Was There a Cambrian Explosion on Land? The Case of Arthropod Terrestrialization
Abstract
Simple Summary
Abstract
1. Introduction
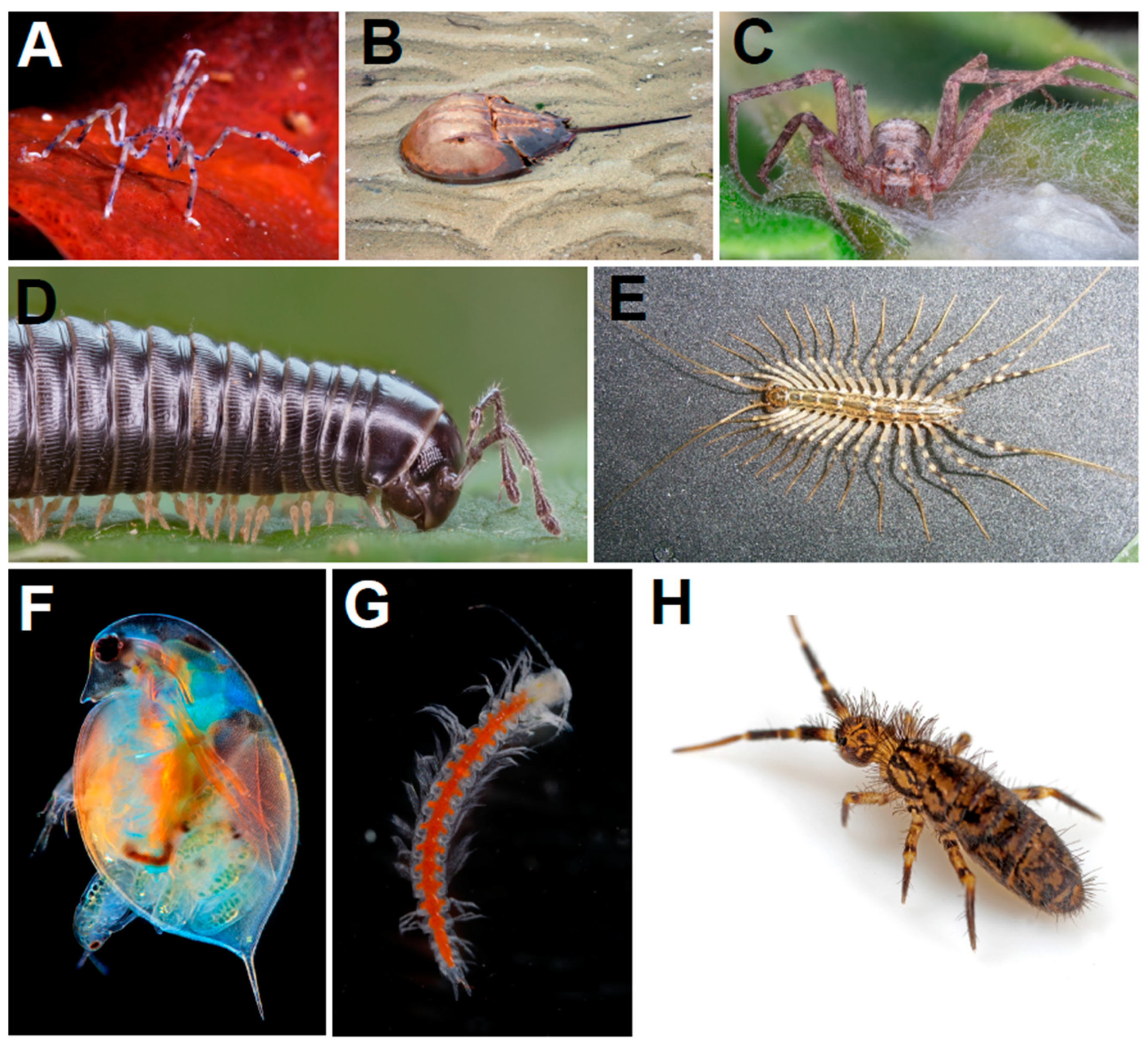
2. Origin and Terrestrialization of Arthropods
2.1. Arthropod Origins
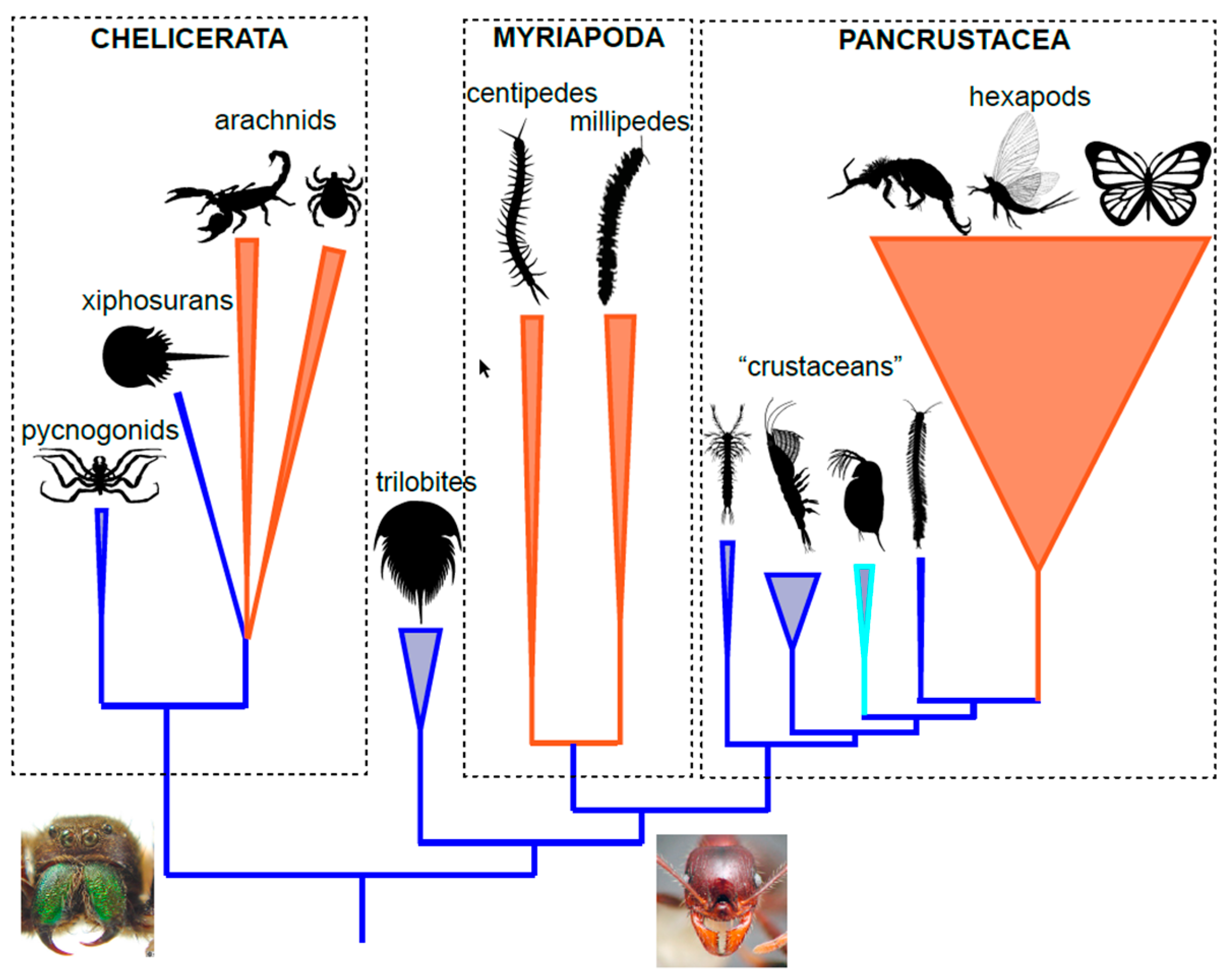
2.2. Arthropod Phylogeny
2.3. Myriapods
2.4. Pancrustacea (Hexapoda)
2.5. Pancrustacea (Isopods)
2.6. Arachnids
3. Pre-Devonian Fossil Record of Terrestrial Arthropods
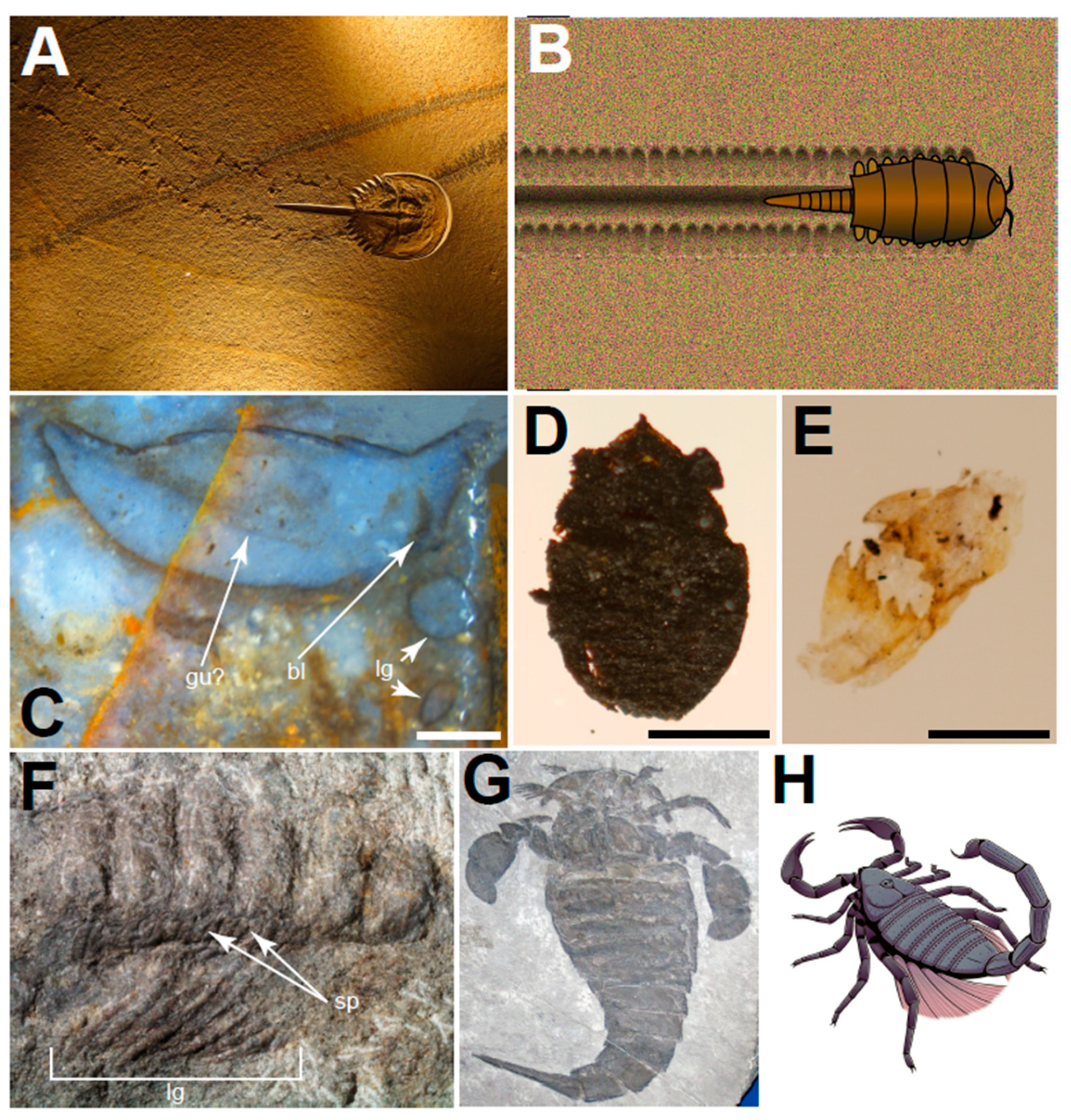
3.1. Trace Fossil Evidence
3.2. Body Fossil Evidence
4. Reconciling Rocks and Clocks
4.1. Methodologies to Build Chronologies
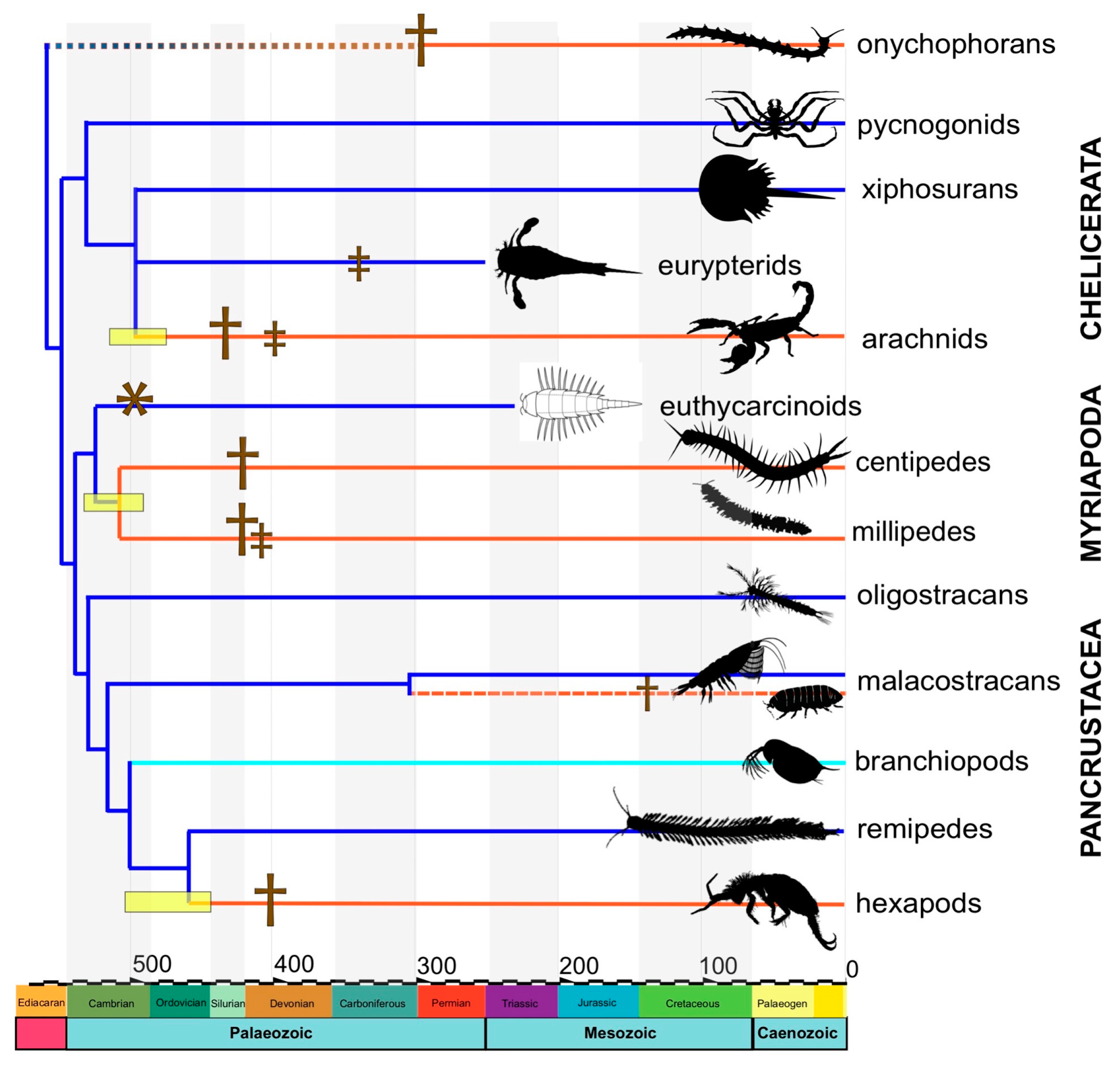
4.2. Dating the Arthropod Terrestrialization
4.3. Reconciling the Fossil and Molecular Evidence
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Betts, H.C.; Puttick, M.N.; Clark, J.W.; Williams, T.A.; Donoghue, P.C.J.; Pisani, D. Integrated Genomic and Fossil Evidence Illuminates Life’s Early Evolution and Eukaryote Origin. Nat. Ecol. Evol. 2018, 2, 1556–1562. [Google Scholar] [CrossRef]
- Schopf, T.J.M. Rates of Evolution and the Notion of “Living Fossils”. Annu. Rev. Earth Planet. Sci. 1984, 12, 245–292. [Google Scholar] [CrossRef]
- Allwood, A.C.; Rosing, M.T.; Flannery, D.T.; Hurowitz, J.A.; Heirwegh, C.M. Reassessing Evidence of Life in 3700-Million-Year-Old Rocks of Greenland. Nature 2018, 563, 241–244. [Google Scholar] [CrossRef]
- Cavalazzi, B.; Lemelle, L.; Simionovici, A.; Cady, S.L.; Russell, M.J.; Bailo, E.; Canteri, R.; Enrico, E.; Manceau, A.; Maris, A.; et al. Cellular Remains in a ~3.42-Billion-Year-Old Subseafloor Hydrothermal Environment. Sci. Adv. 2021, 7, eabf3963. [Google Scholar] [CrossRef]
- Sogin, M.L. The origin of eukaryotes and evolution into Major Kingdoms. In Early Life on Earth; Nobel Symposium No. 84; Columbia University Press: New York, NY, USA, 1994; pp. 181–192. [Google Scholar]
- Zhu, S.; Zhu, M.; Knoll, A.H.; Yin, Z.; Zhao, F.; Sun, S.; Qu, Y.; Shi, M.; Liu, H. Decimetre-Scale Multicellular Eukaryotes from the 1.56-Billion-Year-Old Gaoyuzhuang Formation in North China. Nat. Commun. 2016, 7, 11500. [Google Scholar] [CrossRef]
- Paterson, S.; Vogwill, T.; Buckling, A.; Benmayor, R.; Spiers, A.J.; Thomson, N.R.; Quail, M.; Smith, F.; Walker, D.; Libberton, B.; et al. Antagonistic Coevolution Accelerates Molecular Evolution. Nature 2010, 464, 275–278. [Google Scholar] [CrossRef]
- Erwin, D.H. Evolutionary Uniformitarianism. Dev. Biol. 2011, 357, 27–34. [Google Scholar] [CrossRef]
- Erwin, D.H.; Valentine, J.W. The Cambrian Explosion: The Construction of Animal Biodiversity; Macmillan Learning: Bedford, UK, 2013. [Google Scholar]
- Gaines, R.R. Burgess Shale-Type Preservation and Its Distribution in Space and Time. Paleontol. Soc. Pap. 2014, 20, 123–146. [Google Scholar] [CrossRef]
- Yang, C.; Li, X.-H.; Zhu, M.; Condon, D.J.; Chen, J. Geochronological Constraint on the Cambrian Chengjiang Biota, South China. J. Geol. Soc. 2018, 175, 659–666. [Google Scholar] [CrossRef]
- Littlewood, D.T.J. Marine parasites and the tree of life. In Marine Parasitology; Rohde, K.K., Ed.; CSIRO Publishing: Melbourne, Australia, 2005; pp. 6–10. [Google Scholar]
- Selden, P.A.; Jeram, A.J. Palaeophysiology of Terrestrialisation in the Chelicerata. Earth Environ. Sci. Trans. R. Soc. Edinb. 1989, 80, 303–310. [Google Scholar] [CrossRef]
- De Baets, K.; Dentzien-Dias, P.; Harrison, G.W.M.; Littlewood, D.T.J.; Parry, L.A. Fossil Constraints on the Timescale of Parasitic Helminth Evolution. In The Evolution and Fossil Record of Parasitism: Identification and Macroevolution of Parasites; Topics in Geobiology; De Baets, K., Huntley, J.W., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 231–271. ISBN 978-3-030-42484-8. [Google Scholar]
- Muscente, A.D.; Schiffbauer, J.D.; Broce, J.; Laflamme, M.; O’Donnell, K.; Boag, T.H.; Meyer, M.; Hawkins, A.D.; Huntley, J.W.; McNamara, M.; et al. Exceptionally Preserved Fossil Assemblages through Geologic Time and Space. Gondwana Res. 2017, 48, 164–188. [Google Scholar] [CrossRef]
- Vermeij, G.J.; Watson-Zink, V.M. Terrestrialization in Gastropods: Lineages, Ecological Constraints and Comparisons with Other Animals. Biol. J. Linn. Soc. 2022, 136, 393–404. [Google Scholar] [CrossRef]
- Garwood, R.J.; Edgecombe, G.D.; Charbonnier, S.; Chabard, D.; Sotty, D.; Giribet, G. Carboniferous Onychophora from Montceau-Les-Mines, France, and Onychophoran Terrestrialization. Invert. Biol. 2016, 135, 179–190. [Google Scholar] [CrossRef]
- van Straalen, N.M. Evolutionary Terrestrialization Scenarios for Soil Invertebrates. Pedobiologia 2021, 87–88, 150753. [Google Scholar] [CrossRef]
- Holterman, M.; Schratzberger, M.; Helder, J. Nematodes as Evolutionary Commuters between Marine, Freshwater and Terrestrial Habitats. Biol. J. Linn. Soc. 2019, 128, 756–767. [Google Scholar] [CrossRef]
- Okamura, B.; Gruhl, A.; De Baets, K. Evolutionary Transitions of Parasites between Freshwater and Marine Environments. Integr. Comp. Biol. 2022, 62, 345–356. [Google Scholar] [CrossRef]
- Tchesunov, A.V.; Ivanenko, V.N. What Is the Difference between Marine and Limnetic-Terrestrial Associations of Nematodes with Invertebrates? Integr. Zool. 2022, 17, 481–510. [Google Scholar] [CrossRef]
- Guidetti, R.; Bertolani, R. Paleontology and Molecular Dating. In Water Bears: The Biology of Tardigrades; Zoological Monographs; Schill, R.O., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 131–143. ISBN 978-3-319-95702-9. [Google Scholar]
- Sluys, R. The Evolutionary Terrestrialization of Planarian Flatworms (Platyhelminthes, Tricladida, Geoplanidae): A Review and Research Programme. Zoosyst. Evol. 2019, 95, 543–556. [Google Scholar] [CrossRef]
- Dunlop, J.A.; Scholtz, G.; Selden, P.A. Water-to-Land Transitions. In Arthropod Biology and Evolution: Molecules, Development, Morphology; Minelli, A., Boxshall, G., Fusco, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 417–439. ISBN 978-3-642-36160-9. [Google Scholar]
- Lozano-Fernandez, J.; Carton, R.; Tanner, A.R.; Puttick, M.N.; Blaxter, M.; Vinther, J.; Olesen, J.; Giribet, G.; Edgecombe, G.D.; Pisani, D. A Molecular Palaeobiological Exploration of Arthropod Terrestrialization. Phil. Trans. Roy. Soc. B. 2016, 371, 20150133. [Google Scholar] [CrossRef]
- Felsenstein, J. Phylogenies and the Comparative Method. Am. Nat. 1985, 125, 1–15. [Google Scholar] [CrossRef]
- Benton, M.J. The Origins of Modern Biodiversity on Land. Phil. Trans. R. Soc. B 2010, 365, 3667–3679. [Google Scholar] [CrossRef] [PubMed]
- Román-Palacios, C.; Moraga-López, D.; Wiens, J.J. The Origins of Global Biodiversity on Land, Sea and Freshwater. Ecol. Lett. 2022, 25, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.T.; Warnock, R.C.M.; Donoghue, P.C.J. Establishing a Time-Scale for Plant Evolution. New Phytol. 2011, 192, 266–301. [Google Scholar] [CrossRef]
- Prave, A.R. Life on Land in the Proterozoic: Evidence from the Torridonian Rocks of Northwest Scotland. Geology 2002, 30, 811–814. [Google Scholar] [CrossRef]
- Buatois, L.A.; Davies, N.S.; Gibling, M.R.; Krapovickas, V.; Labandeira, C.C.; MacNaughton, R.B.; Mángano, M.G.; Minter, N.J.; Shillito, A.P. The Invasion of the Land in Deep Time: Integrating Paleozoic Records of Paleobiology, Ichnology, Sedimentology, and Geomorphology. Integr. Comp. Biol. 2022, 62, 297–331. [Google Scholar] [CrossRef]
- Xu, H.; Wang, K.; Huang, Z.; Tang, P.; Wang, Y.; Liu, B.; Yan, W. The Earliest Vascular Land Plants from the Upper Ordovician of China 2022. Biol. Sci. 2022, preprint. [Google Scholar]
- Wilson, H.M.; Anderson, L.I. Morphology and Taxonomy of Paleozoic Millipedes (Diplopoda: Chilognatha: Archipolypoda) from Scotland. J. Paleontol. 2004, 78, 169–184. [Google Scholar] [CrossRef]
- Dunlop, J. A Trigonotarbid Arachnid from the Upper Silurian of Shropshire. Palaeontology 1996, 39, 605–614. [Google Scholar]
- Ward, P.; Labandeira, C.; Laurin, M.; Berner, R.A. Confirmation of Romer’s Gap as a Low Oxygen Interval Constraining the Timing of Initial Arthropod and Vertebrate Terrestrialization. Proc. Natl. Acad. Sci. USA 2006, 103, 16818–16822. [Google Scholar] [CrossRef]
- Davies, N.S.; Rygel, M.C.; Gibling, M.R. Marine Influence in the Upper Ordovician Juniata Formation (Potters Mills, Pennsylvania): Implications for the History of Life on Land. PALAIOS 2010, 25, 527–539. [Google Scholar] [CrossRef]
- Lozano-Fernandez, J.; Tanner, A.R.; Puttick, M.N.; Vinther, J.; Edgecombe, G.D.; Pisani, D. A Cambrian–Ordovician Terrestrialization of Arachnids. Front. Genet. 2020, 11, 182. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.L.; Puttick, M.N.; Clark, J.W.; Edwards, D.; Kenrick, P.; Pressel, S.; Wellman, C.H.; Yang, Z.; Schneider, H.; Donoghue, P.C. The Timescale of Early Land Plant Evolution. Proc. Natl. Acad. Sci. USA 2018, 115, E2274–E2283. [Google Scholar] [CrossRef] [PubMed]
- Edgecombe, G.D.; Strullu-Derrien, C.; Góral, T.; Hetherington, A.J.; Thompson, C.; Koch, M. Aquatic Stem Group Myriapods Close a Gap between Molecular Divergence Dates and the Terrestrial Fossil Record. Proc. Natl. Acad. Sci. USA 2020, 117, 8966–8972. [Google Scholar] [CrossRef] [PubMed]
- Lamsdell, J.C.; McCoy, V.E.; Perron-Feller, O.A.; Hopkins, M.J. Air Breathing in an Exceptionally Preserved 340-Million-Year-Old Sea Scorpion. Curr. Biol. 2020, 30, 4316–4321.e2. [Google Scholar] [CrossRef]
- Royer, D.L.; Donnadieu, Y.; Park, J.; Kowalczyk, J.; Goddéris, Y. Error Analysis of CO2 and O2 Estimates from the Long-Term Geochemical Model GEOCARBSULF. Am. J. Sci. 2014, 314, 1259–1283. [Google Scholar] [CrossRef]
- Schachat, S.R.; Labandeira, C.C.; Saltzman, M.R.; Cramer, B.D.; Payne, J.L.; Boyce, C.K. Phanerozoic p O2 and the Early Evolution of Terrestrial Animals. Proc. R. Soc. Lond. B 2018, 285, 20172631. [Google Scholar]
- Gregor, B. Denudation of the Continents. Nature 1970, 228, 273–275. [Google Scholar] [CrossRef]
- Blatt, H.; Jones, R.L. Proportions of Exposed Igneous, Metamorphic, and Sedimentary Rocks. GSA Bull. 1975, 86, 1085–1088. [Google Scholar] [CrossRef]
- Khain, V.Y.; Ronov, A.B.; Seslavinskiy, K.B. Silurian Lithologic Associations of the World. Int. Geol. Rev. 1978, 20, 249–268. [Google Scholar] [CrossRef]
- Ronov, A.B. The Earth’s Sedimentary Shell (Quantitative Patterns of Its Structure, Compositions, and Evolution). Int. Geol. Rev. 1982, 24, 1313–1363. [Google Scholar] [CrossRef]
- Ronov, A.B.; Khain, V.E.; Balukhovsky, A.N.; Seslavinsky, K.B. Quantitative Analysis of Phanerozoic Sedimentation. Sed. Geol. 1980, 25, 311–325. [Google Scholar] [CrossRef]
- Smith, A.B.; McGowan, A.J. The Shape of the Phanerozoic Marine Palaeodiversity Curve: How Much Can Be Predicted from the Sedimentary Rock Record of Western Europe? Palaeontology 2007, 50, 765–774. [Google Scholar] [CrossRef]
- Robardet, M.; Blaise, J.; Bouyx, E.; Gourvennec, R.; Lardeux, H.; Le Hérissé, A.; Le Menn, J.; Melou, M.; Paris, F.; Plusquellec, Y.; et al. Palaeogeography of Western Europe from the Ordovician to the Devonian. Bull. Soc. Geol. Fr. 1993, 164, 683–695. [Google Scholar]
- Kenrick, P.; Wellman, C.H.; Schneider, H.; Edgecombe, G.D. A Timeline for Terrestrialization: Consequences for the Carbon Cycle in the Palaeozoic. Phil. Trans. R. Soc. B 2012, 367, 519–536. [Google Scholar] [CrossRef]
- Zhang, Z.-Q. Animal Biodiversity: An Outline of Higher-Level Classification and Survey of Taxonomic Richness (Addenda 2013). Zootaxa 2013, 3703, 1–82. [Google Scholar] [CrossRef] [PubMed]
- Maloof, A.C.; Rose, C.V.; Beach, R.; Samuels, B.M.; Calmet, C.C.; Erwin, D.H.; Poirier, G.R.; Yao, N.; Simons, F.J. Possible Animal-Body Fossils in Pre-Marinoan Limestones from South Australia. Nat. Geosci. 2010, 3, 653–659. [Google Scholar] [CrossRef]
- Wolfe, J.M.; Daley, A.C.; Legg, D.A.; Edgecombe, G.D. Fossil Calibrations for the Arthropod Tree of Life. Earth Sci. Rev. 2016, 160, 43–110. [Google Scholar] [CrossRef]
- Edgecombe, G.D. Arthropod Phylogeny: An Overview from the Perspectives of Morphology, Molecular Data and the Fossil Record. Arthropod Struct. Dev. 2010, 39, 74–87. [Google Scholar] [CrossRef]
- Grimaldi, D.; Engel, M.S. Evolution of the Insects, 1st ed.; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Govorushko, S. Economic and Ecological Importance of Termites: A Global Review. Entomol. Sci. 2019, 22, 21–35. [Google Scholar] [CrossRef]
- Brusca, R.C.; Brusca, G.J. Invertebrates, 2nd ed.; Sinauer Associates, Incorporated: Sunderland, MA, USA, 2003; ISBN 978-0-87893-099-9. [Google Scholar]
- Little, C. The Colonisation of Land: Origins and Adaptations of Terrestrial Animals; Cambridge University Press: Cambridge, UK, 1983; ISBN 978-0-521-25218-8. [Google Scholar]
- Jeram, A.J.; Selden, P.A.; Edwards, D. Land Animals in the Silurian: Arachnids and Myriapods from Shropshire, England. Science 1990, 250, 658–661. [Google Scholar] [CrossRef]
- Shear, W.; Selden, P. Eoarthropleura (Arthropoda, Arthropleurida) from the Silurian of Britain and the Devonian of North America. Neues Jahrb. Geol. Palaontol. Abh. 1995, 196, 347–375. [Google Scholar] [CrossRef]
- Shear, W.A.; Jeram, A.J.; Selden, P. Centiped Legs (Arthropoda, Chilopoda, Scutigeromorpha) from the Silurian and Devonian of Britain and the Devonian of North America. Am. Mus. Novit. 1998, 3231, 1–16. [Google Scholar]
- Dunlop, J.A. A Replacement Name for the Trigonotarbid Arachnid Eotarbus Dunlop. Palaeontology 1999, 42, 191. [Google Scholar] [CrossRef]
- Brookfield, M.E.; Catlos, E.J.; Suarez, S.E. Myriapod Divergence Times Differ between Molecular Clock and Fossil Evidence: U/Pb Zircon Ages of the Earliest Fossil Millipede-Bearing Sediments and Their Significance. Hist. Biol. 2020, 10, 2014–2018. [Google Scholar] [CrossRef]
- Marshall, J.E.A. Palynology of the Stonehaven Group, Scotland: Evidence for a Mid Silurian Age and Its Geological Implications. Geol. Mag. 1991, 128, 283–286. [Google Scholar] [CrossRef]
- Wellman, C.H. A Land Plant Microfossil Assemblage of Mid Silurian Age from the Stonehaven Group, Scotland. J. Micropalaeontol. 1993, 12, 47–66. [Google Scholar] [CrossRef]
- Howard, R.J.; Puttick, M.N.; Edgecombe, G.D.; Lozano-Fernandez, J. Arachnid Monophyly: Morphological, Palaeontological and Molecular Support for a Single Terrestrialization within Chelicerata. Arthropod Struct. Devel. 2020, 59, 100997. [Google Scholar] [CrossRef]
- Suarez, S.E.; Brookfield, M.E.; Catlos, E.J.; Stöckli, D.F. A U-Pb Zircon Age Constraint on the Oldest-Recorded Air-Breathing Land Animal. PLoS ONE 2017, 12, e0179262. [Google Scholar] [CrossRef]
- Kutscher, F. Friedrich Beiträge Zur Sedimentation Und Fossilführung Des Hunsrückschiefers 32. Palaeoscorpius devonicus, Ein Devonischer Skorpion. Jahrb. Nassau. Ver. Naturkd. 1971, 101, 191. [Google Scholar]
- Lehmann, W.M. Palaeoscorpius devonicus Ng, n. Sp., Ein Skorpion Aus Dem Rheinischen Unterdevon. N. Jahrb. Geol. Palaontol. Monat. 1944, 7, 177–185. [Google Scholar]
- Kühl, G.; Bergmann, A.; Dunlop, J.; Garwood, R.J.; Rust, J. Redescription and Palaeobiology of Palaeoscorpius devonicus Lehmann, 1944 from the Lower Devonian Hunsrück Slate of Germany. Palaeontology 2012, 55, 775–787. [Google Scholar] [CrossRef]
- Zrzavý, J.; Hypša, V.; Vlášková, M. Arthropod Phylogeny: Taxonomic Congruence, Total Evidence and Conditional Combination Approaches to Morphological and Molecular Data Sets. In Arthropod Relationships; Fortey, R.A., Thomas, R.H., Eds.; The Systematics Association Special Volume Series; Springer: Dordrecht, The Netherlands, 1998; pp. 97–107. ISBN 978-94-011-4904-4. [Google Scholar]
- Giribet, G.; Edgecombe, G.D. The Phylogeny and Evolutionary History of Arthropods. Curr. Biol. 2019, 29, R592–R602. [Google Scholar] [CrossRef] [PubMed]
- Legg, D.A.; Sutton, M.D.; Edgecombe, G.D. Arthropod Fossil Data Increase Congruence of Morphological and Molecular Phylogenies. Nat. Commun. 2013, 4, 2485. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, J.A.; Santibáñez-López, C.E.; Baker, C.M.; Benavides, L.R.; Cunha, T.J.; Gainett, G.; Ontano, A.Z.; Setton, E.V.W.; Arango, C.P.; Gavish-Regev, E.; et al. Comprehensive Species Sampling and Sophisticated Algorithmic Approaches Refute the Monophyly of Arachnida. Mol. Biol. Evol. 2022, 39, msac021. [Google Scholar] [CrossRef] [PubMed]
- Tihelka, E.; Cai, C.; Giacomelli, M.; Lozano-Fernandez, J.; Rota-Stabelli, O.; Huang, D.; Engel, M.S.; Donoghue, P.C.J.; Pisani, D. The Evolution of Insect Biodiversity. Curr. Biol. 2021, 31, R1299–R1311. [Google Scholar] [CrossRef] [PubMed]
- Bäcker, H.; Fanenbruck, M.; Wägele, J.W. A Forgotten Homology Supporting the Monophyly of Tracheata: The Subcoxa of Insects and Myriapods Re-Visited. Zool. Anz. J. Comp. Zool. 2008, 247, 185–207. [Google Scholar] [CrossRef]
- Giribet, G.; Edgecombe, G.D. Reevaluating the Arthropod Tree of Life. Annu. Rev. Entomol. 2012, 57, 167–186. [Google Scholar] [CrossRef]
- Fernández, R.; Edgecombe, G.D.; Giribet, G.; Edgecombe, G.D.; Giribet, G. Phylogenomics Illuminates the Backbone of the Myriapoda Tree of Life and Reconciles Morphological and Molecular Phylogenies. Sci. Rep. 2018, 8, 83. [Google Scholar] [CrossRef]
- Rota-Stabelli, O.; Campbell, L.; Brinkmann, H.; Edgecombe, G.D.; Longhorn, S.J.; Peterson, K.J.; Pisani, D.; Philippe, H.; Telford, M.J. A Congruent Solution to Arthropod Phylogeny: Phylogenomics, MicroRNAs and Morphology Support Monophyletic Mandibulata. Proc. R. Soc. B 2011, 278, 298–306. [Google Scholar] [CrossRef]
- Fernández, R.; Edgecombe, G.D.; Giribet, G. Exploring Phylogenetic Relationships within Myriapoda and the Effects of Matrix Composition and Occupancy on Phylogenomic Reconstruction. Syst. Biol. 2016, 65, 871–889. [Google Scholar] [CrossRef]
- Szucsich, N.U.; Bartel, D.; Blanke, A.; Böhm, A.; Donath, A.; Fukui, M.; Grove, S.; Liu, S.; Macek, O.; Machida, R.; et al. Four Myriapod Relatives—But Who Are Sisters? No End to Debates on Relationships among the Four Major Myriapod Subgroups. BMC Evol. Biol. 2020, 20, 144. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bai, Y.; Zhao, H.; Mu, R.; Dong, Y. Reinvestigating the Phylogeny of Myriapoda with More Extensive Taxon Sampling and Novel Genetic Perspective. PeerJ 2021, 9, e12691. [Google Scholar] [CrossRef] [PubMed]
- Benavides, L.R.; Edgecombe, G.D.; Giribet, G. Re-Evaluating and Dating Myriapod Diversification with Phylotranscriptomics under a Regime of Dense Taxon Sampling. Mol. Phylogenetics Evol. 2022, 178, 107621. [Google Scholar] [CrossRef] [PubMed]
- Schwentner, M.; Combosch, D.J.; Nelson, J.P.; Giribet, G. A Phylogenomic Solution to the Origin of Insects by Resolving Crustacean-Hexapod Relationships. Curr. Biol. 2017, 27, 1818–1824. [Google Scholar] [CrossRef]
- Regier, J.C.; Shultz, J.W.; Zwick, A.; Hussey, A.; Ball, B.; Wetzer, R.; Martin, J.W.; Cunningham, C.W.; Shultz, J.W.; Zwick, A.; et al. Arthropod Relationships Revealed by Phylogenomic Analysis of Nuclear Protein-Coding Sequences. Nature 2010, 463, 1079–1083. [Google Scholar] [CrossRef]
- Lozano-Fernandez, J.; Giacomelli, M.; Fleming, J.F.; Chen, A.; Vinther, J.; Thomsen, P.F.; Glenner, H.; Palero, F.; Legg, D.A.; Iliffe, T.M.; et al. Pancrustacean Evolution Illuminated by Taxon-Rich Genomic-Scale Data Sets with an Expanded Remipede Sampling. Genome Biol. Evol. 2019, 11, 2055–2070. [Google Scholar] [CrossRef]
- Yager, J. Remipedia, a New Class of Crustacea from a Marine Cave in the Bahamas. J. Crustacean Biol. 1981, 1, 328–333. [Google Scholar] [CrossRef]
- Schmalfuss, H. World Catalog of Terrestrial Isopods (Isopoda: Oniscidea). Stuttg. Beitr. Naturkd. A 2003, 654, 341. [Google Scholar]
- Richardson, A.; Araujo, P.B. Lifestyles of Terrestrial Crustacean. In The Natural History of the Crustacea. Lifestyles and Feeding Biology; Oxford University Press: Oxford, UK, 2015; pp. 299–336. [Google Scholar]
- Broly, P.; Deville, P.; Maillet, S. The Origin of Terrestrial Isopods (Crustacea: Isopoda: Oniscidea). Evol. Ecol. 2013, 27, 461–476. [Google Scholar] [CrossRef]
- Elisabeth, H. Evolutionary Adaptation of Oniscidean Isopods to Terrestrial Life: Structure, Physiology and Behavior. Terr. Arthropod Rev. 2011, 4, 95–130. [Google Scholar] [CrossRef]
- Schmidt, C. Phylogeny of the Terrestrial Isopoda (Oniscidea): A Review. Arthr. Syst. Phyl. 2008, 66, 191–226. [Google Scholar]
- Dimitriou, A.C.; Taiti, S.; Sfenthourakis, S. Genetic Evidence against Monophyly of Oniscidea Implies a Need to Revise Scenarios for the Origin of Terrestrial Isopods. Sci. Rep. 2019, 9, 18508. [Google Scholar] [CrossRef] [PubMed]
- Tabacaru, I.; Giurginca, A. The Monophyly and the Classification of the Terrestrial Isopods (Crustacea, Isopoda, Oniscidea). Trav. Inst. Speol. Emile Racovitza 2021, 59, 3–23. [Google Scholar]
- Ballesteros, J.A.; Sharma, P.P. A Critical Appraisal of the Placement of Xiphosura (Chelicerata) with Account of Known Sources of Phylogenetic Error. Syst. Biol. 2019, 68, 896–917. [Google Scholar] [CrossRef]
- Shultz, J.W. A Phylogenetic Analysis of the Arachnid Orders Based on Morphological Characters. Zool. J. Linn. Soc. 2007, 150, 221–265. [Google Scholar] [CrossRef]
- Shultz, J.W. Evolutionary Morphology and Phylogeny of Arachnida. Cladistics 1990, 6, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Bicknell, R.D.C.; Pates, S. Pictorial Atlas of Fossil and Extant Horseshoe Crabs, with Focus on Xiphosurida. Front. Earth Sci. 2020, 8, 98. [Google Scholar] [CrossRef]
- Gould, S.J. Dollo on Dollo’s Law: Irreversibility and the Status of Evolutionary Laws. J. Hist. Biol. 1970, 3, 189–212. [Google Scholar] [CrossRef]
- Lamsdell, J.C. Evolutionary History of the Dynamic Horseshoe Crab. Int. Wader Stud. 2019, 21, 1–15. [Google Scholar]
- Bicknell, R.D.C.; Kimmig, J.; Budd, G.E.; Legg, D.A.; Bader, K.S.; Haug, C.; Kaiser, D.; Laibl, L.; Tashman, J.N.; Campione, N.E. Habitat and Developmental Constraints Drove 330 Million Years of Horseshoe Crab Evolution. Biol. J. Linn. Soc. 2022, 136, 155–172. [Google Scholar] [CrossRef]
- Lozano-Fernandez, J.; Tanner, A.R.; Giacomelli, M.; Carton, R.; Vinther, J.; Edgecombe, G.D.; Pisani, D. Increasing Species Sampling in Chelicerate Genomic-Scale Datasets Provides Support for Monophyly of Acari and Arachnida. Nat. Commun. 2019, 10, 2295. [Google Scholar] [CrossRef] [PubMed]
- MacNaughton, R.B.; Cole, J.M.; Dalrymple, R.W.; Braddy, S.J.; Briggs, D.E.G.; Lukie, T.D. First Steps on Land: Arthropod Trackways in Cambrian-Ordovician Eolian Sandstone, Southeastern Ontario, Canada. Geology 2002, 30, 391–394. [Google Scholar] [CrossRef]
- Collette, J.H.; Hagadorn, J.W. Three-Dimensionally Preserved Arthropods from Cambrian Lagerstätten of Quebec and Wisconsin. J. Paleontol. 2010, 84, 646–667. [Google Scholar] [CrossRef]
- Vaccari, N.E.; Edgecombe, G.D.; Escudero, C.; Edgecombe, G.D.; Escudero, C. Cambrian Origins and Affinities of an Enigmatic Fossil Group of Arthropods. Nature 2004, 430, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Braddy, S.J.; Gass, K.C.; Gass, T.C. Fossils of Blackberry Hill, Wisconsin, USA: The First Animals on Land, 500 Million Years Ago. Geol. Today 2022, 38, 25–31. [Google Scholar] [CrossRef]
- Mángano, M.G.; Buatois, L.A.; Astini, R.; Rindsberg, A.K. Trilobites in Early Cambrian Tidal Flats and the Landward Expansion of the Cambrian Explosion. Geology 2014, 42, 143–146. [Google Scholar] [CrossRef]
- Mángano, M.G.; Buatois, L.A.; Waisfeld, B.G.; Muñoz, D.F.; Vaccari, N.E.; Astini, R.A. Were All Trilobites Fully Marine? Trilobite Expansion into Brackish Water during the Early Palaeozoic. Proc. R. Soc. B 2021, 288, 20202263. [Google Scholar] [CrossRef]
- Suzuki, Y.; Bergström, J. Respiration in Trilobites: A Reevaluation. GFF 2008, 130, 211–229. [Google Scholar] [CrossRef]
- Hou, J.; Hughes, N.C.; Hopkins, M.J. The Trilobite Upper Limb Branch Is a Well-Developed Gill. Sci. Adv. 2021, 7, eabe7377. [Google Scholar] [CrossRef]
- Retallack, G.J. Scoyenia Burrows from Ordovician Palaeosols of the Juniata Formation in Pennsylvania. Palaeontology 2001, 44, 209–235. [Google Scholar] [CrossRef]
- Shillito, A.P.; Davies, N.S. Death near the Shoreline, Not Life on Land: Ordovician Arthropod Trackways in the Borrowdale Volcanic Group, UK. Geology 2018, 47, 55–58. [Google Scholar] [CrossRef]
- Johnson, E.W.; Briggs, D.E.G.; Suthren, R.J.; Wright, J.L.; Tunnicliff, S.P. Non-Marine Arthropod Traces from the Subaerial Ordovician Borrowdale Volcanic Group, English Lake District. Geol. Mag. 1994, 131, 395–406. [Google Scholar] [CrossRef]
- Ortega-Hernández, J.; Legg, D.A.; Tremewan, J.; Braddy, S.J. Euthycarcinoids. Geol. Today 2010, 26, 195–198. [Google Scholar] [CrossRef]
- Collette, J.H.; Gass, K.G.; Hagadorn, J.W. Protichnites eremita Unshelled? Experimental Model-Based Neoichnology and New Evidence for a Euthycarcinoid Affinity for This Ichnospecies. J. Paleontol. 2012, 86, 442–454. [Google Scholar] [CrossRef]
- Gueriau, P.; Lamsdell, J.C.; Wogelius, R.A.; Manning, P.L.; Egerton, V.M.; Bergmann, U.; Bertrand, L.; Denayer, J. A New Devonian Euthycarcinoid Reveals the Use of Different Respiratory Strategies during the Marine-to-Terrestrial Transition in the Myriapod Lineage. R. Soc. Open Sci. 2020, 7, 201037. [Google Scholar] [CrossRef]
- Trewin, N.H.; Gurr, P.R.; Jones, R.B.; Gavin, P. The Biota, Depositional Environment and Age of the Old Red Sandstone of the Island of Kerrera, Scotland. Scott. J. Geol. 2012, 48, 77–90. [Google Scholar] [CrossRef]
- Scourfield, D.J. The Oldest Known Fossil Insect. Nature 1940, 145, 799–801. [Google Scholar] [CrossRef]
- Whalley, P.; Jarzembowski, E.A. A New Assessment of Rhyniella, the Earliest Known Insect, from the Devonian of Rhynie, Scotland. Nature 1981, 291, 317. [Google Scholar] [CrossRef]
- Fayers, S.R.; Trewin, N.H. A Hexapod from the Early Devonian Windyfield Chert, Rhynie, Scotland. Palaeontology 2005, 48, 1117–1130. [Google Scholar] [CrossRef]
- Greenslade, P.; Whalley, P.E.S. The Systematic Position of Rhyniella praecursor (Hirst and Malik, the Earliest Known Hexapod. In Proceedings of the 2nd International Seminar on Apterygota, Siena, Italy, 1 January 1986; Volume 1986, pp. 319–323. [Google Scholar]
- Shear, W.A. An Insect to Fill the Gap. Nature 2012, 488, 34–35. [Google Scholar] [CrossRef]
- Kukalová-Peck, J. New Carboniferous Diplura, Monura, and Thysanura, the Hexapod Ground Plan, and the Role of Thoracic Side Lobes in the Origin of Wings (Insecta). Can. J. Zool. 1987, 65, 2327–2345. [Google Scholar] [CrossRef]
- Hošek, P. Fossil Insect Diversity. Vesmír 1994, 73, 196–200. [Google Scholar]
- Crowson, R.A. Comments on Insecta of the Rhynie Chert. Entomol. Gener. 1985, 97–98. [Google Scholar] [CrossRef]
- Rohdendorf, B.B. Devonskie Eopteridy-Ne Nasekomye a Rakoobraznye Eumalacostraca. Entomol. Oboz. 1972, 51, 96–97. [Google Scholar]
- Schram, F.R. Miscellaneous Late Paleozoic Malacostraca of the Soviet Union. J. Paleontol. 1980, 54, 542–547. [Google Scholar]
- Haug, C.; Haug, J.T. The Presumed Oldest Flying Insect: More Likely a Myriapod? PeerJ 2017, 5, e3402. [Google Scholar] [CrossRef]
- Garrouste, R.; Clément, G.; Nel, P.; Engel, M.S.; Grandcolas, P.; D’Haese, C.; Lagebro, L.; Denayer, J.; Gueriau, P.; Lafaite, P.; et al. A Complete Insect from the Late Devonian Period. Nature 2012, 488, 82–85. [Google Scholar] [CrossRef]
- Hörnschemeyer, T.; Haug, J.T.; Bethoux, O.; Beutel, R.G.; Charbonnier, S.; Hegna, T.A.; Koch, M.; Rust, J.; Wedmann, S.; Bradler, S.; et al. Is Strudiella a Devonian Insect? Nature 2013, 494, E3–E4. [Google Scholar] [CrossRef]
- Haas, F.; Waloszek, D.; Hartenberger, R. Devonohexapodus bocksbergensis, a New Marine Hexapod from the Lower Devonian Hunsrück Slates, and the Origin of Atelocerata and Hexapoda. Org. Divers. Evol. 2003, 3, 39–54. [Google Scholar] [CrossRef][Green Version]
- Kühl, G.; Rust, J. Devonohexapodus Bocksbergensis Is a Synonym of Wingertshellicus backesi (Euarthropoda)—No Evidence for Marine Hexapods Living in the Devonian Hunsrück Sea. Org. Divers. Evol. 2009, 9, 215–231. [Google Scholar] [CrossRef][Green Version]
- Brauckmann, C.; Brauckmann, B.; Groning, E. The Stratigraphical Position of the Oldest Known Pterygota (Insecta. Carboniferous, Namurian). Ann. Soc. Géol. Belg. 1994, 117, 47–56. [Google Scholar]
- Dunlop, J.A.; Erik Tetlie, O.; Prendini, L. Reinterpretation of the Silurian Scorpion Proscorpius osborni (Whitfield): Integrating Data from Palaeozoic and Recent Scorpioans. Palaeontology 2008, 51, 303–320. [Google Scholar] [CrossRef]
- Waddington, J.; Rudkin, D.M.; Dunlop, J.A. A New Mid-Silurian Aquatic Scorpion—One Step Closer to Land? Biol. Lett. 2015, 11, 20140815. [Google Scholar] [CrossRef] [PubMed]
- Wendruff, A.J.; Babcock, L.E.; Wirkner, C.S.; Kluessendorf, J.; Mikulic, D.G. A Silurian Ancestral Scorpion with Fossilised Internal Anatomy Illustrating a Pathway to Arachnid Terrestrialisation. Sci. Rep. 2020, 10, 14. [Google Scholar] [CrossRef]
- Sharma, P.P.; Kaluziak, S.T.; Pérez-Porro, A.R.; González, V.L.; Hormiga, G.; Wheeler, W.C.; Giribet, G. Phylogenomic Interrogation of Arachnida Reveals Systemic Conflicts in Phylogenetic Signal. Mol. Biol. Evol. 2014, 31, 2963–2984. [Google Scholar] [CrossRef]
- Leite, D.J.; Baudouin-Gonzalez, L.; Iwasaki-Yokozawa, S.; Lozano-Fernandez, J.; Turetzek, N.; Akiyama-Oda, Y.; Prpic, N.-M.; Pisani, D.; Oda, H.; Sharma, P.P.; et al. Homeobox Gene Duplication and Divergence in Arachnids. Mol. Biol. Evol. 2018, 35, 2240–2253. [Google Scholar] [CrossRef]
- Ontano, A.Z.; Gainett, G.; Aharon, S.; Ballesteros, J.A.; Benavides, L.R.; Corbett, K.F.; Gavish-Regev, E.; Harvey, M.S.; Monsma, S.; Santibáñez-López, C.E.; et al. Taxonomic Sampling and Rare Genomic Changes Overcome Long-Branch Attraction in the Phylogenetic Placement of Pseudoscorpions. Mol. Biol. Evol. 2021, 38, 2446–2467. [Google Scholar] [CrossRef]
- Claridge, M.F.; Lyon, A.G. Lung-Books in the Devonian Palæocharinidae (Arachnida). Nature 1961, 191, 1190–1191. [Google Scholar] [CrossRef]
- Kamenz, C.; Dunlop, J.A.; Scholtz, G.; Kerp, H.; Hass, H. Microanatomy of Early Devonian Book Lungs. Biol. Lett. 2008, 4, 212–215. [Google Scholar] [CrossRef][Green Version]
- Dunlop, J.A.; Garwood, R.J. Terrestrial Invertebrates in the Rhynie Chert Ecosystem. Phil. Trans. R. Soc. B 2018, 373, 20160493. [Google Scholar] [CrossRef]
- Zuckerkandl, E.; Pauling, L. Evolutionary Divergence and Convergence in Proteins. In Evolving Genes and Proteins; Bryson, V., Vogel, H.J., Eds.; Academic Press: Cambridge, MA, USA, 1965; pp. 97–166. ISBN 978-1-4832-2734-4. [Google Scholar]
- Parham, J.F.; Donoghue, P.C.; Bell, C.J.; Calway, T.D.; Head, J.J.; Holroyd, P.A.; Inoue, J.G.; Irmis, R.B.; Joyce, W.G.; Ksepka, D.T. Best Practices for Justifying Fossil Calibrations. Syst. Biol. 2011, 61, 346–359. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Rannala, B. Bayesian Estimation of Species Divergence Times under a Molecular Clock Using Multiple Fossil Calibrations with Soft Bounds. Mol. Biol. Evol. 2006, 23, 212–226. [Google Scholar] [CrossRef] [PubMed]
- dos Reis, M.; Thawornwattana, Y.; Angelis, K.; Telford, M.J.; Donoghue, P.C.J.; Yang, Z. Uncertainty in the Timing of Origin of Animals and the Limits of Precision in Molecular Timescales. Curr. Biol. 2015, 25, 2939–2950. [Google Scholar] [CrossRef] [PubMed]
- Mongiardino Koch, N.; Parry, L.A. Death Is on Our Side: Paleontological Data Drastically Modify Phylogenetic Hypotheses. Syst. Biol. 2020, 69, 1052–1067. [Google Scholar] [CrossRef] [PubMed]
- Mongiardino Koch, N.; Garwood, R.J.; Parry, L.A. Inaccurate Fossil Placement Does Not Compromise Tip-Dated Divergence Times. bioRxiv 2022. preprint. [Google Scholar] [CrossRef]
- Wright, A.M.; Bapst, D.W.; Barido-Sottani, J.; Warnock, R.C.M. Integrating Fossil Observations into Phylogenetics Using the Fossilized Birth–Death Model. Ann. Rev. Earth Planet. Sci. 2022, 53, 12. [Google Scholar] [CrossRef]
- Klopfstein, S. The Age of Insects and the Revival of the Minimum Age Tree. Austral. Entomol. 2021, 60, 138–146. [Google Scholar] [CrossRef]
- Rota-Stabelli, O.; Lartillot, N.; Philippe, H.; Pisani, D. Serine Codon-Usage Bias in Deep Phylogenomics: Pancrustacean Relationships as a Case Study. Syst. Biol. 2013, 62, 121–133. [Google Scholar] [CrossRef]
- Rehm, P.; Meusemann, K.; Borner, J.; Misof, B.; Burmester, T. Phylogenetic Position of Myriapoda Revealed by 454 Transcriptome Sequencing. Mol. Phylogenet. Evol. 2014, 77, 25–33. [Google Scholar] [CrossRef]
- Miyazawa, H.; Ueda, C.; Yahata, K.; Su, Z.-H.; Ueda, C.; Yahata, K.; Su, Z.-H. Molecular Phylogeny of Myriapoda Provides Insights into Evolutionary Patterns of the Mode in Post-Embryonic Development. Sci. Rep. 2014, 4, 4127. [Google Scholar] [CrossRef]
- Ronquist, F.; Klopfstein, S.; Vilhelmsen, L.; Schulmeister, S.; Murray, D.L.; Rasnitsyn, A.P. A Total-Evidence Approach to Dating with Fossils, Applied to the Early Radiation of the Hymenoptera. Syst. Biol. 2012, 61, 973–999. [Google Scholar] [CrossRef]
- Howard, R.J.; Edgecombe, G.D.; Legg, D.A.; Pisani, D.; Lozano-Fernandez, J. Exploring the Evolution and Terrestrialization of Scorpions (Arachnida: Scorpiones) with Rocks and Clocks. Org. Divers. Evol. 2019, 19, 71–86. [Google Scholar] [CrossRef]
- Su, D.; Yang, L.; Shi, X.; Ma, X.; Zhou, X.; Hedges, S.B.; Zhong, B. Large-Scale Phylogenomic Analyses Reveal the Monophyly of Bryophytes and Neoproterozoic Origin of Land Plants. Mol. Biol. Evol. 2021, 38, 3332–3344. [Google Scholar] [CrossRef] [PubMed]
- Parry, L.A.; Smithwick, F.; Nordén, K.K.; Saitta, E.T.; Lozano-Fernandez, J.; Tanner, A.R.; Caron, J.-B.; Edgecombe, G.D.; Briggs, D.E.G.; Vinther, J. Soft-Bodied Fossils Are Not Simply Rotten Carcasses—Toward a Holistic Understanding of Exceptional Fossil Preservation. BioEssays 2018, 40, 1700167. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tihelka, E.; Howard, R.J.; Cai, C.; Lozano-Fernandez, J. Was There a Cambrian Explosion on Land? The Case of Arthropod Terrestrialization. Biology 2022, 11, 1516. https://doi.org/10.3390/biology11101516
Tihelka E, Howard RJ, Cai C, Lozano-Fernandez J. Was There a Cambrian Explosion on Land? The Case of Arthropod Terrestrialization. Biology. 2022; 11(10):1516. https://doi.org/10.3390/biology11101516
Chicago/Turabian StyleTihelka, Erik, Richard J. Howard, Chenyang Cai, and Jesus Lozano-Fernandez. 2022. "Was There a Cambrian Explosion on Land? The Case of Arthropod Terrestrialization" Biology 11, no. 10: 1516. https://doi.org/10.3390/biology11101516
APA StyleTihelka, E., Howard, R. J., Cai, C., & Lozano-Fernandez, J. (2022). Was There a Cambrian Explosion on Land? The Case of Arthropod Terrestrialization. Biology, 11(10), 1516. https://doi.org/10.3390/biology11101516







