Life History and Fishing Aspects of the Deep-Sea Silver Scabbardfish Lepidopus caudatus in the Azores
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.1.1. Scientific Surveys
2.1.2. Commercial Catches
2.1.3. Official Commercial Landings
2.2. Data Analyses
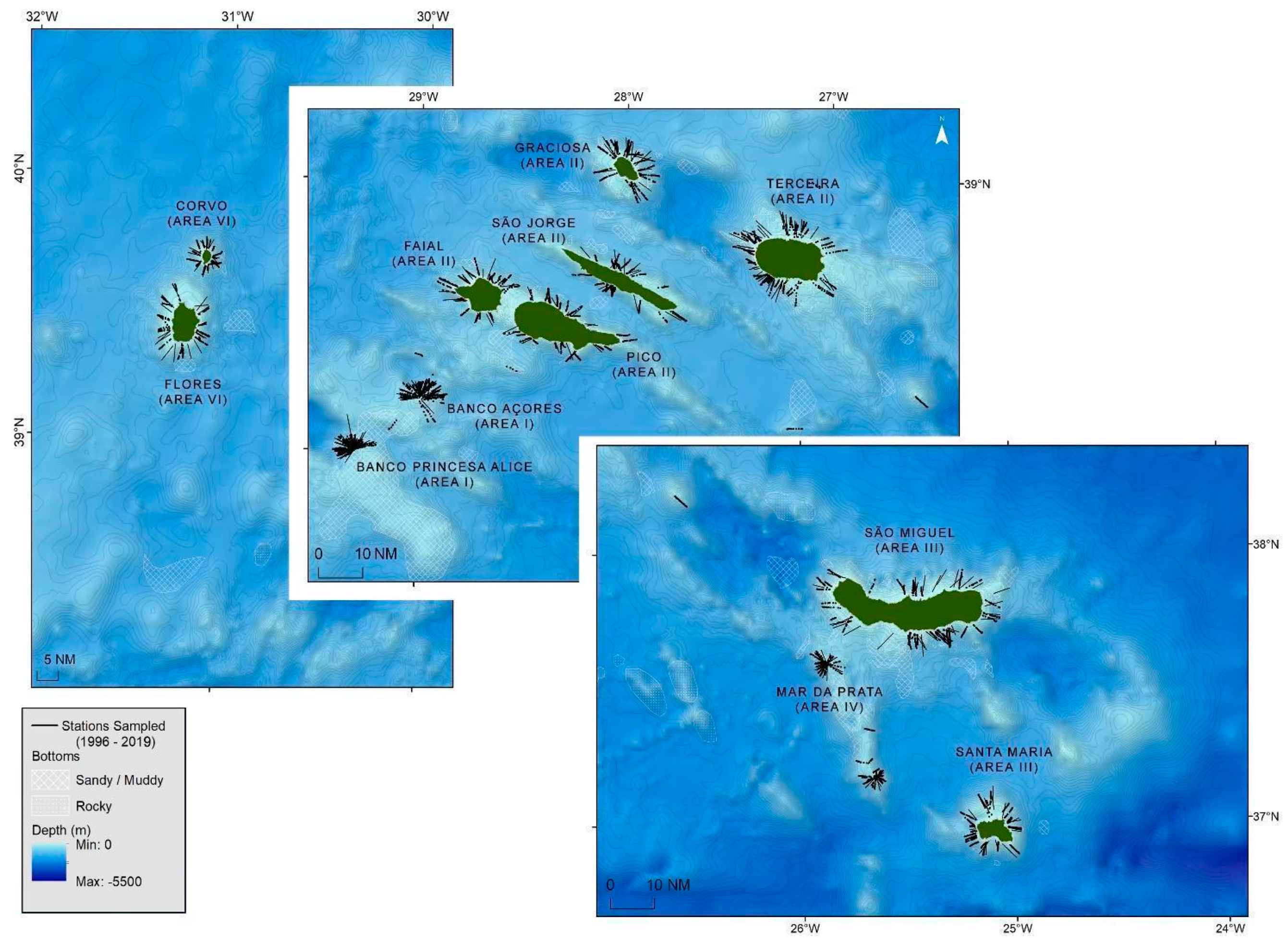
2.2.1. Spatial Distribution
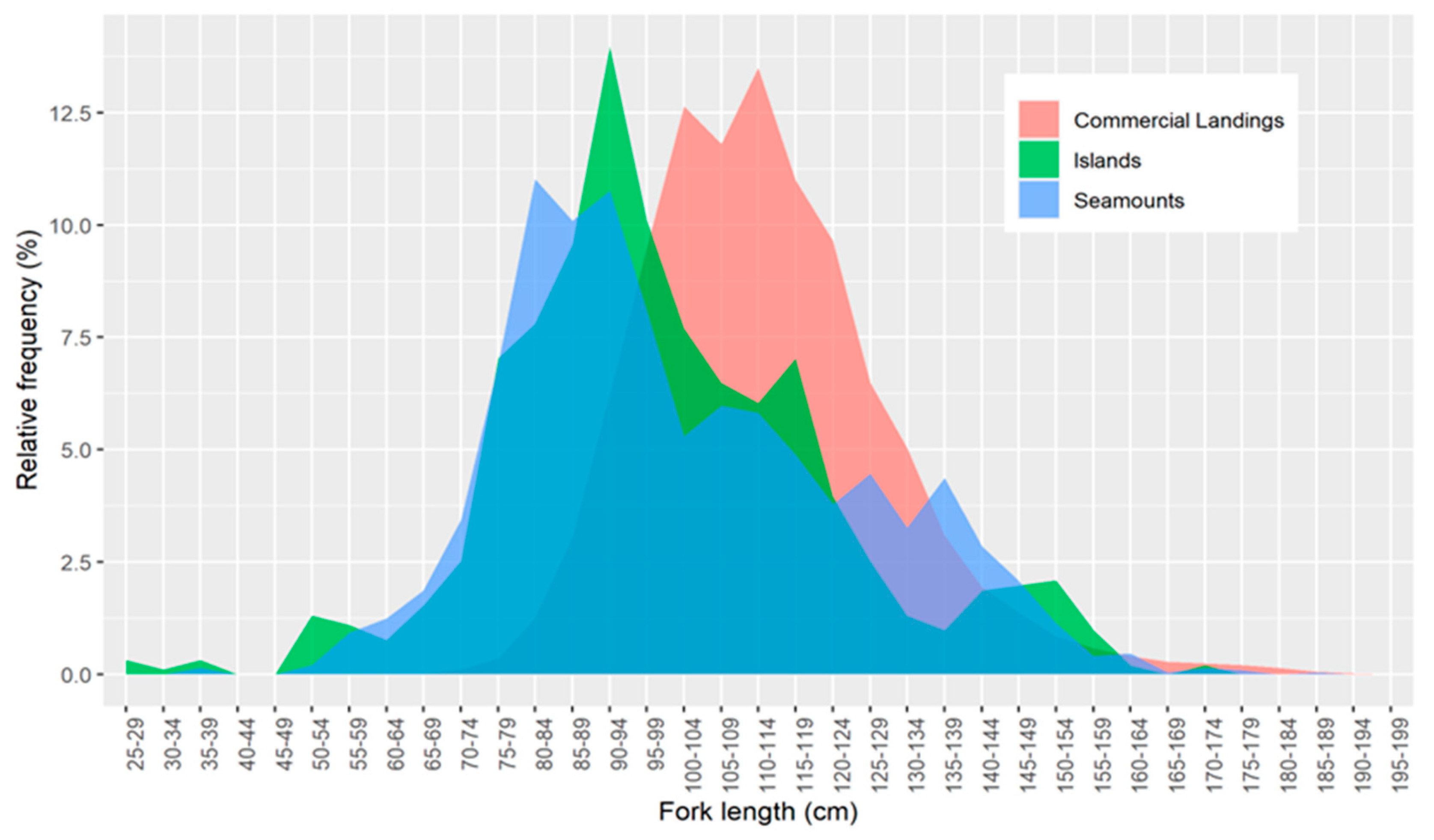
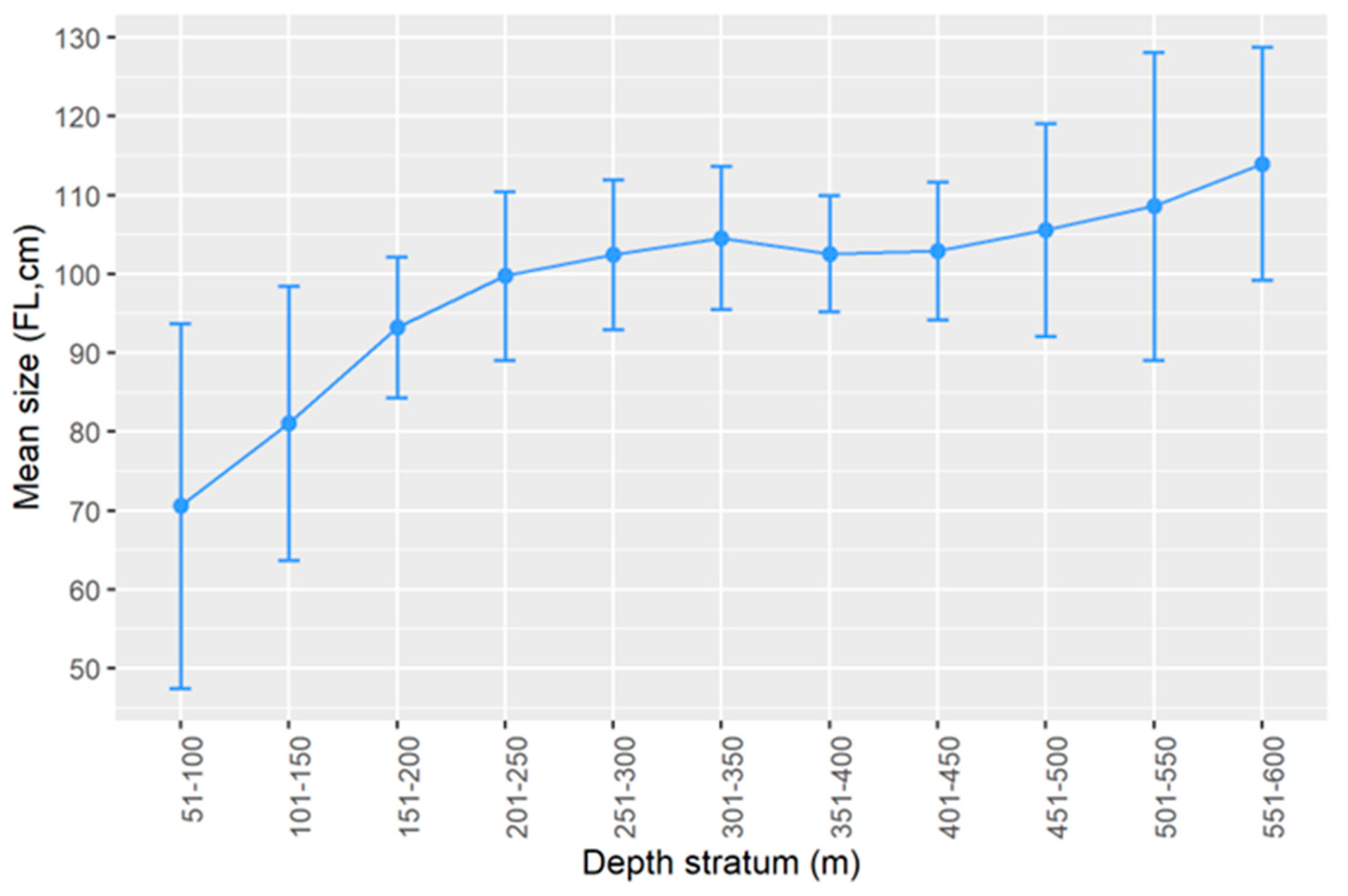
2.2.2. Size Structure
2.2.3. Sex Ratio
2.2.4. Growth Parameters
2.2.5. Mortality Parameters
2.2.6. Trends in Abundance
3. Results
3.1. Spatial Distribution
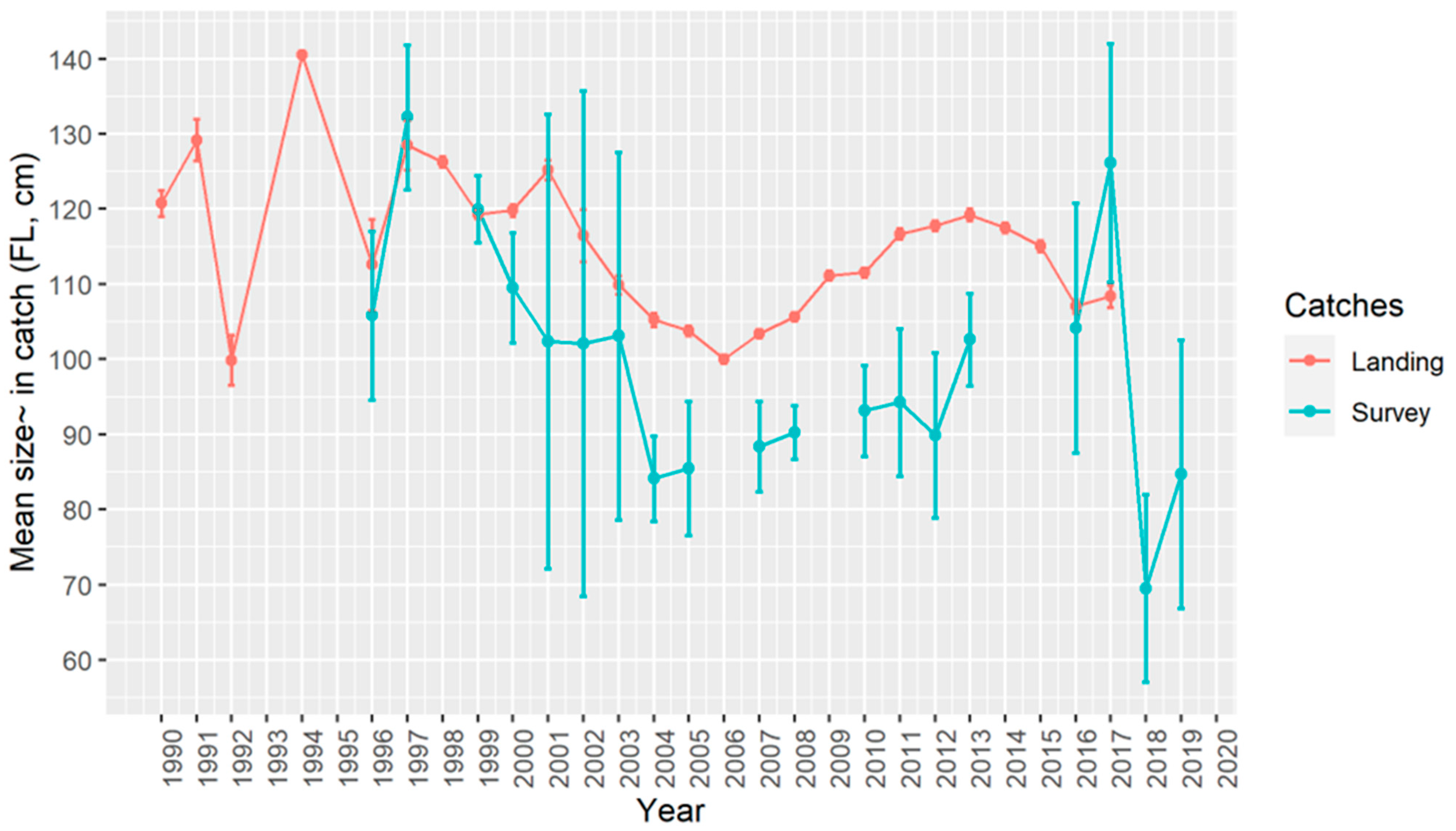
3.2. Size Structure
3.3. Sex Ratio
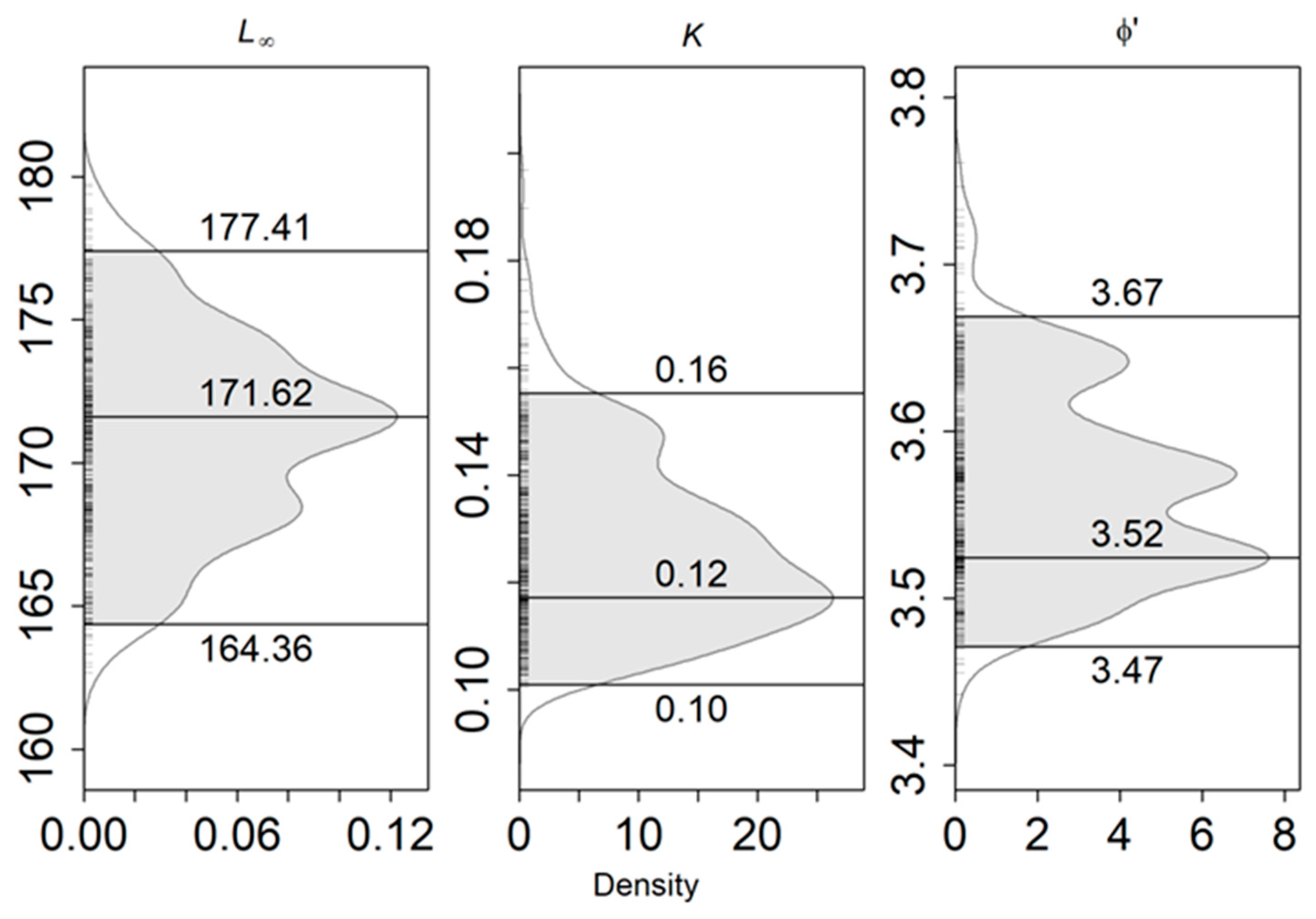
3.4. Growth Parameters
3.5. Mortality Rates
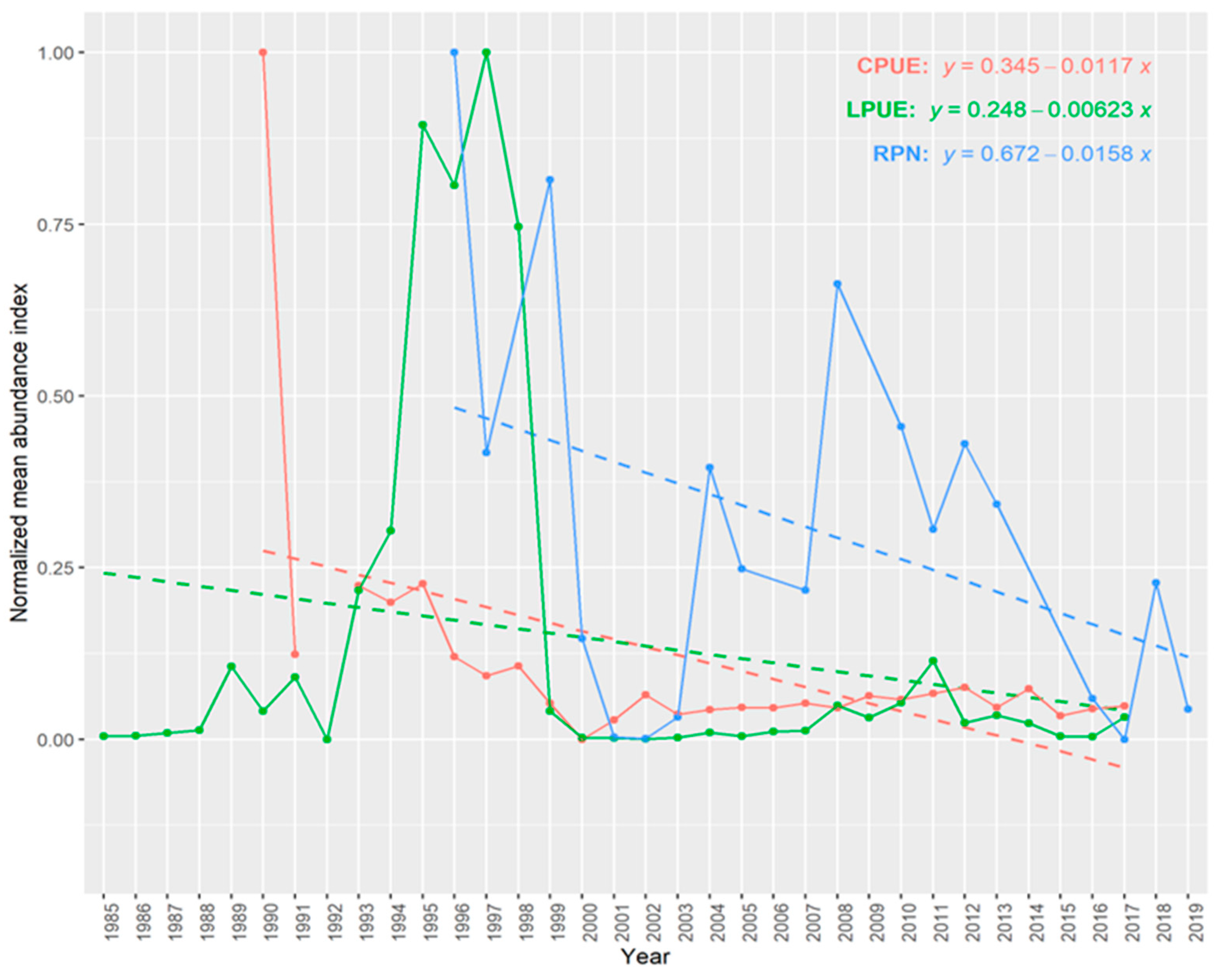
3.6. Trends in Abundance and Catch
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clark, M.; Althaus, F.; Schlacher, T.A.; Williams, A.; Bowden, D.A.; Rowden, A.A. The Impacts of Deep-Sea Fisheries on Benthic Communities: A Review. ICES J. Mar. Sci. 2016, 73, i51–i69. [Google Scholar] [CrossRef]
- Molenaar, E.J. Global, Regional and Unilateral Approaches to Unregulated Deep-Sea Fisheries. In Deep Sea 2003: Conference on the Governance and Management of Deep-Sea Fisheries; Shotton, R., Ed.; Queenstown, New Zealand, 1–5 December 2003, FAO Fisheries Proceedings Nº 3/1; FAO: Rome, Italy, 2005; pp. 415–432. ISBN 92-5-105402-9. [Google Scholar]
- FAO. El Estado Mundial de La Pesca y La Acuicultura 2020 La Sostenibilidad En Acción; FAO: Rome, Italy, 2020; ISBN 978-92-5-132756-2. [Google Scholar]
- Priede, I.G. Deep-Sea Fishes Biology, Diversity, Ecology and Fisheries, 1st ed.; Cambridge University Press: Cambridge, UK, 2017; ISBN 9781107083820. [Google Scholar]
- Morato, T.; Watson, R.; Pitcher, T.; Pauly, D. Fishing down the Deep. Fish Fish. 2006, 7, 24–34. [Google Scholar] [CrossRef]
- Norse, E.A.; Brooke, S.; Cheung, W.W.L.; Clark, M.R.; Ekeland, I.; Froese, R.; Gjerde, K.M.; Haedrich, R.L.; Heppell, S.S.; Morato, T.; et al. Sustainability of Deep-Sea Fisheries. Mar. Policy 2012, 36, 307–320. [Google Scholar] [CrossRef] [Green Version]
- Clark, M. Are Deepwater Fisheries Sustainable?—The Example of Orange Roughy (Hoplostethus atlanticus) in New Zealand. Fish. Res. 2001, 51, 123–135. [Google Scholar] [CrossRef]
- Santos, R.; Medeiros-Leal, W.; Pinho, M. Synopsis of Biological, Ecological and Fisheries-Related Information on Priority Marine Species in the Azores Region. Arquipel. Life Mar. Sci. 2020, 12, 1–138. [Google Scholar]
- Nakamura, I.; Parin, N. Snake Mackerels and Cutlassfishes of the World (Families Gempylidae and Trichiuridae). FAO Species Cat. 1993, 15, 1–136. [Google Scholar]
- Mytilineou, C.; Politou, C.Y.; Papaconstantinou, C.; Kavadas, S.; D’Onghia, G.; Sion, L. Deep-Water Fish Fauna in the Eastern Ionian Sea. Belg. J. Zool. 2005, 135, 229–233. [Google Scholar]
- Demestre, M.; Moli, B.; Recasens, L.; Sánchez, P. Life History and Fishery of Lepidopus caudatus (Pisces: Trichiuridae) in the Catalan Sea (Northwestern Mediterranean). Mar. Biol. 1993, 115, 23–32. [Google Scholar] [CrossRef]
- Falsone, F.; Scannella, D.; Geraci, M.L.; Gancitano, V.; Vitale, S.; Fiorentino, F. How Fishery Collapses: The Case of Lepidopus caudatus (Pisces: Trichiuridae) in the Strait of Sicily (Central Mediterranean). Front. Mar. Sci. 2021, 7, 5–8. [Google Scholar] [CrossRef]
- FAO. Fishery and Aquaculture Statistics. Global Capture Production 1950–2019 (FishstatJ). Available online: www.fao.org/fishery/statistics/software/fishstatj/en (accessed on 15 June 2022).
- Portuguese National Institute of Statistics. Annual Nominal Catches by Landed Port and Species. Available online: http://www.ine.pt (accessed on 20 June 2022).
- Santos, R.; Medeiros-Leal, W.; Pinho, M. Stock Assessment Prioritization in the Azores: Procedures, Current Challenges and Recommendations. Arquipelago. Life Mar. Sci. 2020, 37, 45–64. [Google Scholar]
- Figueiredo, C.; Diogo, H.; Pereira, J.G.; Higgins, R.M. Using Information-Based Methods to Model Age and Growth of the Silver Scabbardfish, Lepidopus caudatus, from the Mid-Atlantic Ocean. Mar. Biol. Res. 2015, 11, 37–41. [Google Scholar] [CrossRef]
- ICES. Azores Ecoregion––Fisheries Overviews. In Report of the ICES Advisory Committee; ICES: Copenhagen, Denmark, 2020; ICES Advice 2020, Section 3.2. [Google Scholar]
- Pinho, M.; Medeiros-Leal, W.; Sigler, M.; Santos, R.; Novoa-Pabon, A.; Menezes, G.; Silva, H. Azorean Demersal Longline Survey Abundance Estimates: Procedures and Variability. Reg. Stud. Mar. Sci. 2020, 39, 101443. [Google Scholar] [CrossRef]
- EC. Council Regulation (EC) No. 199/2008 of 25 February 2008 Concerning the Establishment of a Community Framework for the Collection, Management and Use of Data in the Fisheries Sector and Support for Scientific Advice Regarding the Common Fisheries Policy. Off. J. Eur. Union L 2008, 60, 1–12. [Google Scholar]
- Grüss, A.; Yemane, D.; Fairweather, T.P. Exploring the Spatial Distribution Patterns of South African Cape Hakes Using Generalised Additive Models. Afr. J. Mar. Sci. 2016, 38, 395–409. [Google Scholar] [CrossRef]
- Guisan, A.; Edwards, T.C.; Hastie, T. Generalized Li.inear and Generalized Additive Models in Studies of Species Distributions: Setting the Scene. Ecol. Model. 2002, 157, 89–100. [Google Scholar] [CrossRef]
- Maravelias, C.D.; Tserpes, G.; Pantazi, M.; Peristeraki, P. Habitat Selection and Temporal Abundance Fluctuations of Demersal Cartilaginous Species in the Aegean Sea (Eastern Mediterranean). PLoS ONE 2012, 7, e35474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, S.N. Generalized Additive Models: An Introduction with R; Chapman and Hall/CRC: New York, NY, USA, 2006; ISBN 9780429093159. [Google Scholar]
- Barry, S.C.; Welsh, A.H. Generalized Additive Modelling and Zero Inflated Count Data. Ecol. Model. 2002, 157, 179–188. [Google Scholar] [CrossRef]
- Ridout, M.; Demetrio, C.G.B.; Hinde, J. Models for Count Data with Many Zeros. Int. Biom. Conf. 1998, 19, 179–192. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Games, P.A.; Howell, J.F. Pairwise Multiple Comparison Procedures with Unequal N’s and/or Variances: A Monte Carlo Study. J. Educ. Stat. 1976, 1, 113–125. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows, version 28.0; IBM Corp: Armonk, NY, USA, 2021. [Google Scholar]
- von Bertalanffy, L. A Quantitative Theory of Organic Growth (Inquiries on Growth Laws II). Hum. Biol. 1938, 10, 183–213. [Google Scholar]
- Sparre, P.; Venema, S. Introduction to Tropical Fish Stock Assessment. Part 1: Manual; FAO: Rome, Italy, 1998; ISBN 92-5-103996-8. [Google Scholar]
- Munro, J.L.; Pauly, D. A Simple Method for Comparing the Growth of Fishes and Invertebrates. Fishbyte 1983, 1, 5–6. [Google Scholar]
- Schwamborn, R.; Mildenberger, T.K.; Taylor, M.H. Assessing Sources of Uncertainty in Length-Based Estimates of Body Growth in Populations of Fishes and Macroinvertebrates with Bootstrapped ELEFAN. Ecol. Model. 2019, 393, 37–51. [Google Scholar] [CrossRef] [Green Version]
- Mildenberger, T.K.; Taylor, M.H.; Wolff, M. TropFishR an R Package for Fisheries Analysis with Length-frequency Data. Methods Ecol. Ogy Evol. 2017, 8, 1520–1527. [Google Scholar] [CrossRef] [Green Version]
- Taylor, M.H.; Mildenberger, T.K. Extending Electronic Length Frequency Analysis in R. Fish. Manag. Ecol. 2017, 24, 330–338. [Google Scholar] [CrossRef]
- Pauly, D.; Munro, J.L. One More on the Comparison of Growth in Fish and and Invertebrates. Fishbyte 1984, 2, 21. [Google Scholar]
- Santos, R.; Peixoto, U.; Medeiros-Leal, W.; Novoa-Pabon, A.; Pinho, M. Growth Parameters and Mortality Rates Estimated for Seven Data-Deficient Fishes from the Azores Based on Length-Frequency Data. Life 2022, 12, 778. [Google Scholar] [CrossRef] [PubMed]
- Pauly, D. Fish Population Dynamics in Tropical Waters: A Manual for Use with Programmable Calculators; WorldFish: George Town, Malaysia, 1984; Volume 8, ISBN 9711022044. [Google Scholar]
- Gulland, J.A. The Fish Resources of the Ocean; Fishing News (Books), Ltd.: West Byfleet, UK, 1971; Volume 255. [Google Scholar]
- Santos, R.; Crespo, O.; Medeiros-Leal, W.; Novoa-Pabon, A.; Pinho, M. Error Distribution Model to Standardize LPUE, CPUE and Survey-Derived Catch Rates of Target and Non-Target Species. Modelling 2021, 3, 1–13. [Google Scholar] [CrossRef]
- Lenth, R.V. Least-Squares Means: The R Package Lsmeans. J. Stat. Softw. 2016, 69, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Andrade, J.M.; Estévez-Pérez, M.G. Statistical Comparison of the Slopes of Two Regression Lines: A Tutorial. Anal. Chim. Acta 2014, 838, 1–12. [Google Scholar] [CrossRef]
- Goldberg, D.E.; Scheiner, S.M. ANOVA and ANCOVA: Field Competition Experiments. In Design and Analysis of Experiments; Scheiner, S.M., Gurevitch, J., Eds.; Oxford University Press: Oxford, UK, 2001; pp. 77–98. ISBN 9781119130536. [Google Scholar]
- McDonald, J.H. Analysis of Covariance. In Handbook of Biological Statistics; Sparky House Publishing: Baltimore, MD, USA, 2014; pp. 220–228. [Google Scholar]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; SAGE Publications, Inc: Thousand Oaks, CA, USA, 2018. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [Green Version]
- Froese, R.; Binohlan, C. Empirical Relationships to Estimate Asymptotic Length, Length at First Maturity and Length at Maximum Yield per Recruit in Fishes, with a Simple Method to Evaluate Length Frequency Data. J. Fish. Biol. 2000, 56, 758–773. [Google Scholar] [CrossRef]
- Santos, R.; Medeiros-Leal, W.; Crespo, O.; Novoa-Pabon, A.; Pinho, M. Contributions to Management Strategies in the NE Atlantic Regarding the Life History and Population Structure of a Key Deep-Sea Fish (Mora moro). Biology 2021, 10, 522. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.; Medeiros-Leal, W.; Novoa-Pabon, A.; Crespo, O.; Pinho, M. Biological Knowledge of Thornback Ray (Raja clavata) from the Azores: Improving Scientific Information for the Effectiveness of Species-Specific Management Measures. Biology 2021, 10, 676. [Google Scholar] [CrossRef]
- Damalas, D.; Maravelias, C.D.; Katsanevakis, S.; Karageorgis, A.P.; Papaconstantinou, C. Seasonal Abundance of Non-Commercial Demersal Fish in the Eastern Mediterranean Sea in Relation to Hydrographic and Sediment Characteristics. Estuar. Coast. Shelf Sci. 2010, 89, 107–118. [Google Scholar] [CrossRef]
- Macpherson, E. Species Range Size Distributions for Some Marine Taxa in the Atlantic Ocean. Effect of Latitude and Depth. Biol. J. Linn. Soc. 2003, 80, 437–455. [Google Scholar] [CrossRef] [Green Version]
- Parra, H.E.; Pham, C.K.; Menezes, G.M.; Rosa, A.; Tempera, F.; Morato, T. Predictive Modeling of Deep-Sea Fish Distribution in the Azores. Deep Sea Res. 2 Top Stud. Oceanogr. 2017, 145, 49–60. [Google Scholar] [CrossRef]
- Macpherson, E.; Duarte, C.M. Bathymetric Trends in Demersal Fish Size: Is There a General Relationship? Mar. Ecol. Prog. Ser. 1991, 71, 103–112. [Google Scholar] [CrossRef]
- D’Onghia, G.; Mastrototaro, F.; Maiorano, P. Biology of Silver Scabbard Fish, Lepidopus caudatus (Trichiuridae), from the Ionian Sea (Eastern-Central Mediterranean). Cybium 2000, 24, 249–262. [Google Scholar]
- Tuset, V.M.; González, J.A.; Santana, J.I.; Moreno-López, A.; García-Díaz, M.M. Reproductive Pattern and Growth in Lepidopus caudatus (Osteichthyes, Trichiuridae) From the Canary Islands (Easterncentral Atlantic). Electron. J. Ichthyol. 2006, 1, 26–37. [Google Scholar]
- Pauly, D.; Christensen, V.; Dalsgaard, J.; Froese, R.; Torres, F., Jr. Fishing down Marine Food Webs. Science 1998, 279, 860–863. [Google Scholar] [CrossRef]
- Pauly, D. Focusing One’s Microscope. Sci. Chron. 2011, 2011, 4–7. [Google Scholar]
- Stergiou, K.I.; Tsikliras, A.C. Fishing down, Fishing through and Fishing up: Fundamental Process versus Technical Details. Mar. Ecol. Prog. Ser. 2011, 441, 295–301. [Google Scholar] [CrossRef]
- Shin, Y.J.; Rochet, M.J.; Jennings, S.; Field, J.G.; Gislason, H. Using Size-Based Indicators to Evaluate the Ecosystem Effects of Fishing. ICES J. Mar. Sci. 2005, 62, 384–396. [Google Scholar] [CrossRef] [Green Version]
- Jennings, S.; Greenstreet, S.P.R.; Reynolds, J.D. Structural Change in an Exploited Fish Community: A Consequence of Differential Fishing Effects on Species with Contrasting Life Histories. J. Anim. Ecol. 1999, 68, 617–627. [Google Scholar] [CrossRef]
- Estácio, S.; Mendonça, A.; Krug, H.; Menezes, G.M.; Pinho, M.R. Aspects of the Reproduction of Six Exploited Demersal Fish Species in the Azores Archipelago. Arquipélago. Life Mar. Sci. 2001, 2, 83–94. [Google Scholar]
- Danovaro, R.; Corinaldesi, C.; Dell’Anno, A.; Snelgrove, P.V.R. The Deep-Sea under Global Change. Curr. Biol. 2017, 27, R461–R465. [Google Scholar] [CrossRef] [Green Version]
- Wirtz, P.; Morato, T. Unequal Sex Ratios in Longline Catches. J. Mar. Biol. Assoc. UK 2001, 81, 187–188. [Google Scholar] [CrossRef]
- Horn, P.L. Age Determination of Frostfish (Lepidopus caudatus) off West Coast South Island. N. Z. Fish. Assess. Rep. 2013, 21, 1–24. [Google Scholar]
- Wang, K.; Zhang, C.; Sun, M.; Xu, B.; Ji, Y.; Xue, Y.; Ren, Y. Fishing Pressure and Lifespan Affect the Estimation of Growth Parameters Using ELEFAN. Fish. Res. 2021, 238, 105903. [Google Scholar] [CrossRef]
- Townsend, C.; Begon, M.; Harper, J.L. From Populations to Communities. In Essentials of Ecology; Blackwell Publishing: Oxford, UK, 2008; pp. 281–307. ISBN 978-1-4051-5658-5. [Google Scholar]
- Santos, R.; Medeiros-Leal, W.; Novoa-Pabon, A.; Silva, H.; Pinho, M. Long-Term Changes in the Diversity, Abundance and Size Composition of Deep Sea Demersal Teleosts from the Azores Assessed through Surveys and Commercial Landings. Aquat. Living Resour. 2019, 32, 25. [Google Scholar] [CrossRef] [Green Version]
- Diogo, H.; Pereira, J.G.; Higgins, R.M.; Canha, Â.; Reis, D. History, Effort Distribution and Landings in an Artisanal Bottom Longline Fishery: An Empirical Study from the North Atlantic Ocean. Mar. Policy 2015, 51, 75–85. [Google Scholar] [CrossRef]
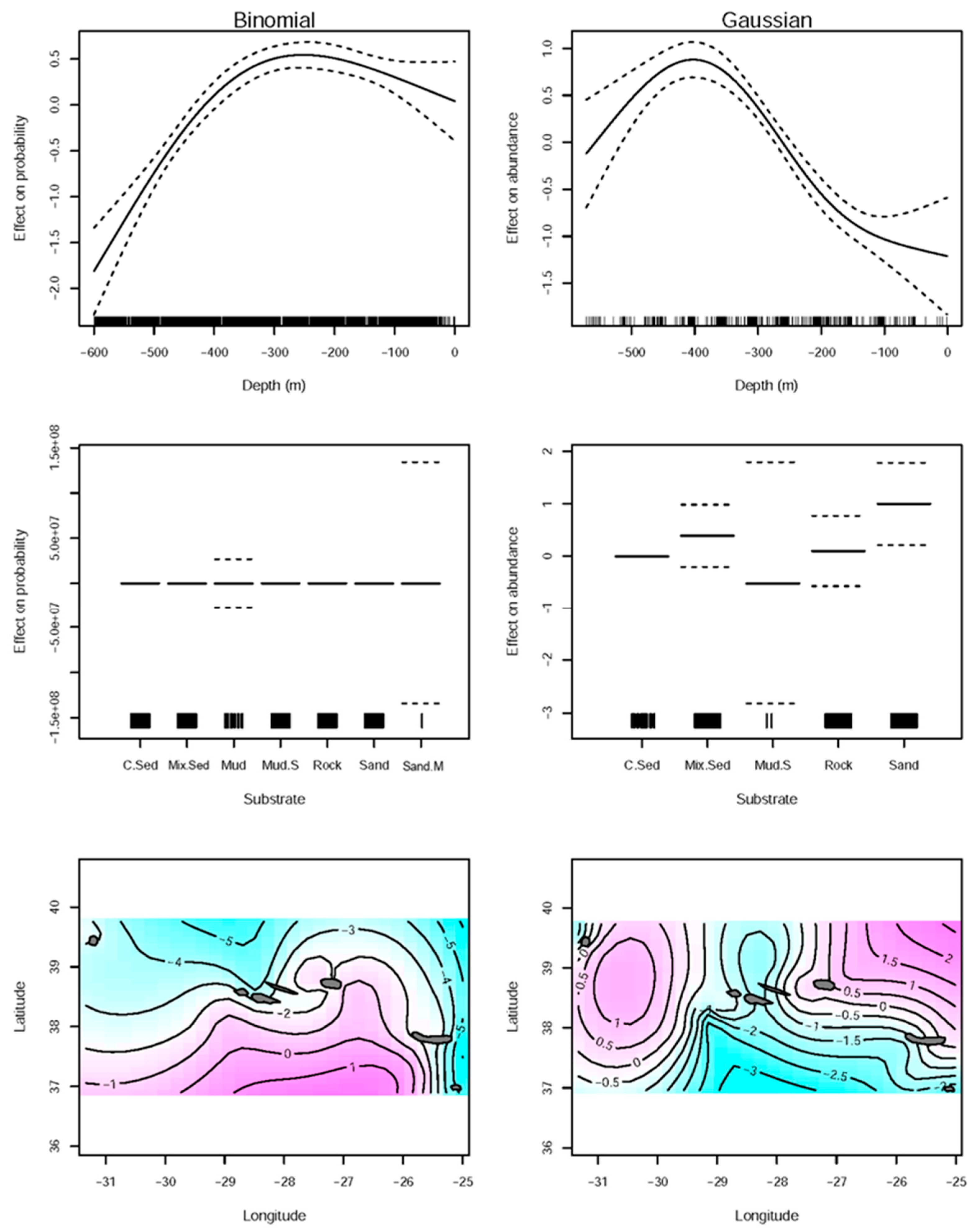


| Family | Link Function | Formula | Adjusted R2 | Deviance Explained | |||||
|---|---|---|---|---|---|---|---|---|---|
| Binomial | logit | RPN.Bi ~ s (longitude, latitude) + s(depth, k = 4) + substrate | 0.0963 | 14.9% | |||||
| Gaussian | identity | RPN ~ s (longitude, latitude) + s(depth, k = 4) + substrate | 0.347 | 38% | |||||
| Binomial | Gaussian | ||||||||
| Parametric coefficients | |||||||||
| Estimate | Std. Error | z Value | Pr (>|z|) | Estimate | Std. Error | z Value | Pr (>|z|) | ||
| (Intercept) | −2.909 × 100 | 2.414 × 10−1 | −12.049 | <2 × 1016 *** | (Intercept) | −1.6603 | 0.2943 | −5.642 | 2.8 × 10−8 *** |
| SubstrateMix.Sed | 2.087 × 10−2 | 2.425 × 10−1 | 0.086 | 0.931 | SubstrateMix.Sed | 0.3895 | 0.2963 | 1.315 | 0.1892 |
| SubstrateMud | −3.901 × 101 | 1.342 × 107 | 0.000 | 1.000 | Substrate Mud.S | −0.5146 | 1.1527 | −0.446 | 0.6555 |
| Substrate Mud.S | 3.360 × 10−1 | 8.086 × 10−1 | 0.415 | 0.678 | Substrate Rock | 0.1021 | 0.3341 | 0.306 | 0.7601 |
| Substrate Rock | 9.064 × 10−2 | 2.636 × 10−1 | 0.344 | 0.731 | Substrate Sand | 0.9959 | 0.3911 | 2.546 | 0.0112 * |
| Substrate Sand | −1.124 × 10−1 | 2.983 × 10−1 | −0.377 | 0.706 | |||||
| Substrate Sand.M | −3.394 × 101 | 6.711 × 107 | 0.000 | 1.000 | |||||
| Approximate significant smooth terms | |||||||||
| edf | Ref. df | F | p-Value | edf | Ref. df | F | p-Value | ||
| s(longitude, latitude) | 22.660 | 25.956 | 269.82 | <2 × 10−16 *** | s(longitude, latitude) | 20.630 | 24.713 | 6.412 | <2 × 10−16 *** |
| s(depth) | 2.688 | 2.929 | 90.51 | <2 × 10−16 *** | s(depth) | 2.926 | 2.995 | 37.265 | <2 × 10−16 *** |
| Reference | Equation | Result |
|---|---|---|
| Beverton and Holt (1959) | 0.22 | |
| Taylor (1960) | 0.13 | |
| Tanaka (1960) | 0.13 | |
| Alverson and Carney (1975) | 0.34 | |
| Pauly (1980) | 0.53 | |
| Hoening (1983) | 0.13 | |
| Alagajara (1984) | 0.20 | |
| Djabali (1993) | 0.21 | |
| Pauly and Binohlan (1996) | 0.23 | |
| Jensen (1996) | 0.21 | |
| Jensen (1996) | 0.20 | |
| Cubillos et al. (1999) | 0.18 | |
| Frisk (2001) | 0.18 | |
| Hewitt and Hoening (2005) | 0.18 | |
| Mean | 0.22 |
| Mean Abundance ~ Year + Abundance Index + Year * Abundance Index | ||||
| Sum Sq | Df | F Value | Pr(>F) | |
|---|---|---|---|---|
| (Intercept) | 0.437 | 1 | 7.41 | 0.008 ** |
| Year | 0.231 | 1 | 3.91 | 0.052 |
| Abundance index | 0.242 | 2 | 2.06 | 0.135 |
| Year:Abundance index | 0.077 | 2 | 0.66 | 0.521 |
| Residuals | 4.300 | 73 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariño-Briceño, G.; Medeiros-Leal, W.; Peixoto, U.I.; Pinho, M.; Santos, R. Life History and Fishing Aspects of the Deep-Sea Silver Scabbardfish Lepidopus caudatus in the Azores. Biology 2022, 11, 1619. https://doi.org/10.3390/biology11111619
Mariño-Briceño G, Medeiros-Leal W, Peixoto UI, Pinho M, Santos R. Life History and Fishing Aspects of the Deep-Sea Silver Scabbardfish Lepidopus caudatus in the Azores. Biology. 2022; 11(11):1619. https://doi.org/10.3390/biology11111619
Chicago/Turabian StyleMariño-Briceño, Gloria, Wendell Medeiros-Leal, Ualerson Iran Peixoto, Mário Pinho, and Régis Santos. 2022. "Life History and Fishing Aspects of the Deep-Sea Silver Scabbardfish Lepidopus caudatus in the Azores" Biology 11, no. 11: 1619. https://doi.org/10.3390/biology11111619
APA StyleMariño-Briceño, G., Medeiros-Leal, W., Peixoto, U. I., Pinho, M., & Santos, R. (2022). Life History and Fishing Aspects of the Deep-Sea Silver Scabbardfish Lepidopus caudatus in the Azores. Biology, 11(11), 1619. https://doi.org/10.3390/biology11111619







