.M.; Walker, P.M.; Marshall, J.C. Let the cells speak: Neutrophils as biologic markers of the inflammatory response. Sepsis 1998, 2, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.C.; Malam, Z.; Jia, S.H. Modulating neutrophil apoptosis. Novartis Found Symp. 2007, 280, 53–72. [Google Scholar]
- Zanza, C.; Romenskaya, T.; Thangathurai, D.; Ojetti, V.; Saviano, A.; Abenavoli, L.; Robba, C.; Cammarota, G.; Franceschi, F.; Piccioni, A.; et al. Microbiome in Critical Care: An Unconventional and Unknown Ally. Curr. Med. Chem. 2022, 29, 3179–3188. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.W.; Rotstein, O.D.; Parodo, J.; Bitar, R.; Marshall, J.C. The interleukin-1 beta converting enzyme (caspase-1) inhibits apoptosis of inflammatory neutrophils through activation of IL-1b. J. Immunol. 1998, 161, 957–962. [Google Scholar]
- Jia, S.H.; Li, Y.; Parodo, J.; Kapus, A.; Fan, L.; Rotstein, O.D.; Marshall, J.C. Pre-B cell colony-enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis. J. Clin. Investig. 2004, 113, 1318–1327. [Google Scholar] [CrossRef]
- Lukaszewicz, A.C.; Grienay, M.; Resche-Rigon, M.; Pirracchio, R.; Faivre, V.; Boval, B.; Payen, D. Monocytic HLA-DR expression in intensive care patients: Interest for prognosis and secondary infection prediction. Crit. Care Med. 2009, 37, 2746–2752. [Google Scholar]
- Venet, F.; Tissot, S.; Debard, A.L.; Faudot, C.; Crampé, C.; Pachot, A.; Ayala, A.; Monneret, G. Decreased monocyte human leukocyte antigen-DR expression after severe burn injury: Correlation with severity and secondary septic shock. Crit. Care Med. 2007, 35, 1910–1917. [Google Scholar] [CrossRef]
- Docke, W.D.; Randow, F.; Syrbe, U.; Krausch, D.; Asadullah, K.; Reinke, P.; Volk, H.D.; Kox, W. Monocyte deactivation in septic patients: Restoration by IFN-gamma treatment. Nature Med. 1997, 3, 678–681. [Google Scholar] [CrossRef]
- Longhitano, Y.; Zanza, C.; Thangathurai, D.; Taurone, S.; Kozel, D.; Racca, F.; Audo, A.; Ravera, E.; Migneco, A.; Piccioni, A.; et al. Gut Alterations in Septic Patients: A Biochemical Literature Review. Rev. Recent. Clin. Trials. 2020, 15, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Koffel, R.; Meshcheryakova, A.; Warszawska, J.; Hennig, A.; Wagner, K.; Jorgl, A.; Gubi, D.; Moser, D.; Hladik, A.; Hoffmann, U.; et al. Monocytic cell differentiation from band-stage neutrophils under inflammatory conditions via MKK6 activation. Blood 2014, 124, 2713–2724. [Google Scholar] [CrossRef] [Green Version]
- Ostrand-Rosenberg, S.; Sinha, P. Myeloid-derived suppressor cells: Linking inflammation and cancer. J. Immunol. 2009, 182, 4499–4506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sander, L.E.; Sackett, S.D.; Dierssen, U.; Beraza, N.; Linke, R.P.; Muller, M.; Blander, J.M.; Tacke, F.; Trautwein, C. Hepatic acute-phase proteins control innate immune responses during infection by promoting myeloid-derived suppressor cell function. J. Exp. Med. 2010, 207, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- Derive, M.; Bouazza, Y.; Alauzet, C.; Gibot, S. Myeloid-derived suppressor cells control microbial sepsis. Intensive Care Med. 2012, 38, 1040–1049. [Google Scholar] [CrossRef]
- Guerin, E.; Orabona, M.; Raquil, M.A.; Giraudeau, B.; Bellier, R.; Gibot, S.; Bene, M.C.; Lacombe, F.; Droin, N.; Solary, E.; et al. Circulating immature granulocytes with T-cell killing functions predict sepsis deterioration. Crit. Care Med. 2014, 42, 2007–2018. [Google Scholar] [CrossRef]
- Dinauer, M.C. Disorders of neutrophil function: An overview. Methods Mol. Biol 2007, 412, 489–504. [Google Scholar]
- Brown, K.A.; Brain, S.D.; Pearson, J.D.; Edgeworth, J.D.; Lewis, S.M.; Treacher, D.F. Neutrophils in development of multiple organ failure in sepsis. Lancet 2006, 368, 157–169. [Google Scholar] [CrossRef]
- Hoesel, L.M.; Neff, T.A.; Neff, S.B.; Younger, J.G.; Olle, E.W.; Gao, H.; Pianko, M.J.; Bernacki, K.D.; Sarma, J.V.; Ward, P.A. Harmful and protective roles of neutrophils in sepsis. Shock 2005, 24, 40–47. [Google Scholar] [CrossRef]
- Taneja, R.; Parodo, J.; Kapus, A.; Rotstein, O.D.; Marshall, J.C. Delayed neutrophil apoptosis in sepsis is associated with maintenance of mitochondrial transmembrane potential (DYM) and reduced caspase-9 activity. Crit. Care Med. 2004, 32, 1460–1469. [Google Scholar] [CrossRef]
- Jimenez, M.F.; Watson, R.W.; Parodo, J.; Evans, D.; Foster, D.; Steinberg, M.; Rotstein, O.D.; Marshall, J.C. Dysregulated expression of neutrophil apoptosis in the systemic inflammatory response syndrome. Arch. Surg. 1997, 132, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.H.; Parodo, J.; Charbonney, E.; Tsang, J.L.; Jia, S.Y.; Rotstein, O.D.; Kapus, A.; Marshall, J.C. Activated neutrophils induce epithelial cell apoptosis through oxidant-dependent tyrosine dephosphorylation of caspase-8. Am. J. Pathol. 2014, 184, 1030–1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.F.; Li, J.B.; Zhao, Y.J.; Yi, W.J.; Bian, J.J.; Wan, X.J.; Zhu, K.M.; Deng, X.M. Up-regulation of programmed cell death 1 ligand 1 on neutrophils may be involved in sepsis-induced immunosuppression: An animal study and a prospective case-control study. Anesthesiology 2015, 122, 852–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wenzel, R.P.; Edmond, M.B. Septic shock—Evaluating another failed treatment. N. Engl. J. Med. 2012, 366, 2122–2124. [Google Scholar] [CrossRef]
- Gomez, H.; Ince, C.; De Backer, D.; Pickkers, P.; Payen, D.; Hotchkiss, J.; Kellum, J.A. A unified theory of sepsis-induced acute kidney injury: Inflammation, micro-circulatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock 2014, 41, 3–11. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Immunosuppression in sepsis: A novel understanding of the disorder and a new therapeutic approach. Lancet Infect. Dis. 2013, 13, 260–268. [Google Scholar] [CrossRef] [Green Version]
- Zahorec, R. Ratio of neutrophil to lymphocyte counts: Rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl. Lek. Listy 2001, 102, 5–14. [Google Scholar]
- Tschaikowsky, K.; Hedwig-Geissing, M.; Schiele, A.; Bremer, F.; Schywalsky, M.; Schuttler, J. Coincidence of pro- and anti-inflammatory responses in the early phase of severe sepsis: Longitudinal study of mononuclear histocompatibility leukocyte antigen-DR expression, procalcitonin, C-reactive protein, and changes in T-cell subsets in septic and postoperative patients. Crit. Care Med. 2002, 30, 1015–1023. [Google Scholar]
- Holub, M.; Kluckova, Z.; Helcl, M.; Prihodov, J.; Rokyta, R.; Beran, O. Lymphocyte subset numbers depend on the bacterial origin of sepsis. Clin. Microbiol Infect. 2003, 9, 202–211. [Google Scholar] [CrossRef] [Green Version]
- Kelly-Scumpia, K.M.; Scumpia, P.O.; Weinstein, J.S.; Delano, M.J.; Cuenca, A.G.; Nacionales, D.C.; Wynn, J.L.; Lee, P.Y.; Kumagai, Y.; Efron, P.A.; et al. B cells enhance early innate immune responses during bacterial sepsis. J. Exp. Med. 2011, 208, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Monserrat, J.; de Pablo, R.; Diaz-Martin, D.; Rodriguez-Zapata, M.; de la Hera, A.; Prieto, A.; Alvarez-Mon, M. Early alterations of B cells in patients with septic shock. Crit. Care 2013, 17, R105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Sepsis-induced immunosuppression: From cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 2013, 13, 862–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, R.P.; Christou, N.V.; Meakins, J.L.; Nohr, C. Humoral immunity in surgical patients with and without trauma. Arch. Surg. 1991, 126, 143–148. [Google Scholar] [CrossRef]
- Rode, H.N.; Christou, N.V.; Gordon, J.; Meakins, J.L.; MacLean, L.D. Anergy in surgical patients: Is the failure at the afferent or the efferent limb? Surg. Forum 1979, 30, 41–43. [Google Scholar]
- Walton, A.H.; Muenzer, J.T.; Rasche, D.; Boomer, J.S.; Sato, B.; Brownstein, B.H.; Pachot, A.; Brooks, T.L.; Deych, E.; Shannon, W.D.; et al. Reactivation of multiple viruses in patients with sepsis. PLoS ONE 2014, 9, e98819. [Google Scholar] [CrossRef] [Green Version]
- Monneret, G.; Debard, A.L.; Venet, F.; Bohe, J.; Hequet, O.; Bienvenu, J.; Lepape, A. Marked elevation of human circulating CD4 þ CD25þ regulatory T cells in sepsis-induced immunoparalysis. Crit. Care Med. 2003, 31, 2068–2071. [Google Scholar] [CrossRef]
- Chang, K.C.; Burnham, C.A.; Compton, S.M.; Rasche, D.P.; Mazuski, R.J.; McDonough, J.S.; Unsinger, J.; Korman, A.J.; Green, J.M.; Hotchkiss, R.S. Blockade of the negative co-stimulatory molecules PD-1 and CTLA-4 improves survival in primary and secondary fungal sepsis. Crit. Care 2013, 17, R85. [Google Scholar] [CrossRef] [Green Version]
- Venet, F.; Chung, C.S.; Kherouf, H.; Geeraert, A.; Malcus, C.; Poitevin, F.; Bohe, J.; Lepape, A.; Ayala, A.; Monneret, G. Increased circulating regulatory T cells (CD4+CD25+CD127−) contribute to lymphocyte anergy in septic shock patients. Intensive Care Med. 2009, 35, 678–686. [Google Scholar] [CrossRef]
- Boomer, J.S.; To, K.; Chang, K.C.; Takasu, O.; Osborne, D.F.; Walton, A.H.; Bricker, T.L.; Jarman, S.D., II; Kreisel, D.; Krupnick, A.S.; et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 2011, 306, 2594–2605. [Google Scholar] [CrossRef]
- Drewry, A.M.; Samra, N.; Skrupky, L.P.; Fuller, B.M.; Compton, S.M.; Hotchkiss, R.S. Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock 2014, 42, 383–391. [Google Scholar] [CrossRef] [Green Version]
- Hotchkiss, R.S.; Karl, I.E. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 2003, 348, 138–150. [Google Scholar] [CrossRef] [Green Version]
- Hotchkiss, R.S.; Swanson, P.E.; Freeman, B.D.; Tinsley, K.W.; Cobb, J.P.; Matuschak, G.M.; Buchman, T.G.; Karl, I.E. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit. Care Med. 1999, 27, 1230–1251. [Google Scholar] [CrossRef] [PubMed]
- Pearce, E.L.; Pearce, E.J. Metabolic pathways in immune cell activation and quiescence. Immunity 2013, 38, 633–643. [Google Scholar] [CrossRef] [Green Version]
- Losser, M.R.; Damoisel, C.; Payen, D. Bench-to-bedside review: Glucose and stress conditions in the intensive care unit. Crit. Care 2010, 14, 231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Losser, M.R.; Bernard, C.; Beaudeux, J.L.; Pison, C.; Payen, D. Glucose modulates hemodynamic, metabolic, and inflammatory responses to lipopolysaccharide in rabbits. J. Appl. Physiol. 1997, 83, 1566–1574. [Google Scholar] [CrossRef]
- Van den Berghe, G.; Wilmer, A.; Hermans, G.; Meersseman, W.; Wouters, P.J.; Milants, I.; Van Wijngaerden, E.; Bobbaers, H.; Bouillon, R. Intensive insulin therapy in the medical ICU. N. Engl. J. Med. 2006, 354, 449–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Berghe, G.; Wouters, P.; Weekers, F.; Verwaest, C.; Bruyninckx, F.; Schetz, M.; Vlasselaers, D.; Ferdinande, P.; Lauwers, P.; Bouillon, R. Intensive insulin therapy in critically ill patients. N. Engl. J. Med. 2001, 345, 1359–1367. [Google Scholar] [CrossRef]
- Hoeboer, S.H.; Groeneveld, A.B. Changes in circulating procalcitonin versus Creactive protein in predicting evolution of infectious disease in febrile, critically ill patients. PLoS ONE 2013, 8, e65564. [Google Scholar] [CrossRef]
- Uzzan, B.; Cohen, R.; Nicolas, P.; Cucherat, M.; Perret, G.Y. Procalcitonin as a diagnostic test for sepsis in critically ill adults and after surgery or trauma: A systematic review and meta-analysis. Crit. Care Med. 2006, 34, 1996–2003. [Google Scholar] [CrossRef]
- Masson, S.; Caironi, P.; Spanuth, E.; Thomae, R.; Panigada, M.; Sangiorgi, G.; Fumagalli, R.; Mauri, T.; Isgrò, S.; Fanizza, C.; et al. Presepsin (soluble CD14 subtype) and procalcitonin levels for mortality prediction in sepsis: Data from the Albumin Italian Outcome Sepsis trial. Crit. Care 2014, 18, R6. [Google Scholar] [CrossRef] [Green Version]
- Luz Fiusa, M.M.; Costa-Lima, C.; de Souza, G.R.; Vigorito, A.C.; Penteado Aranha, F.J.; Lorand-Metze, I.; Annichino-Bizzacchi, J.M.; de Souza, C.A.; De Paula, E.V. A high angiopoietin-2/angiopoietin-1 ratio is associated with a high risk of septic shock in patients with febrile neutropenia. Crit. Care 2013, 17, R169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorente, L.; Martín, M.M.; Varo, N.; Borreguero-León, J.M.; Solé-Violán, J.; Blanquer, J.; Labarta, L.; Díaz, C.; Jiménez, A.; Pastor, E.; et al. Association between serum soluble CD40 ligand levels and mortality in patients with severe sepsis. Crit. Care 2011, 15, R97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huttunen, R.; Syrjänen, J.; Vuento, R.; Laine, J.; Hurme, M.; Aittoniemi, J. Apoptosis markers soluble Fas (sFas), Fas Ligand (FasL) and sFas/FasL ratio in patients with bacteremia: A prospective cohort study. J. Infect. 2012, 64, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Venet, F.; Pachot, A.; Debard, A.L.; Bohe, J.; Bienvenu, J.; Lepape, A.; Powell, W.S.; Monneret, G. Human CD4+CD25+ regulatory T lymphocytes inhibit lipopolysaccharide-induced monocyte survival through a Fas/Fas ligand-dependent mechanism. J. Immunol. 2006, 177, 6540–6547. [Google Scholar] [CrossRef] [Green Version]
- Duong, S.; Condotta, S.A.; Rai, D.; Martin, M.D.; Griffith, T.S.; Badovinac, V.P. Polymicrobial sepsis alters antigen-dependent and -independent memory CDT cell functions. J. Immunol. 2014, 192, 3618–3625. [Google Scholar] [CrossRef] [Green Version]
- Patschan, S.A.; Patschan, D.; Temme, J.; Korsten, P.; Wessels, J.T.; Koziolek, M.; Henze, E.; Müller, G.A. Endothelial progenitor cells (EPC) in sepsis with acute renal dysfunction (ARD). Crit. Care 2011, 15, R94. [Google Scholar] [CrossRef] [Green Version]
- Trimmel, H.; Luschin, U.; Köhrer, K.; Anzur, C.; Vevera, D.; Spittler, A. Clinical outcome of critically ill patients cannot be defined by cutoff values of monocyte human leukocyte antigen-DR expression. Shock 2012, 37, 140–144. [Google Scholar] [CrossRef]
- Wu, J.F.; Ma, J.; Chen, J.; Ou-Yang, B.; Chen, M.Y.; Li, L.F.; Liu, Y.J.; Lin, A.H.; Guan, X.D. Changes of monocyte human leukocyte antigen-DR expression as a reliable predictor of mortality in severe sepsis. Crit. Care 2011, 15, R220. [Google Scholar] [CrossRef]
- Forel, J.M.; Chiche, L.; Thomas, G.; Mancini, J.; Farnarier, C.; Cognet, C.; Guervilly, C.; Daumas, A.; Vély, F.; Xéridat, F.; et al. Phenotype and functions of natural killer cells in critically-ill septic patients. PLoS ONE 2012, 7, e50446. [Google Scholar] [CrossRef] [Green Version]
- Kjaergaard, A.G.; Nielsen, J.S.; Tønnesen, E.; Krog, J. Expression of NK cell and monocyte receptors in critically ill patients’ potential biomarkers of sepsis. Scand. J. Immunol. 2015, 81, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Hanna, M.O.F.; Abdelhameed, A.M.; Abou-Elalla, A.A.; Hassan, R.M.; Kostandi, I. Neutrophil and monocyte receptor expression in patients with sepsis: Implications for diagnosis and prognosis of sepsis. Pathog Dis. 2019, 77, ftz055. [Google Scholar] [CrossRef] [PubMed]
- Loupy, A.; Lefaucheur, C.; Vernerey, D.; Chang, J.; Hidalgo, L.G.; Beuscart, T.; Verine, J.; Aubert, O.; Dubleumortier, S.; Duong van Huyen, J.P.; et al. Molecular microscope strategy to improve risk stratification in early antibody-mediated kidney allograft rejection. J. Am. Soc. Nephrol 2014, 25, 2267–2277. [Google Scholar] [CrossRef] [Green Version]
- Song, Z.; Song, Y.; Yin, J.; Shen, Y.; Yao, C.; Sun, Z.; Jiang, J.; Zhu, D.; Zhang, Y.; Shen, Q.; et al. Genetic variation in the TNF gene is associated with susceptibility to severe sepsis, but not with mortality. PLoS ONE 2012, 7, e46113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Payen, D.; Lukaszewicz, A.C.; Belikova, I.; Faivre, V.; Gelin, C.; Russwurm, S.; Launay, J.M.; Sevenet, N. Gene profiling in human blood leucocytes during recovery from septic shock. Intensive Care Med. 2008, 34, 1371–1376. [Google Scholar] [CrossRef] [PubMed]
- Cajander, S.; Bäckman, A.; Tina, E.; Stralin, K.; Söderquist, B.; Källman, J. Preliminary results in quantitation of HLA-DRA by real-time PCR: A promising approach to identify immunosuppression in sepsis. Crit. Care 2013, 17, R223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicodeme, E.; Jeffrey, K.L.; Schaefer, U.; Beinke, S.; Dewell, S.; Chung, C.W.; Chandwani, R.; Marazzi, I.; Wilson, P.; Coste, H.; et al. Suppression of inflammation by a synthetic histone mimic. Nature 2010, 468, 1119–1123. [Google Scholar] [CrossRef] [Green Version]
- Tacke, F.; Roderburg, C.; Benz, F.; Cardenas, D.V.; Luedde, M.; Hippe, H.J.; Frey, N.; Vucur, M.; Gautheron, J.; Koch, A.; et al. Levels of circulating miR-133a are elevated in sepsis and predict mortality in critically ill patients. Crit. Care Med. 2014, 42, 1096–1104. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Vilanova, D.; Atalar, K.; Delfour, O.; Edgeworth, J.; Ostermann, M.; Hernandez-Fuentes, M.; Razafimahatratra, S.; Michot, B.; Persing, D.H.; et al. Genome-wide sequencing of cellular microRNAs identifies a combinatorial expression signature diagnostic of sepsis. PLoS ONE 2013, 8, e75918. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, P.; Chen, W.; Feng, D.; Jia, Y.; Xie, L. SerummicroRNAsignatures identified by Solexa sequencing predict sepsis patients’ mortality: A prospective observational study. PLoS ONE 2012, 7, e38885. [Google Scholar]
- Camussi, G.; Deregibus, M.C.; Bruno, S.; Cantaluppi, V.; Biancone, L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010, 78, 838–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azevedo, L.C.; Janiszewski, M.; Pontieri, V.; Pedro Mde, A.; Bassi, E.; Tucci, P.J.; Laurindo, F.R. Platelet-derived exosomes from septic shock patients induce myocardial dysfunction. Crit. Care 2007, 11, R120. [Google Scholar] [CrossRef] [Green Version]
- Gambim, M.H.; do Carmo Ade, O.; Marti, L.; Veríssimo-Filho, S.; Lopes, L.R.; Janiszewski, M. Platelet-derived exosomes induce endothelial cell apoptosis through peroxynitrite generation: Experimental evidence for a novel mechanism of septic vascular dysfunction. Crit. Care 2007, 11, R107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sprung, C.L.; Annane, D.; Keh, D.; Moreno, R.; Singer, M.; Freivogel, K.; Weiss, Y.G.; Benbenishty, J.; Kalenka, A.; Forst, H.; et al. Hydrocortisone therapy for patients with septic shock. N. Engl. J. Med. 2008, 358, 111–124. [Google Scholar] [CrossRef] [Green Version]
- Marti-Carvajal, A.J.; Sola, I.; Lathyris, D.; Cardona, A.F. Human recombinant activated protein C for severe sepsis. Cochrane Database Syst. Rev. 2012, 3, CD004388. [Google Scholar]
- Yekebas, E.F.; Eisenberger, C.F.; Ohnesorge, H.; Saalmuller, A.; Elsner, H.A.; Engelhardt, M.; Gillesen, A.; Meins, J.; The, M.; Strate, T.; et al. Attenuation of sepsisrelated immunoparalysis by continuous veno-venous hemofiltration in experimental porcine pancreatitis. Crit. Care Med. 2001, 29, 1423–1430. [Google Scholar] [CrossRef]
- Ono, S.; Tsujimoto, H.; Matsumoto, A.; Ikuta, S.; Kinoshita, M.; Mochizuki, H. Modulation of human leukocyte antigen-DR on monocytes and CD16 on granulocytes in patients with septic shock using hemoperfusion with polymyxin B-immobilized fiber. Am. J. Surg. 2004, 188, 150–156. [Google Scholar] [CrossRef]
- Payen, D.M.; Guilhot, J.; Launey, Y.; Lukaszewicz, A.C.; Kaaki, M.; Veber, B.; Pottecher, J.; Joannes-Boyau, O.; Martin-Lefevre, L.; Jabaudon, M.; et al. Early use of polymyxin B hemoperfusion in patients with septic shock due to peritonitis: A multicenter randomized controlled trial. Intensive Care Med. 2015, 41, 975–984. [Google Scholar] [CrossRef] [Green Version]
- Kumagai, T.; Takeyama, N.; Yabuki, T.; Harada, M.; Miki, Y.; Kanou, H.; Inoue, S.; Nakagawa, T.; Noguchi, H. Apheresis of activated leukocytes with an immobilized polymyxin B filter in patients with septic shock. Shock 2010, 34, 461–466. [Google Scholar] [CrossRef]
- Srisawat, N.; Tungsanga, S.; Lumlertgul, N.; Komaenthammasophon, C.; Peerapornratana, S.; Thamrongsat, N.; Tiranathanagul, K.; Praditpornsilpa, K.; Eiam-Ong, S.; Tungsanga, K.; et al. The effect of polymyxin B hemoperfusion on modulation of human leukocyte antigen DR in severe sepsis patients. Crit. Care 2018, 22, 279. [Google Scholar] [CrossRef] [Green Version]
- Kaynar, A.M.; McLaughlin, J.N.; Zhu, L. Leukocyte capture and modulation of cell-mediated immunity during human sepsis: An ex vivo study. Crit. Care 2013, 17, R59. [Google Scholar]
- Peng, Z.Y.; Carter, M.J.; Kellum, J.A. Effects of hemoadsorption on cytokine removal and short-term survival in septic rats. Crit. Care Med. 2008, 36, 1573–1577. [Google Scholar] [CrossRef]
- Peng, Z.Y.; Bishop, J.V.; Singbartl, K. Modulation of chemokine gradients by apheresis redirects leukocyte trafficking to different compartments during sepsis, studies in a rat model. Crit. Care 2014, 18, R141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronco, C.; Brendolan, A.; Lonnemann, G.; Bellomo, R.; Piccinni, P. A pilot study of coupled plasma filtration with adsorption in septic shock. Crit. Care Med. 2002, 30, 1250–1255. [Google Scholar] [CrossRef] [PubMed]
- Cantaluppi, V.; Weber, V.; Segoloni, G.P. Protective effect of resin adsorption on septic plasma-induced tubular injury. Crit. Care 2010, 14, R4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgera, S.; Haase, M.; Kox, W.J. High permeability haemofiltration improves peripheral blood mononuclear cell proliferation in septic patients with acute renal failure. Nephrol. Dial. Transplant. 2003, 18, 2570–2576. [Google Scholar] [CrossRef] [Green Version]
- Morgera, S.; Haase, M.; Beck, W. Intermittent high-permeability hemofiltration modulates inflammatory response in septic patients with multiorgan failure. Nephron Clin. Pract. 2003, 94, c75–c80. [Google Scholar] [CrossRef]
- Leentjens, J.; Kox, M.; Pickkers, P. Reversal of immunoparalysis in humans in vivo: A double-blind, placebo-controlled, randomized pilot study. Am. J. Respir. Crit. Care Med. 2012, 186, 838–845. [Google Scholar] [CrossRef]
- Meisel, C.; Schefold, J.C.; Zuckermann, H. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: A double-blind, randomized, placebo-controlled multicenter trial. Am. J. Respir. Crit. Care Med. 2009, 180, 640–648. [Google Scholar] [CrossRef]
- Chang, K.; Svabek, C.; Green, J. Targeting the programmed cell death 1: Programmed cell death ligand 1 pathway reverses T cell exhaustion in patients with sepsis. Crit. Care 2014, 18, R3. [Google Scholar] [CrossRef] [Green Version]
- Venet, F.; Foray, A.P.; Monneret, G. IL-7 restores lymphocyte functions in septic patients. J. Immunol. 2012, 189, 5073–5081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, P.; Qie, S.; Liu, Z. Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: A single arm meta-analysis. J. Med. Virol 2020, 92, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Zanza, C.; Racca, F.; Longhitano, Y.; Piccioni, A.; Franceschi, F.; Artico, M.; Abenavoli, L.; Maiese, A.; Passaro, G.; Volonnino, G.; et al. Risk Management and Treatment of Coagulation Disorders Related to COVID-19 Infection. Int. J. Environ. Res. Public Health 2021, 18, 1268. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.J.; Ni, Z.Y.; Hu, Y. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, Y.; Xu, J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef] [Green Version]
- Gu, J.; Han, B.; Wang, J. COVID-19: Gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology 2020, 158, 1518–1519. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.D.; Zhang, S.P.; Wei, Q.Z. COVID-19 complicated with DIC: 2 cases report and literatures review. Zhonghua Xue Ye Xue Za Zhi 2020, 41, E001. [Google Scholar]
- Cheng, Y.; Luo, R.; Wang, K. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020, 97, 829–838. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.L.; Wang, X.G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Longhitano, Y.; Zanza, C.; Romenskaya, T.; Saviano, A.; Persiano, T.; Leo, M.; Piccioni, A.; Betti, M.; Maconi, A.; et al. Single-Breath Counting Test Predicts Non-Invasive Respiratory Support Requirements in Patients with COVID-19 Pneumonia. J. Clin. Med. 2021, 11, 179. [Google Scholar] [CrossRef]
- Huang, C. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Behrens, E.M.; Koretzky, G.A. Review: Cytokine storm syndrome: Looking toward the precision medicine era. Arthritis Rheumatol 2017, 69, 1135–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teijaro, J.R.; Walsh, K.B.; Cahalan, S. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell 2011, 146, 980–991. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhou, Y.H.; Yang, Z.Q. The cytokine storm of severe influenza and development of immunomodulatory therapy. Cell Mol. Immunol. 2016, 13, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronco, C.; Tetta, C.; Mariano, F.; Wratten, M.L. Interpreting the mechanisms of continuous renal replacement therapy in sepsis: The peak concentration hypothesis. Artif. Organs 2003, 27, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Angus, D.C.; van der Poll, T. Severe sepsis and septic shock. N. Engl. J. Med. 2013, 369, 840–851. [Google Scholar] [CrossRef]
- Zanza, C.; Tassi, M.F.; Romenskaya, T.; Piccolella, F.; Abenavoli, L.; Franceschi, F.; Piccioni, A.; Ojetti, V.; Saviano, A.; Canonico, B.; et al. Lock, Stock and Barrel: Role of Renin-Angiotensin-Aldosterone System in Coronavirus Disease 2019. Cells 2021, 10, 1752. [Google Scholar] [CrossRef]
- Cha, R.H.; Joh, J.S.; Jeong, I. Critical Care Team of National Medical Center. Renal Complications and their prognosis in Korean patients with middle east respiratory syndrome-coronavirus from the central MERS-CoV designated hospital. J. Korean Med. Sci. 2015, 30, 1807–1814. [Google Scholar] [CrossRef]
- Network, V.; Palevsky, P.M.; Zhang, J.H. Intensity of renal support in critically ill patients with acute kidney injury. N. Engl. J. Med. 2008, 3, 7–20. [Google Scholar]
- Bellomo, R.; Cass, A. Intensity of continuous renal-replacement therapy in critically ill patients. N. Engl. J. Med. 2009, 361, 1627–1638. [Google Scholar]
- Joannes-Boyau, O.; Honore, P.M.; Perez, P. High-volume versus standard-volume haemofiltration for septic shock patients with acute kidney injury (IVOIRE study): A multicentre randomized controlled trial. Intensive Care Med. 2013, 39, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Padala, S.A.; Vakiti, A.; White, J.J.; Mulloy, L.; Mohammed, A. First Reported Use of Highly Adsorptive Hemofilter in Critically Ill COVID-Patients in the USA. J. Clin. Med. Res. 2020, 12, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Monard, C.; Rimmelé, T.; Ronco, C. Extracorporeal Blood Purification Therapies for Sepsis. Blood Purif 2019, 47 (Suppl. 3), 1–14. [Google Scholar] [CrossRef] [PubMed]

| GENERAL VARIABLES | CUT-OFF |
|---|---|
| Fever | >38.3 °C |
| Hypotermia | <36 °C |
| Heart Rate | >90 min or more than two S.D. above the normal value for age |
| Tachypnea | >20 rr/min |
| Altered Mental Status | impairment |
| Significant Edema or positive fluid balance | >20 ml/kg over 24 h |
| Hyperglycemia | pGluc > 140 mg/dl in the absence of diabetes |
| INFLAMMATORY VARIABLES | CUT-OFF |
| Leukocytosis | WBC count > 12,000/µL |
| Leukopenia | WBC count < 12,000/µL |
| DISEASE | DEFINITION (SEPSIS-3) |
| Sepsis | Suspected/confirmed infection+≥2 criteria of SOFA |
| Septic Shock | Sepsis+ fluid refractory Hypotension_
|
| SOFA | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| RESPIRATION (P/F) ** | ≥400 | <400 | <300 | <200 # | <100 # |
| COAGULATION (plts) | ≥150 | <150 | <100 | <50 | 20 |
| LIVER, BILIRUBIN (mg/dL) | 1.2 | 1.2–1.9 | 2.0–5.9 | 6–11.9 | >12 |
| CARDIOVASULAR | Map+ ≥ 70 | Map+ < 70 | any dose dpx or dbx | dpx > 5 or epi ≤ 0.1 or norepi ≤ 0.1 | dpx > 15 or epi > 0.1 or norepi ≤ 0.1 |
| GLASGOW COMA SCORE | 15 | 13–14 | 10–12 | 6–9 | <6 |
| CREATININA (mg/dL) | <1.4 | 1.4–1.9 | 2.0–3.4 | 3.5–4.9 | >5.0 |
| Extracorporeal Blood Purification | |||
|---|---|---|---|
| Convection Therapies | Adsorption Therapies | Combination Therapies | Other Therapies |
| Continuous Renal Replacement (CRRT) | Immobilized Polimixin B (PMX) | Coupled Plasma Filtration Adsorption (CPFA) | Plasma exchange |
| High-Volume Hemofiltration (HVHF) | Hemadsorption (e.g., CytoSorb) | Combined Filtration and Adsorption (e.g., oXiris) | Renal Assist Device (RAD) |
| High Cut-Off Membranes (HCO) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanza, C.; Caputo, G.; Tornatore, G.; Romenskaya, T.; Piccioni, A.; Franceschi, F.; Artico, M.; Taurone, S.; Savioli, G.; Longhitano, Y. Cellular Immuno-Profile in Septic Human Host: A Scoping Review. Biology 2022, 11, 1626. https://doi.org/10.3390/biology11111626
Zanza C, Caputo G, Tornatore G, Romenskaya T, Piccioni A, Franceschi F, Artico M, Taurone S, Savioli G, Longhitano Y. Cellular Immuno-Profile in Septic Human Host: A Scoping Review. Biology. 2022; 11(11):1626. https://doi.org/10.3390/biology11111626
Chicago/Turabian StyleZanza, Christian, Giorgia Caputo, Gilda Tornatore, Tatsiana Romenskaya, Andrea Piccioni, Francesco Franceschi, Marco Artico, Samanta Taurone, Gabriele Savioli, and Yaroslava Longhitano. 2022. "Cellular Immuno-Profile in Septic Human Host: A Scoping Review" Biology 11, no. 11: 1626. https://doi.org/10.3390/biology11111626
APA StyleZanza, C., Caputo, G., Tornatore, G., Romenskaya, T., Piccioni, A., Franceschi, F., Artico, M., Taurone, S., Savioli, G., & Longhitano, Y. (2022). Cellular Immuno-Profile in Septic Human Host: A Scoping Review. Biology, 11(11), 1626. https://doi.org/10.3390/biology11111626








