Quantifications of Mandibular Trabecular Bone Microstructure Using Cone Beam Computed Tomography for Age Estimation: A Preliminary Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
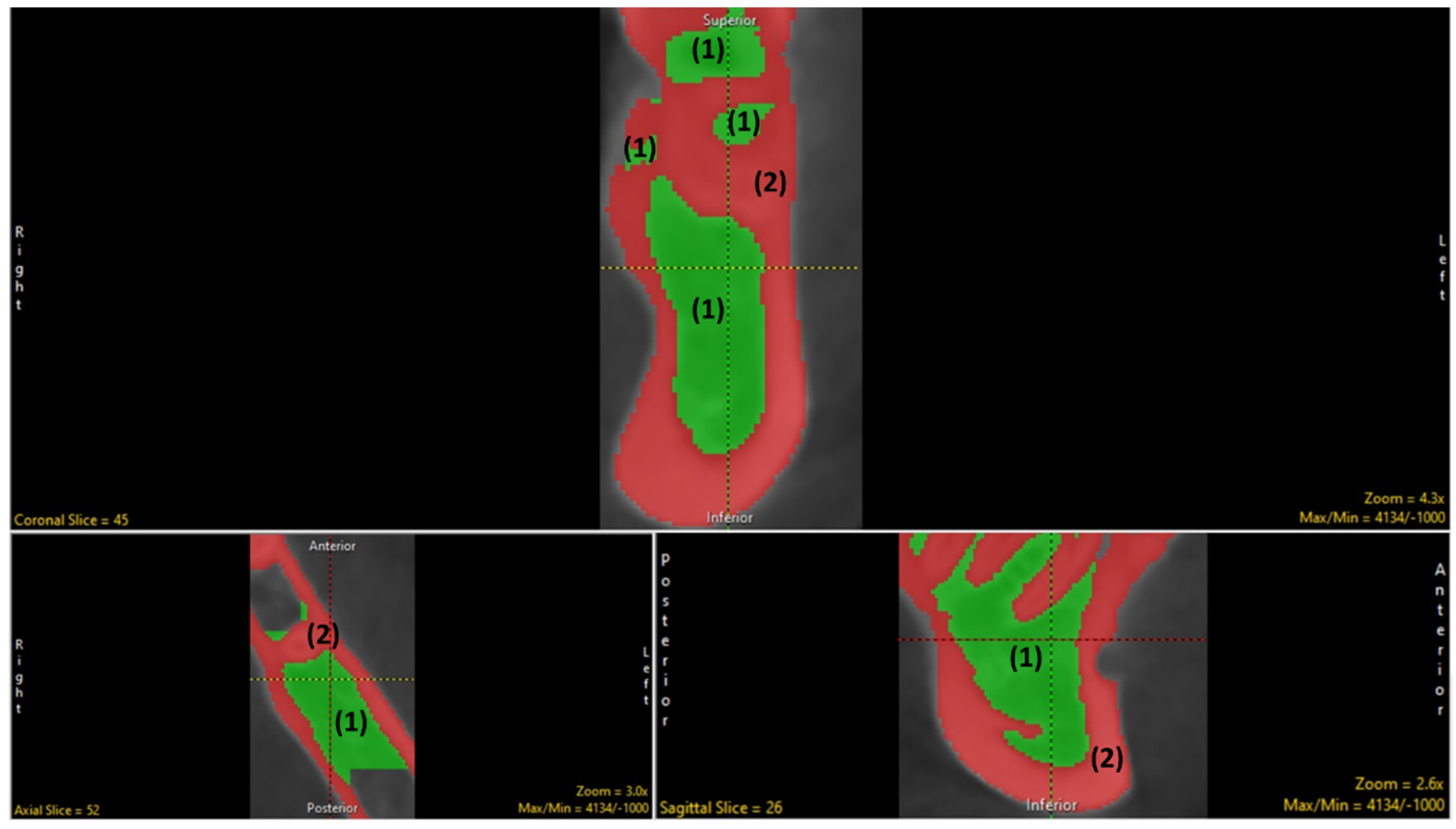
2.1. Selection of Region of Interest (ROI)
2.2. Data Preprocessing
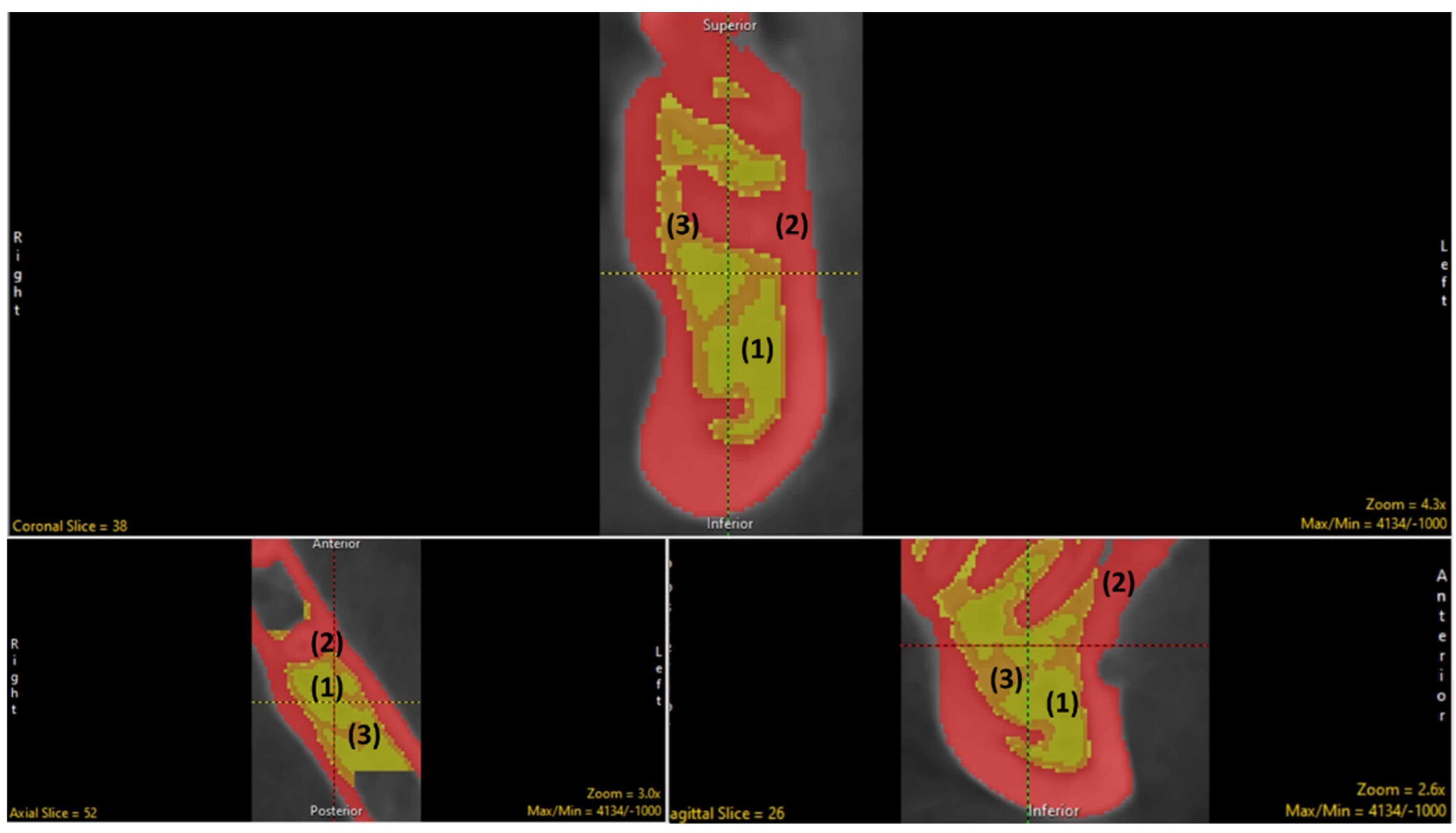
2.3. Segmentation (Manual and Semi-Automatic)
2.4. Measurement and Tabulations via BMA
2.5. Intraobserver Reliability
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, W.; Zhong, L.; Yao, L.; Wei, Y.; Gui, T.; Li, Z.; Kim, H.; Holdreith, N.; Jiang, X.; Tong, W.; et al. Bone Marrow Adipogenic Lineage Precursors Promote Osteoclastogenesis in Bone Remodeling and Pathologic Bone Loss. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef]
- Yip, J.K.; Borrell, L.N.; Cho, S.-C.; Francisco, H.; Tarnow, D.P. Association between Oral Bisphosphonate Use and Dental Implant Failure among Middle-Aged Women. J. Clin. Periodontol. 2012, 39, 408–414. [Google Scholar] [CrossRef]
- Wang, F.; Zheng, L.; Theopold, J. Methods for Bone Quality Assessment in Human Bone Tissue: A Systematic Review. J. Orthop. Surg. Res. 2022, 17, 174. [Google Scholar] [CrossRef]
- Wade, A.; Nelson, A.; Garvin, G.; Holdsworth, D.W. Preliminary Radiological Assessment of Age-Related Change in the Trabecular Structure of the Human Os Pubis. J. Forensic Sci. 2011, 56, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.F.; Othman, A.; Mustafa, N.S.; Kashmoola, M.A.; Mustafa, B.E.; Mohd Yusof, M.Y.P. Accuracy of Different Dental Age Assessment Methods to Determine Chronological Age among Malay Children. J. Phys. Conf. Ser. 2018, 1028, 012102. [Google Scholar] [CrossRef]
- Vom Scheidt, A.; Hemmatian, H.; Püschel, K.; Krause, M.; Amling, M.; Busse, B. Bisphosphonate Treatment Changes Regional Distribution of Trabecular Microstructure in Human Lumbar Vertebrae. Bone 2019, 127, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, J.S.; Niklassen, A.S.; Ebbesen, E.N.; Brüel, A. Age-related changes of vertical and horizontal lumbar vertebral trabecular 3D bone microstructure is different in women and men. Bone 2013, 57, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, J.S.; Jensen, M.V.; Niklassen, A.S.; Ebbesen, E.N.; Brüel, A. Age-Related Changes in Vertebral and Iliac Crest 3D Bone Microstructure—Differences and Similarities. Osteoporos. Int. 2014, 26, 219–228. [Google Scholar] [CrossRef]
- Singh, H.; Aggarwal, A.; Gupta, T.; Kaur, H.; Sahni, D.; Sodhi, L.; Chawla, K. Estimation of Age from Symphyseal Surface of Cadaveric Pubic Bones of Northwest Indian Male Adults. J. Postgrad. Med. Educ. Res. 2016, 50, 176–180. [Google Scholar] [CrossRef]
- Silva, M.J. Characterization of Trabecular Bone Structure. In Skeletal Aging and Osteoporosis: Biomechanics and Mechanobiology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; pp. 39–46. [Google Scholar]
- Shapiro, F.; Wu, J.Y. Woven Bone Overview: Structural Classification Based on Its Integral Role in Developmental, Repair and Pathological Bone Formation throughout Vertebrate Groups. Eur. Cells Mater. 2019, 38, 137–167. [Google Scholar] [CrossRef] [PubMed]
- Sattarath, P.; Wantanajittikul, K.; Prasitwattanaseree, S.; Settakorn, J.; Mekjaidee, K. Age Related Lumbar Trabecular Bone in a Thai Population. Chiang Mai Univ. J. Nat. Sci. 2021, 20, e2021069. [Google Scholar] [CrossRef]
- Pauwels, R.; Jacobs, R.; Singer, S.R.; Mupparapu, M. CBCT-Based Bone Quality Assessment: Are Hounsfield Units Applicable? Dentomaxillofac. Radiol. 2015, 44, 20140238. [Google Scholar] [CrossRef]
- Parsa, A.; Ibrahim, N.; Hassan, B.; Stelt, P.; Wismeijer, D. Bone Quality Evaluation at Dental Implant Site Using Multislice CT, Micro-CT, and Cone Beam CT. Clin. Oral Implant. Res. 2013, 26, e1–e7. [Google Scholar] [CrossRef] [PubMed]
- Parra-Torres, A.Y.; Valdés-Flores, M.; Velázquez-Cruz, L.O. A Molecular Aspects of Bone Remodeling. In Topics in Osteoporosis; IntechOpen: London, UK, 2013. [Google Scholar]
- Newman, M.G.; Takei, H.; Klokkevold, P.R.; Carranza, F.A. Newman and Carranza’s Clinical Periodontology E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Nackaerts, O.; Depypere, M.; Zhang, G.; Vandenberghe, B.; Maes, F.; Jacobs, R. Segmentation of Trabecular Jaw Bone on Cone Beam CT Datasets. Clin. Implant. Dent. Relat. Res. 2014, 17, 1082–1091. [Google Scholar] [CrossRef] [PubMed]
- Von Wowern, N. General and Oral Aspects of Osteoporosis: A Review. Clin. Oral Investig. 2001, 5, 71–82. [Google Scholar] [CrossRef] [PubMed]
- McGivern, H.; Greenwood, C.; Márquez-Grant, N.; Kranioti, E.F.; Xhemali, B.; Zioupos, P. Age-Related Trends in the Trabecular Micro-Architecture of the Medial Clavicle: Is It of Use in Forensic Science? Front. Bioeng. Biotechnol. 2020, 7, 467. [Google Scholar] [CrossRef]
- Mağat, G.; Özcan Şener, S. The Morphological Changes in the Mandible Bone: The Effects of Age, Sex and Dental Status. Meandros Med. Dent. J. 2018, 19, 111–120. [Google Scholar] [CrossRef]
- Ott, S.M. Bone Strength: More than Just Bone Density. Kidney Int. 2016, 89, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Lu, J. Advanced Methods for the Quantification of Trabecular Bone Structure and Density in Micro Computed Tomography Images. Doctoral Dissertation, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany, 2011. [Google Scholar]
- Ling, H.; Yang, X.; Li, P.; Megalooikonomou, V.; Xu, Y.; Yang, J. Cross Sex–Age Trabecular Texture Analysis in Cone Beam CT. Dentomaxillofacial Radiol. 2014, 43, 20130324. [Google Scholar] [CrossRef] [PubMed]
- Leversha, J.; McKeough, G.; Myrteza, A.; Skjellrup-Wakefiled, H.; Welsh, J.; Sholapurkar, A. Age and Sex Correlation of Gonial Angle, Ramus Height and Bigonial Width in Dentate Subjects in a Dental School in Far North Queensland. J. Clin. Exp. Dent. 2016, 8, e49–e54. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; Riggs, B.L.; Atkinson, E.J. Effects of Sex and Age on Bone Microstructure at the Ultradistal Radius: A Population-Based Noninvasive In Vivo Assessment. J. Bone Miner. Res. 2005, 21, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Key, Y.W.; Ng, Z.; Al-Namnam, N.M.; Nambiar, P.; Ngeow, W.C.; Chai, W.L.; Lim, Z.Y. The Location of the Mental Foramen in Relation to the Biometrics of the Lower Dentition and Mandibular Arch: A Cross-Sectional Study. Ital. J. Anat. Embryol. 2022, 125, 103–119. [Google Scholar] [CrossRef]
- Kang, S.R.; Bok, S.C.; Choi, S.C.; Lee, S.S.; Heo, M.S.; Huh, K.H.; Kim, T.I.; Yi, W.J. The Relationship between Dental Implant Stability and Trabecular Bone Structure Using Cone-Beam Computed Tomography. J. Periodontal. Implant. Sci. 2016, 46, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Van Dessel, J.; Huang, Y.; Depypere, M.; Rubira-Bullen, I.; Maes, F.; Jacobs, R. A Comparative Evaluation of Cone Beam CT and Micro-CT on Trabecular Bone Structures in the Human Mandible. Dentomaxillofacial Radiol. 2013, 42, 20130145. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.; Parsa, A.; Hassan, B.; Stelt, P.; Aartman, I.H.A.; Wismeijer, D. The Effect of Scan Parameters on Cone Beam CT Trabecular Bone Microstructural Measurements of the Human Mandible. Dentomaxillofacial Radiol. 2013, 42, 20130206. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.; Parsa, A.; Hassan, B.; Stelt, P.; Wismeijer, D. Diagnostic Imaging of Trabecular Bone Microstructure for Oral Implants: A Literature Review. Dentomaxillofacial Radiol. 2013, 42, 20120075. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.; Parsa, A.; Hassan, B.; Stelt, P.; Aartman, I.H.A.; Wismeijer, D. Accuracy of Trabecular Bone Microstructural Measurement at Planned Dental Implant Sites Using Cone-Beam CT Datasets. Clin. Oral Implant. Res. 2013, 25, 941–945. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Dessel, J.V.; Depypere, M. Validating Cone-Beam Computed Tomography for Peri-Implant Bone Morphometric Analysis. Bone Res. 2014, 2, 14010. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhao, B.; Deng, C.; Shang, D.; Zhang, C. Assessment of Implant Cumulative Survival Rates in Sites with Different Bone Density and Related Prognostic Factors: An 8-Year Retrospective Study of 2684 Implants. Int. J. Oral Maxillofac. Implant. 2015, 30, 360–371. [Google Scholar] [CrossRef]
- Guo, Z.; Du, X.; Wang, L. Measurements of volumetric bone mineral density in the mandible do not predict spinal osteoporosis. Dentomaxillofacial Radiol. 2020, 49, 20190280. [Google Scholar] [CrossRef] [PubMed]
- Giordano, V.; Franco, J.S.; Koch, H.A.; Labronici, P.J.; Pires, R.E.S.; Amaral, N.P.D. Age-related changes in bone architecture. Rev. Colégio Bras. Cir. 2016, 43, 276–285. [Google Scholar] [CrossRef]
- Felsenberg, D.; Boonen, S. The Bone Quality Framework: Determinants of Bone Strength and Their Interrelationships, and Implications for Osteoporosis Management. Clin. Ther. 2005, 27, 1–11. [Google Scholar] [CrossRef]
- Dias, D.R.; Leles, C.R.; Batista, A.C.; Lindh, C.; Ribeiro-Rotta, R.F. Agreement between Histomorphometry and Microcomputed Tomography to Assess Bone Microarchitecture of Dental Implant Sites. Clin. Implant. Dent. Relat. Res. 2013, 17, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Dessel, J.; Ferreira, L.; Nicolielo, P. Accuracy and Reliability of Different Cone Beam Computed Tomography (CBCT) Devices for Structural Analysis of Alveolar Bone in Comparison with Multislice CT and Micro-CT. Eur. J. Oral. Implantol. 2017, 10, 95–105. [Google Scholar]
- Dessel, J.; Ferreira, L.; Nicolielo, P. Quantification of bone quality using different cone beam computed tomography devices: Accuracy assessment for edentulous human mandibles. Eur. J. Oral. Implantol. 2016, 9, 411–424. [Google Scholar] [PubMed]
- Deguette, C.; Ramond-Roquin, A.; Rougé-Maillart, C. Relationships between Age and Microarchitectural Descriptors of Iliac Trabecular Bone Determined by MicroCT. Morphologie 2017, 101, 64–70. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, X.; Fujita, H.; Onozuka, M.; Kubo, K.-Y. Age-Related Changes in Trabecular and Cortical Bone Microstructure. Int. J. Endocrinol. 2013, 2013, 213234. [Google Scholar] [CrossRef] [PubMed]
- Castillo, R.F.; Ubelaker, D.H.; Djorojevic, M. Age Estimation through Histological Study of Trabecular Volume and Cortical Bone Width of the Iliac Crest. Science 2012, 52, 177–180. [Google Scholar] [CrossRef]
- Bridge, P.; Fielding, A.; Rowntree, P.; Pullar, A. Intraobserver Variability: Should We Worry? J. Med. Imaging Radiat. Sci. 2016, 47, 217–220. [Google Scholar] [CrossRef]
- Bhardwaj, D. Radiographic Evaluation of Mandible to Predict the Sex and Age. J. Clin. Diagn. Res. 2014, 8, ZC66. [Google Scholar] [CrossRef]
- Andronowski, J.M.; Crowder, C.; Martinez, M.S. Recent Advancements in the Analysis of Bone Microstructure: New Dimensions in Forensic Anthropology. Forensic Sci. Res. 2018, 3, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Andronowski, J.M.; Cole, M.E. Current and emerging histomorphometric and imaging techniques for assessing age-at-death and cortical bone quality. WIREs Forensic Sci. 2020, 3, 1–37. [Google Scholar] [CrossRef]



| Sex | N (%) |
|---|---|
| Male | 11 (55%) |
| Female | 9 (45%) |
| Age | |
| Mean ± SD | 26.6 ± 5.9 |
| Range | 22–43 years |
| Variable | Single Measure ICC | Average Measure ICC |
|---|---|---|
| TV | 0.567 | 0.724 |
| BV | 0.318 | 0.482 |
| BS | 0.411 | 0.583 |
| Tb. N | 0.386 | 0.557 |
| Tb. Th | 0.052 | 0.100 |
| Tb. Sp | 0.092 | 0.169 |
| Tb. Th. SD | 0.037 | 0.071 |
| Tb. Sp. SD | 0.434 | 0.606 |
| Parameter | Mean ± SD |
|---|---|
| TV | 1647.85 ± 934.78 |
| BV | 741.65 ± 544.19 |
| BS | 2653.45 ± 1453.22 |
| BV/TV | 44.40 ± 14.77 |
| BS/TV | 1.75 ± 0.64 |
| BS/BV | 4.00 ± 1.21 |
| Tb. N | 0.443 ± 0.15 |
| Tb. Th | 1.25 ± 0.55 |
| Tb. Sp | 2.05 ± 0.75 |
| Tb. Th. SD | 0.35 ± 0.49 |
| Tb. Sp. SD | 0.90 ± 0.45 |
| Parameter | Age | Sex | ||
|---|---|---|---|---|
| r Value | p Value | r Value | p Value | |
| TV | 0.076 | 0.749 | −0.156 | 0.512 |
| BV | 0.051 | 0.832 | −0.0258 | 0.272 |
| BS | −0.079 | 0.740 | −0.260 | 0.267 |
| BV/TV | −0.152 | 0.523 | −0.402 | 0.079 |
| BS/TV | −0.527 | 0.017 * | −0.283 | 0.227 |
| BS/BV | −0.219 | 0.354 | 0.255 | 0.278 |
| Tb. N | −0.489 | 0.029 * | −0.379 | 0.099 |
| Tb. Th | 0.081 | 0.736 | −0.234 | 0.320 |
| Tb. Sp | 0.016 | 0.946 | 0.075 | 0.754 |
| Tb. Th. SD | −0.167 | 0.483 | −0.032 | 0.895 |
| Tb. Sp. SD | 0.004 | 0.987 | −0.023 | 0.923 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabassum, A.; Chainchel Singh, M.K.; Ibrahim, N.; Ramanarayanan, S.; Mohd Yusof, M.Y.P. Quantifications of Mandibular Trabecular Bone Microstructure Using Cone Beam Computed Tomography for Age Estimation: A Preliminary Study. Biology 2022, 11, 1521. https://doi.org/10.3390/biology11101521
Tabassum A, Chainchel Singh MK, Ibrahim N, Ramanarayanan S, Mohd Yusof MYP. Quantifications of Mandibular Trabecular Bone Microstructure Using Cone Beam Computed Tomography for Age Estimation: A Preliminary Study. Biology. 2022; 11(10):1521. https://doi.org/10.3390/biology11101521
Chicago/Turabian StyleTabassum, Arshiya, Mansharan Kaur Chainchel Singh, Norliza Ibrahim, Subramaniam Ramanarayanan, and Mohd Yusmiaidil Putera Mohd Yusof. 2022. "Quantifications of Mandibular Trabecular Bone Microstructure Using Cone Beam Computed Tomography for Age Estimation: A Preliminary Study" Biology 11, no. 10: 1521. https://doi.org/10.3390/biology11101521
APA StyleTabassum, A., Chainchel Singh, M. K., Ibrahim, N., Ramanarayanan, S., & Mohd Yusof, M. Y. P. (2022). Quantifications of Mandibular Trabecular Bone Microstructure Using Cone Beam Computed Tomography for Age Estimation: A Preliminary Study. Biology, 11(10), 1521. https://doi.org/10.3390/biology11101521







