HDAC1 in the Ovarian Granulosa Cells of Tan Sheep Improves Cumulus Cell Expansion and Oocyte Maturation Independently of the EGF-like Growth Factors
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Methods
2.2.1. Immunohistochemistry
2.2.2. Immunofluorescence
2.2.3. Follicle Dissection and Follicular Fluid Collection
2.2.4. Extraction and Culture of Primary Granulosa Cells
2.2.5. Oocyte Collection and Culture
2.2.6. Western Blotting
2.2.7. The Criteria for Expansion of Cumulus Cells
2.2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.2.9. Cellular Immunofluorescence
2.2.10. Statistical Analysis
3. Results
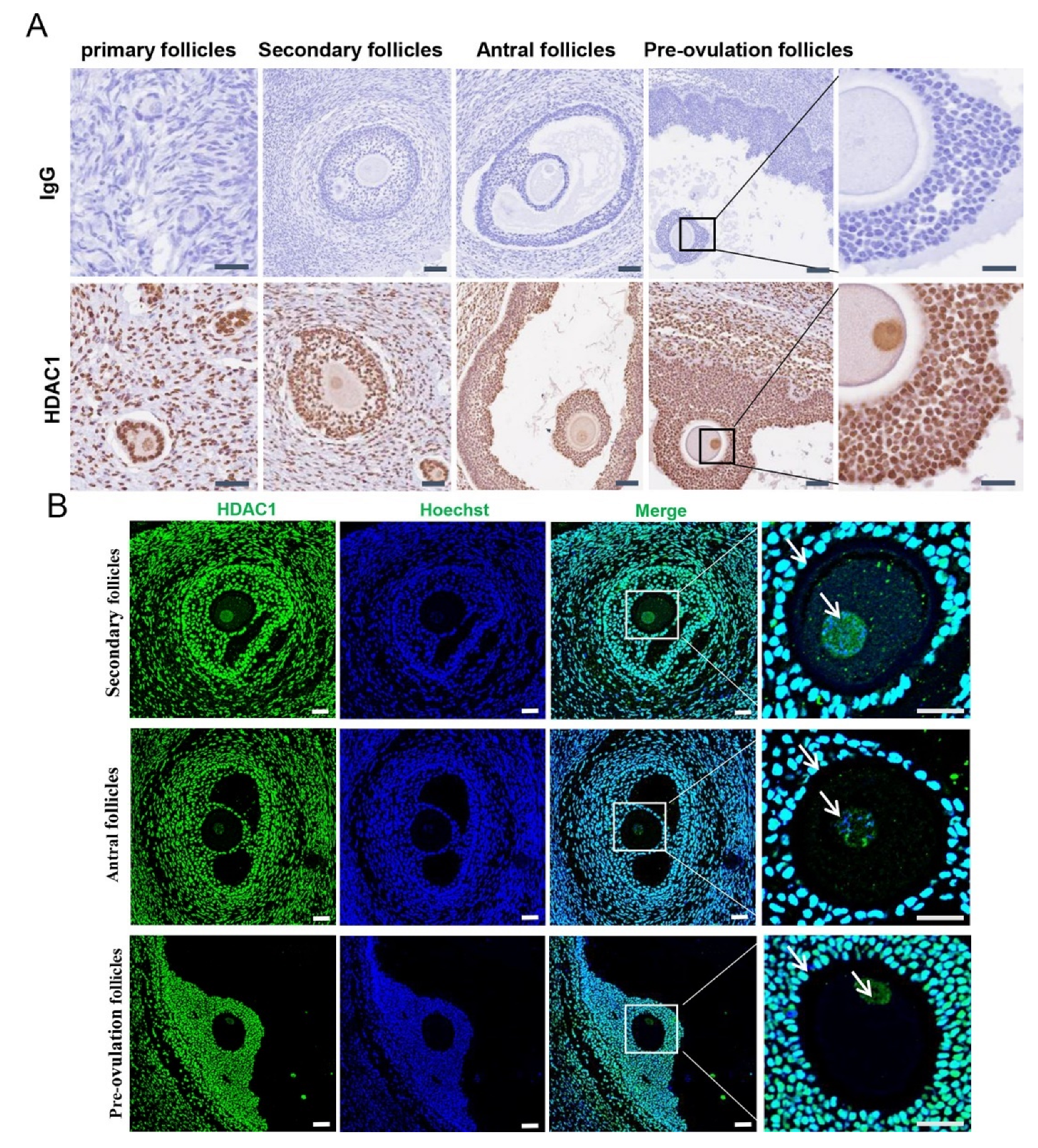
3.1. HDAC1 Expression in the Growing Follicles Resided in the Nuclei of Both Germ Cells and Ovarian Somatic Cells in the Tan Sheep
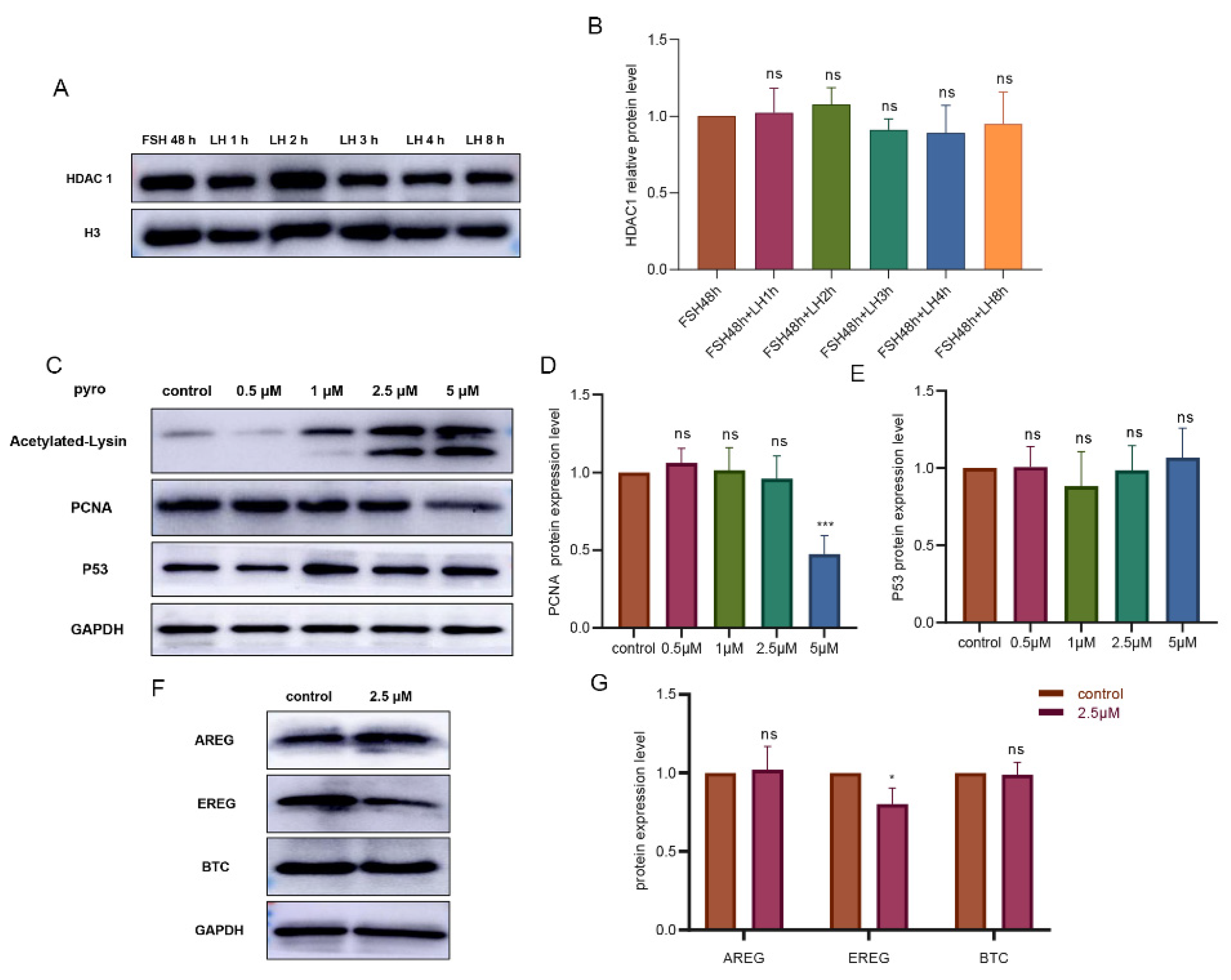
3.2. The HDAC1 Expression Was Dependent on Follicular Growth in Freshly Collected Follicles of the Tan Sheep
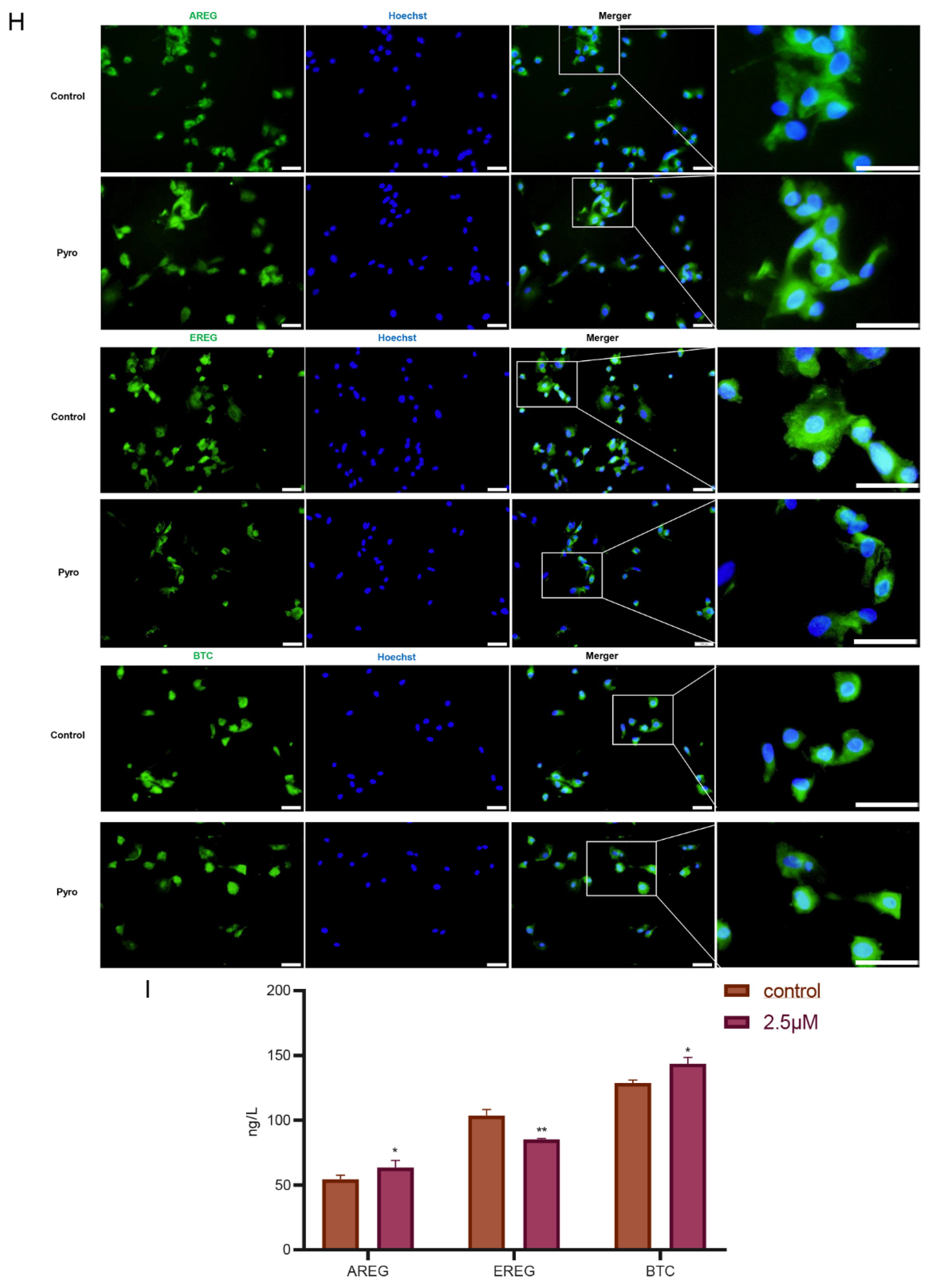
3.3. Inhibition of HDAC1 in Granulosa Cells Had Mild Influence on Production of EGF-like Growth Factors
3.4. HDAC1 Was Indispensable for Cumulus Cell Expansion and Oocyte Maturation for the Tan Sheep In Vitro
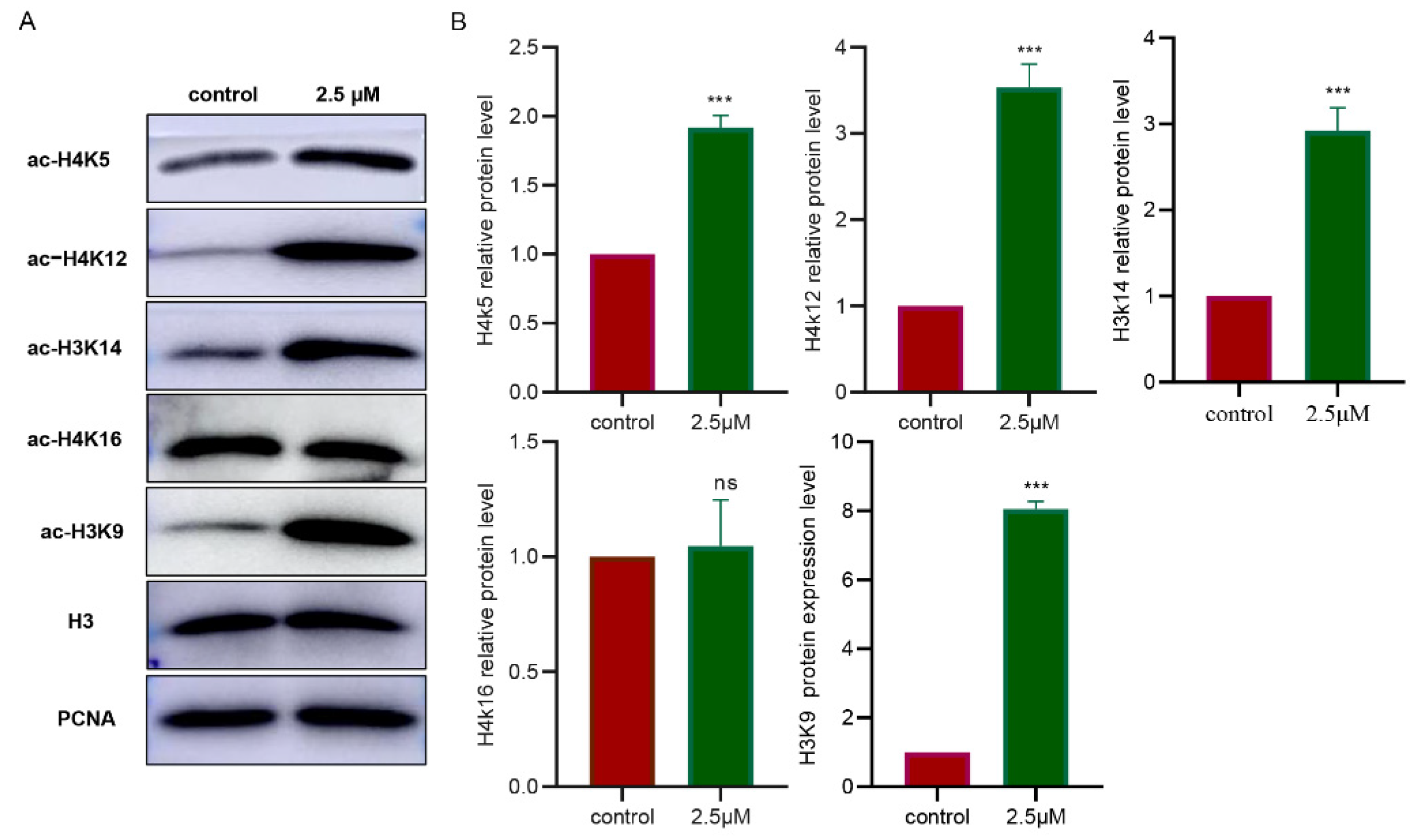
3.5. Inhibition of HDAC1 Affects Multiple Acetylation Sites in the Granulosa Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| English Abbreviation | Full English Name |
| HDACs | Histone deacetylases |
| HDAC1 | Histone deacetylase 1 |
| HAT | Histone acetyltransferase |
| EGF | Epidermal growth factor |
| AREG | Amphiregulin |
| EREG | Epithelial regulatory protein |
| BTC | Betacellulin |
| FSH | Follicle-stimulating hormone |
| LH | Luteinizing hormone |
| COCs | Cumulus oocyte complexes |
| PBS | Phosphate-buffered saline |
| FBS | Fetal bovine serum |
| PB1 | First polar body |
| ELISA | Enzyme-linked immunosorbent assay |
| PCNA | Proliferating cell nuclear antigen |
| P53 | Tumor protein p53 |
| PVDF | Polyvinylidene fluoride |
| GV | Germinal vesicle |
| AI | Anaphase I |
| MI | Metaphase I |
| MII | Metaphase II |
| IVM | In vitro maturation |
References
- McGee, E.A.; Hsueh, A.J. Initial and cyclic recruitment of ovarian follicles. Endocr. Rev. 2000, 21, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Brower, P.T.; Schultz, R.M. Intercellular communication between granulosa cells and mouse oocytes: Existence and possible nutritional role during oocyte growth. Dev. Biol. 1982, 90, 144–153. [Google Scholar] [CrossRef]
- Jaffe, L.A.; Egbert, J.R. Regulation of Mammalian Oocyte Meiosis by Intercellular Communication Within the Ovarian Follicle. Annu. Rev. Physiol. 2017, 79, 237–260. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.X.; Zhang, Y.; Li, Y.Y.; Liu, X.M.; Wang, X.X.; Zhang, C.L.; Hao, C.F.; Deng, S.L. Regulation of follicular development and differentiation by intra-ovarian factors and endocrine hormones. Front. Biosci. 2019, 24, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, A.; Kim, B.; Yeh, J. Luteinizing Hormone Action in Human Oocyte Maturation and Quality: Signaling Pathways, Regulation, and Clinical Impact. Reprod. Sci. 2020, 27, 1223–1252. [Google Scholar] [CrossRef]
- Schneider, M.R.; Antsiferova, M.; Feldmeyer, L.; Dahlhoff, M.; Bugnon, P.; Hasse, S.; Paus, R.; Wolf, E.; Werner, S. Betacellulin regulates hair follicle development and hair cycle induction and enhances angiogenesis in wounded skin. J. Investig. Dermatol. 2008, 128, 1256–1265. [Google Scholar] [CrossRef]
- Tsafriri, A.; Cao, X.; Ashkenazi, H.; Motola, S.; Popliker, M.; Pomerantz, S.H. Resumption of oocyte meiosis in mammals: On models, meiosis activating sterols, steroids and EGF-like factors. Mol. Cell Endocrinol. 2005, 234, 37–45. [Google Scholar] [CrossRef]
- Wang, H.; Cai, H.; Wang, X.; Zhang, M.; Liu, B.; Chen, Z.; Yang, T.; Fang, J.; Zhang, Y.; Liu, W.; et al. HDAC3 maintains oocyte meiosis arrest by repressing amphiregulin expression before the LH surge. Nat. Commun. 2019, 10, 5719. [Google Scholar] [CrossRef]
- Gallinari, P.; Di Marco, S.; Jones, P.; Pallaoro, M.; Steinkühler, C. HDACs, histone deacetylation and gene transcription: From molecular biology to cancer therapeutics. Cell Res. 2007, 17, 195–211. [Google Scholar] [CrossRef]
- Bolden, J.E.; Peart, M.J.; Johnstone, R.W. Anticancer activities of histone deacetylase inhibitors. Nat. Rev. Drug Discov. 2006, 5, 769–784. [Google Scholar] [CrossRef]
- de Ruijter, A.J.; van Gennip, A.H.; Caron, H.N.; Kemp, S.; van Kuilenburg, A.B. Histone deacetylases (HDACs): Characterization of the classical HDAC family. Biochem. J. 2003, 370, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Ropero, S.; Esteller, M. The role of histone deacetylases (HDACs) in human cancer. Mol. Oncol. 2007, 1, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, N.; Seto, E. Regulation of histone deacetylase activities. J. Cell Biochem. 2004, 93, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Cueto, M.A.; Asselbergs, F.; Atadja, P. Cloning and functional characterization of HDAC11, a novel member of the human histone deacetylase family. J. Biol. Chem. 2002, 277, 25748–25755. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Liu, Y.; XU, Y.; Yang, Y.; Liu, X.; Wang, C. Expression characteristics and correlation analysis of Hdac6 and AREG of different developmental stages follicles inTan sheep ovarian (Ovis aries). J. Agric. Biotechnol. 2022, 30, 1524–1533. [Google Scholar]
- Ma, P.; Pan, H.; Montgomery, R.L.; Olson, E.N.; Schultz, R.M. Compensatory functions of histone deacetylase 1 (HDAC1) and HDAC2 regulate transcription and apoptosis during mouse oocyte development. Proc. Natl. Acad. Sci. USA 2012, 109, E481–E489. [Google Scholar] [CrossRef]
- Ma, P.; Schultz, R.M. HDAC1 and HDAC2 in mouse oocytes and preimplantation embryos: Specificity versus compensation. Cell Death Differ. 2016, 23, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, R.L.; Davis, C.A.; Potthoff, M.J.; Haberland, M.; Fielitz, J.; Qi, X.; Hill, J.A.; Richardson, J.A.; Olson, E.N. Histone deacetylases 1 and 2 redundantly regulate cardiac morphogenesis, growth, and contractility. Genes Dev. 2007, 21, 1790–1802. [Google Scholar] [CrossRef] [PubMed]
- LeBoeuf, M.; Terrell, A.; Trivedi, S.; Sinha, S.; Epstein, J.A.; Olson, E.N.; Morrisey, E.E.; Millar, S.E. Hdac1 and Hdac2 act redundantly to control p63 and p53 functions in epidermal progenitor cells. Dev Cell. 2010, 19, 807–818. [Google Scholar] [CrossRef]
- Xu, H.; Khan, A.; Zhao, S.; Wang, H.; Zou, H.; Pang, Y.; Zhu, H. Effects of Inhibin A on Apoptosis and Proliferation of Bovine Granulosa Cells. Animals 2020, 10, 367. [Google Scholar] [CrossRef]
- Guo, X.; Jia, K.; Han, Q.; Ma, X.; Dang, W.; Wang, K.; Li, Y.; Li, P.; lu, L. Study on the expression pattern of Smad9 in bovine ovarian follicles. Chin. J. Anim. Sci. 2021, 57, 145–148+153. [Google Scholar]
- Vanderhyden, B.C. Species differences in the regulation of cumulus expansion by an oocyte-secreted factor(s). J. Reprod. Fertil. 1993, 98, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Virant-Klun, I.; Bauer, C.; Ståhlberg, A.; Kubista, M.; Skutella, T. Human oocyte maturation in vitro is improved by co-culture with cumulus cells from mature oocytes. Reprod. Biomed. Online 2018, 36, 508–523. [Google Scholar] [CrossRef] [PubMed]
- Wright, L.H.; Menick, D.R. A class of their own: Exploring the nondeacetylase roles of class IIa HDACs in cardiovascular disease. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H199–H206. [Google Scholar] [CrossRef]
- Seto, E.; Yoshida, M. Erasers of histone acetylation: The histone deacetylase enzymes. Cold Spring Harb Perspect. Biol. 2014, 6, a018713. [Google Scholar] [CrossRef]
- Benedetti, R.; Conte, M.; Altucci, L. Targeting Histone Deacetylases in Diseases: Where Are We? Antioxid. Redox. Signal. 2015, 23, 99–126. [Google Scholar] [CrossRef]
- Li, Y.; Seto, E. HDACs and HDAC Inhibitors in Cancer Development and Therapy. Cold Spring Harb. Perspect. Med. 2016, 6, a026831. [Google Scholar] [CrossRef]
- Lakshmaiah, K.C.; Jacob, L.A.; Aparna, S.; Lokanatha, D.; Saldanha, S.C. Epigenetic therapy of cancer with histone deacetylase inhibitors. J. Cancer Res. Ther. 2014, 10, 469–478. [Google Scholar] [CrossRef]
- Pontelo, T.P.; Rodrigues, S.A.D.; Kawamoto, T.S.; Leme, L.O.; Gomes, A.; Zangeronimo, M.G.; Franco, M.M.; Dode, M.A.N. Histone acetylation during the in vitro maturation of bovine oocytes with different levels of competence. Reprod Fertil Dev. 2020, 32, 690–696. [Google Scholar] [CrossRef]
- Wang, H.; Cui, W.; Meng, C.; Zhang, J.; Li, Y.; Qian, Y.; Xing, G.; Zhao, D.; Cao, S. MC1568 Enhances Histone Acetylation During Oocyte Meiosis and Improves Development of Somatic Cell Nuclear Transfer Embryos in Pig. Cell Reprogram. 2018, 20, 55–65. [Google Scholar] [CrossRef]
- Iwashita, J.; Kodama, A.; Konno, Y.; Abe, T.; Murata, J. Histone deacetylase induces accelerated maturation in Xenopus laevis oocytes. Dev. Growth Differ. 2013, 55, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Pei, Y.; Zhou, G.B.; Suo, L.; Wang, Y.P.; Wu, G.Q.; Fu, X.W.; Hou, Y.P.; Zhu, S.E. Histone deacetyltransferase1 expression in mouse oocyte and their in vitro-fertilized embryo: Effect of oocyte vitrification. Cryo. Letters 2011, 32, 13–20. [Google Scholar] [PubMed]
- Lorenzo, P.L.; Liu, I.K.; Carneiro, G.F.; Conley, A.J.; Enders, A.C. Equine oocyte maturation with epidermal growth factor. Equine Vet. J. 2002, 34, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J.; Ying Wang, C.; Yan Kwok, A.H.; Leung, F.C. Epidermal growth factor (EGF) receptor ligands in the chicken ovary: I. Evidence for heparin-binding EGF-like growth factor (HB-EGF) as a potential oocyte-derived signal to control granulosa cell proliferation and HB-EGF and kit ligand expression. Endocrinology 2007, 148, 3426–3440. [Google Scholar] [CrossRef] [PubMed]
- Procházka, R.; Srsen, V.; Nagyová, E.; Miyano, T.; Flechon, J.E. Developmental regulation of effect of epidermal growth factor on porcine oocyte-cumulus cell complexes: Nuclear maturation, expansion, and F-actin remodeling. Mol. Reprod. Dev. 2000, 56, 63–73. [Google Scholar] [CrossRef]
- Zamah, A.M.; Hsieh, M.; Chen, J.; Vigne, J.L.; Rosen, M.P.; Cedars, M.I.; Conti, M. Human oocyte maturation is dependent on LH-stimulated accumulation of the epidermal growth factor-like growth factor, amphiregulin. Hum. Reprod. 2010, 25, 2569–2578. [Google Scholar] [CrossRef]
- Gu, L.; Wang, Q.; Sun, Q.Y. Histone modifications during mammalian oocyte maturation: Dynamics, regulation and functions. Cell Cycle 2010, 9, 1942–1950. [Google Scholar] [CrossRef]
- Tang, L.S.; Wang, Q.; Xiong, B.; Hou, Y.; Zhang, Y.Z.; Sun, Q.Y.; Wang, S.Y. Dynamic changes in histone acetylation during sheep oocyte maturation. J. Reprod. Dev. 2007, 53, 555–561. [Google Scholar] [CrossRef]
- Ma, P.; Schultz, R.M. Histone deacetylase 1 (HDAC1) regulates histone acetylation, development, and gene expression in preimplantation mouse embryos. Dev. Biol. 2008, 319, 110–120. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, T.; Zhang, P.; Zhang, S.; Guan, J.; Ma, X.; Yin, Y.; Zhang, J.; Tang, B.; Li, Z. Histone deacetylase 1 down-regulation on developmental capability and histone acetylation in bovine oocytes and parthenogenetic embryos. Reprod. Domest Anim. 2011, 46, 1022–1028. [Google Scholar] [CrossRef]


| Group | FSH | FSH + LH | FSH + Pyroxamide | FSH + LH + Pyroxamide |
|---|---|---|---|---|
| 0–2 level (%) | 5.93±2.53 | 5.24±2.01 ns | 92.36±2.23 *** | 93.75±2.41 *** |
| 3–4 level (%) | 94.07±2.6 | 94.43±2.37 ns | 7.636±2.24 *** | 6.25±2.41 *** |
| Group | Number of First Polar Body Emission (Pieces) | Total Number of Oocytes (Pieces) | First Polar Body Discharge Rate (%) |
|---|---|---|---|
| FSH | 43 | 143 | 29.4 ± 6.3 |
| FSH + LH | 84 | 148 | 56.1 ± 4.8 *** |
| FSH + pyroxamide | 38 | 129 | 29.25 ± 6.65 ns |
| FSH + LH + pyroxamide | 41 | 130 | 31.6 ± 5 ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Fan, S.; Liu, Y.; Shi, J.; Xie, X.; Wang, X.; Wang, C.; Liu, X.; Xia, G. HDAC1 in the Ovarian Granulosa Cells of Tan Sheep Improves Cumulus Cell Expansion and Oocyte Maturation Independently of the EGF-like Growth Factors. Biology 2022, 11, 1464. https://doi.org/10.3390/biology11101464
Xu Y, Fan S, Liu Y, Shi J, Xie X, Wang X, Wang C, Liu X, Xia G. HDAC1 in the Ovarian Granulosa Cells of Tan Sheep Improves Cumulus Cell Expansion and Oocyte Maturation Independently of the EGF-like Growth Factors. Biology. 2022; 11(10):1464. https://doi.org/10.3390/biology11101464
Chicago/Turabian StyleXu, Yaxiu, Shanshan Fan, Yujun Liu, Jiaqi Shi, Xianguo Xie, Xiangyan Wang, Chao Wang, Xinfeng Liu, and Guoliang Xia. 2022. "HDAC1 in the Ovarian Granulosa Cells of Tan Sheep Improves Cumulus Cell Expansion and Oocyte Maturation Independently of the EGF-like Growth Factors" Biology 11, no. 10: 1464. https://doi.org/10.3390/biology11101464
APA StyleXu, Y., Fan, S., Liu, Y., Shi, J., Xie, X., Wang, X., Wang, C., Liu, X., & Xia, G. (2022). HDAC1 in the Ovarian Granulosa Cells of Tan Sheep Improves Cumulus Cell Expansion and Oocyte Maturation Independently of the EGF-like Growth Factors. Biology, 11(10), 1464. https://doi.org/10.3390/biology11101464





